Abstract
The mucin MUC1 is typically aberrantly glycosylated by epithelial cancer cells manifested by truncated O-linked saccharides. The resultant glycopeptide epitopes can bind cell surface major histocompatibility complex (MHC) molecules and are susceptible to recognition by cytotoxic T lymphocytes (CTLs), whereas aberrantly glycosylated MUC1 protein on the tumor cell surface can be bound by antibodies to mediate antibody-dependent cell-mediated cytotoxicity (ADCC). Efforts to elicit CTLs and IgG antibodies against cancer-expressed MUC1 have not been successful when nonglycosylated MUC1 sequences were used for vaccination, probably due to conformational dissimilarities. Immunizations with densely glycosylated MUC1 peptides have also been ineffective due to impaired susceptibility to antigen processing. Given the challenges to immuno-target tumor-associated MUC1, we have identified the minimum requirements to consistently induce CTLs and ADCC-mediating antibodies specific for the tumor form of MUC1 resulting in a therapeutic response in a mouse model of mammary cancer. The vaccine is composed of the immunoadjuvant Pam3CysSK4, a peptide Thelper epitope and an aberrantly glycosylated MUC1 peptide. Covalent linkage of the three components was essential for maximum efficacy. The vaccine produced CTLs, which recognized both glycosylated and nonglycosylated peptides, whereas a similar nonglycosylated vaccine gave CTLs which recognized only nonglycosylated peptide. Antibodies elicited by the glycosylated tripartite vaccine were significantly more lytic compared with the unglycosylated control. As a result, immunization with the glycosylated tripartite vaccine was superior in tumor prevention. Besides its own aptness as a clinical target, these studies of MUC1 are likely predictive of a covalent linking strategy applicable to many additional tumor-associated antigens.
Keywords: cancer vaccine, multicomponent, chemical synthesis, Tn antigen
A large number of carcinomas of breast, ovary, colon, rectum, pancreas, and prostate exhibit a striking overexpression of MUC1 resulting in a loss of polarization and altered glycosylation (1, 2). MUC1 is a heavily glycosylated type 1 transmembrane mucin that is expressed on the apical surface of glandular epithelial cells at low levels and at very high levels following transformation. Human MUC1 is composed of a cytoplasmic signaling peptide, a transmembrane domain, and an ectodomain composed of a variable number tandem repeats of twenty amino acids. Each repeat contains five potential O-glycosylation sites. The glycosylation pattern depends on the tissue of origin and the physiological state of the tissue (1, 3). Tumor-associated MUC1 is aberrantly glycosylated due to a lack of core 1,3-galactosyltransferase (T-synthase) (4), producing truncated carbohydrate structures such as Tn (αGalNAc-Thr), STn (αNeu5Ac-(2,6)-αGalNAc-Thr), and Thomsen–Friedenreich (TF) antigen (βGal-(1,3)-αGalNAc-Thr). Recently, the NCI Translational Research Working Group prioritized cancer vaccine targets based on therapeutic function, immunogenicity, role of Ag in oncogenicity, specificity, expression level, stem cell expression, percentage of patients with antigen positive cancer, and cellular location (5). MUC1 was ranked second of 75 tumor-associated antigens. In this respect, MUC1 displays nearly ubiquitous expression in a wide variety of tumor types: It is found on cancer stem cells and has a functional role in tumorigenesis.
Humoral responses to MUC1 have been observed in benign diseases and carcinoma patients and the presence of circulating antibodies against MUC1 at the time of cancer diagnosis has been correlated with a favorable disease outcome in breast cancer patients (6, 7). The MUC1-derived peptide sequences RPAPGS, PPAHGVT, and PDTRP have been identified as the most frequent minimal epitopes (8, 9). Furthermore, modification of the peptides with αGalNAc (Tn-antigen) led to stronger antibody binding. It has been proposed that the improved binding is due to saccharide induced conformational change of the peptide backbone (10–12).
Cytotoxic T lymphocytes (CTLs) isolated from patients with breast carcinoma can recognize epitopes present on MUC1 tandem repeat peptide (13). It has been proposed that T cell epitopes from the MUC1 core domain are packaged within tumor cells in their truncated glycosylation state into major histocompatibility complex (MHC) class I molecules, leading to natural MHC-restricted recognition of “hypoglycosylated” epitopes (14–17). Several MUC1-derived HLA-A2-binding peptides have been identified including STAPPAHGV and SAPDTRPAPG (13, 18, 19).
