Abstract
BACKGROUND
Neoadjuvant chemotherapy reduces tumor size prior to surgery in women with breast cancer. We assessed the ability of mammography and ultrasound to predict residual tumor size following neoadjuvant chemotherapy.
METHODS
In a retrospective review of consecutive breast cancer patients treated with neoadjuvant chemotherapy, residual tumor size estimated by diagnostic imaging was compared with residual tumor size determined by surgical pathology.
RESULTS
192 patients with 196 primary breast cancers were studied. Of 104 tumors evaluated by both imaging modalities, ultrasound was able to size 91.3%, however, mammography was able to size only 51.9% of tumors (chi-square, p<.001). Ultrasound also was more accurate than mammography in estimating residual tumor size (62/104 [59.6%] versus 33/104 [31.7%], p < .001). There was little difference in the ability of mammography and ultrasound to predict pathologic complete response (ROC= 0.741 versus 0.784).
CONCLUSIONS
Breast ultrasound was more accurate than mammography in predicting residual tumor size following neoadjuvant chemotherapy. Likelihood of a complete pathological response was 80% when both imaging modalities demonstrated no residual disease.
Keywords: breast carcinoma, breast ultrasound mammography, neoadjuvant chemotherapy
INTRODUCTION
Neoadjuvant chemotherapy has become an accepted component of the multidisciplinary treatment of clinical stage II and III breast cancer [1, 2]. The main advantage of administering chemotherapy prior to surgery is to decrease tumor size allowing for an increased percentage of women to undergo breast conservation therapy [3-6]. A second benefit of neoadjuvant chemotherapy is ability to directly monitor the response to the therapeutic regimen administered. Although, randomized studies do not demonstrate overall improved survival with neoadjuvant chemotherapy, complete pathologic response after neoadjuvant chemotherapy is a surrogate marker for patient outcome [7,8,9]. Meta-analysis has shown equivalent or improved disease-free survival with the use of neoadjuvant chemotherapy [10, 11].
Precise measurement of residual tumor size following neoadjuvant chemotherapy is essential for surgical decision-making. Furthermore, the ability to identify the subgroup of patients with a pathologic complete response to neoadjuvant chemotherapy would help identify those patients who may not require additional induction therapy from those patients who may benefit from additional chemotherapy [9]. Modalities that have been used to assess residual tumor size following neoadjuvant chemotherapy include physical examination, mammography, breast ultrasound, and magnetic resonance imaging [13-21].
Mammography and breast ultrasound are the most commonly used diagnostic imaging modalities to estimate primary tumor size at the time of diagnosis [22-27]. Although there is clear evidence that these modalities are accurate in measuring tumor size at the time of diagnosis [22-24, 27], there are concerns regarding the accuracy of these modalities to measure residual tumor size following neoadjuvant chemotherapy. The most significant concerns are related to the fact that the primary tumor response to chemotherapy may vary, resulting in fibrosis [28], fragmentation [29], and/or change in the density of malignant tissue [30], all of which may affect the estimation of residual tumor size. In this study, we sought to determine the accuracy of mammography and breast ultrasound in assessing the residual tumor size and pathologic complete response after neoadjuvant chemotherapy in women with clinical stage II or III breast cancer.
METHODS
Patient Population
A cohort of all breast cancer patients who underwent neoadjuvant chemotherapy between January, 2000 and June, 2004 at the Alvin J. Siteman Cancer Center at Washington University in St. Louis was identified. Retrospective chart review was conducted to collect information about patients’ demographics, diagnostic imaging and surgical pathology. All patients had clinical stage II or III invasive breast cancer with a primary tumor size greater than 2.0 cm. Four patients were diagnosed with bilateral breast cancer for a total of 196 tumors evaluated. In all patients, breast cancer was diagnosed by core needle biopsy. Standard staging studies were performed to exclude patients with distant disease. Although the precise regimen of neoadjuvant chemotherapy varied, all patients received between 4-8 cycles of chemotherapy before definitive surgical therapy at the discretion of the treating medical oncologist per the institutional standard of care. The study was approved by the Institutional Review Board at Washington University School of Medicine.
