Abstract
Forest ecosystems store approximately 45% of the carbon found in terrestrial ecosystems, but they are sensitive to climate-induced dieback. Forest die-off constitutes a large uncertainty in projections of climate impacts on terrestrial ecosystems, climate–ecosystem interactions, and carbon-cycle feedbacks. Current understanding of the physiological mechanisms mediating climate-induced forest mortality limits the ability to model or project these threshold events. We report here a direct and in situ study of the mechanisms underlying recent widespread and climate-induced trembling aspen (Populus tremuloides) forest mortality in western North America. We find substantial evidence of hydraulic failure of roots and branches linked to landscape patterns of canopy and root mortality in this species. On the contrary, we find no evidence that drought stress led to depletion of carbohydrate reserves. Our results illuminate proximate mechanisms underpinning recent aspen forest mortality and provide guidance for understanding and projecting forest die-offs under climate change.
Keywords: carbon starvation, ecosystem shift, biosphere–atmosphere feedbacks, drought impacts, global change ecology
Forests are important carbon sinks, yet they are threatened by global change (1, 2). In the past decade, widespread forest mortality related to drought or temperature stress has been documented in multiple biomes and on all vegetated continents (3–6). In temperate North America, some of these events have been linked to “global change-type droughts,” defined as severe drought coupled with elevated summer temperatures (6–9). Such mortality events can radically transform regional land cover and affect biodiversity, fire risk, ecosystem function, land–atmosphere interactions, and ecosystem services (10–12). Furthermore, forest diebacks can lead to dramatic decreases in net primary production and carbon sequestration, driving these ecosystems to become CO2 sources and to have a positive feedback to climate warming (11, 13–17). Climate-mediated die-off of pine forests caused by insect outbreak in Canada led to estimated carbon emissions of 990 Mt CO2e (CO2 equivalent) over a 20-y period, equivalent to 5 y of Canada's annual transportation sector emissions (200 Mt CO2e/y) (15). The response of forests to climate warming remains a large uncertainty in climate change impacts projections on terrestrial ecosystems, climate-ecosystem feedbacks, and climate policy (7, 15–20).
We currently lack understanding of the mechanisms that underlie drought-induced forest mortality (4). Such mechanisms are critical to inform projections of mortality events with future climate change, but have been difficult to test directly and in situ. A recent synthesis put forth two broad non–pathogen-mediated and nonexclusive physiological hypotheses that could underlie drought-induced (soil water depletion or high evaporative demand) forest mortality (4). The carbon starvation hypothesis posits that drought drives stomatal closure and/or other effects on photosynthesis, leading to a negative carbon balance that depletes carbohydrate reserves and ends in tissue-level carbohydrate starvation (4). The hydraulic failure hypothesis suggests that drought increases an individual tree's water stress to the point at which the water transport system that supplies leaves is impaired (4). This occurs when the water column in xylem conduits breaks, or cavitates, as a result of excessively negative xylem sap pressure, leading to tissue dehydration.
However, tree mortality during drought is likely to be a complex phenomenon involving interacting and interconnected mechanisms and systems with feedbacks that may not be captured by these two hypotheses (21). Thus, although measuring the fluxes of carbon and water (e.g., photosynthesis, respiration, transpiration) that occur during drought can be informative, directly measuring the carbon/water status in the system (e.g., carbohydrate reserves, hydraulic conductivity) holds great potential to shed light on the mechanisms and processes that occur as a result of drought stress (22). Almost all previous research has focused on measuring fluxes (23) and used them to infer mechanisms but has not directly measured the state of system stress (24). We provide a direct examination of carbon stress and hydraulic stress during ongoing drought-induced widespread forest die-off. These can be considered tests of carbon starvation and hydraulic failure, as posited by McDowell et al. (4). However, more importantly, we suggest that they provide insight into the processes that occur during drought and tree health decline. Carbon and hydraulic changes during plant mortality are highly interrelated and likely interact, and although we present an initial approximation of their respective roles, they should not be viewed in isolation (Results and Discussion).