Early efforts to develop MUC1-based cancer vaccines focused on the use of unglycosylated MUC1 tandem repeat peptides of different lengths, conjugated to different carriers and/or administered with an adjuvant (8, 20–27). In general, these strategies have failed to elicit effective immune responses to MUC1-expressing cancer cells, probably due to the conformational disparities between nonglycosylated vaccine sequences and tumor-expressed, aberrantly glycosylated MUC1 (10–12). The immunogenicity of carbohydrate epitopes (Tn- or sialosyl-Tn) conjugated to an antigenically irrelevant carrier protein has been examined in mice, however, these constructs elicited only modest IgM and IgG antibody responses (28–31). Such vaccine candidates suffer from immune suppression by the carrier protein and, in addition, cannot activate CTL responses. A synthetic 60-mer MUC1 tandem-repeat peptide, which was glycosylated by polypeptide GalNAc transferases to give saturating O-glycan occupancy (five sites per repeat), elicited only modest antibody responses (32). Recent clarifying studies have shown that a densely glycosylated MUC1 glycopeptide cannot be processed by antigen-presenting cells (APCs) (17), thereby compromising the presentation of class I and class II glycopeptides; consequently, Thelper cells and CTLs will not be activated. Interestingly, glycopeptides carrying the Tn- or TF-antigens have been used to induce a carbohydrate-specific cytotoxic T cell response in mice (33). Two-component vaccines, consisting of an MHC I glycopeptide and a Thelper epitope, have shown promise in tumor models (34). However, these vaccine candidates do not induce antibody responses. Thus, a MUC1-based cancer vaccine that consistently elicits relevant humoral and cellular immunity has not yet been developed.
We show here that a glycosylated MUC1-derived glycopeptide covalently linked to a Toll-like receptor (TLR) agonist can elicit potent humoral and cellular immune responses and is efficacious in reversing tolerance and generating a therapeutic response. The examination of a number of control compounds demonstrate that the therapeutic effect of the three-component vaccine is due to nonspecific antitumor responses elicited by the adjuvant, and specific humoral and cellular immune responses elicited by the MUC1-derived glycopeptide. It has been found that glycosylation of the MUC1 peptide and covalent attachment of the TLR agonist is critical for inducing optimal immune responses.
Results
Antigen Design and Tumor Challenge Studies.
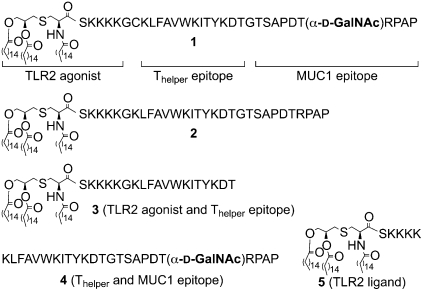
The efficacy of liposomal preparations of compounds 1, 2, 3, a mixture of 4 and 5, and 5 alone (Fig. 1) were examined in a well established mouse model for mammary cancer (35). The multicomponent vaccine candidate 1 contains a tumor-associated glycopeptide derived from MUC1 (1, 3), the well-documented murine MHC class II restricted Thelper epitope KLFAVWKITYKDT derived from polio virus (36), and the lipopeptide Pam3CysSK4, which is a potent agonist of Toll-like receptor 2 (TLR2) (37). Previously, the MUC1-derived glycopeptide SAPDTαGalNAc)RPAP, was identified as the antigenic-dominant domain of the tandem repeat of MUC1 (8, 9). Furthermore, this epitope can also be presented in complex with MHC class I (Kb) resulting in the activation of CTLs (38). The MHC class II restricted Thelper epitope of 1 was expected to induce a class switch from IgM to IgG antibody production and facilitate the presentation of exogenous glycopeptides on MHC class 1. Finally, the Pam3CysSK4 moiety of 1 will function as an inbuilt adjuvant by eliciting relevant cytokines and chemokines (37). To determine the importance of the carbohydrate moiety of 1, construct 2 was examined, which has a similar structure as 1 except that the threonine of the MUC1 peptide is not glycosylated. Compound 3 lacks the MUC1 (glyco)peptide epitope of 1 and 2 and was examined to account for possible therapeutic effects due to immune activation by the adjuvant. Finally, a mixture of the glycopeptide 4 and adjuvant Pam3CysSK4 5 was examined to establish the importance of covalent attachment of the adjuvant to the MUC1 glycopeptide and Thelper epitope.
Fig. 1.
Chemical structures of synthetic antigens.
