Abstract
There is a serious need to develop effective mitigators against accidental radiation exposures. In radiation accidents, many people may receive nonuniform whole-body or partial-body irradiation. The lung is one of the more radiosensitive organs, demonstrating pneumonitis and fibrosis that are believed to develop at least partially because of radiation-induced chronic inflammation. Here we addressed the crucial questions of how damage to the lung can be mitigated and whether the response is affected by irradiation to the rest of the body. We examined the widely used dietary supplement genistein given at two dietary levels (750 or 3750 mg/kg) to Fischer rats irradiated with 12 Gy to the lung or 8 Gy to the lung + 4 Gy to the whole body excluding the head and tail (whole torso). We found that genistein had promising mitigating effects on oxidative damage, pneumonitis and fibrosis even at late times (36 weeks) when drug treatment was initiated 1 week after irradiation and stopped at 28 weeks postirradiation. The higher dose of genistein showed no greater beneficial effect. Combined lung and whole-torso irradiation caused more lung-related severe morbidity resulting in euthanasia of the animals than lung irradiation alone.
INTRODUCTION
Exposure of civilian populations to radiation due to accident, war or terrorist act is a significant concern, and our knowledge of the best clinical management of such victims remains uncertain (1–6). Comparisons of signs, symptoms and clinical effects from radiation exposures in Hiroshima/Nagasaki, the Marshall Islands, the South Urals, Wismut and Chernobyl have been reported in the literature (7). The extent of the effects from radiation exposures associated with the recent earthquake and tsunami at the Fukushima nuclear reactors remains to be determined. Radiation damage to the bone marrow is a major concern and may be treatable by bone marrow transplantation, but the gastrointestinal tract is also radiation sensitive; another organ of similar sensitivity is the lung. These and other organs may become of significant concern in patients exposed to partial-body or nonuniform exposures, particularly in the context of multi-organ effects that can significantly affect treatment outcome (8–10). Appropriate strategies to mitigate or treat such exposures are currently very limited (11–15). However, the development of such strategies may also help to reduce normal tissue complications associated with the radiation treatment of cancers.
For victims of radiation accidents or terrorist-instigated radiation exposure, agents to reduce the effects need to be effective when administered after the exposure (i.e., they must mitigate against or treat the radiation injury), while for rescue workers a possibility exists to provide protection prior to exposure. In either case any agent proposed for treatment of victims must have low toxicity and must be widely available. Genistein is such an agent (16). It is an isoflavone, a major component of soy products, and has been reported to have a wide range of biological activities, including anti-tyrosine kinase activity, antioxidant activity and inhibition of the activation of NFκB, one of the major transcription factors involved in inflammatory responses (17–19). Previous studies have shown that injection of genistein 24 h prior to irradiation can provide partial protection of mice against whole-body irradiation in terms of both bone marrow response and lung response (20, 21). Our studies have shown that administering genistein in the diet (750 mg/kg of diet) after irradiation can provide partial mitigation of radiation-induced lung damage in Sprague-Dawley rats and C3H mice given lung irradiation only (22–24). Here we studied the effects in Fischer rats given lung plus whole-torso (whole body less head and tail) irradiation of increasing the dietary dose of genistein to determine if a larger dose would provide greater mitigation. We studied the combination of whole-lung and whole-torso irradiation to examine how nonlethal multi-organ damage, a possible scenario if there is a radiation accident, might affect lung response. We chose Fischer rats for the study since they are more widely used for long-term studies and have been recommended for long-term radiation studies (13, 25). We gave treatment starting 1 week after irradiation, since many accident victims might not be able to be given treatment immediately after exposure. We terminated the treatment at 28 weeks to determine if stopping would decrease efficacy at later times.
METHODS
Animals and Drugs
Female Fischer 344 rats (Harlan Teklad, Madison, WI) were used throughout the studies and housed in animal facilities accredited by the Canadian Council on Animal Care (CCAC) and treated in accordance with approved protocols. The rats were 12–14 weeks old and weighed 200–220 g at the start of the study. In the study there were two radiation-only groups that received either whole-lung irradiation or whole-lung plus whole-torso irradiation, two genistein plus whole-lung irradiation groups, and two genistein plus whole-lung plus whole-torso irradiation groups (8 rats/group). There were also two control groups (4 rats/group); one was euthanized at the time of irradiation (12-week control) and the other was maintained throughout the study until they were euthanized at 36 weeks postirradiation (36-week control). The genistein-treated animals were fed a genistein diet (750 mg/kg or 3750 mg/kg) initiated 1 week after irradiation and maintained until 28 weeks postirradiation. The AIN-76A diet (Harlan Teklad, Madison, WI), a semi-purified casein-based diet containing no detectable phytoestrogens (limit of detection 5 pmol/liter), was used as the control diet. The genistein diet was formulated from the control diet by supplementing with 750 mg/kg or 3750 mg/kg of genistein. The genistein was chemically synthesized (Toronto Research Chemicals Inc., Toronto, Ontario) and incorporated into the AIN-76A diet at Harlan Teklad. Measurements of dietary consumption in all the treatment groups of rats at the start of treatment indicated the food intake was 24 g/day (Supplementary Fig. 1; http://dx.doi.org/10.1667/RR2562.1.S1). Over the course of the experiment the animals lost some weight in the weeks immediately after irradiation and consumed less food. This was greatest in the animals given the larger dose of genistein and reduced the intake of genistein particularly in these two groups (Supplementary Fig. 1; http://dx.doi.org/10.1667/RR2562.1.S1). From these measurements the calculated intake of genistein ranged from 70–90 mg/kg/day for animals on the 750 mg/kg/day diet and from 275–450 mg/kg/day for animals on the 3750 mg/kg/day diet. Assuming an oral bioavailability of approximately 12% (26), the rats on the 750 mg/kg diet should have absorbed a dose of about 8.5–11 mg/kg/day and the rats on the 3750 mg/kg diet a dose of about 33–55 mg/kg/day.
