Abstract
Thiol-yne-methacrylate and thiol-yne-acrylate ternary systems were investigated for polymerization kinetics and material properties and compared to the analogous pure thiol-yne and (meth)acrylate systems. Both thiol-yne-methacrylate and thiol-yne-acrylate systems were demonstrated to reduce polymerization induced shrinkage stress while simultaneously achieving high glass transition temperatures (Tg) and modulius. Formulations with 70 wt% methacrylate increased the Tg from 51 ± 2 to 75 ± 1 °C and the modulus from 1800 ± 100 to 3200 ± 400 MPa (44% increase) over the pure thiol-yne system. Additionally, the shrinkage stress was 1.2 ± 0.2 MPa, which is lower than that of the pure methacrylate, binary thiol-yne and thiol-ene-methacrylate control systems which are all > 2 MPa. Interestingly, with increasing methacrylate or acrylate concentration, a decrease and subsequent increase in the shrinkage stress values were observed. A minimum shrinkage stress value (1.0 ± 0.2 MPa) was observed in the 50 wt% methacrylate and 70 wt% acrylate systems. This tunable behavior results from the competitive reaction kinetics of the methacrylate or acrylate homopolymerization versus chain transfer to thiol and the accompanying thiol-yne step-growth polymerization. The crosslinking density of the networks and the amount of volumetric shrinkage that occurs prior to gelation relative to the total volumetric shrinkage were determined as two key factors that control the final shrinkage stress of the ternary systems.
Introduction
Methacrylate and acrylate resins make up the majority of photocuring systems and exhibit numerous desirable properties that include ambient cure, cure on demand, high energy efficiency and require minimal or no solvents. These systems also have limitations such as oxygen inhibition and high shrinkage stress.1 The polymerization induced shrinkage and shrinkage stress are caused by the reduction of free volume during the formation of covalent bonds in the crosslinking networks. Shrinkage and shrinkage stress result in a variety of limitations for these systems that often result in the formation of cracks and/or delamination at the interface between the polymer and substrate.2 Various methods have been used to reduce the shrinkage stress of these resin systems. Cationic ring opening polymerizations, such as oxiranes3, siloranes4 or epoxides5, are alternative systems that reduce shrinkage stress due to their relatively low shrinkage per mole of functional groups that react. Low shrinkage additives (pre-polymers)6; reactive nanogels7, phase separating systems8, hyper-branched polymers9 and dendrimers10 have all been added to resins to reduce both the shrinkage and shrinkage stress without compromising mechanical properties. Stress reductions have also been achieved by photo-induced addition fragmentation reactions that conserve the same network connectivity and properties.11
Thiol-ene polymerization reactions, which proceed via a radical mediated step-growth polymerization mechanism, have also been demonstrated to maintain lower shrinkage stress due to the chain transfer step delaying gelation during the polymerization.12 The step-growth polymerization mechanism proceeds by thiyl radical addition across a carbon-carbon double bond to form a carbon-centered radical. The carbon-centered radical subsequently abstracts a hydrogen from another thiol functional group, regenerating the thiyl radical.13 These alternating addition and chain transfer reactions form the basis for the step growth polymerization which results in slow and uniform network formation that leads to delayed gelation and reduced shrinkage stress as compared to chain growth methacrylate or acrylate polymerizations. Thiol-ene reactions, which are one type of click reaction, have the advantages of high selectivity and high yield with minimal side reactions while also exhibiting little sensitivity to oxygen or moisture.14 Recently, thiol-yne reactions have also been demonstrated to follow a radical step growth polymerization mechanism. The alkyne group first reacts with a thiyl radical to generate a carbon-centered radical, which abstracts a hydrogen from a thiol monomer to generate a vinyl sulfide moiety. The double bond on the vinyl sulfide moiety reacts with another thiyl radical to generate another carbon-centered radical followed again by chain transfer to thiol. In this reaction, each alkyne group reacts twice with thiyl radicals thereby achieving a higher crosslink density and increased mechanical properties relative to other comparable thiol-ene systems.15,16,17 Moreover, compared with chain growth methacrylate or acrylate systems, both thiol-ene and thiol-yne systems follow a step growth mechanism which leads to a more homogenous network with a narrower glass transition regime; as a result, a different conversion-dependent profile of modulus and shrinkage stress build up with conversion after vitrification.14,16,17
Ternary polymerization systems enable a way to directly tailor the network material properties because of the unique competitive reaction kinetics and network formation process. A range of previous studies has evaluated the polymerization mechanism, kinetics, and ensuing material properties of ternary (meth)acrylate-thiol-ene systems. When a methacrylate or acrylate is added to thiol-ene systems, the methacrylate or acrylate homopolymerization is accompanied with chain transfer to thiol and thiol-ene step-growth polymerization.18 A model for the reaction kinetics of thiol-ene-methacrylate ternary systems was built and evaluated the ratio of homopolymerization and chain transfer kinetic parameters.19 Due to the high amount of methacrylate homopolymerization, a pseudo two-stage hybrid polymerization was reported in thiol-ene-methacrylate systems that also resulted in the reduction of shrinkage stress.2 Moreover, it has also been reported that by adding methacrylates with a bisphenol-A structure and varying the stoichiometric ratio of thiol to ene, the shrinkage stress was further reduced while maintaining modulus and strength at values close to the bulk methacrylate systems.20,21 The glass transition temperature (Tg) and the heterogeneity of thiol-ene-acrylate networks were able to be controlled by varying the amount and chemical structure of the acrylate monomers.22 The use of addition-fragmentation reactions has also been combined with thiol-ene-methacrylate ternary systems to achieve reduced shrinkage stress.23
Due to the higher modulus and Tg of thiol-yne systems as compared with thiol-enes and the tunable ternary system properties, thiol-yne-methacrylate systems have significant potential to reduce shrinkage stress while maintaining or improving further the mechanical properties. Moreover, it is interesting to investigate the mechanism of the reduction of shrinkage stress in this complex system. This work evaluated a thiol-yne model system which also incorporared methacrylate or acrylate monomers with rigid bis-phenol A core structures. The pure methacrylate, binary thiol-yne and thiol-ene-methacrylate systems were also studied for comparison. The reaction kinetics, gel point, and mechanical properties (crosslinking density, Tg, modulus) along with shrinkage and shrinkage stress were studied, and the relationship between the mechanism of shrinkage stress reduction and unique network formation was evaluated.