Mammography and Breast Ultrasound
Diagnostic imaging following neoadjuvant chemotherapy was performed to evaluate tumor size after the last cycle of chemotherapy, 1-4 weeks prior to surgery. Mammography or breast ultrasound or both imaging modalities were used to estimate tumor size for surgical decision making as part of routine patient care at the discretion of the breast surgeon. Thus, not all patients were evaluated by both mammography and breast ultrasound. MRI is not routinely used as part of routine patient care at our center; therefore, it was not included in this study. All diagnostic imaging was performed at the Breast Health Center at the Siteman Cancer Center, Washington University School of Medicine. The Breast Health Center is fully certified according to the Federal Mammography Quality Standards Act (MQSA). Breast imaging examinations were performed using standard techniques by one of five accredited radiologists whose sole practice is breast imaging using various imaging modalities. To evaluate the maximum tumor diameter, mammographic evaluation included, but was not necessarily limited to, standard craniocaudal and mediolateral oblique views. Mammographic findings associated with malignancy typically included nodular densities with poorly defined or spiculated margins, clustered or pleomorphic calcifications, or architectural distortion. Real-time ultrasonography of the tumor also was performed by these Breast Health Center radiologists. Ultrasonographic findings associated with malignancy typically included hypoechoic solid masses with poorly defined margins, shadowing, or acoustical enhancement.
Criteria for Assessment of Tumor Size
In all cases, the largest tumor dimension documented for each mammogram or breast ultrasound performed was used in the analyses. The following categories were used to characterize the ability of mammography and/or breast ultrasound to definitively size residual breast tumors following neoadjuvant chemotherapy: (1) tumor size is defined: the tumor was visualized and size dimensions were assigned; (2) tumor size unable to be defined: the tumor was visualized, but size dimensions could not be assigned because of the imaging characteristics of the tumor; (3) no residual tumor: no diagnostic imaging abnormalities corresponding to the tumor were visualized.
Pathologic Analysis
Surgical pathology reports were reviewed retrospectively. Surgical pathologic analysis was performed per the standard of care with microscopic examination of routine hematoxylin and eosin staining of paraffin-embedded tissues. In all cases, the largest tumor dimension documented was used for subsequent analyses. If no residual tumor was observed by microscopic analysis, the case was classified as a pathologic complete response.
Statistical Analysis
The ability of mammography and breast ultrasound to predict tumor size was assessed by calculating the difference (in centimeters) in residual tumor size as predicted by the imaging modalities compared to surgical pathology. We categorized the accuracy of these assessments into three groups, which is similar to other studies (14-17): (1) accurate within one centimeter; (2) overestimation of tumor size by diagnostic imaging by more than one centimeter; and (3) underestimation of tumor size by diagnostic imaging by more than one centimeter. Agreement between the accuracy of tumor size assessments between imaging modalities was determined using kappa statistics.
The sensitivity, specificity, and associated 95% confidence intervals of mammography and breast ultrasound to predict pathologic complete response were calculated. The uncorrected McNemar test was used to determine statistical differences in sensitivity and specificity between the two imaging modalities. Positive and negative predictive values were calculated for each imaging modality. Positive predictive value was calculated as the percentage of pathologic complete responses observed when a complete response was predicted by mammography or breast ultrasound. Negative predictive value was calculated as the percentage without pathologic complete response observed when residual tumor was predicted by mammography or breast ultrasound. Likelihood ratios of a positive and negative test for both imaging modalities were also calculated. Positive and negative likelihood ratios describe the discriminatory properties of positive and negative imaging results (i.e., how many times more likely a correct imaging test is observed in breast tumors with a complete pathologic response than those with a non-complete pathologic response). Positive likelihood ratios above 10 and negative likelihood ratios below 0.1 are considered convincing diagnostic evidence, whereas positive likelihood ratios above 5 and negative likelihood ratios below 0.2 give strong diagnostic evidence.
Cohen’s kappa statistic was used to measure the agreement between pathologic and imaging data. Because kappa is influenced by systematic differences between both data sources and by the frequency of pathologic response, the Bias Index and the Prevalence Index were also calculated [31, 32]. The Bias Index ranges from −1 to 1, with 0 indicating no difference and −1 or 1 representing the maximum differences between both data sources in the proportions of agreement between pathologic complete response and complete response according to the imaging modality. The Prevalence Index ranges from −1 to 1 and is 0 when the two-sourced-averaged proportion of “yes” is 50%, is negative when the proportion is lower than 50%, and is positive when the proportion is over 50%. We defined kappa or prevalence and bias adjusted kappa (PABAK) values <0.40 as poor or fair agreement, 0.40-0.60 as moderate agreement, 0.61-0.80 as substantial agreement, and 0.81-1.00 as almost perfect agreement [33].