Mass Spectrometry for Quantification of Serum Levels of Genistein
Genistein present in rat plasma was quantified using LC-MS after selective enzymatic hydrolysis as described by Holder et al. (27). Briefly, 50 μl of the internal standard 3,6-dihydroxyflavone solution (250 ng/ml) was added to 0.1 ml of plasma sample. Then 2.5 ml of methyl-t-butyl ether was added and the tubes were shaken. The tubes were centrifuged and the supernatants were evaporated to dryness at room temperature. The extracts were reconstituted with 0.25 ml of methanol/water (1:1) and 10 μl was injected onto the HPLC-MS/MS system for quantitative analysis. The HPLC system (Shimadzu Corporation, Columbia, MD) was interfaced to an MDS Sciex triple quadrupole mass spectrometer (API 3200, Applied Biosystems, Foster City, CA) that was operated in the positive-ion electrospray ionization mode. For multiple-reaction monitoring, the transitions monitored were m/z 271.1 to 153.1 for genistein and m/z 255.2 to 181.2 for the internal standard. The standard curve ranges in plasma were 2.0 to 500.0 ng/ml for genistein. The data collection, peak integration and calculation were performed using Applied Biosystem MDS Analyst 1.4.2 software. We compared the plasma levels of free genistein from the two different diets using plasma samples taken at 16 and 28 weeks postirradiation. We found (Fig. 1) that at both 16 and 28 weeks the plasma level in rats given the 750 mg/kg diet in both the whole-lung irradiation and whole-lung plus whole-torso irradiation groups were similar. The rats receiving the oral dose of 3750 mg/kg with whole-lung irradiation showed 3–6-fold higher plasma concentrations than those receiving 750 mg/kg, consistent with the difference in the levels of consumed genistein (Supplementary Fig. 1; http://dx.doi.org/10.1667/RR2562.1.S1). The data also indicate that the whole-lung plus whole-torso irradiation rats on the higher dose had lower values, and for these rats the plasma values at 28 weeks were reduced. Since levels of consumed genistein were similar in the whole-lung irradiation and whole-lung plus whole-torso irradiation groups, these results may reflect reduced uptake in whole-lung plus whole-torso irradiated rats (Supplementary Fig. 1; http://dx.doi.org/10.1667/RR2562.1.S1). These results for the whole-lung irradiation treated Fischer rats are estimated to be approximately 6-fold lower than those reported for Wistar rats (16) and for pregnant Sprague-Dawley rats (28). The reasons for these differences are unclear, although the doses and conditions for delivery of the genistein were not identical to ours in these studies.
FIG. 1.
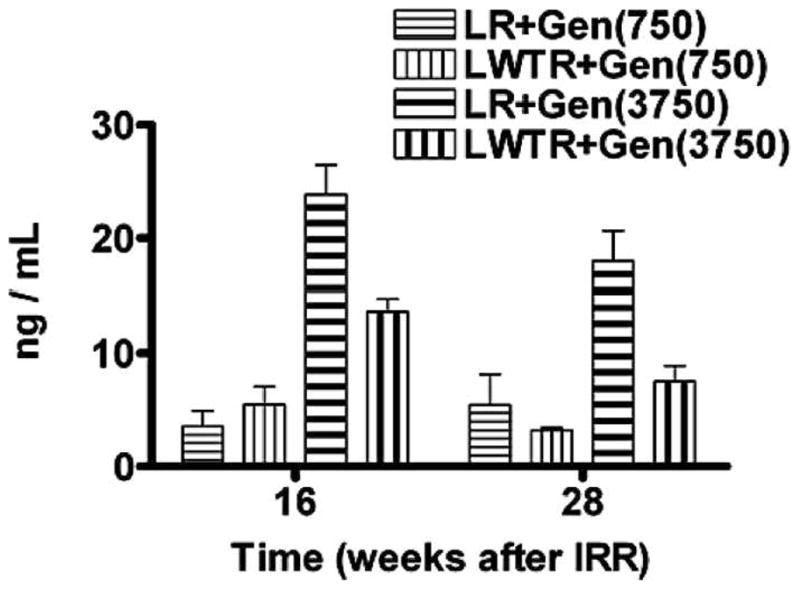
Analysis of free plasma genistein levels (ng/ml) using mass spectrometry at 16 and 28 weeks after 8 Gy whole-lung + 4 Gy whole-torso (LWTR) irradiation or 12 Gy whole-lung (LR) irradiation in rats given a diet containing 750 mg/kg or 3750 mg/kg of genistein.
Irradiation
An image-guided microirradiator (X-RAD 225Cx, Precision X-ray Inc., North Branford, CT) was used for targeting and irradiating the desired volume in each animal. The details of imaging characterization of the unit have been described previously (29). The unit was calibrated at 225 kVp, 13 mA for the purpose of irradiation, following the AAPM TG-61 protocol (30), and its output is verified by routine quality assurance. Further dosimetry was done for the geometry of the whole-lung and whole-torso irradiation by using EBT Gafchromic films in solid water. The dose rates at 225 kVp, 13 mA (HVL: 0.93 mm Cu, added filtration: 0.3 mm Cu) were estimated as 3.51 Gy/min and 3.15 Gy/min at depth of 1.5 cm in solid water for the whole-lung and whole-torso irradiation, respectively. For whole-lung irradiation, the animals were anesthetized by isofluorane inhalation and immobilized in a Lucite jig. Isofluorane fed to tubing inside the jig maintained the anesthetic condition during irradiation. Two circular lead surface collimators (OD 4.9 cm and ID 4 cm) were inserted on the surfaces of the jig to further facilitate targeting of the lung volume with minimized diaphragm in the radiation field. Animals were imaged and adjusted inside the jig for targeting the whole lung inside the lead surface collimators. The total imaging dose was estimated as less than 1 cGy. The animal then received either 8 Gy or 12 Gy with anterior-posterior (a-p) and p-a beams. The midplane of the animal, at a depth of 1.5 cm, was placed at the isocenter. For whole-torso irradiation, the animals were anesthetized by isofluorane inhalation and placed on the couch inside the irradiator. A source collimator was used for targeting and irradiating a volume from the neck to the base of tail. The field size on the midplane of the animal, depth of 1.5 cm, was 11 × 8 cm at extended source-to-surface distance. Images were taken and the couch, which is controlled by a PC, was adjusted for targeting the desired volume. Animals were treated with a-p and p-a beams for a total dose of 4 Gy. Animals were then set up for whole-lung irradiation (8 Gy), which was delivered within 5 min of the end of the whole-torso irradiation.
Breathing Rate Measurement
We measured the breathing frequency of rats using a respiration rate monitor (Columbus Instruments, Columbus, OH). The rats were acclimatized to the method several times in the 2 weeks preceding the start of the experiment. Breathing frequencies were then measured at 2, 4 and 6 weeks and then weekly from 7 to 36 weeks postirradiation. The breathing rate of each animal was measured for 2 min after an initial 45-s acclimatization period. Breathing rate was determined by taking the mean of a maximum of five 6-s intervals of calm breathing within the 2-min measurement period.