A previous study examined carbon starvation and hydraulic failure in an experimental manipulation of Pinus edulis under global change-type droughts and found earlier mortality under elevated temperatures, which stimulated respiration and could deplete carbon reserves (23). However, carbohydrate reserves were not measured, and elevated temperatures could have also increased stress on the hydraulic system—making it difficult to distinguish between these alternative interpretations of carbon and hydraulic stress (24, 25). Two recent studies have measured carbohydrate reserves and found mixed support for root or sapwood carbohydrate depletion during drought, but current evidence for carbon starvation is quite limited (26–28). To our knowledge, no previous studies on drought-induced forest mortality have directly measured both the carbon and hydraulic stress in mature and drought-stressed trees while they were dying to examine mechanisms that induce mortality (22, 24).
We present here a series of observations and experiments that examine carbon and hydraulic stress as proximate mechanisms of forest mortality. We measure hydraulic stress through use of standard plant water relations metrics of xylem pressure and hydraulic conductivity. We assess carbon stress through the metric of tissue nonstructural carbohydrate (NSC) reserves, which are considered to be important throughout tree ontogeny (29). This definition of carbon starvation (presented in ref. 4) includes all the stored carbon reserves that are employable for plant growth and maintenance. Depletion of this pool is therefore a parsimonious test of carbon starvation. Although we have performed analyses that suggest that pathogens play little role in inciting this die-off (SI Methods), we do not examine here other potential pathways of carbon-related mortality, such as mechanisms related to impaired carbon production, mobilization, transport, and utilization, or carbon–hydraulic interactions (21, 22) (Results and Discussion).
We examine a recent climate-induced widespread mortality in trembling aspen (Populus tremuloides) in the western United States. This die-off event, termed sudden aspen decline (SAD), began following severe drought from 2000 to 2004 and affects an estimated 17% of aspen forests in many western states, and similar die-off has been observed in parts of Canada (9, 30). Potential carbon emissions from areas of aspen die-off in Canada alone were estimated as equivalent to 7% of Canada's entire annual emissions (30). Following this severe drought, SAD continued through 2010 with SAD ramets in the process of dying, allowing direct measurements of physiological stresses inducing the die-off.
Results and Discussion
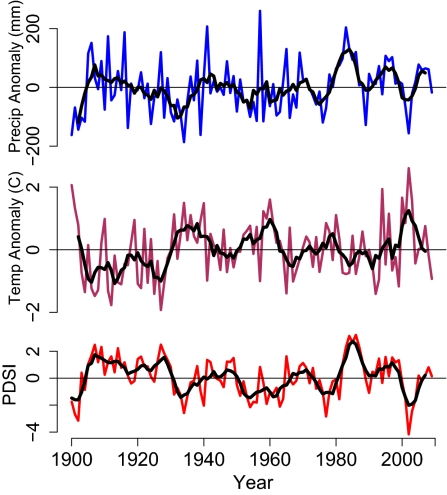
We examined regional climate records at 51 aspen field sites across western Colorado from 1900 to 2009. The 2000 to 2004 period was the most severe drought on record at these sites, largely as a result of high summertime temperatures rather than precipitation anomaly (Fig. 1). Thus, this drought has been considered a global change-type drought that is likely to become more frequent with future climate change (6, 23).
Fig. 1.
Precipitation and temperature deviations from 1900 to 1999 average, especially the severe drought of 2000 to 2004, at 51 aspen field sites across Colorado. (Top) Annual rainfall anomaly (blue) during 1900 to 2009 and 5-y average (black). (Middle) Summer temperature anomaly (maroon) during 1900 to 2009 and 5-y average (black). (Bottom) Annual Palmer Drought Severity Index (red) and 5-y average from 1900 to 2009 (black).