The discriminatory power of both imaging modalities to predict a pathologic complete response was also determined based on the area under the receiver operating characteristic (ROC) curve as calculated from logistic regression models using the c-statistic. Higher values of the c-statistic indicate better discriminatory power.
RESULTS
Patient Population
During the study period, 192 consecutive patients (196 tumors) were treated with neoadjuvant chemotherapy. Mean age of the sample population was 51 (range 22-89) years, and 69% of patients were white. Of the 196 tumors, 144 (73.5%) were evaluated by mammography, 110 (56.1%) were evaluated by breast ultrasound, and 46 (23.5%) were not evaluated by either imaging modality; 104 (53.1%) were evaluated by both mammography and breast ultrasound. Tumor characteristics of the entire sample and the subset of 104 tumors are shown in Table 1.
Table I. Tumor characteristics.
| Characteristic | Total number of tumors N = 196 (%) |
Tumors imaged by both mammography and breast ultrasound n = 104 (%) |
|---|---|---|
| Histology | ||
| Invasive ductal carcinoma | 155 (79.1) | 75 (72.1) |
| Invasive lobular carcinoma | 22 (11.2) | 16 (15.4) |
| Invasive mammary carcinoma | 16 (8.2) | 11 (10.6) |
| Others | 3 (1.5) | 2 (2.0) |
| Tumor grade | ||
| I | 19 (9.7) | 8 (7.7) |
| II | 50 (25.5) | 29 (27.9) |
| III | 122 (62.2) | 63 (60.6) |
| Unknown | 5 (2.6) | 4 (3.8) |
| Estrogen-receptor status | ||
| Positive | 96 (49.0) | 50 (48.1) |
| Negative | 99 (50.5) | 54 (51.9) |
| Unknown | 1 (0.5) | 0 (0.0) |
| Progesterone-receptor status | ||
| Positive | 73 (37.2) | 38 (36.5) |
| Negative | 121 (61.7) | 65 (62.5) |
| Unknown | 2 (1.0) | 1 (1.0) |
| Her-2-neu status | ||
| Positive | 59 (30.1) | 32 (30.8) |
| Negative | 136 (69.4) | 72 (69.2) |
Measuring residual tumor size
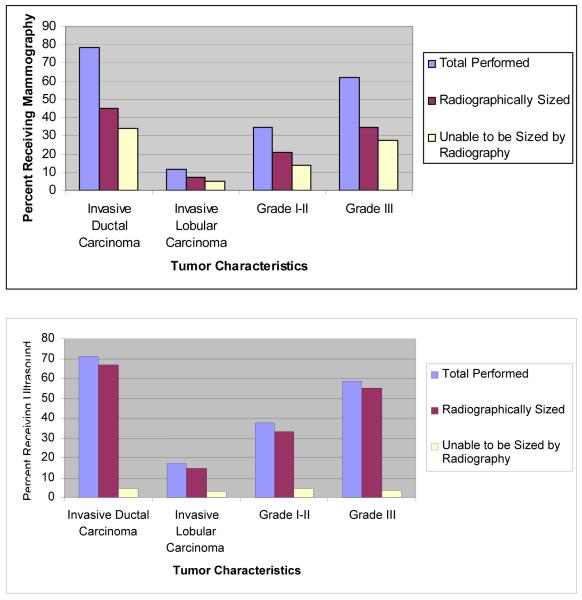
Of all imaged tumors, size was unable to be defined in 60 (41.7%) of the 144 tumors imaged by mammography and in nine (8.2%) of 110 tumors imaged by breast ultrasound. Of 104 tumors evaluated by both imaging modalities, ultrasound was able to size 91.3%, however, mammography was able to size only 51.9% of tumors (chi-square, p<.001). Eight of 104 tumors (7.7%) that were imaged by both mammography and breast ultrasound could not be sized by either imaging modality. The ability of mammography or ultrasound to define a tumor size was not significantly related to type of cancer or grade (Figure 1).
Figure 1.
Characteristics of tumors able to be sized by imagining modality
A. 144 tumors overall were imaged by mammography
B. 110 tumors overall were imaged by sonography
All subsequent analyses were conducted using data about the 104 tumors imaged by both mammogram and ultrasound. Of these104 tumors (Table 2), residual tumor size was more likely to be accurately measured (within 1.0 cm of the pathologic size) by breast ultrasound than mammography (59.6% versus 31.7%). The inability to measure residual tumor size was more likely to occur with mammographic imaging than with breast ultrasound (48.1% versus 8.7%; chi-square p < .001). Despite the difference in the ability of mammography and breast ultrasound to accurately size residual tumors, when the tumor size was defined by either mammographic or ultrasound imaging (n = 54 for mammography and n = 95 for breast ultrasound), the two imaging modalities were similarly likely to be accurate within 1.0 cm of pathologic tumor size (33/54 [61.1%] accurate for mammography and 62/95 [65.3%] accurate for breast ultrasound).