Lung Extraction and Immunohistochemistry
We used the whole left lung for immunohistochemical analysis while the lobes of right lung were used for the hydroxyproline assay and TBARS assay, respectively. For the lung tissue removal from the animals, α-MEM supplemented with antibiotics was perfused through the right ventricle of the hearts of deeply anaesthetized (ketamine/xylazine) animals to remove as much blood in the lungs as possible. The lungs were removed and the lobes of the right lung were immediately frozen in liquid nitrogen and stored frozen until analysis. In the majority of the animals a volume (0.5–1.0 ml) of 10% buffered formalin was injected into the left lobe of the lung to expand the alveoli and the lobes were placed in 10% formalin for at least 48 h for fixation. The whole left lobe of lungs was embedded in paraffin and sections 5 μm thick were cut and placed on slides in preparation for immunohistochemical staining by the research pathology laboratory in our facility. The primary antibodies used were activated macrophage marker ED-1 (MCA341, 1:100, AbD Serotec, Oxford, UK), cytokines IL-1α (sc-1254, 1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA), IL-1β (AAR15G, 1: 1000, AbD Serotec), IL-6 (sc-1265, 1:200, Santa Cruz Biotechnology), TNF-α (sc-1357, 1:200, Santa Cruz Biotechnology), TGF-beta (MCA797, 1:50, AbD Serotec), and for 8-hydroxy-2-deoxyguanosine (8-OHdG) (MOG-110P, 1:1000, JaICA, Shizuoka, Japan). Secondary antibodies included biotinylated anti-mouse immunoglobulin G, IgG (BA-1000, 1:200, Vector Laboratories), biotinylated anti-rabbit IgG (BA-1000, 1: 200, Vector Laboratories) and biotinylated anti-goat IgG (BA-5000, 1: 300, Vector Laboratories) for 30 min at room temperature.
Assessment of Fibrosis in Lungs
To image fibrosis, sections from the left lung were stained with the Masson’s trichrome procedure. Fibrosis was also assessed by analyzing part of the upper right lung for hydroxyproline content using a colorimetric assay on papain-digested lung tissue (P3125, Sigma-Aldrich Canada, Oakville, ON, Canada). Lung tissue (100 mg) was digested at 60°C for 48 h and then subjected to acid hydrolysis for 18 h at 110°C. Free hydroxyproline was released from protein and peptides into the solution, which was then neutralized. The hydroxyproline was oxidized into a pyrrole with chloramine T (857319, Sigma-Aldrich). This intermediate turns pink in color with the addition of Ehrlich’s Reagent (4-dimethylaminobenzaldehyde) (156477, Sigma-Aldrich Canada). The samples were loaded into a 96-well microplate and the absorbance was measured at 560 nm using a plate reader. The concentrations of the samples were determined from a standard curve using cis-4-hydroxy-L-proline (H1637, Sigma-Aldrich Canada).
Sircol Assay for Estimation of Recently Synthesized Collagen
The Sircol collagen assay (Biocolor Ltd., Belfast, UK), which measures recently synthesized collagen, was performed following the manufacturer’s instructions. It is a colorimetric procedure that uses a dye reagent containing Sirius red in picric acid, which specifically binds to soluble collagen. Part of the upper right lung (25 mg) was used for the analysis. A standard curve was derived from the kit and used to determine the collagen content of samples.
TBARS Assay to Measure Malondialdehyde (MDA)
A part of the right lung (25 mg) of each rat was analyzed for MDA levels using a TBARS assay kit (Cayman Chemical Company, Ann Arbor, MI). The flash-frozen sample was thawed, sonicated for 15 s in RIPA buffer with protease inhibitors (Roche Applied Science, Laval, Quebec), and then centrifuged (1600g). The supernatant containing the lipid fraction was used for the assay. A standard curve was derived from the kit and used to determine the MDA content of samples.
Image Analysis
After antibody staining, the slides were scanned using the ScanScope XT (Aperio Technologies, Vista, CA). This is a brightfield scanner that digitizes the whole microscope slide at 20× and 40× magnification and provides high-resolution images. The images can then be viewed with ImageScope (Aperio Technologies) for quantitative analysis. Using the Positive Pixel Algorithm, the whole lung section was analyzed and the number of positive pixels/number of positive and negative pixels × 100 was recorded (% positivity). The positive pixel count algorithm is used to quantify the amount of a specific stain present in a scanned slide image. The algorithm has a set of default input parameters preconfigured for brown color quantification in three intensity ranges (220–175, 175–100 and 100–0). Pixels that do not fall into the positive-color specification are considered negative; these pixels are counted as well, so that the fraction of positive to total stained pixels is determined. Air spaces were excluded. We analyzed at least one section from each rat for each time.
Statistical Analysis
Multiple linear regressions and Tukey’s method for the adjustment of least-squares means in multiple comparisons were used for analysis of the data sets. The radiation-only group was set as the primary comparison group for these analyses since the purpose of the study was to determine the efficacy of treatments relative to this group. A P value less than 0.05 was considered as significantly different. Mixed modeling was used to examine time trends in the breathing-rate data.