SAD has been found in previous studies predominantly at the lower elevation edge of aspen's distribution (9). Thus, if carbon starvation is associated with aspen dieback, we predict that stored total NSC reserves should decline at lower elevational margins of aspen's range. To examine this, we measured root NSC reserves, considered to be the main storage tissue in aspen (27), in 51 aspen forest sites across western Colorado. Total NSC reserves as well as individual carbohydrate levels did not vary across aspect, elevation, and level of stand mortality (defined as the average ramet crown mortality across all ramets surveyed in the stand; ANOVA, F = 0.12; P = 0.97; Fig. 2A and SI Methods: Mortality Measurements), although they did vary seasonally and with defoliation treatments in nondroughted trees in other experiments. Although this correlative analysis could have missed ramets that already died of NSC depletion, we test carbohydrate depletion in additional tissues and with more mechanistic tests (as detailed later), and our results are corroborated by a study that found increases in aspen seedling's root carbohydrate reserves with simulated drought (27).
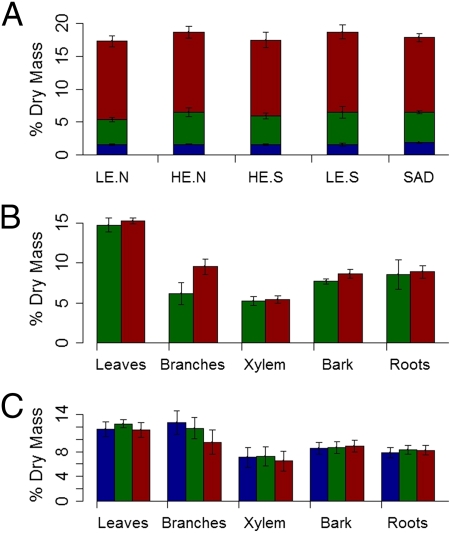
Fig. 2.
(A) Percent dry-mass (mean ± SE) of glucose (blue), sucrose (green), and starch (red) in root tissues across elevation, aspect, and stand health. Treatments are low elevation/north-facing (LE-N), high elevation/north-facing (HE-N), high elevation/south-facing (HE-S), low elevation/south-facing (LE-S), and SAD stands. (B) Percent dry mass of starch in five tissues from two isolated plots of mature aspen ramets after experimental drought (control, green; drought, red). (C) Percent dry mass of starch in seven clones on a gradient of stand mortality (healthy ramets in healthy area, blue; healthy ramets in SAD area, green; SAD ramets in SAD area, red).
We next examined NSC concentrations in all major tissues (leaves, branches, bole xylem, bole bark, and roots) in two induced-drought experiments during June to August 2010. If carbon stress induces mortality, we would expect declines in carbohydrate reserves during experimental drought, although reserves might initially increase as a result of changes in carbon allocation to growth (4). In the first induced-drought experiment, we monitored 22 potted aspen trees, 12 under drought stress. In the second, we constructed a rainfall exclusion experiment to reduce the water supply to one of two isolated 216-m2 plots of mature aspen forest in southwestern Colorado. In both experiments, we found no evidence of carbohydrate decreases between drought and control treatments in any tissue or carbohydrate (Fig. 2B and Figs. S1 and S2). In the mature forest experiment, drought led to increases in stored starch reserves in many tissues, especially branches, relative to control ramets, perhaps through declines in growth (Fig. 2 and Fig. S2). Sucrose and glucose concentrations also largely increased in the drought treatment, with no evidence of depletion in any tissue (Figs. S1 and S2).
Because SAD is still occurring, we measured carbohydrate reserves of native trees during the process of dying, which complements the carbohydrate measurements during experimental drought. We examined native ramets in three categories of health between healthy and SAD-affected (along a gradient of stand mortality; SI Methods: Mortality Gradient Methods) within the same clone to assess NSC reserves up to ramet death. Severe reduction of carbohydrate reserves before death in at least one tissue would indicate potential carbon starvation. We analyzed NSC reserves in all major tissues across categories of (i) SAD-affected ramets in a SAD area, (ii) healthy ramets in a SAD area, and (iii) healthy ramets in a healthy area, replicated in seven stands in the San Juan National Forest. A total of 68% of the SAD-affected ramets died during summer 2010. We found no evidence of significant carbohydrate decreases or depletion in SAD-affected trees in any carbohydrate or in any tissue (Fig. 2C). It is possible, at least in principle, that drought could have led to carbon starvation in the past, triggering eventual ramet death, and then carbohydrate levels recovered before death. This would require, however, a long-term and substantial carbon investment in restoration of carbohydrate reserves in the context of failing tree health. These results cannot eliminate the possibility of failure to mobilize or translocate these carbon reserves, but it is clear that dying plants still had abundant carbohydrate reserves in all major tissues.