Experimental
Materials
1-hydroxy-cyclohexyl-phenyl-ketone (Irgacure 184), pentaerythritol tetra(3-mercaptopropionate) (PETMP), and ethoxylated bisphenol A dimethacrylate (EO/phenol 1.5, which implies there are 3 ethoxylate groups per monomer) (EBPADMA) were donated by BASF Corp., Evans Chemetics and Esstech Inc., respectively. 1,6-heptadiyne (HDY), 1,6-heptadiene (HDE) and ethoxylated bisphenol A diacrylate (EO/phenol 1.5, which means there are 3 ethoxylate groups per monomer) (EBPADA) were purchased from Aldrich.
FTIR
Fourier transform infrared spectroscopy (FTIR) (Magna 750, series II, Nicolet Instrument Corp., Madison, WI) combined with a vertical UV-light source (Acticure, EFOS, Mississaugua, Ontario, Canada) was utilized to measure the real time conversion during curing.24 The samples were cured with 365 nm light at 10 mW/cm2 in the FTIR chamber. Mid-IR was employed to study the reaction kinetics with ~10 μm thick samples between NaCl plates. The conversion of alkyne functional groups was determined by the C-H stretch at 3288 cm−1, conversion of thiol functional groups was determined by the S-H stretching at 2570 cm−1, and (meth)acrylate functional group conversion was determined by the C=C vibration at 1637 cm−1. To couple with various mechanical property measurements, near-IR was utilized to evaluate functional group conversions in polymerizations of 1 mm thick samples polymerized between glass slides. The alkyne and (meth)acrylate conversions were monitored by the C-H vibration peak at 6505 cm−1 and the C=C vibration peak at 6163 cm−1, respectively.
Mechanical Measurements
A dynamic mechanical analyzer DMA Q800 (TA Instruments) was utilized to measure the glass transition temperatures and moduli of samples with 1 × 5 × 10 mm rectangular dimensions. Multi-frequency strain mode was utilized by applying a sinusoidal stress of 1 Hz frequency with the temperature ramping from −40 to 160 °C at 3 °C/minute. The Tg was determined as the maximum of the tan delta curve. The modulus values at ambient temperature and well into the rubbery state were measured at 25 °C and at Tg + 50 °C, respectively. For the pure methacrylate or acrylate systems, the modulus values for the rubbery state were read at Tg + 100 °C due to the breadth of the tan delta peak. Tg1/2width was taken as the half width of tan delta peak at half maximum value.
Shrinkage Stress Measurement
A cantilever mode tensometer (American Dental Association Health Foundation) combined with a vertical UV-light source (Acticure, EFOS, Mississaugua, Ontario, Canada) and the horizontal remote FTIR optical fibers (Magna 750, series II, Nicolet Instrument Corp., Madison, WI) was utilized to simultaneously measure the shrinkage stress and conversion during polymerization.25 The samples were disk shapes with 6 mm diameter and 1 mm thickness. An aluminum beam with 4.76 μm/N compliance was chosen to enable both the sensitive measurement of the low shrinkage stress values achieved in many of the ternary systems while simultaneously enabling accurate evaluation of stress values up to 2 MPa. Under these conditions, stress values greater than 2.0 MPa cannot be accurately measured and are indicated throughout the text as simply being > 2 MPa.
Gelation Measurement
A rheometer (ARES 4400, TA Instruments) combined with a vertical UV-light source (Acticure, EFOS, Mississaugua, Ontario, Canada) and FTIR measurement achieved via optical fibers (Magna 750, series II, Nicolet Instrument Corp., Madison, WI) was utilized to measure the conversion at gelation during the polymerization.26 Parallel plate geometry with 22 mm diameter plates and 0.3 mm thickness and dynamic stress with 20% strain was utilized. The gel point is the point at which tan δ becomes frequency independent. In this work, we have utilized the common technique of taking the G′/G″ crossover point as the approximate gel point.27,28
Results and discussion
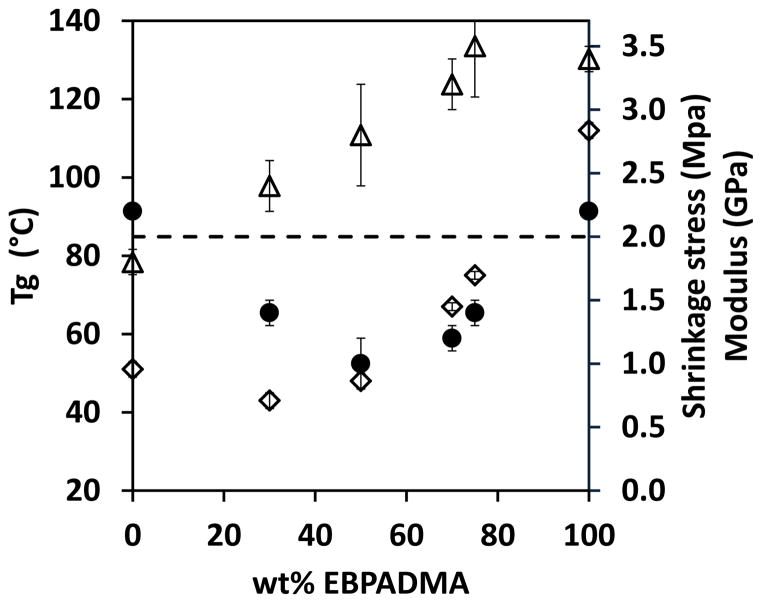
Figure 1 shows the Tg, modulus and shrinkage stress values of a polymerizing system with stoichiometric thiol-yne mixture (i.e., two thiol functional groups for each yne functional group) and varying amounts of methacrylate (i.e., the ratio of functional groups of thiol:yne:methacrylate is 2:1:x). These results demonstrate the feasibility of adding a methacrylate component to the thiol-yne system, and that this approach is able to increase the Tg and modulus while maintaining relatively low shrinkage stress. With increasing methacrylate ratio in this ternary system, the Tg and modulus are increased from 51 ± 2 to 75 ± 1 °C and from 1800 ± 100 to 3200 ± 400 MPa (44% increase), respectively. The increase in mechanical properties is resultant from the increased amount of methacrylate homopolymerization and incorporation of the rigid bis-phenol structure into the polymer network. Due to the chain transfer to thiol that results in the mixed mode step-chain growth polymerization mechanism, the Tg2/1width, which is related to the network heterogeneity, is reduced from 75 ± 2 °C for the pure methacrylate to 34 ± 1 °C for the ternary system that still contains 70% methacrylate. At the same time, the shrinkage stress is reduced from >2 MPa to 1.2 ± 0.2 MPa. If the chain transfer step were the primary factor that results in reduced shrinkage stress, the pure thiol-yne system, which has the greatest amount of chain transfer, would be expected to have the lowest shrinkage stress. However, the shrinkage stress of the ternary systems is much lower than either the pure methacrylate or binary thiol-yne systems. This result implies that additional factors, besides chain transfer, also contribute to the overall shrinkage stress in the thiol-yne-methacrylate ternary systems. These factors include the reaction mechanism, conversion, reaction rate, gel point, and network evolution. We hypothesize that a combination of the competitive reaction kinetics and the unique network formation results in the reduction of shrinkage stress in the thiol-yne-methacrylate ternary systems.