RESULTS
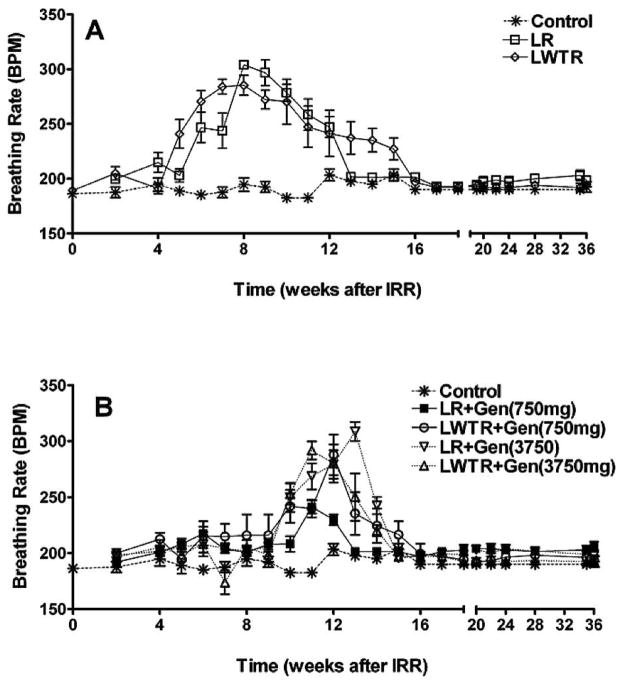
Breathing Rate Changes in Irradiated Rats
Radiation-induced lung damage is typically separated into two phases. Radiation pneumonitis usually occurs between 2–4 months after irradiation and fibrosis tends to develop after 4–6 months (31). These effects reduce the functional capacity of lung and, depending on their severity and extent, can be lethal. Increases in breathing rate are widely used in rodents as an indicator of pneumonitis caused by radiation. We examined the effects of the two different levels of genistein in the diet on the breathing rate measured over a 36-week period after lung irradiation (12 Gy) or lung (8 Gy) plus whole-torso irradiation (4 Gy). Figure 2A shows that the average breathing rate increased in the rats after irradiation both in whole-lung and whole-lung plus whole-torso irradiated groups after 4/5 weeks and returned to the normal level at 13 weeks for the whole-lung irradiation group but not until 16 weeks for the whole-lung plus whole-torso irradiation group (P < 0.05). In contrast, Fig. 2B shows that no rise in breathing rate was observed until 10 weeks for the groups of rats on a genistein diet (750 mg/kg or 3750 mg/kg) with whole-lung irradiation and whole-lung plus whole-torso irradiation, returning to control levels by 16 weeks (P < 0.05 relative to radiation-only groups). The group receiving whole-lung irradiation with the 750 mg/kg genistein diet showed the smallest rise in breathing rate at 10–11 weeks compared to the other treated groups and returned to the control level at 14 weeks (P < 0.05 relative to the other radiation + genistein-treated groups). The other three radiation plus genistein treatment groups were not significantly different.
FIG. 2.
Breathing rate (mean breaths per minute) as a function of time after irradiation. Panels A and B: Control = no irradiation (soy-free diet); LR = whole-lung irradiation (12 Gy); LWTR = whole-torso (4 Gy) + whole-lung irradiation (8 Gy); Gen = genistein diet at 750 mg/kg or 3750 mg/kg. The drug treatments were started 1 week postirradiation and terminated at 28 weeks, and the rats were euthanized at 36 weeks. Each point represents the mean ± SEM for all rats available for analysis at different times.
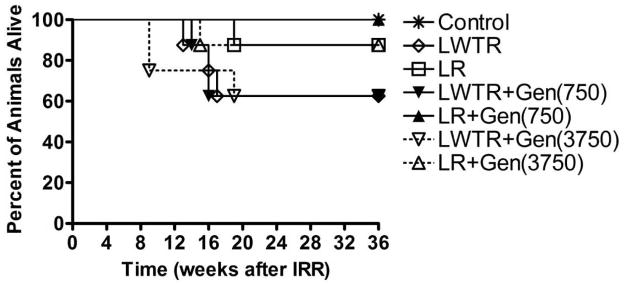
Survival of Irradiated Rats
That the radiation treatment given to the rats was at the maximum level of their tolerance was demonstrated by the fact that a proportion of the irradiated rats had to be euthanized due to excessive morbidity in the 6–18-week (pneumonitis) time window (Fig 3). The rats given whole-lung plus whole-torso irradiation (taken as a group) showed significantly (P < 0.01) decreased levels of survival at 20–36 weeks (15/24 = 62.5%) compared to those given whole-lung irradiation (22/24 = 92%), suggesting that the rats were less able to tolerate this combination treatment. However, the differences were not statistically significant for the individual treatment groups. Almost all the rats that received whole-lung irradiation with genistein survived until the termination of the study at 36 weeks. There was an initial decrease of body weight in irradiated rats during the pneumonitis stage. However, after the pneumonitis stage, the irradiated rats gained weight similarly to the other rats, although the animals always had a lower weight than the controls and those on the diet with the higher level of genistein did not gain as much weight as those on the diet with the lower concentration (Supplementary Fig. 1; http://dx.doi.org/10.1667/RR2562.1.S1).
FIG. 3.
Survival of rats as a function of time after irradiation. Labeling of the treatment groups is as indicated in the legend to Fig. 2.
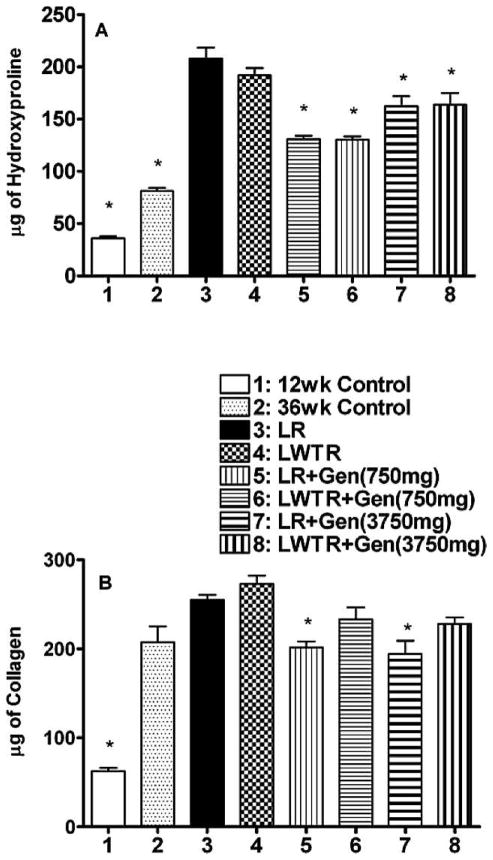
Lung Fibrosis
Fibrosis tends to develop 4–6 months after irradiation and is reflective of excess collagen deposition stimulated by profibrotic cytokines such as TGF-β (31–40). Masson’s trichrome stains collagen fibers and is widely used as a marker of collagen deposition and fibrosis in lung studies. A panel of photomicrographs illustrating the Masson’s trichrome staining is shown in Supplementary Fig. 2 (http://dx.doi.org/10.1667/RR2562.1.S1) and shows increased staining levels in the irradiated lungs, with reduced levels in the animals given the genistein diet. Since this staining is only semi-quantitative, we measured the amount of hydroxyproline in the lungs as a quantitative measure of collagen content. Figure 4A shows the hydroxyproline content of lung tissue at 36 weeks. Both whole-lung irradiation and whole-lung plus whole-torso irradiation caused a significant (P < 0.01) increase in hydroxyproline content of the lung tissue relative to both the 12-week and 36-week controls. Both doses of genistein caused a significant decrease in hydroxyproline content in both whole-lung irradiation and whole-lung plus whole-torso irradiation groups of rats (P < 0.05), with the 750 mg/kg diet showing a greater reduction in hydroxyproline content than the 3750 mg/kg diet (P < 0.05). To assess collagen production in the lungs, we measured recently synthesized (RS) collagen using the Sircol assay (Fig. 4B). The recently synthesized collagen was increased by both whole-lung plus whole-torso irradiation and whole-lung irradiation by similar amounts relative to the 36-week control (P < 0.05). For the whole-lung irradiation groups, both genistein diets resulted in a significant decrease (P < 0.05) relative to whole-lung irradiation, but for the whole-lung plus whole-torso irradiation groups, there was only a trend for the genistein diets to cause a decrease.