Table II. Accuracy of mammography and breast ultrasound in measuring residual tumor size following neoadjuvant chemotherapy compared with surgical pathology measurement (n=104).
| Imaging Modality |
Accurate ± 1 cm n (%) |
Overestimation > 1 cm n (%) |
Underestimation > 1 cm n (%) |
Unable to be Sized n (%) |
|---|---|---|---|---|
| Mammogram | 33 (31.7) | 6 (5.8) | 15 (14.4) | 50 (48.1) |
| Ultrasound | 62 (59.6) | 17 (16.3) | 16 (15.4) | 9 (8.7) |
Note: Numbers in parentheses are percentages of the row total of 104 tumors, which were measured by both mammography and breast ultrasound. Chi-square p < .001.
Measuring pathologic complete response
Twenty-four (23.1%) of 104 tumors evaluated by both mammographic and ultrasound imaging modalities were undetectable pathologically after neoadjuvant chemotherapy and were considered a pathologic complete response. Pathologic complete response differed significantly by grade (grade I: 0/8 [0%]; grade II: 3/29 [10.3%]; grade III: 19/63 [30.2%]; grade not determined: 2/4 [50.0%]; p=0.037); by estrogen receptor status (positive: 4/50 [8.0%]; negative: 20/54 [37.0%]; p<0.001); and by progesterone receptor status (positive: 2/38 [5.3%]; negative: 22/65 [33.8%]; p=0.001). The sensitivity and specificity of mammography in predicting pathologic complete response was 54.2% and 86.3%, respectively (Table 3). The sensitivity and specificity of breast ultrasound in predicting a pathologic complete response was 45.8% and 93.8%, respectively. The sensitivity and specificity between the two imaging modalities were not statistically different (p=0.480, and p=0.083, respectively). Agreement between pathologic and imaging data was considered to be fair to moderate for both mammography (kappa=0.40) and breast ultrasound (kappa=0.45). These kappas did not differ statistically from each other (p=0.726). Prevalence and bias-adjusted kappa levels (PABAK) improved for both breast ultrasound and mammography. The likelihood ratio of a positive test was higher for breast ultrasound (7.3) than for mammography (3.9), but the likelihood ratios of a negative test were similar for both imaging modalities indicating that both modalities were equally accurate at predicting the presence of residual disease, however sonography was better at predicting a pathologic complete response.
Table III. Test characteristics of mammography and breast ultrasound in predicting pathologic complete response following neoadjuvant chemotherapy (n=104).
| Test characteristic | Mammography (95% CI) |
Breast Ultrasound (95% CI) |
|---|---|---|
| Sensitivity | 54.2 (34.3–73.0) | 45.8 (25.6–67.2) |
| Specificity | 86.3 (77.4–92.6) | 93.8 (86.7–97.7) |
| Predictive value positive | 54.2 (34.3–73.0) | 68.8 (43.7–87.5) |
| Predictive value negative | 86.3 (77.4–92.6) | 85.2 (76.6–91.5) |
| Likelihood ratio of a positive test | 3.9 | 7.3 |
| Likelihood ratio of a negative test | 0.53 | 0.58 |
| Kappa | 0.40 (0.18–0.62) | 0.45 (0.24–0.66) |
| PABAK | 0.58 | 0.65 |
| Probability of a pCR based on an imaging CR | 54.3 | 68.9 |
| Probability of a pCR based on imaging residual disease |
13.8 | 14.8 |
95% CI: 95 percent confidence interval; PABAK: Prevalence and bias-adjusted kappa; pCR: pathologic complete response
Using the pretest probability of 23.1% for a pathologic complete response and the above likelihood ratios, we calculated the gain in certainty of observing a pathologic complete response based on a positive or negative imaging complete response result for either imaging modality. As shown in Table 3, the gain in certainty of a pathologic complete response following a breast ultrasound indicating a complete response is slightly higher than the gain in certainty by a mammogram indicating a complete response (68.9% versus 54.3%, respectively). There was little difference in using either imaging modality when the results were negative (14.8% versus 13.8%, respectively).