The multicomponent vaccine 1 was prepared by liposome-mediated native chemical ligation of the thiobenzyl ester of Pam3CysSK4 (39) and the glycopeptide CKLFAVWKITYKDTGTSAPDT(αGalNAc)RPAP (11, SI Appendix, Fig. S1) followed by purification by reverse phase C-4 column chromatography. Compounds 2, 3, and 4 were synthesized by a linear solid phase peptide synthesis (SPPS) protocol using a Rink amide AM resin, Fmoc protected amino acids, and Fmoc-Thr-(3,4,6-triO-acetyl-α-d-GalNAc). The resulting compounds were incorporated into phospholipid-based small unilamellar vesicles (SUVs) by hydration of a thin film of the synthetic compounds, egg phosphatidylcholine, phosphatidylglycerol, and cholesterol in a Hepes buffer (10 mM, pH 7.4) containing NaCl (145 mM) followed by extrusion through a 100 nm Nuclepore polycarbonate membrane. Groups of MUC1.Tg mice (C57BL/6; H-2b) that express human MUC1 were immunized three times at biweekly intervals with liposomal preparations of compounds 1, 2, 3, a mixture of 4 and 5, and 5 alone. After 35 d, the mice were challenged with Mouse Mammary Tumor (MMT) cells (positive for MUC1 and Tn) followed by one more boost after one week. One week after the last immunization, the mice were killed and the efficacy of the vaccines determined by tumor weight. Furthermore, the robustness of humoral immune responses was assessed by titers of MUC1-specific antibodies and the ability of the antisera to lyse MUC1-bearing tumor cells. In addition, cellular immune responses were evaluated by determining the number of IFN-γ producing CD8+ T cells and the ability of these cells to lyse cells.
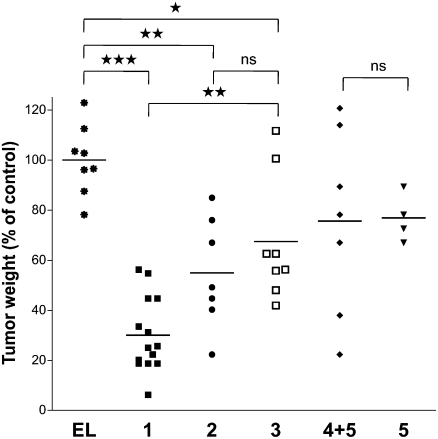
Immunization with multicomponent vaccine candidate 1 led to a significant reduction in tumor burden compared with empty liposomes or treatment with compound 3, which does not contain a MUC1 glycopeptide epitope (Fig. 2). Interestingly, immunizations with compound 3 led to somewhat smaller tumors compared with the application of empty liposomes, indicating antitumor properties due to nonspecific adjuvant effects. Unglycosylated multicomponent vaccine candidate 2 and a mixture of compounds 4 and 5 did not exhibit a significant improvement of anti-cancer properties compared with control immunizations. In these cases, large dispersion in tumor weights was observed whereas immunization with compound 1 led to substantial reduction in tumor weight in all mice.
Fig. 2.
Glycosylated multicomponent vaccine reduces MMT tumor burden in MUC1.Tg mice. MUC1.Tg mice were immunized with empty liposomes (EL) as control or with liposomes containing 1, 2, 3, 4 + 5, or 5. Data are presented as percentage of control (mice vaccinated with empty liposomes). Each data point represents an individual mouse and the horizontal lines indicate the mean for the group of mice.
Humoral Immunity.
Anti-MUC1 antibody titers were determined by coating microtiter plates with the MUC1-derived glycopeptide CTSAPDT(αGalNAc)RPAP conjugated to maleimide-modified BSA. Compound 1 had elicited robust IgG antibody responses, and subtyping of the antibodies indicated a mixed Th1/Th2 response (Table 1 and SI Appendix, Fig. S2). Mice immunized with 1 but not challenged with MMT tumor cells elicited similar titers of antibodies, indicating that immune suppression by cancer cells was probably reversed. Inhibition ELISA using the MUC1-derived (glyco)peptides SAPDT(αGalNAc)RPAP (6) and SAPDTRPAP (7) as inhibitors showed that the polyclonal sera had slightly higher affinities for the glycosylated MUC1 epitope (SI Appendix, Table S1 and Fig. S3). Furthermore, low titers of antibodies against the Thelper epitope were measured indicating that the candidate vaccine does not suffer from immune suppression. Although compound 2 does not contain a carbohydrate moiety, the resulting antisera could recognize the CTSAPDT(αGalNAc)RPAP epitope. However, in this case, no IgG3 antibodies were detected, consistent with an absence of carbohydrate in the vaccine. Interestingly, the mixture of compounds 4 and 5 had elicited low titers of antibodies, highlighting the importance of covalent attachment of the Pam3CysSK4 to the glycopeptide epitope for robust antigenic responses. As expected, the controls that did not contain a MUC1-derived epitope (3 and 5) did not elicit anti-MUC1 antibody responses.