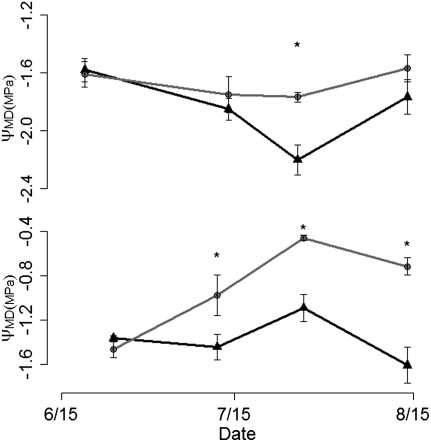
To examine the role of hydraulic failure, we measured xylem pressures and hydraulic conductivities of stems and roots in the potted-tree induced-drought experiment detailed earlier. Hydraulic failure implies that experimental drought should lead to more negative xylem pressures and to decreased xylem conductivity (i.e., partial to total hydraulic failure). Midday xylem pressure was more negative than −1.6 MPa in potted trees and −2.2 MPa in mature ramets under experimental drought (Fig. 3). Aspen branches exhibit 50% loss of conductivity between −1.0 and −2.5 MPa (31), suggesting that soils dried sufficiently to stress the water transport system and push midday xylem pressures to critically negative values for xylem cavitation. We measured hydraulic conductance in terminal branch networks (n = 30) and root segments (n = 33) before and after removing embolisms by vacuum infiltration (32). Branch conductance was measured before and after drought experiment, whereas root conductance was measured only after drought application to avoid damage to trees. Branches from droughted trees had significantly higher levels of cavitation than their paired predrought baseline, whereas control trees did not (Student t test, Pdrought = 0.04; Pcontrol = 0.39; Fig. 4B). Root segments from droughted trees had approximately 400% higher levels of cavitation than did those of control trees (P = 0.01; Fig. 4C). Thus, although induced-drought did not significantly stress carbon reserves, it led to notable increases in loss of hydraulic function in this experiment, especially in roots (Fig. S3).
Fig. 3.
Midday xylem pressure (mean ± SE) for induced-drought experiment in mature aspen ramets (Upper) and potted trees (Lower) over the experiment (black, drought; gray, control). Asterisks indicate statistically significant differences (P < 0.05).
Fig. 4.
(A) Percent loss of conductance of branches in mature healthy (Healthy), healthy in SAD areas (Healthy-SAD), and SAD ramets. (B) Pair-wise difference in percent loss of conductance (i.e. treatment minus baseline) in branches of control potted trees (Cont Branch) and drought potted trees (Drt Branch) after experiment. (C) Percent loss of conductance in roots of control trees (Cont Root) and drought trees (Drt Root) after experiment in potted trees. Asterisks indicate statistically significant differences (P < 0.05).
We found the same patterns of partial hydraulic failure in the stand mortality gradient detailed earlier in native stands. SAD-affected trees exhibited approximately 70% loss of conductivity, compared with 17% for healthy trees in healthy areas (ANOVA, F = 7.03; P = 0.01; Fig. 4A). Aspen seedlings have been found to exhibit leaf mortality at approximately 50% loss of conductivity and have complete crown mortality at approximately 90% loss (33). These hydraulic changes in dying ramets provide additional evidence for the role of hydraulic impairment in recent aspen forest die-off. SAD-affected ramets had lower native levels of branch conductance, and midsummer loss of conductance weakly predicted the observed increases in SAD ramet crown mortality at the end of the summer (r2 = 0.41; P = 0.088; Fig. S4).