In our series, the probability of correctly predicting a pathologic complete response based on the sequence of imaging was maximized when breast ultrasound is performed first and mammography is only performed when the breast ultrasound is interpreted as a complete response. The combined sensitivity of both imaging modalities was 45.8%, the specificity was 93.8%, and the probability of a pathologic complete response is 68.8%. If both imaging modalities were interpreted as a complete response, then the probability of a pathologic complete response was 80.0%. However, if only one imaging modality, either mammography or sonography, was interpreted as a complete response then the probability of a pathologic complete response was 53.3%. The area under the ROC curve as a measure of discriminatory power of a test was 0.67 for mammography, 0.71 for breast ultrasound and 0.77 for both imaging modalities combined. This indicates reasonable discrimination in the presence or absence of disease by using either or both imaging modalities.
DISCUSSION
Neoadjuvant chemotherapy has become an established component of the multidisciplinary treatment of breast cancer [1, 2, 34]. Accurate prediction of pathologic residual tumor size is essential for surgical decision making and monitoring response to therapy [35, 36]. In this study, we compared the accuracy of two commonly used diagnostic imaging modalities in predicting residual tumor size and pathologic complete response.
Tumor size at diagnosis is commonly assessed by physical examination and diagnostic imaging. It is not clear which modality is superior for estimating tumor size. Several studies have reported that breast ultrasound is superior to mammography and physical examination in estimating primary tumor size [22, 23, 26]. However, other studies have concluded that the three modalities perform equally well in measuring the primary tumor size [25, 37], or that mammography out performs physical examination or breast ultrasound [22, 38].
Predicting residual tumor size after neoadjuvant chemotherapy is considered to be even more challenging [17, 39], with no clear consensus in the literature regarding the best method for the accurate assessment of residual tumor size. Although a few studies reported physical examination measured residual tumor size accurately [14, 16, 40], other studies have determined that physical examination alone can be inaccurate in determining the amount of residual disease [30, 41-44]. Physical examination of breast tumors < 2 cm is often difficult depending on the density of the breast [45]. Furthermore, physical examination may be inaccurate when the tumor is irregular, has poorly defined margins, or when neoadjuvant chemotherapy results in residual fibrosis and/or necrosis [43, 46].
Measuring residual tumor size using mammography or breast ultrasound after neoadjuvant chemotherapy has been reported in the literature, however these studies did not directly compare the two imaging modalities [30, 47, 48]. The accuracy of mammography in predicting residual tumor size may depend on the initial mammographic appearance of the tumor. Huber et al. found that mammography was accurate in predicting residual tumor size for those tumors whose margins could be defined by more than 50% on the baseline mammogram [47]. Other studies have reported breast ultrasound to be superior to mammography in estimating residual tumor size [17, 49].
In the present study, we found that a greater proportion of tumors were able to be sized using ultrasound than using mammography and that, overall, breast ultrasound was more accurate than mammography in predicting residual tumor size following neoadjuvant chemotherapy. Our study also demonstrated that if the residual tumor can be sized radiographically, then mammography and breast ultrasound performed equally well in accurately measuring the residual tumor. In a retrospective analysis of 100 patients undergoing neoadjuvant chemotherapy for breast cancer, Herrada et. al. found that physical exam alone was more accurate than mammography or ultrasound at assessing the size of residual tumor and that physical exam combined with mammography was more accurate than physical exam combined with ultrasound [16]. Fiorentino et. al., in a similar series, also concluded that physical examination was more accurate than either mammography or ultrasound and that prediction of pathological outcome was not improved by the addition of either modality. Mammography was slightly more accurate than ultrasound in assessing tumor size [14]. In a smaller study comparing MRI to these modalities, MRI and ultrasound were superior to mammography in detecting residual disease [17]. Esserman et. al. showed that MRI was superior to mammography in the accurate prediction of anatomic extent of breast cancer [50]
Approximately 8% of the tumors could not be sized by either mammography or ultrasonography. Imaging by MRI has been reported to be equal or superior to ultrasound and mammography in the evaluation of tumor size after neoadjuvant chemotherapy when compared with pathologic tumor size [51, 52]. MRI is very useful for evaluating residual disease for the subset of tumors that cannot be sized using ultrasound or mammography.
It has been reported that predicting pathologic complete response is not highly accurate with either mammography or breast ultrasound [16, 20, 53]. Pathologic complete response has been reported to correlate with physical examination, mammography, and breast ultrasound in only 13-25% of cases [16, 20, 53]. In our study, we found no difference in the ability of mammography or breast ultrasound to predict pathologic complete response. However, when both mammography and breast ultrasound demonstrated no residual disease, then the likelihood of a pathologic complete response was 80%, which is similar to results recently reported by Peintinger et al [54].