Figure 1.
Glass transition temperature (◇), modulus (△) and shrinkage stress (●) of PETMP-HDY-EBPADMA (2:1:x) initiated with 1 wt% Irgacure 184 and cured by 365 nm light at 10 mW/cm2. Note: 2 MPa is the maximum stress accurately measured by the tensometer under these conditions. Stress values greater than this threshold are indicative of greater than 2 MPa stress rather than exact values.
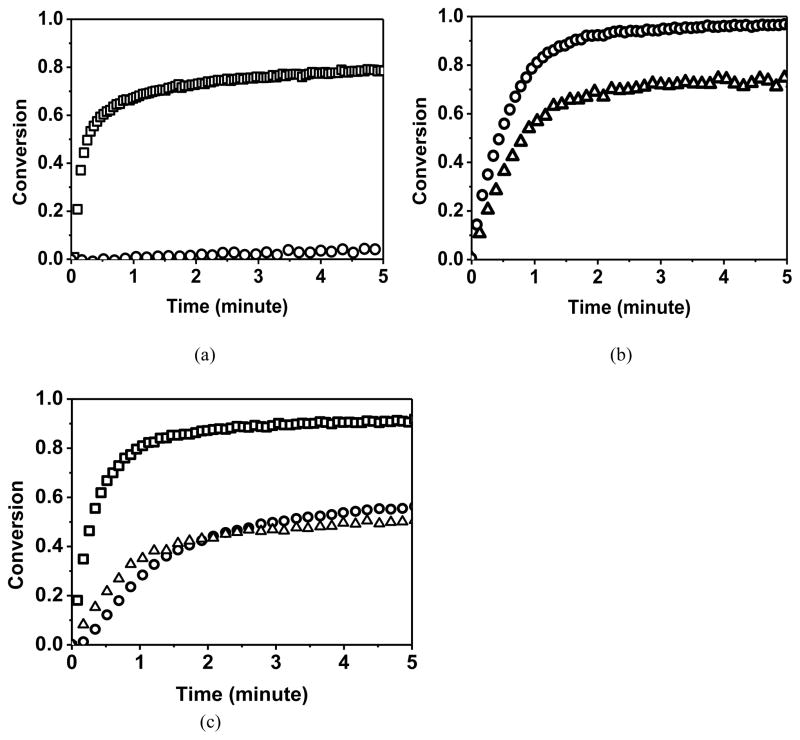
In the thiol-yne-methacrylate ternary systems, the vinyl sulfide peak cannot be separated from the methacrylate peak accurately. While for the heptadiyne monomer, the reactivity of the thiyl radicals towards vinylsufides is much higher than the reactivity towards alkynes, thus the concentration of vinylsulfide moieties remains low and the polymerizations are effectively monitored by following only the yne conversion.29 When added to a thiol-yne reaction, the methacrylate simultaneously participates in both homopolymerization of the double bond and chain transfer to thiol. Figure 2(a) indicates that in the HDY-EBPADMA control system in the absence of any thiol, the alkyne group is effectively unreactive, achieving only 4% conversion, while the methacrylate functional groups achieve 80% conversion due to the homopolymerization of EBPADMA. In the pure thiol-yne control system (PETMP-HDY, Figure 2b), the thiol conversion is slightly lower than the yne conversion during the reaction because not all of the transiently formed vinyl sulfide functional groups have reacted and these vinyl sulfide groups barely react with alkyne groups29. For the PETMP-HDY-EBPADMA ternary system (Figure 2c), there are multiple competitive reactions including the methacrylate homopolymerization, chain transfer to the thiol from the various radicals and propagation through the yne/vinyl sulfide by the thiyl radical. Initially, the thiol conversion exceeds that of the alkyne due to chain transfer from the methacrylic radical. At the latter stages of the polymerization, when the methacrylate has reached higher conversions, the thiol-yne reaction becomes the primary reaction and the yne conversion exceeds that of the thiol as in the pure thiol-yne system. The overall reaction rate of methacrylate is much faster than the thiol and yne, therefore the vitrification is controlled by methacrylate conversion and the system vitrified before complete thiol and yne conversions were achieved. The final conversions of both thiol and yne are reduced, relative to the binary system, due to the early vitrification that results from the additional crosslinking associated with the larger amounts of multimethacrylate present. Moreover, Figure 2(c) also indicates the total reaction rate of methacrylate including both homopolymerization and chain transfer is higher than the thiol-yne step-growth reaction rate. However, the difference in the reaction rates is not as significant as observed in thiol-ene-methacrylate systems2,21. Due to the reduced conversion of the yne functional groups in the stoichiometric thiol-yne ternary systems, off stoichiometric ratios of thiol-yne with fixed methacrylate amounts in the PETMP-YNE-EBPADMA resins were also studied (Table 1) in which the functional group ratio of thiol:yne:methacrylate was x:y:2.7. Increasing the thiol ratio while keeping the amount of methacrylate constant serves to increase the amount of methacrylate chain transfer to thiol and further reduces the shrinkage stress. The overall shrinkage stress was reduced from 1.2 ± 0.1 MPa for the 2:1 thiol:yne stoichiometric system to 1.0 ± 0.1 for the 2.5:0.5 thiol:yne off stoichiometric system with the same methacrylate content. Again, the reduction of the shrinkage stress is strongly affected by a combination of factors as explored in the remainder of this work.
Figure 2.