Table 1.
ELISA anti-MUC1 and anti-Thelper antibody titers after four immunizations with various preparations
| Immunization* | IgG total MUC1 | IgG1 MUC1 | IgG2a MUC1 | IgG2b MUC1 | IgG3 MUC1 | IgM MUC1 | IgG total Thelper |
| 1 (no tumor induced) | 31,900 | 10,600 | 10,000 | 15,500 | 3,900 | 100 | 2,100 |
| 1 | 30,200 | 16,000 | 6,600 | 10,700 | 3,900 | 50 | 3,000 |
| 2 | 12,900 | 10,400 | 4,100 | 4,500 | 700 | 100 | 1,000 |
| 3 | 1,300 | 0 | 100 | 900 | 0 | 0 | 50 |
| 4 + 5 | 300 | 0 | 0 | 200 | 0 | 0 | 1,000 |
| 5 | 0 | 0 | 200 | 0 | 0 | 50 | 50 |
Anti-MUC1 and anti-Thelper antibody titers are presented as median values for groups of 4–13 mice. ELISA plates were coated with BSA-MI-CTSAPDT(αGalNAc)RPAP conjugate for anti-MUC1 antibody titers or NeutrAvidin-biotin-Thelper for anti-Thelper antibody titers. Titers were determined by linear regression analysis, with plotting of dilution versus absorbance. Titers are defined as the highest dilution yielding an optical density of 0.1 or greater relative to normal control mouse sera.
*Liposomal preparations were used. MMT tumors were induced between the third and fourth immunization.
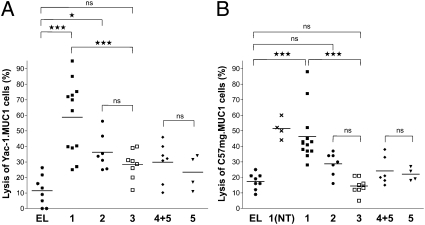
Antibody-dependent cell-mediated cytotoxity (ADCC) was examined by labeling two MUC1- expressing cancer cell types with 51Cr, followed by the addition of antisera and cytotoxic effector cells (NK cells) and measurement of released 51Cr. The antisera obtained by immunization with 1 was able to significantly increase cancer cell lysis compared with the control compound 3 (Fig. 3 A and B). Importantly, antibodies elicited by compound 2 were significantly less efficacious in cell lysis compared with compound 1, highlighting the importance of glycosylation for relevant antigenic responses. As expected, the antisera derived from a mixture of 4 and 5 and the control derivatives lacking the MUC1 glycopeptide did not induce significant cell lysis.
Fig. 3.
Induction of antibody-dependent cell-mediated cytotoxicity (ADCC). Tumor cells, Yac-1.MUC1 (A) and C57mg.MUC1 (B), were labeled with chromium and incubated with serum obtained from mice immunized with empty liposomes (EL) or liposomes containing 1, 2, 3, 4 + 5, or 5 with or without (NT) tumor induction. The tumor cells were then incubated with effector cells (NK cells KY-1 clone). Spontaneous release was below 20% of complete release. Each data point represents an individual mouse and the horizontal lines indicate the mean for the group of mice.
Cellular Immunity.
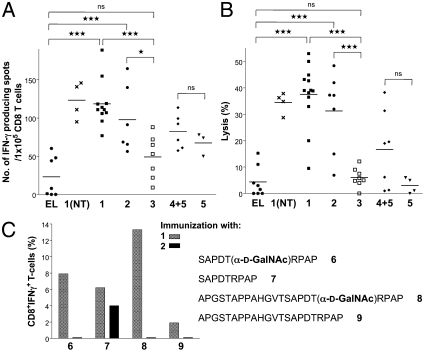
To assess the ability of the vaccine candidates to activate CTLs, CD8+ T cells from lymph nodes of the mice were isolated by magnetic cell sorting and incubated with irradiated dendritic cells (DCs) pulsed with the immunizing peptides on ELISPOT plates. As expected, vaccine candidates 1 and 2 exhibited robust CD8+ responses compared with control (Fig. 4A, 1 and 2 vs. 3). Interestingly, a mixture of glycopeptides 4 and adjuvant 5 (Pam3CysSK4) induced the activation of a smaller number of CD8+, indicating that covalent attachment of the MUC1 and Thelper epitope to the adjuvant is important for optimal activation of CTLs.
Fig. 4.