There are several limitations to our study. First, the central focus of this study was on a subset of 104 tumors that were evaluated by both mammography and breast ultrasound imaging modalities at one institution. Ninety-two tumors were excluded from these analyses because they were not evaluated by both imaging modalities. Reasons for exclusion included patients who chose not to have breast conserving surgery or who were not considered candidates for breast conservation therapy due to multifocal disease, disease progression, or poor response to therapy. We do not believe that the exclusion of these tumors biased our results, since the purpose of examining the residual tumor size by radiographic imaging was to estimate tumor size prior to definitive surgery. Moreover, the subset of 104 tumors that were imaged by both modalities was representative of all the tumors being evaluated for breast conservation therapy following neoadjuvant chemotherapy. In addition, very few tumors were examined using sonography alone, precluding reliable estimates of the accuracy of measuring residual tumor size using this modality alone compared with mammography alone or with both types of imaging.
In conclusion, our retrospective analysis of the ability of mammography and breast ultrasound to accurately measure residual tumor size following neoadjuvant chemotherapy compared with surgical pathology measurement of the residual tumor demonstrated that 59.6% of residual tumors were sized accurately within 1.0 cm using breast ultrasound compared with 31.7% using mammography. Our results underscore the importance of breast ultrasound in surgical decision making following neoadjuvant chemotherapy but also serve as an important reminder of the limitations of employing a single breast imaging modality in this clinical context. Furthermore, the use of both imaging modalities improved the accuracy of predicting a pathological complete response to neoadjuvant chemotherapy in a greater percentage of cases than use of either modality alone. For surgical decision-making, the use of ultrasound in combination with mammography can predict amount of residual disease and complete pathological response.
Summary.
In this study, we retrospectively review a series of patients with breast cancer who received neoadjuvant chemotherapy. Residual tumor size after chemotherapy was assessed by ultrasound and mammography. Ultrasound was found to be more accurate than mammography in assessing residual tumor size.
ACKNOWLEDGMENTS
The authors thank Kathy Sorenson for help with data collection and the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Health Behavior and Outreach Core, which provided data management, epidemiological, and statistical services. The Siteman Cancer Center is supported in part by a National Cancer Institute Cancer Center Support Grant (#P30 CA91842).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufmann M, von Minckwitz G, Smith R, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21(13):2600–2608. doi: 10.1200/JCO.2003.01.136. [DOI] [PubMed] [Google Scholar]
- 2.Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol. 2000;18(7):1558–1569. doi: 10.1200/JCO.2000.18.7.1558. [DOI] [PubMed] [Google Scholar]
- 3.Makris A, Powles TJ, Ashley SE, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol. 1998;9(11):1179–1184. doi: 10.1023/a:1008400706949. [DOI] [PubMed] [Google Scholar]
- 4.Rouzier R, Mathieu MC, Sideris L, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101(5):918–925. doi: 10.1002/cncr.20491. [DOI] [PubMed] [Google Scholar]
- 5.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998 Aug;16(8):2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 8.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999 Feb;17(2):460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 9.Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma:effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002 Sep;236(3):295–302. doi: 10.1097/01.SLA.0000027526.67560.64. discussion 302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey LA, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97(15):1137–1142. doi: 10.1093/jnci/dji206. [DOI] [PubMed] [Google Scholar]
- 11.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 12.Reitsamer R, Peintinger F, Prokop E, Hitzl W. Pathological complete response rates comparing 3 versus 6 cycles of epidoxorubicin and docetaxel in the neoadjuvant setting of patients with stage II and III breast cancer. Anticancer Drugs. 2005;16(8):867–870. doi: 10.1097/01.cad.0000173475.59616.b4. [DOI] [PubMed] [Google Scholar]