Functional group conversion (methacrylate (□), thiol (△) and yne (○)) as a function of time for (a) HDY-EBPADMA (1:2.7), (b) PETMP-HDY (2:1) and (c) PETMP-HDY-EBPADMA (2:1:2.7) resins initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2.
Table 1.
Shrinkage stress values for PETMP-HDY-EBPADMA cured resins with off stoichiometric thiol-yne ratios and fixing methacrylate amount (the functional group of thiol:yne:methacrylate = x:y:2.7). The systems were initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2
| Functional group ratio Thiol: Yne: Methacrylate | Shrinkage stress (MPa) |
|---|---|
| 1.5: 1: 2.7 | 1.4 ± 0.01 |
| 2: 1: 2.7 | 1.2 ± 0.01 |
| 2.5: 1: 2.7 | 1.1 ± 0.01 |
|
| |
| 1.5: 1.5: 2.7 | 1.3 ± 0.01 |
| 2: 1: 2.7 | 1.2 ± 0.01 |
| 2.5: 0.5: 2.7 | 1.0 ± 0.01 |
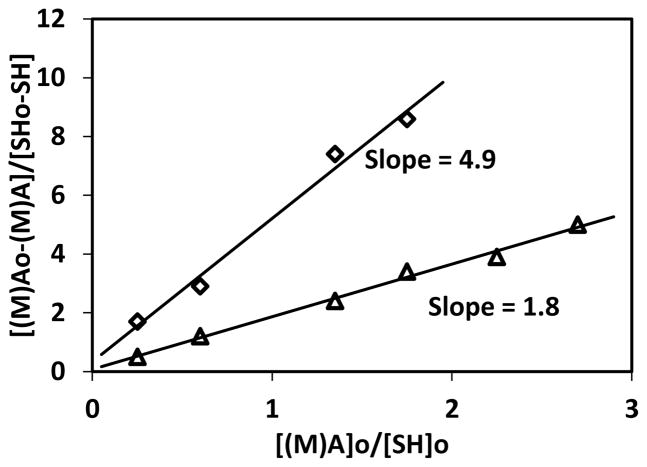
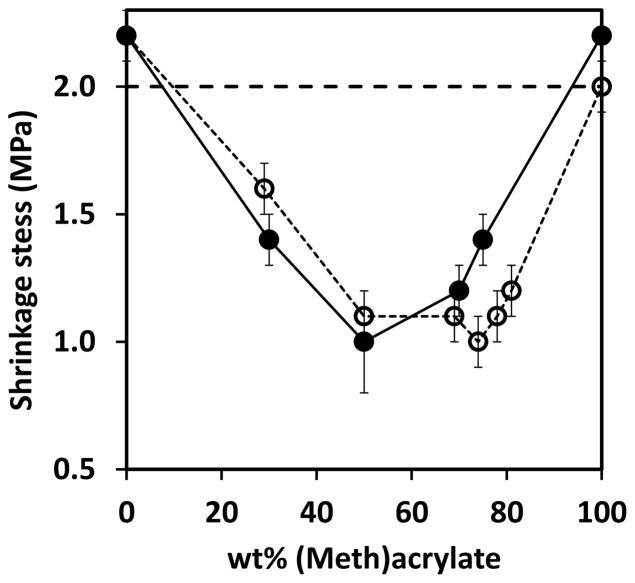
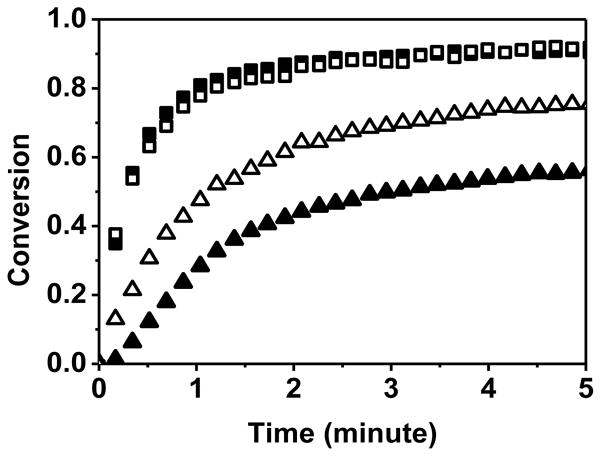
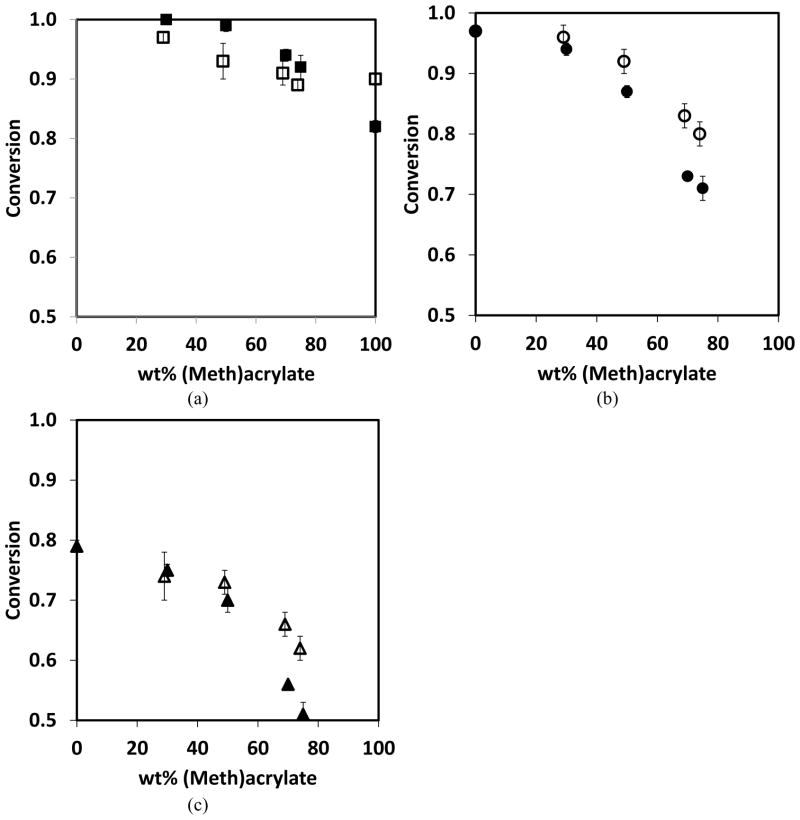
Thiol-yne-acrylate systems (PETMP-HDY-EBPADA) follow a similar reaction mechanism to the thiol-yne-methacrylate systems; however, the chain transfer reaction to the thiol is more competitive and of comparable rate to the acrylate homopolymerization, even at relatively low acrylate conversions. To compare the extent of chain transfer to thiol between the thiol-yne-methacrylate and the thiol-yne-acrylate systems, double bond consumption per thiol was calculated in the first ten seconds of the reaction. In this regime which represents relatively low conversions, it is assumed that the reaction kinetics are not affected by any diffusional limitations associated with vitrification. The double bond consumption per thiol normalized by the initial ratio of (meth)acrylate to thiol is shown in Figure 3 where the slope approximately represents the kinetic parameter ratio of (meth)acrylate homopolymerization to chain transfer.18 The ratio for the thiol-yne-acrylate systems is 1.8 compared to 4.9 in the thiol-yne-methacrylate systems, indicating that the chain transfer reaction is more competitive with homopolymerization in the acrylate system. In this case, if reductions in shrinkage stress were primarily resultant from chain transfer, the shrinkage stress of the thiol-yne-acrylate systems would be expected to be lower than that of the thiol-yne-methacrylate systems. Moreover, if all the other factors are equal, compared with the pure methacrylate, the pure acrylate has a lower shrinkage stress due to the lower Tg. However, the shrinkage values of these two systems are very similar and are in the range of 1.0 – 1.5 MPa as shown in Figure 4. To evaluate further this interesting phenomenon, the polymerization kinetics of each component were studied. As seen in Figure 5, at early stages of the polymerization, there is a greater difference between methacrylate and yne conversion than there is between acrylate and yne conversion. This difference implies that when the (meth)acrylate reaches higher conversions, the thiol and yne components remain at relatively lower conversions. The thiol and yne oligomers are still relatively small molecules with enough mobility to behave as a plasticizing solvent in the early stages of the polymerization and result in reduced shrinkage stress development.21 In the thiol-yne-acrylate systems, a reduced solvation effect appears to counter balance the increased chain transfer, thereby resulting in similar overall behavior. This outcome is further evidence that the reduction of shrinkage stress in the ternary resins is resultant from a combination of both reaction kinetics and the complex network evolution. Figure 6 shows the final conversions of each component in the ternary systems; the final conversion of both methacrylate and acrylate double bonds (Figure 6 (a)) are above 0.9 and do not significantly change with increasing methacrylate or acrylate content. In contrast, the final conversions of both the alkyne (Figure 6b) and thiol (Figure 6c) decrease with increasing methacrylate or acrylate content due to earlier vitrification of the networks with higher (meth)acrylic compositions. The thiol conversion is slightly lower than the yne conversion indicating that vinyl sulfide has not fully reacted with the thiol. Moreover, in the thiol-yne-acrylate systems, both the thiol and yne achieve higher final conversion than those in the thiol-yne-methacrylate systems due to the general reduction in glass transition temperatures found in acrylic polymers as compared to methacrylic polymers.