FIG. 4.
Measurements of fibrosis in the lungs of the rats at time of euthanasia at 36 weeks after irradiation. Panel A: Hydroxyproline (μg of hydroxyproline/100 mg of wet lung tissue) content of the lung tissue. Panel B: Recently synthesized collagen (μg of soluble collagen/600 μg of wet lung tissue). Each bar represents the mean ± SEM for all rats available for analysis. Labeling of the treatment groups is as indicated in the legend to Fig. 2. Twelve-week controls animals were euthanized at the start of the experiment; 36-week controls were euthanized at 36 weeks postirradiation. The asterisks indicate groups that are significantly different from the whole-lung irradiation groups in each panel.
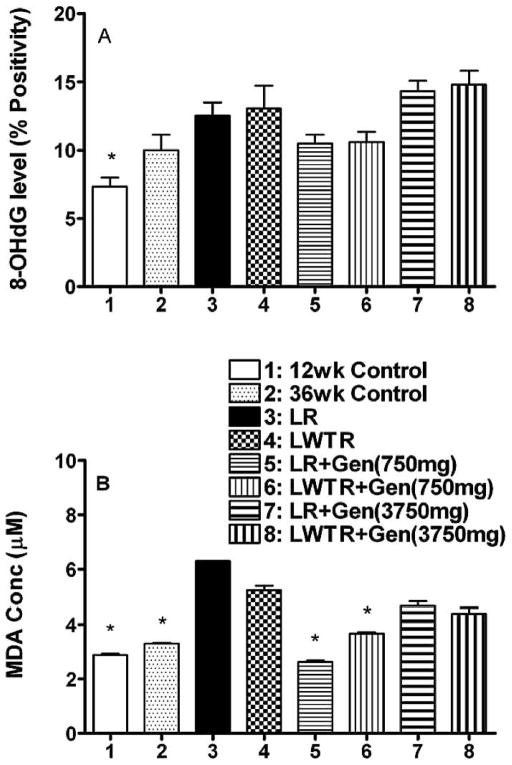
Oxidative Damage
It has been proposed that radiation-induced late effects are caused in part by chronic oxidative stress and inflammation. Increased production of reactive oxygen species, which leads to lipid peroxidation and oxidation of DNA and proteins, as well as activation of pro-inflammatory factors, has been observed in vitro and in vivo after irradiation (15, 33, 41). Consequently we assessed the levels of oxidative damage in the lung tissue using two different measures, levels of 8-hydroxy-2-deoxyguanosine (8-OHdG), a marker of oxidative damage in the DNA (42), and levels of malondialdehyde (MDA), a measure of lipid peroxidation. A panel of micrographs showing the 8-OHdG staining is shown in Supplementary Fig. 3 (http://dx.doi.org/10.1667/RR2562.1.S1). The relative expression of 8-OHdG staining was determined by analyzing a whole lung section for each rat and the number of positive pixels/number of positive and negative pixels × 100 (% positivity) recorded (see the Methods section for details). We observed (Fig. 5A) that there was a trend for an increase in relative expression (% positivity of staining) of 8-OHdG in whole-lung or whole-lung plus whole-torso irradiated rats at 36 weeks relative to the 36-week controls. The treatment with the 750 mg/kg genistein diet showed a trend for a decrease in 8-OHdG levels, but this did not occur in rats that received the 3750 mg/kg genistein diet. There were no differences in the whole-lung irradiation and whole-lung plus whole-torso irradiation groups. Results from the TBARS assay to quantify the MDA levels in the lungs are shown in Fig. 5B. The MDA concentration showed an increase in whole-lung (P < 0.05) and whole-lung plus whole-torso (P = 0.09) irradiated rats relative to the 36-week controls. The MDA concentration was significantly decreased by the treatment of genistein with the 750 mg/kg diet (P < 0.05), but there was only a trend for lower levels with the 3750 mg/kg diet.
FIG. 5.
Measures of oxidative damage in the lungs of the rats at time of euthanasia at 36 weeks after irradiation. Panel A: Analysis of 8-OHdG staining as percentage positivity (the ratio of positive pixels/total number of positive and negative pixels in the tissue section with air spaces excluded); Panel B: Analysis of MDA levels (μM) in the lung tissue. Each bar represents the mean ± SEM for all rats available for analysis. Labeling of the treatment groups is as indicated in the legend to Fig. 2. Twelve-week controls animals were euthanized at the start of the experiment; 36-week controls were euthanized at 36 weeks postirradiation. The asterisks indicate groups that are significantly different from the whole-lung irradiation groups in each panel.
Levels of Inflammatory Cytokines and Activated Macrophages
Oxidative stress in the lung after irradiation is generally thought to be due to a chronic inflammatory response. Thus we assessed levels of inflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α) and levels of activated macrophages using immunohistochemistry. We also assessed levels of TGF-β, since it is one of the key players in the formation of radiation-induced fibrosis in lung tissue. The IL-1α levels were elevated in the lung tissue at 36 weeks after both whole-lung irradiation and whole-lung plus whole-torso irradiation but the genistein diets had no effect on these levels (Supplementary Fig. 4; http://dx.doi.org/10.1667/RR2562.1.S1). A similar result was obtained for IL-6. There was a trend for the lower genistein diet (750 mg/kg) to give reduced values relative to radiation alone for IL-1β and TNF-α, but these effects were not significant (Supplementary Fig. 4; http://dx.doi.org/10.1667/RR2562.1.S1).