We also tested whether root cavitation induced by drought led to increased root mortality, which has been documented in SAD in other studies (9). Coarse root (>2 mm in diameter) mortality increased significantly in the drought treatments in both experiments, as detailed later and in Fig. S3. After one summer of induced drought, potted trees exhibited approximately 8% coarse-root and approximately 20% fine-root mortality, compared with 0% and 2% in control trees, assuming no pretreatment differences. The loss of conductivity in living coarse roots among potted trees was a significant predictor of fine-root mortality (r2 = 0.68; P = 0.033). This suggests a mechanistic link between hydraulic failure and tissue senescence and between experimental drought and observed SAD patterns. Root death may also explain the lack of substantial resprouting in SAD forests (9), which has significant implications for the regrowth of aspen ecosystems, effects on biodiversity, and long-term carbon losses.
These results indicate that hydraulic impairment of distal branches and roots plays an important role in recent drought-driven aspen die-off, with root mortality possibly preceding canopy mortality. As ramet and stand mortality are multiyear processes and can lag drought stress, loss of hydraulic conductivity may not be abrupt and may be influenced by other physiological processes and feedbacks. Hydraulic failure could be catastrophic or gradual within a given branch or root but likely involves processes such as fine root mortality that mediate the multiyear decline (33). Although refilling of cavitated xylem vessels could reverse partial hydraulic failure, previous research suggests that aspens do not consistently refill, and, if they do refill, exhibit substantial increases in vulnerability to future cavitation (i.e., “cavitation fatigue”) (31). Other physiological processes that could play a role in this forest die-off include phloem impairment (21), loss of the ability to use stored carbon reserves (21, 22), and loss of connectivity between aspen ramets within a clone, which would exacerbate hydraulic stress. Our results suggest that further research should explore interconnections and interactions between carbon stress and hydraulic stress, such as the feedback from carbon stress-induced declines in xylem growth leading to greater hydraulic vulnerability. Additionally, research should examine what degree of changes in NSC or hydraulics lead to plant health impairment or death.
These results focus on one forest type dominated by a single species and might not directly apply to other forest die-backs. Still, the combination of observational and experimental tests presented here, especially direct measurements of carbohydrates and hydraulic impairment during experimental or natural stress events coupled to landscape patterns of physiology and mortality, could be useful to examine carbon and hydraulic stress in other systems. Physiological mechanisms provide a foundation to understand, predict, and model threshold events that may dominate certain ecosystem responses to climate change and allows us to better project the uncertain future of forest ecosystems in a warming climate. Although more research is needed to better incorporate drought-induced vegetation mortality into models, our results provide insight into the processes that occur during drought stress. They suggest that processes surrounding hydraulic impairment and potential hydraulic thresholds, rather than carbohydrate pools, may be more relevant to modeling the effects of drought on these forests. Thus, physiological studies can be used to guide the processes included in current and future vegetation and land-surface models to better capture the effects of extreme climate events such as drought on ecosystems (34).
Methods
We collected tissue samples from 20 healthy aspen stands across a range of elevations and aspects in the San Juan National Forest, Colorado, and from 31 SAD-affected stands across four National Forests in Colorado (SI Methods: Observational Patterns of Root Carbohydrates). At these sites, we calculated the observed drought stress in these aspen forests from PRISM 800-m-gridded annual precipitation anomaly, summer temperature anomaly, and a dataset of high resolution Palmer Drought Severity Index (35). To examine native root carbohydrate reserves, we collected 612 root tissue samples from these sites during July through August 2009. We analyzed NSC concentrations via enzyme digestions to measure glucose, sucrose, and starch (SI Methods: Observational Patterns of Root Carbohydrates). In addition, we identified seven clones that exhibited a gradient of stand mortality from areas of high levels of ramet mortality to healthy areas. During moderate seasonal water stress in June and July 2010, we collected branch samples for hydraulic measurements and tissue samples for carbohydrate measurements of all major tissues from three ramets in each of three treatments in these clones (SI Methods: Mortality Gradient Methods).