Figure 3.
(Meth)acrylate functional group consumption per thiol functional group versus the initial ratio of (meth)acrylate to thiol functional group concentrations. PETMP-HDY-EBPADMA (2:1:x) (◇) and PETMP-HDY-EBPADA (2:1:x) (△) systems initiated with 1 wt% Irgacure 184 and cured by 365nm at 10mW/cm2
Figure 4.
Shrinkage stress of PETMP-HDY-EBPADMA (filled symbols) and PETMP-HDY-EBPADA (open symbols) with stoichiometric thiol:yne and changing (meth)acrylate content in which the functional groups of thiol:yne:(meth)acrylate was 2:1:x. The systems were initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2. Note: 2 MPa is the maximum range of the tensometer measurement. The stress values above this line indicate higher than 2 MPa rather than the exact values.
Figure 5.
Real time conversions of PETMP-HDY-EBPADMA with the functional group ratio of thiol:yne:MA of 2:1:2.7 (filled symbols) and PETMP-HDY-EBPADA with the functional group ratio of thiol:yne:acrylate of 2:1:2.7 (open symbols) ((meth)acrylate (□) and yne (△)). The systems were initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2.
Figure 6.
PETMP-HDY-EBPADMA (filled symbols) and PETMP-HDY-EBPADA (open symbols) with stoichiometric thiol:yne and changing (meth)acrylate content in which the functional group ratio of thiol:yne:(meth)acrylate was 2:1:x. (a) (Meth)acrylate, (b) yne, and (c) thiol conversion. The systems were initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2.
The crosslinking density (ρx) was determined at the end of the polymerization for the range of ternary systems studied from rubber elasticity phantom theory (Equation 1)30
| Equation 1 |
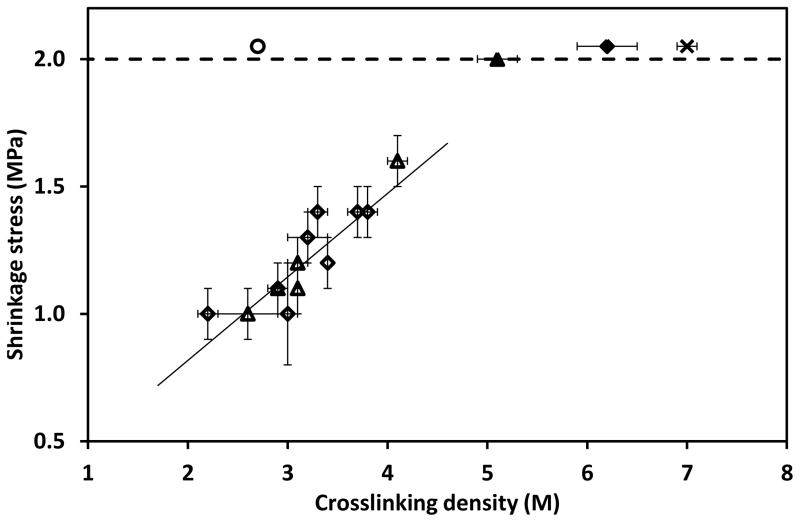
in which, E is the modulus in the rubbery state, R is the gas constant, T is the absolute temperature, and γ is the Poisson’s ratio which is assumed to be 0.5 for incompressible networks. The shrinkage stress as a function of the crosslinking density for stoichiometric thiol:yne with varying stoichiometric (meth)acrylates compositions (2:1:x), off stoichiometric thiol:yne systems with fixed relative methacrylate content (x:y:2.7) and a thiol-ene-methacrylate system (1.5:1.5:2.7) used as a control is plotted in Figure 7. The positive correlation between shrinkage stress and crosslinking density indicates that the crosslinking density plays an important role in controlling the final shrinkage stress of the thiol-yne-(meth)acrylate ternary systems. This plot also indicates that the shrinkage stress of the pure (meth)acrylate and thiol-yne polymer networks are much higher than the ternary systems because of the higher crosslinking density of the systems. EBPADMA (◆), EBPADA (▲) and PETMP-HDY (✗) systems achieve crosslinking densities of 6.2 ± 0.3, 5.1 ± 0.2 and 7.0 ± 0.1 M, respectively; while the crosslinking density of the ternary systems (⋄, ▵) are in the range of 2 – 4 M. Moreover, to demonstrate the importance of the increased yne functionality relative to the ene, thiol-ene-methacrylate systems with a stoichiometric ratio of thiol to ene and excess methacrylate (PETMP-HDE-DBPADMA 1.5:1.5:2.7) with similar molecular structure were studied as a control system in Figure 7. At the same crosslinking density value (2.7 M), the thiol-ene-methacrylate system (○) has a much higher shrinkage stress value (> 2 MPa) as compared with the thiol-yne-(meth)acrylate systems (~ 1.1 MPa) demonstrating the advantages of the thiol-yne-(meth)acrylate systems to reduce shrinkage stress.