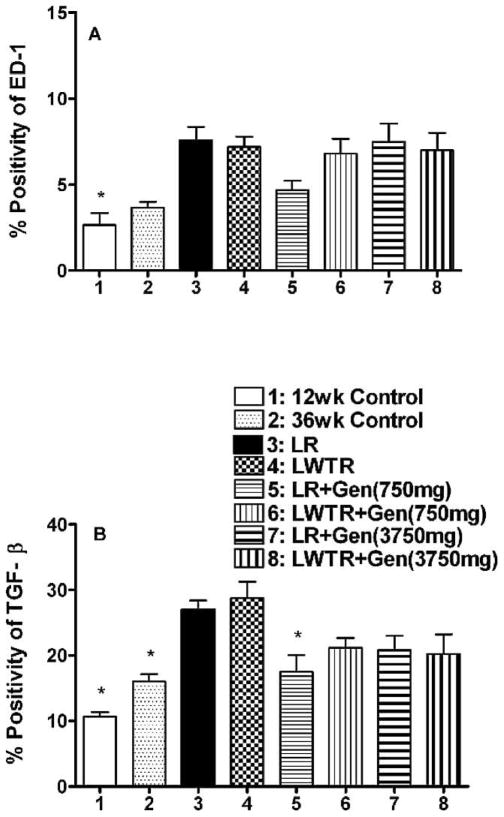
Panels of micrographs showing the staining for activated macrophages (ED-1) and TGF-β at 36 weeks postirradiation are shown in Supplementary Figs. 5 and 6 (http://dx.doi.org/10.1667/RR2562.1.S1). The relative expression of the staining was determined as described previously, and levels of activated macrophages are shown in Fig. 6A. Levels of ED-1 staining were elevated in the whole-lung and whole-lung plus whole-torso irradiated groups relative to the 12-week controls (P < 0.05) and 36-week controls (P = 0.1), but only the animals given the 750 mg/kg genistein diet after whole-lung irradiation showed a trend for a reduction in levels of staining. TGF-β staining levels are shown in Fig. 6B. Both whole-lung irradiation and whole-lung plus whole-torso irradiation resulted in significantly (P < 0.05) increased levels of TGF-β expression. Treatment with both genistein diets showed decreases in TGF-β expression, but only the result for whole-lung irradiation + 750 mg/kg diet was statistically significant (P < 0.05). A comparison of these data with those for the ED-1 staining in Fig. 6A shows a similar pattern of expression for the different treatments, although the treatments did not decrease levels of ED-1 staining to the same extent as TGF-β.
FIG. 6.
Analysis of macrophage activation and TGF-β levels in the lungs of the rats at time of euthanasia at 36 weeks after irradiation. Panel A: Analysis of macrophage activation (ED-1 staining) as percentage positivity (the ratio of positive pixels/total number of positive and negative pixels in the tissue section with air spaces excluded). Panel B: Analysis of TGF-β staining as percentage positivity. Each bar represents the mean ± SEM for all rats available for analysis. Labeling of the treatment groups is as indicated in the legend to Fig. 2. Twelve-week controls animals were euthanized at the start of the experiment; 36-week controls were euthanized at 36 weeks postirradiation. The asterisks indicate groups that are significantly different from the whole-lung irradiation groups in each panel.
DISCUSSION
In the work presented here we focused on the combination of whole-lung and whole-torso irradiation since this is a possible scenario if there is a radiation accident and there is a possibility of interacting effects in lung relating to multi-organ damage (8–10). We were particularly interested in the effects of genistein as a mitigator of radiation-induced lung damage because of its antioxidant and anti-NFκB activity and its wide availability and low toxicity (16–18, 43). We previously found that genistein (750 mg/kg diet) could provide some mitigation against radiation-induced lung injury out to 28 weeks in Sprague-Dawley rats even when treatment ceased at 14 weeks, but there was a limited reduction of fibrosis (22). Consequently we were interested in examining a higher dose of dietary genistein to determine if this would provide greater mitigation and to determine if the effects would be observed in a different strain of rats. Genistein is also of interest therapeutically for cancer patients because it has been reported to inhibit invasion, metastasis and angiogenesis in vitro and in vivo in a number of cancers, although recent data have raised questions about this effect in prostate cancers (44–52).
We observed an increased breathing rate after irradiation both in whole-lung and whole-lung plus whole-torso irradiated rats in the period 4–16 weeks after irradiation (the pneumonitis time window). We found that treatment with genistein caused damping and shifting of the increase in breathing rate that was particularly apparent for the animals on the 750 mg/kg diet. The higher dietary dose did not result in a greater effect. Interestingly, there was no secondary elevation of breathing rate at 20–40 weeks as observed in Sprague-Dawley rats in our previous study (22) and consistent with other studies (53). This suggests that Fischer rats may be more resistant to radiation-induced fibrosis. However, our analyses of collagen content in the lungs of the rats demonstrated significant increases in collagen content (consistent with fibrosis) at 36 weeks postirradiation. Possibly the extent of lung fibrosis was not severe enough to cause functional deficit to a critical level. Studies by others using high doses (28 Gy) to the right lung only have also demonstrated the development of severe fibrosis in the lungs of Fischer rats (54–56). In those studies the increase in breathing rate was more prolonged, extending out to 24 weeks, possibly related to the larger dose of radiation delivered in those studies.
The results for both Masson’s trichrome staining and hydroxyproline content showed that treatment with genistein resulted in a reduction of fibrosis in the rats (Fig. 4a and Supplementary Fig. 2; http://dx.doi.org/10.1667/RR2562.1.S1). Disappointingly, it was again found that the higher dose of genistein in the diet did not result in a greater level of mitigation. It should be noted that these measurements were made 2 months after the cessation of the drug treatment, indicating prolonged mitigation of the radiation effect, consistent with our previous study in Sprague-Dawley rats when treatment was stopped at 14 weeks after irradiation (57). Levels of recently synthesized collagen were observed to be still elevated at these late times, and the drug treatments showed evidence for a modest reduction of the increased levels (Fig. 4B).
We have previously reported that, after partial-lung irradiation, DNA damage can be observed both in and out of the radiation field (58, 59) and that this damage can be largely suppressed by genistein or ROS scavengers given after irradiation (22–24, 60). This supports the concept that DNA damage in the lung can be caused by the action of inflammatory cytokines and the resultant production of ROS. In the current study the drug treatments reduced levels of oxidative damage in the lungs of the rats for both DNA oxidation and lipid peroxidation, and again there was a trend for a slightly greater effect with the lower dietary treatment (Fig. 5 and Supplementary Fig. 3; http://dx.doi.org/10.1667/RR2562.1.S1). These results on oxidative damage are of particular interest because, depending on repair and turnover rates, the lesions measured should reflect ongoing levels of oxidative stress rather than that induced at the time of irradiation. The higher levels in the irradiated groups thus suggest that increased levels of oxidative stress are prolonged in the irradiated animals. Since the drug treatments were stopped at 28 weeks whereas the analyses were performed at 36 weeks after irradiation, the results imply that the earlier drug treatments were able to mitigate the ongoing levels of chronic oxidative stress in the lungs.