We performed two induced-drought experiments to examine carbohydrate and hydraulic changes in aspen trees under drought. In the first experiment, we acquired 30 six-year-old (∼3 m height) aspen trees and placed 12 under water stress from June through August 2010 at a site in the San Juan National Forest, monitoring 10 as control trees. We excluded all natural rainfall and carefully controlled the water these trees received, and collected tissue samples for hydraulic and carbohydrate analysis at multiple points (SI Methods: Induced Drought Experiments). In the second experiment, we sought to test the effects of drought on a section of mature aspen clone. We located a mature aspen clone at 2,740 m elevation and within 0.5 km from the potted tree drought experiment on a slight northern aspect. We trenched down to bedrock around two adjacent 12 × 18-m rectangles of forest, severing the control and drought treatment areas from the clonal root system to prevent transfer of water or carbohydrates. As in a typical throughfall exclusion experiment, we constructed clear troughs (2 m width, 19 m length, 0.2 m above ground) that drained downhill away from the plots and covered 50% of the plot surface area in each treatment. We perforated small holes in the control treatment's troughs to allow water to pass through unimpeded while still creating the same microenvironment as the drought treatment. We placed the troughs in both treatments and induced drought from June 20 to August 25, 2010. At the end of the experiment, we removed the troughs and collected the tissue samples of all tissues for NSC analysis. We statistically analyzed changes in NSC concentration by calculating the ramet-specific difference between predrought and postdrought values for control and drought treatments, testing for normality, and performing either a t test or Wilcoxon signed-rank test for each tissue, with a Bonferroni correction for multiple hypothesis testing.
Supplementary Material
Acknowledgments
We thank E. Callaway, M. Anderegg, M. Love, C. Sherman, A. Nees, D. Karp, J. Sprague, E. Sprague, C. Sprague, and G. Robinson for assistance with fieldwork and E. Pringle, T. Raab, C. Sherman, A. Winslow, A. Hausladen, N. Bitler, A. Lunny, W. Lagrandeur, J. Burr, A. Hines, M. Erviti, G. Griffin, M. Dini, and S. Shin for assistance in laboratory work. We thank E. Hadly, D. Karp, and two anonymous reviewers for helpful comments on the manuscript and J. Abatzoglou for providing Palmer Drought Severity Index data. W.R.L.A. thanks the Bill Lane Center for the American West, Morrison Institute, Phi Beta Kappa Northern California Association, Jasper Ridge Biological Preserve, and Stanford Biology small grants program for research funding and equipment. W.R.L.A. was supported in part by an award from the Department of Energy (DOE) Office of Science Graduate Fellowship Program (SCGF). The DOE SCGF Program was made possible in part by the American Recovery and Reinvestment Act of 2009. The DOE SCGF program is administered by the Oak Ridge Institute for Science and Education (ORISE) for the DOE. ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE Contract DE-AC05-06OR23100. All opinions expressed in this paper are the authors' and do not necessarily reflect the policies and views of DOE, ORAU, or ORISE.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107891109/-/DCSupplemental.