Figure 7.
Shrinkage stress versus crosslinking density of PETMP-HDY-EBPADMA systems. PETMP-HDY-EBPADMA systems with stoichiometric thiol to yne ratio with varying methacrylate amount (functional group ratio of thiol:yne:methacrylate was 2:1:x) and off stoichiometric thiol to yne ratios with fixed methacrylate content (functional group ratio of thiol:yne:methacrylate was x:y:2.7 listed in Table 1) (◇), PETMP-HDY-EBPADA systems with stoichiometric thiol to yne ratio with varying acrylate amount (functional group ratio of thiol:yne:acrylate was 2:1:x) (△), EBPADMA (◆), EBPADA (▲), PETMP-HDY (2:1) (✗) and PETMP-HDE-EBPADMA (1.5:1.5:2.7) (○). All resins were initiated with 1 wt% Irgacure 184 and were cured by 365nm light at 10mW/cm2. Note: 2 MPa is the maximum stress accurately measured by the tensometer under these conditions. Stress values greater than this threshold are indicative of greater than 2 MPa stress rather than exact values.
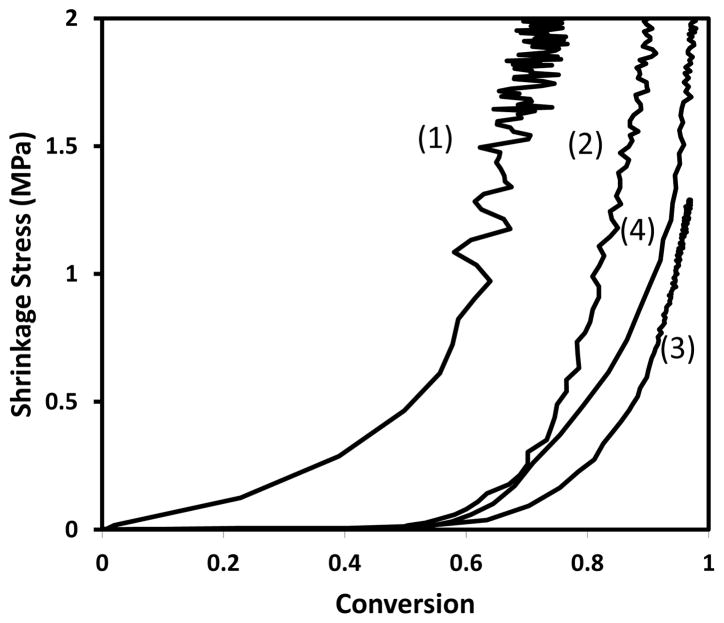
The competitive reaction kinetics in thiol-yne-(meth)acrylate ternary systems leads to a unique network formation process. The radical mediated step-growth mechanism of the thiol-yne reaction and the (meth)acrylate chain transfer to thiol delay gelation during the polymerization. Figure 8 shows how the shrinkage stress develops with conversion for different reaction mechanisms. The pure dimethacrylate system (1) builds up the stress at a very low conversion and reaches a high final stress value (> 2 MPa) due to the early gelation and subsequent vitrification associated with this homopolymerization. Prior to gelation, the resin systems are able to accommodate for the polymerization induced shrinkage through material flow, thus generating minimal stress. The shrinkage stress is primarily built up after gelation due to the restricted mobility and relaxation of the gel accompanied by the subsequent vitrification of the network.2 However, this theory does not fully account for the behavior of the thiol-yne binary system as evidenced in Figure 8. The thiol-yne binary system (2) follows the step-growth polymerization mechanism and the gelation is significantly delayed, but this polymerization still reaches a high final stress (> 2 MPa) resulting from the high final crosslinking density (7.0 ± 0.1 M). The 2PETMP-1HDY-2.7EBPADMA thiol-yne-methacrylate system (3) builds up the stress later resulting from the mixed mode step-chain growth polymerization; and obtains a relatively lower crosslinking density (3.4 ± 0.1 M) and final stress (1.2 ± 0.1 MPa). As a control system, the thiol-ene-methacrylate (1.5PETMP-1.5HDE-2.7EBPADMA) (4) builds up stress slightly earlier than thiol-yne-methacrylate system, since each alkene reacts with only a single thiol. Also the shrinkage stress of the thiol-ene-methacrylate builds up faster than the thiol-yne-methacrylate system due to the faster reaction rate at the same curing condition, which means the faster the reaction occurs, the less time the resin remains in the pre-gelation state with enough mobility to release shrinkage stress. Therefore, this behavior indicates that delaying gelation contributes to, but is not the dominant factor controlling the reduction of shrinkage stress. Moreover, the shape of these curves relating to the network formation and stress development was investigated. The different reaction mechanisms lead to different network heterogeneity and shrinkage stress evolution with conversion. The shrinkage stress value for the pure methacrylate system increases gradually with increasing conversion because the chain growth mechanism creates a very heterogeneous network with a broad glass transition region (Tg1/2width ~ 75 ± 1 °C) enabling a greater amount of polymerization to occur after vitrification. In contrast, the step-growth mechanism of the thiol-yne binary system leads to a more homogeneous network with a narrow Tg1/2width (17 ± 1 °C) so that less polymerization occurs after vitrification, and the stress builds up rapidly after 50% conversion of the alkyne groups. The Tg1/2width of the thiol-yne-methacrylate (2:1:2.7) is 34 ± 1 °C indicating that the heterogeneity of the network is between that of the pure methacrylate and thiol-yne systems. Thus, the shape of the stress curve is a combination of these behaviors as well.