- 13.Denis F, Desbiez-Bourcier AV, Chapiron C, et al. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol. 2004;30(10):1069–1076. doi: 10.1016/j.ejso.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino C, Berruti A, Bottini A, et al. Accuracy of mammography and echography versus clinical palpation in the assessment of response to primary chemotherapy in breast cancer patients with operable disease. Breast Cancer Res Treat. 2001;69(2):143–151. doi: 10.1023/a:1012277325168. [DOI] [PubMed] [Google Scholar]
- 15.Forouhi P, Walsh JS, Anderson TJ, Chetty U. Ultrasonography as a method of measuring breast tumour size and monitoring response to primary systemic treatment. Br J Surg. 1994;81(2):223–225. doi: 10.1002/bjs.1800810221. [DOI] [PubMed] [Google Scholar]
- 16.Herrada J, Iyer R, Atkinson E, et al. Relative value of physical examination, mammography, and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clinical Cancer Research. 1997;9:1565–1569. [PubMed] [Google Scholar]
- 17.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. European Radiology. 2004;14(8):1371–1379. doi: 10.1007/s00330-004-2246-z. [DOI] [PubMed] [Google Scholar]
- 18.Moskovic E, Mansi J, King D, et al. Mammography in the assessment of response to medical treatment of large primary breast cancer. Clinical Radiology. 1993;47:339–344. doi: 10.1016/s0009-9260(05)81451-5. [DOI] [PubMed] [Google Scholar]
- 19.Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181(5):1275–1282. doi: 10.2214/ajr.181.5.1811275. [DOI] [PubMed] [Google Scholar]
- 20.Vinnicombe SJ, MacVicar AD, Guy RL, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. 1996;198(2):333–340. doi: 10.1148/radiology.198.2.8596827. [DOI] [PubMed] [Google Scholar]
- 21.Yeh E, Slanetz P, Kopans DB, et al. Prospective Comparison of Mammography, Sonography, and MRI in Patients Undergoing Neoadjuvant Chemotherapy for Palpable Breast Cancer. Am J Roentgenol. 2005;184(3):868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 22.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 23.Bosch AM, Kessels AG, Beets GL, et al. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol. 2003;48(3):285–292. doi: 10.1016/s0720-048x(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 24.Hieken TJ, Harrison J, Herreros J, Velasco JM. Correlating sonography, mammography, and pathology in the assessment of breast cancer size. The American Journal of Surgery. 2001;182(4):351–354. doi: 10.1016/s0002-9610(01)00726-7. [DOI] [PubMed] [Google Scholar]
- 25.Kald BA, Boiesen P, Ronnow K, et al. Preoperative assessment of small tumours in women with breast cancer. Scand J Surg. 2005;94(1):15–20. doi: 10.1177/145749690509400105. [DOI] [PubMed] [Google Scholar]
- 26.Madjar H, Ladner HA, Sauerbrei W, et al. Preoperative staging of breast cancer by palpation, mammography and high-resolution ultrasound. Ultrasound Obstet Gynecol. 1993;3(3):185–190. doi: 10.1046/j.1469-0705.1993.03030185.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang WT, Lam WW, Cheung H, et al. Sonographic, magnetic resonance imaging, and mammographic assessments of preoperative size of breast cancer. J Ultrasound Med. 1997;16(12):791–797. doi: 10.7863/jum.1997.16.12.791. [DOI] [PubMed] [Google Scholar]
- 28.Sinn HP, Schmid H, Junkermann H, et al. [Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy] Geburtshilfe Frauenheilkd. 1994;54(10):552–558. doi: 10.1055/s-2007-1022338. [DOI] [PubMed] [Google Scholar]
- 29.El-Didi MH, Moneer MM, Khaled HM, Makarem S. Pathological assessment of the response of locally advanced breast cancer to neoadjuvant chemotherapy and its implications for surgical management. Surg Today. 2000;30(3):249–254. doi: 10.1007/s005950050054. [DOI] [PubMed] [Google Scholar]
- 30.Huber S, Medl M, Vesely M, et al. Ultrasonographic tissue characterization in monitoring tumor response to neoadjuvant chemotherapy in locally advanced breast cancer (work in progress) J Ultrasound Med. 2000;19(10):677–686. doi: 10.7863/jum.2000.19.10.677. [DOI] [PubMed] [Google Scholar]
- 31.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–429. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 32.Hoehler FK. Bias and prevalence effects on kappa viewed in terms of sensitivity and specificity. J Clin Epidemiol. 2000;53(5):499–503. doi: 10.1016/s0895-4356(99)00174-2. [DOI] [PubMed] [Google Scholar]
- 33.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33(2):363–374. [PubMed] [Google Scholar]