References
- 1.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 2.Sabine CL, et al. In: The Global Carbon Cycle: Integrating Humans, Climate, and the Natural World. Field CB, Raupach MR, editors. Washington, DC: Island; 2004. [Google Scholar]
- 3.Allen CD, Breshears DD. Climate-induced forest dieback as an emergent global phenomenon. Eos Trans AGU. 2007;88:47–54. [Google Scholar]
- 4.McDowell N, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259:660–684. [Google Scholar]
- 6.Breshears DD, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan JA, Régnière J, Powell J. Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ. 2003;1:130–137. [Google Scholar]
- 8.Shaw JD, Steed BE, DeBlander LT. Forest inventory and analysis (FIA) annual inventory answers the question: What is happening to pinyon-juniper woodlands? J For. 2005;103:280–288. [Google Scholar]
- 9.Worrall JJ, et al. Effects and etiology of sudden aspen decline in southwestern Colorado. For Ecol Manage. 2010;250:638–652. [Google Scholar]
- 10.Dale VH, Joyce LA, McNulty S, Neilson RP. The interplay between climate change, forests, and disturbances. Sci Total Environ. 2000;262:201–204. doi: 10.1016/s0048-9697(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 11.Breshears DD, Allen CD. The importance of rapid, disturbance-induced losses in carbon management and sequestration. Glob Ecol Biogeogr. 2002;11:1–14. [Google Scholar]
- 12.Narisma GT, et al. The role of biospheric feedbacks in the simulation of the impact of historical land cover change on the Australian January climate. Geophys Res Lett. 2003;30:2168–2172. [Google Scholar]
- 13.Clark DA. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos Trans R Soc Lond B Biol Sci. 2004;359:477–491. doi: 10.1098/rstb.2003.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischlin A, et al. Ecosystems, their properties, goods, and services. In: van der Linden PJ, Hanson E, editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge Univ Press; 2007. pp. 211–272. [Google Scholar]
- 15.Kurz WA, et al. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- 16.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323:1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SL, Brando PM, Phillips OL, van der Heijden GM, Nepstad D. The 2010 Amazon drought. Science. 2011;331:554. doi: 10.1126/science.1200807. [DOI] [PubMed] [Google Scholar]
- 18.Kurz WA, Stinson G, Rampley GJ, Dymond CC, Neilson ET. Risk of natural disturbances makes future contribution of Canada's forests to the global carbon cycle highly uncertain. Proc Natl Acad Sci USA. 2008;105:1551–1555. doi: 10.1073/pnas.0708133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox PM, et al. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theor Appl Climatol. 2004;78:137–151. [Google Scholar]
- 20.Sitch S, et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs) Glob Change Biol. 2008;14:2015–2030. [Google Scholar]
- 21.Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann H. Will a 385 million year-struggle for light become a struggle for water and for carbon? – How trees may cope with more frequent climate change-type drought events. Glob Change Biol. 2011;17:642–655. [Google Scholar]
- 23.Adams HD, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA. 2009;106:7063–7066. doi: 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala A. Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proc Natl Acad Sci USA. 2009;106:E68. doi: 10.1073/pnas.0904580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuzinger S, Bigler C, Wolf A, Körner C. Poor methodology for predicting large-scale tree die-off. Proc Natl Acad Sci USA. 2009;106:E106. doi: 10.1073/pnas.0908053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell NG, Sevanto S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010;186:264–266. doi: 10.1111/j.1469-8137.2010.03232.x. [DOI] [PubMed] [Google Scholar]
- 27.Galvez DA, Landhäusser SM, Tyree MT. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011;31:250–257. doi: 10.1093/treephys/tpr012. [DOI] [PubMed] [Google Scholar]
- 28.Galiano L, Martínez-Vilalta J, Lloret F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 2011;190:750–759. doi: 10.1111/j.1469-8137.2010.03628.x. [DOI] [PubMed] [Google Scholar]
- 29.Niinemets U. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance, and acclimation. For Ecol Manage. 2010;260:1623–1637. [Google Scholar]
- 30.Michaelian M, Hogg EH, Hall RJ, Arsenault E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob Change Biol. 2011;17:2084–2094. [Google Scholar]
- 31.Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol. 2001;125:779–786. doi: 10.1104/pp.125.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperry JS, Donnelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988;11:35–45. [Google Scholar]
- 33.Lu Y, Equiza MA, Deng X, Tyree MT. Recovery of Populus tremuloides seedlings following severe drought causing total leaf mortality and extreme stem embolism. Physiol Plant. 2010;140:246–257. doi: 10.1111/j.1399-3054.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- 34.Leuzinger S, Quinn Thomas R. How do we improve Earth system models? Integrating Earth system models, ecosystem models, experiments and long-term data. 1st INTERFACE workshop, Captiva Island, FL, USA, 28 February-3 March 2011. New Phytol. 2011;191:15–18. doi: 10.1111/j.1469-8137.2011.03778.x. [DOI] [PubMed] [Google Scholar]
- 35.Kangas RS, Brown TJ. Characteristics of US drought and pluvials from a high-resolution spatial dataset. Intn'l Journal of Climate. 2007;27:1303–1321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.