Figure 8.
Shrinkage stress versus methacrylate conversion of EBPADMA (1), PETMP-HDY-EBPADMA 2:1:2.7 (3), PETMP-HDE-EBPADMA 1.5:1.5:2.7 (4); and versus yne conversion of PETMP-HDY 2:1 (2). The systems were initiated with 1 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2.
Here, we introduce a new parameter, the ratio of the volumetric shrinkage at gelation to the final volumetric shrinkage (VS(gel)/VS(total)). This parameter is representative of the network formation and correlates to shrinkage stress. The polymerization induced shrinkage stress is related to the volumetric shrinkage of the resin during network formation. Shrinkage stress primarily results from volume shrinkage that occurs post gelation and the final volumetric shrinkage. This ratio is used instead of the difference between the two to provide an indication of the fraction of the shrinkage in the system that occurs following gelation. The volumetric shrinkage of the resin systems before gelation is theoretically calculated based on the conversion of each component at the gel point and the total volumetric shrinkage is calculated based on the final conversion of each component by equation (2).31
| Equation 2 |
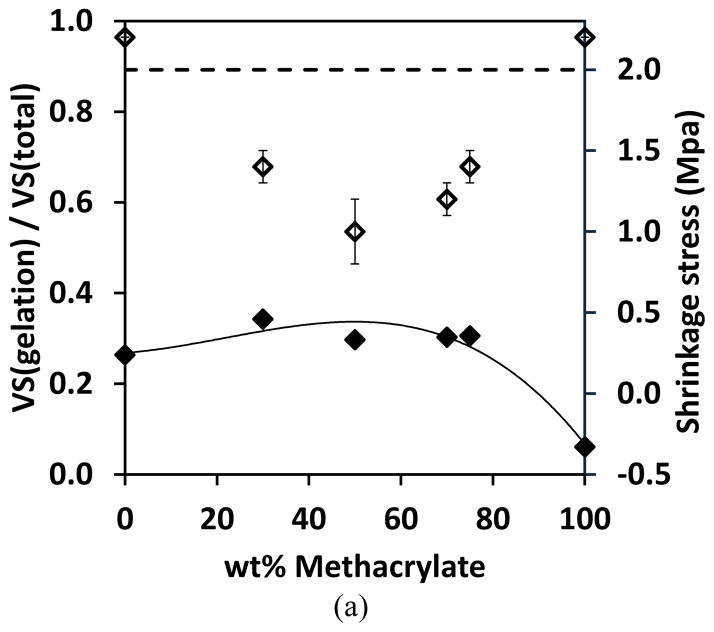
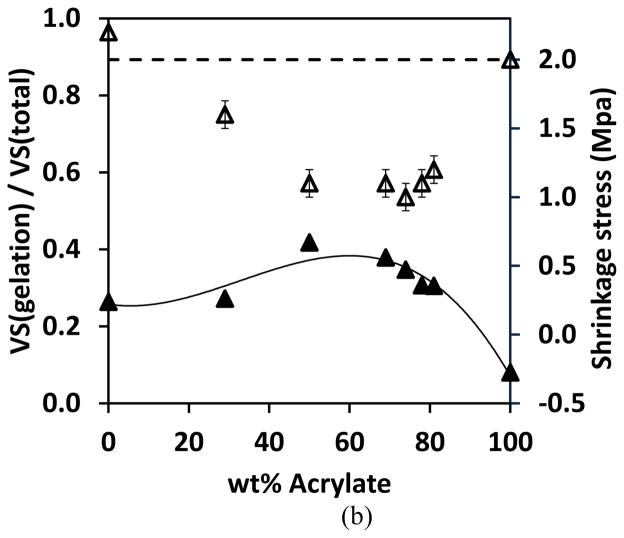
VS is volumetric shrinkage, C0 is the initial concentration of the functional group, X is conversion, SF is the shrinkage factor (listed in Table 2) and i represents each functional group in the resin system. The percentage of volumetric shrinkage before gelation relative to the total shrinkage was calculated and plotted versus (meth)acrylate content in Figure 9. The shrinkage ratio of both thiol-yne-methacrylate and thiol-yne-acrylate systems are seen to increase and then decrease with increasing methacrylate or acrylate content, which inversely correlates to the final shrinkage stress. The higher value indicates that a larger portion of shrinkage occurs before gelation which facilitates the alleviation of a greater amount of the potential shrinkage stress. The reduced shrinkage that occurs after gelation leads to a reduced overall shrinkage stress. This inversely correlated trend shown in Figure 9 demonstrates that the ratio of the volumetric shrinkage before gelation to the total volumetric shrinkage is a significant factor in reducing the shrinkage stress in the ternary systems. The lowest shrinkage stress was observed in the systems containing 50 wt% methacrylate or 70 wt% acrylate (Figure 9), which indicates that at these double bond concentrations, the competitive reaction kinetics result in a maximum volumetric shrinkage that occurs before gelation relative to the total volumetric shrinkage.
Table 2.
Shrinkage factors of thiol-yne-(meth)acrylate systems
| Mixed reaction mechanisms in the ternary systems | Shrinkage factor (mL/mol) | Notes |
|---|---|---|
| (Meth)acrylate homopolymerization | 22.6 | Reference31 |
| (Meth)acrylate chain transfer to thiol | 12.6 | Estimated as a thiol-ene reaction31 |
| Yne reacting with thiol once | 12.6 | Estimated as a thiol-ene reaction31 |
| Yne reacting with thiol twice | 21.0 | Measured by butyl 3-mercaptopropionate with 1-octyne |
Figure 9.