Despite these observations, the actual measurements of inflammatory cytokines in the lung tissue at these late times showed limited evidence that the drug treatments had affected their expression (Supplementary Fig. 4; http://dx.doi.org/10.1667/RR2562.1.S1), although the lower dose of dietary genistein did cause reductions in the level of activated macrophages in the tissue (Fig. 6 and Supplementary Fig. 5; http://dx.doi.org/10.1667/RR2562.1.S1). Previous studies have reported that genistein given before whole-body irradiation (7 Gy) resulted in an elevation of IL-6 in the serum of mice at 4 h after irradiation (61). Our previous studies with Sprague-Dawley rats demonstrated that dietary genistein significantly modified the effects of radiation on TNF-α levels in the lung postirradiation but had limited effects on radiation-induced IL-1a, IL-1b, IL-6 and TGF-β levels at times out to 6 months after irradiation [(24) and unpublished data].
One of the best known protectors for administration before irradiation is amifostine (WR 2721), which is a thiophosphate compound that is converted to an aminothiol in vivo by alkaline phosphatase. When given before irradiation it has been reported to reduce radiation-induced pneumonitis, fibrosis and plasma TGF-β level (62). This agent has also been reported to protect humans against lung injury during radiotherapy (31, 63), but there is little available information about its potential to mitigate or treat the development of injury. Other pharmacological agents that have been found to be partially effective for lung protection after irradiation include pentoxifylline, ACE inhibitors (such as captopril), and angiotensin receptor blockers (12, 36, 64). Again, however, there is limited information about these agents as mitigators of radiation-induced lung damage, although captopril has been reported to mitigate survival, vascular damage and breathing rate increases in irradiated rat lung (65).
Previous studies using antioxidants to modify radiation-induced lung damage have mostly initiated treatment prior to irradiation. However, Rabbani et al. (56) have found that chronic administration of small-molecular-weight catalytic metalloporphyrin antioxidants over a 10-week period after irradiation to female Fischer rats mitigated the effects of a single dose of 28 Gy given to the hemithorax. A more recent study (66) using the same model also reported mitigation with treatments of small-molecular-weight catalytic metalloporphyrin antioxidants that started at various times after irradiation. These authors measured effects at 10 weeks after irradiation and found that maximal mitigation occurred when the treatment was initiated within the first 12 h after irradiation. Both these studies support our findings of mitigation with genistein, although we observed equivalent effects when treatment was initiated 1 week postirradiation.
Figure 3 demonstrates that the animals given whole-torso plus whole-lung irradiation had more severe morbidity than animals given whole-lung irradiation only. That this severe morbidity occurred during the pneumonitis time window suggests effects of damage to other organs on lung response (9). The dose to the other organs was too low to cause lethality in itself. Previous studies in mice have reported a similar result in which the lethal dose for lung irradiation was lower in mice exposed to a whole-body dose of radiation (67, 68). This was thought to be related to the effects of radiation on circulating T cells in the animals. In our breathing rate measurements the group treated with whole-lung plus whole-torso irradiation had a prolonged increase relative to the whole-lung irradiation group, and there was a suggestion that the genistein diet (750 mg/kg) mitigated the increase to a greater extent in the whole-lung irradiation group relative to the whole-lung plus whole-torso irradiation group. However, none of the other measurements made on the lungs at the time of euthanasia of the animals demonstrated significant evidence of a greater effect in the whole-lung plus whole-torso irradiation group relative to the whole-lung irradiation group.
SUMMARY
In this study we used a simulated model of accidental radiation exposure (whole torso and whole lung) to investigate mitigation of radiation-induced lung damage. Animals given whole-torso plus whole-lung irradiation had more severe lung-related morbidity and pneumonitis than animals given whole-lung irradiation only. The lower dose of genistein (750 mg/kg in the diet) showed significant but partial mitigation of lung damage (out to 36 weeks) even when the drug treatments were initiated 1 week after irradiation and stopped at 28 weeks after irradiation. The larger dose of genistein (3750 mg/kg in the diet) was not more efficient and in some cases was less efficient in this regard. Other pharmacological agents that have been found to be effective for lung protection in rodents include statins, ACE inhibitors and angiotensin II receptor blockers (12, 13, 65). Combining genistein with one of these drugs could open new avenues to further mitigate radiation-induced lung damage.
Supplementary Material
Acknowledgments
The authors thank Dr. Patricia Lindsay with help with the dosimetry. This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and by funds from the Canadian Institutes of Health Research (#144089). Partial support was also provided by the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
The online version of this article (DOI: 10.1667/RR2562.1) contains supplementary information that is available to all authorized users.