- 34.Davidson NE, Morrow M. Sometimes a great notion--an assessment of neoadjuvant systemic therapy for breast cancer. J Natl Cancer Inst. 2005;97(3):159–161. doi: 10.1093/jnci/dji049. [DOI] [PubMed] [Google Scholar]
- 35.Buchholz TA, Tucker SL, Masullo L, et al. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol. 2002;20(1):17–23. doi: 10.1200/JCO.2002.20.1.17. [DOI] [PubMed] [Google Scholar]
- 36.Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103(4):689–695. doi: 10.1002/cncr.20815. [DOI] [PubMed] [Google Scholar]
- 37.Pain JA, Ebbs SR, Hern RP, et al. Assessment of breast cancer size: a comparison of methods. Eur J Surg Oncol. 1992;18(1):44–48. [PubMed] [Google Scholar]
- 38.Golshan M, Fung BB, Wiley E, et al. Prediction of breast cancer size by ultrasound, mammography and core biopsy. The Breast. 2004;13(4):265–271. doi: 10.1016/j.breast.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Warren R, Bobrow L, Earl H, et al. Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy? British Journal of Cancer. 2004;90:1349–1360. doi: 10.1038/sj.bjc.6601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loehberg CR, Lux MP, Ackermann S, et al. Neoadjuvant chemotherapy in breast cancer: which diagnostic procedures can be used? Anticancer Res. 2005;25(3c):2519–2525. [PubMed] [Google Scholar]
- 41.Fornage B, Toubas O, Morel M. Clinical, mammographic, and sonographic determination of preoperative breast cancer size. Cancer. 1987;60:765–771. doi: 10.1002/1097-0142(19870815)60:4<765::aid-cncr2820600410>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Helvie MA, Joynt LK, Cody RL, et al. Locally advanced breast carcinoma: accuracy of mammography versus clinical examination in the prediction of residual disease after chemotherapy. Radiology. 1996;198(2):327–332. doi: 10.1148/radiology.198.2.8596826. [DOI] [PubMed] [Google Scholar]
- 43.Moneer M, El-Didi M, Khaled H. Breast conservative surgery: is it appropriate for locally advanced breast cancer following downstaging by neoadjuvant chemotherapy? A pathological assessment. Breast. 1999;8(6):315–319. doi: 10.1054/brst.1999.0079. [DOI] [PubMed] [Google Scholar]
- 44.Sauven P. The surgical management of patients following neoadjuvant chemotherapy for locally advanced breast cancer. Eur J Cancer. 2002;38(18):2371–2374. doi: 10.1016/s0959-8049(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 45.Kuerer HM, Singletary SE, Buzdar AU, et al. Surgical conservation planning after neoadjuvant chemotherapy for stage II and operable stage III breast carcinoma. Am J Surg. 2001;182(6):601–608. doi: 10.1016/s0002-9610(01)00793-0. [DOI] [PubMed] [Google Scholar]
- 46.Cocconi G, Di Blasio B, Alberti G, et al. Problems in evaluating response of primary breast cancer to systemic therapy. Breast Cancer Res Treat. 1984;4(4):309–313. doi: 10.1007/BF01806044. [DOI] [PubMed] [Google Scholar]
- 47.Huber S, Wagner M, Zuna I, et al. Locally advanced breast carcinoma: evaluation of mammography in the prediction of residual disease after induction chemotherapy. Anticancer Res. 2000;20(1B):553–558. [PubMed] [Google Scholar]
- 48.Seymour MT, Moskovic EC, Walsh G, et al. Ultrasound assessment of residual abnormalities following primary chemotherapy for breast cancer. Br J Cancer. 1997;76(3):371–376. doi: 10.1038/bjc.1997.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tardivon AA, Ollivier L, El Khoury C, Thibault F. Monitoring therapeutic efficacy in breast carcinomas. Eur Radiol. 2006;16(11):2549–2558. doi: 10.1007/s00330-006-0317-z. [DOI] [PubMed] [Google Scholar]
- 50.Esserman L, Hylton N, Yassa L, et al. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol. 1999 Jan;17(1):110–9. doi: 10.1200/JCO.1999.17.1.110. [DOI] [PubMed] [Google Scholar]
- 51.Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three-dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J. 2006 Mar-Apr;12(2):130–7. doi: 10.1111/j.1075-122X.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 52.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004 Aug;14(8):1371–9. doi: 10.1007/s00330-004-2246-z. [DOI] [PubMed] [Google Scholar]
- 53.von Minckwitz G, Costa SD, Eiermann W, et al. Maximized reduction of primary breast tumor size using preoperative chemotherapy with doxorubicin and docetaxel. J Clin Oncol. 1999;17(7):1999–2005. doi: 10.1200/JCO.1999.17.7.1999. [DOI] [PubMed] [Google Scholar]
- 54.Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–1449. doi: 10.1245/s10434-006-9086-9. [DOI] [PubMed] [Google Scholar]