The fraction of volumetric shrinkage that occurs prior to gelation relative to the total volume shrinkage (filled symbols) and shrinkage stress (open symbols) versus (meth)acrylate content. (a) PETMP-HDY-EBPADMA 2:1:x and (b) PETMP-HDY-EBPADA 2:1:x. The systems were initiated with 1.0 wt% Irgacure 184, and cured by 365nm light at 10mW/cm2. Note: 2 MPa is the maximum stress accurately measured by the tensometer under these conditions. Stress values greater than this threshold are indicative of greater than 2 MPa stress rather than exact values.
Conclusions
Thiol-yne-methacrylate and thiol-yne-acrylate ternary systems are demonstrated as resin systems that achieve high glass transition temperatures and moduli while maintaining low shrinkage stress. The reduction of shrinkage stress is affected by a combination of factors; after evaluating these factors, the crosslinking density and the percentage of volumetric shrinkage occurring before gelation relative to the total shrinkage were investigated as factors that control the final shrinkage stress. The shrinkage stress of the ternary systems is lower than both the pure (meth)acrylate and pure thiol-yne resins; and the lowest shrinkage stress was observed in the ternary system with 50 wt% methacrylate or 70 wt% acrylate. This tunable behavior is resultant from the competitive reaction kinetics and unique network formation process. When (meth)acrylate is added to the thiol-yne step growth system, gelation is delayed causing more volumetric shrinkage to occur before gelation relative to the total volumetric shrinkage; thereby reducing the shrinkage stress.
Acknowledgments
The authors gratefully acknowledge the National Science Foundation CBET 0626023 and National Institutes of Health NIH/NIDCR Grants DE10959 and DE018233-0142 for funding this work.
Contributor Information
Sheng Ye, Email: sheng.ye@colorado.edu.
Neil B. Cramer, Email: neil.cramer@colorado.edu.
Ian R. Smith, Email: ian.smith@colorado.edu.
Katerina R. Voigt, Email: katerina.voigt@colorado.edu.
Christopher N. Bowman, Email: christopher.bowman@colorado.edu.
References
- 1.Fouassier JP, Rabek JF, editors. Radiation Curing in Polymer Science and Technology (Volume I) Elsevier Science Publishers Ltd; Essex: 1993. pp. 7–9. [Google Scholar]
- 2.Lee TY, Carioscia J, Smith Z, Bowman CN. Macromolecules. 2007;40:1473–1479. [Google Scholar]
- 3.Eick JD, Kostoryz EL, Rozzi SM, Jacobs DW, Oxman JD, Chappelow CC, Glaros aG, Yourtee DM. Dent Mater. 2002;18:413–21. doi: 10.1016/s0109-5641(01)00071-9. [DOI] [PubMed] [Google Scholar]
- 4.Van Ende A, De Munck J, Mine A, Lambrechts P, Van Meerbeek B. Dent Mater. 2010;26:215–22. doi: 10.1016/j.dental.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Oxman JD, Jacobs DW. 9362410. US Patent. 2000
- 6.Cao X, Lee LJ. J Appl Polym Sci. 2003;90:1486–1496. [Google Scholar]
- 7.Moraes RR, Garcia JW, Barros MD, Lewis SH, Pfeifer CS, Liu J, Stansbury JW. Dent Mater. 2011;27:509–19. doi: 10.1016/j.dental.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer NB, Stansbury JW, Bowman CN. J Dent Res. 2011;90:402–16. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodiuk-Kenig H, Lizenboim K, Eppelbaum I, Zalsman B, Kenig S. J Adhes Sci Technol. 2004;18:1723–1737. [Google Scholar]
- 10.Mezzenga R. Compos Sci Technol. 2001;61:787–795. [Google Scholar]
- 11.Scott TF, Draughon RB, Bowman CN. Adv Mater. 2006;18:2128–2132. [Google Scholar]
- 12.Bowman CN, Kloxin CJ. AIChE J. 2008;54 [Google Scholar]
- 13.Cramer NB, Bowman CN. J Polym Sci Pol Chem. 2001;39:3311–3319. [Google Scholar]
- 14.Hoyle CE, Lee TY, Roper T. J Polym Sci Pol Chem. 2004;42:5301–5338. [Google Scholar]
- 15.Fairbanks BD, Sims Ea, Anseth KS, Bowman CN. Macromolecules. 2010;43:4113–4119. [Google Scholar]
- 16.Chan JW, Shin J, Hoyle CE, Bowman, Lowe AB. Macromolecules. 2010;43:4937–4942. [Google Scholar]
- 17.Chan JW, Zhou H, Hoyle CE, Lowe AB. Chem Mater. 2009;21:1579–1585. [Google Scholar]
- 18.Reddy SK, Cramer NB, Bowman CN. Macromolecules. 2006;39:3681–3687. [Google Scholar]
- 19.Reddy SK, Cramer NB, Kalvaitas M, Lee TY, Bowman CN. Aust J Chem. 2006;59:586. [Google Scholar]
- 20.Cramer NB, Couch CL, Schreck KM, Boulden JE, Wydra R, Stansbury JW, Bowman CN. Dent Mater. 2010;26:799–806. doi: 10.1016/j.dental.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer NB, Couch CL, Schreck KM, Carioscia Ja, Boulden JE, Stansbury JW, Bowman CN. Dent Mater. 2010;26:21–28. doi: 10.1016/j.dental.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senyurt AF, Wei H, Hoyle CE, Piland SG, Gould TE. Macromolecules. 2007;40:4901–4909. [Google Scholar]
- 23.Park HY, Kloxin CJ, Scott TF, Bowman CN. Dent Mater. 2010;26:1010–6. doi: 10.1016/j.dental.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovell LG, Berchtold Ka, Elliott JE, Lu H, Bowman CN. Polym Advan Technol. 2001;12:335–345. [Google Scholar]
- 25.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. J Mater Sci Mater Med. 2004;15:1097–103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Polymer. 2011;52:3295–3303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter HH. Polym Eng Sci. 1987;27:1698–1702. [Google Scholar]
- 29.Fairbanks BD, Scott TF, Kloxin CJ, Anseth KS, Bowman CN. Macromolecules. 2009;42:211–217. doi: 10.1021/ma801903w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flory PJ. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY: 1953. [Google Scholar]
- 31.Lu H, Carioscia Ja, Stansbury JW, Bowman CN. Dent Mater. 2005;21:1129–36. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]