References
- 1.Drouet M, Herodin F. Radiation victim management and the haematologist in the future: time to revisit therapeutic guidelines? Int J Radiat Biol. 2010;86:636–48. doi: 10.3109/09553001003789604. [DOI] [PubMed] [Google Scholar]
- 2.Vlasov PA, Kvacheva Iu E. The pathomorphology of the pulmonary infectious complications in acute radiation sickness based on the autopsy data from persons who died as a result of the accident at the Chernobyl Atomic Electric Power Station. Ter Arkh. 1996;68:23–6. [PubMed] [Google Scholar]
- 3.Goans RE, Wald N. Radiation accidents with multi-organ failure in the United States. BJR Suppl. 2005;27:41–6. [Google Scholar]
- 4.Hirama T, Akashi M. Multi-organ involvement in the patient who survived the Tokai-mura criticality accident. BJR Suppl. 2005;27:17–20. [Google Scholar]
- 5.Svendsen ER, Kolpakov IE, Stepanova YI, Vdovenko VY, Naboka MV, Mousseau TA, et al. 137Cesium exposure and spirometry measures in Ukrainian children affected by the Chernobyl nuclear incident. Environ Health Perspect. 2010;118:720–5. doi: 10.1289/ehp.0901412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M, et al. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. BJR Suppl. 2005;27:13–6. [Google Scholar]
- 7.Densow D. Are there ‘common denominators’ in different radiation exposure scenarios as a target for predictive assessment? Stem Cells. 1995;13 (Suppl 1):307–17. doi: 10.1002/stem.5530130738. [DOI] [PubMed] [Google Scholar]
- 8.Densow D, Kindler H, Baranov AE, Tibken B, Hofer EP, Fliedner TM. Criteria for the selection of radiation accident victims for stem cell transplantation. Stem Cells. 1997;15 (Suppl 2):287–97. doi: 10.1002/stem.5530150738. [DOI] [PubMed] [Google Scholar]
- 9.Fliedner TM, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR Suppl. 2005;27:1–8. [Google Scholar]
- 10.Williams JP, McBride WH. After the bomb drops: A new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87:851–68. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Pollard JM, Norris AJ, McDonald JT, Sun Y, Micewicz E, et al. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin Cancer Res. 2009;15:7238–45. doi: 10.1158/1078-0432.CCR-09-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17:141–8. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386–94. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol. 2009;2:122–33. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 16.Michael McClain R, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 18.Choi C, Cho H, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFkappaB activation in RAW 264.7 macrophages. Biosci Biotechnol Biochem. 2003;67:1916–22. doi: 10.1271/bbb.67.1916. [DOI] [PubMed] [Google Scholar]
- 19.Kang JL, Lee HW, Lee HS, Pack IS, Chong Y, Castranova V, et al. Genistein prevents nuclear factor-kappa B activation and acute lung injury induced by lipopolysaccharide. Am J Respir Crit Care Med. 2001;164:2206–12. doi: 10.1164/ajrccm.164.12.2104017. [DOI] [PubMed] [Google Scholar]
- 20.Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–85. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 21.Day RM, Barshishat-Kupper M, Mog SR, McCart EA, Prasanna PG, Davis TA, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat Res (Tokyo) 2008;49:361–72. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173:602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Para AE, Bezjak A, Yeung IW, Van Dyk J, Hill RP. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92:500–10. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Hill RP, Zaidi A, Mahmood J, Jelveh S. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011 doi: 10.1016/j.radonc.201106.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko T, Tahara S, Tanno M, Taguchi T. Effect of age on the induction of 8-oxo-2′-deoxyguanosine-releasing enzyme in rat liver by gamma-ray irradiation. Arch Gerontol Geriatr. 2003;36:23–35. doi: 10.1016/s0167-4943(02)00056-0. [DOI] [PubMed] [Google Scholar]
- 26.Coldham NG, Zhang AQ, Key P, Sauer MJ. Absolute bioavail-ability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet. 2002;27:249–58. doi: 10.1007/BF03192335. [DOI] [PubMed] [Google Scholar]
- 27.Holder CL, Churchwell MI, Doerge DR. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ES-MS. J Agric Food Chem. 1999;47:3764–70. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- 28.Soucy NV, Parkinson HD, Sochaski MA, Borghoff SJ. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol Sci. 2006;90:230–40. doi: 10.1093/toxsci/kfj077. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson R, Lindsay PE, Ansell S, Wilson G, Jelveh S, Hill RP, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2011;38:845–56. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, et al. AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys. 2001;28:868–93. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 31.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 32.Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28:621–31. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 33.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Haiping Z, Takayama K, Uchino J, Harada A, Adachi Y, Kura S, et al. Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther. 2006;13:864–72. doi: 10.1038/sj.cgt.7700959. [DOI] [PubMed] [Google Scholar]
- 35.Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J. Predicting risk of radiation-induced lung injury. J Thorac Oncol. 2007;2:864–74. doi: 10.1097/JTO.0b013e318145b2c6. [DOI] [PubMed] [Google Scholar]
- 36.Molteni A, Wolfe LF, Ward WF, Ts’ao CH, Molteni LB, Veno P, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 37.Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, et al. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165–74. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 39.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–42. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 40.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, et al. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem. 2004;279:15167–76. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- 41.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 42.van Loon B, Markkanen E, Hubscher U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair (Amst) 2010;9:604–16. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Baxa DM, Yoshimura FK. Genistein reduces NF-kappa B in T lymphoma cells via a caspase-mediated cleavage of I kappa B alpha. Biochem Pharmacol. 2003;66:1009–18. doi: 10.1016/s0006-2952(03)00415-5. [DOI] [PubMed] [Google Scholar]
- 44.Chambers AF. Influence of diet on metastasis and tumor dormancy. Clin Exp Metastasis. 2009;26:61–6. doi: 10.1007/s10585-008-9164-4. [DOI] [PubMed] [Google Scholar]
- 45.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69:3695–703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farina HG, Pomies M, Alonso DF, Gomez DE. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep. 2006;16:885–91. [PubMed] [Google Scholar]
- 47.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–32. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 48.Magee PJ, McGlynn H, Rowland IR. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004;208:35–41. doi: 10.1016/j.canlet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, Dharmawardhane S. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27:465–80. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011;6:e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006;66:1851–8. doi: 10.1158/0008-5472.CAN-05-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65:3396–403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 53.van Eerde MR, Kampinga HH, Szabo BG, Vujaskovic Z. Comparison of three rat strains for development of radiation-induced lung injury after hemithoracic irradiation. Radiother Oncol. 2001;58:313–6. doi: 10.1016/s0167-8140(00)00301-7. [DOI] [PubMed] [Google Scholar]
- 54.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–9. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, et al. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87:889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int J Radiat Oncol Biol Phys. 1998;40:467–76. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 59.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 60.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79:231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Singh VK, Grace MB, Parekh VI, Whitnall MH, Landauer MR. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int Immunopharmacol. 2009;9:1401–10. doi: 10.1016/j.intimp.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Vujaskovic Z, Feng QF, Rabbani ZN, Anscher MS, Samulski TV, Brizel DM. Radioprotection of lungs by amifostine is associated with reduction in profibrogenic cytokine activity. Radiat Res. 2002;157:656–60. doi: 10.1667/0033-7587(2002)157[0656:rolbai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Antonadou D, Throuvalas N, Petridis A, Bolanos N, Sagriotis A, Synodinou M. Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57:402–8. doi: 10.1016/s0360-3016(03)00590-x. [DOI] [PubMed] [Google Scholar]
- 64.Ozturk B, Egehan I, Atavci S, Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2004;58:213–9. doi: 10.1016/s0360-3016(03)01444-5. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, et al. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75:1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 68.McBride WH, Vegesna V. The role of T-cells in radiation pneumonitis after bone marrow transplantation. Int J Radiat Biol. 2000;76:517–21. doi: 10.1080/095530000138529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.