Induction of cytotoxic T cell responses. (A) IFN-γ producing CD8+ T cells in MUC1.Tg mice. CD8+ T cells isolated from lymph nodes of mice immunized with empty liposomes (EL) or liposomes containing 1, 2, 3, 4 + 5, or 5 with or without (NT) tumor induction were analyzed for MUC1-specific IFN-γ spot formation without in vitro stimulation. (B) Induction of CD8+ cytolytic T cells in MUC1.Tg mice. CD8+ T cells were isolated from lymph nodes of mice immunized with empty liposomes (EL) or liposomes containing 1, 2, 3, 4 + 5, or 5 with or without (NT) tumor induction and subjected to a 51Cr-release assay without any in vitro stimulation. DCs pulsed with glycopeptide SAPDT(αGalNAc)RPAP (6) for 1 (NT), 1, 3, 4 + 5, and 5, peptide SAPDTRPAP (7) for 2, or unpulsed for EL were used as targets. Spontaneous release was below 15% of complete release. Each data point represents an individual mouse and the horizontal lines indicate the mean for the group of mice. (C) Epitope requirements of CD8+ T cells. Mice were immunized with liposomes containing 1 or 2. Lymph node derived T cells expressing low levels of CD62L were obtained by cell sorting and cultured for 14 d in the presence of DCs pulsed with glycopeptide 6 for 1 or peptide 7 for 2. The resulting cells were analyzed by ICC for the presence of CD8+IFNγ+ T cells after exposure to DCs pulsed with (glyco)peptides 6–9.
The lytic activity of the isolated CD8+ cells without in vitro stimulation was examined by a 51Cr-release assay in which DCs were pulsed with the MUC1-derived glycopeptide SAPDT(αGalNAc)RPAP (6) or the peptide SAPDTRPAP (7) in the case of immunization 2. CTLs activated by compounds 1 and 2 exhibited significantly greater cytotoxicity compared with controls (Fig. 4B). Furthermore, mice immunized with a mixture of 4 and 5 exhibited a reduced lytic activity, further demonstrating the importance of covalent attachment of the various epitopes.
To investigate in detail the epitope requirements of the CD8+ cells, groups of five MUC1.Tg were immunized with liposomal preparations of compounds 1 and 2, followed by sorting CD62Low T cells from lymph nodes, which were stimulated in vitro for 2 d by DCs pulsed with glycopeptide SAPDT(αGalNAc)RPAP (6) and peptide SAPDTRPAP (7), respectively and then allowed to expand for 14 d by culturing with IL-2, IL-7, and IL-15. The percentage of IFN-γ producing CD8+ cells was established after pulsing DCs with MUC1-derived (glyco)peptides 6-9. Compound 1 had activated a diverse range of CTL that could be activated by glycosylated and nonglycosylated structures, whereas those obtained by immunization with 2 only showed responsiveness with unglycosylated peptide 7. Furthermore, CD8+ cells obtained from immunizing with 1 could lyse DCs pulsed with glycosylated and unglycosylated structures (Fig. 4C).
These results indicate that CTLs activated by immunizations with 1 recognize a wider range of structures including glycosylated and unglycosylated MUC1-derived peptides whereas CTLs obtained from compound 2 exhibit a strong preference for unglycosylated peptides.
Cytokine Induction.
The lipopeptide moiety of the three-component vaccine is required for initiating the production of necessary cytokines and chemokines by interacting with TLR2 on the surface of mononuclear phagocytes (37, 40, 41). To examine the activity of the TLR2 moiety of the vaccine candidates, primary DCs obtained by an established method (42) were exposed over a wide range of concentrations to the compounds 1-3 and Escherichia coli 055:B5 LPS and the supernatants examined for mouse TNF-α, IFN-β, RANTES, IL-6, IL-1β, IL-10, IP-10, IL-12p70, and IL-12/23p40 using commercial or in-house developed capture ELISAs (SI Appendix, Tables S2 and S3 and Fig. S4). The compounds induced the secretion of TNF-α, RANTES, IL-6, IL-1β, and IL-12/23p40 with similar efficacies and potencies indicating that attachment of the carbohydrate did not affect activity. The compounds did not induce the secretion of immunosuppressant IL-10, and furthermore, IFN-β and IP-10 were not detected, which is in agreement with TRIF-dependent cellular activation of these cytokines (43).
Discussion
Evidence is emerging that a successful cancer vaccine should be multimodal and activate several aspects of the immune system at once (44). Although cellular and humoral immune responses against MUC1 have been observed in some cancer patients, it has been difficult to design cancer vaccine candidates that can elicit both of these responses (2). Previously, we found that a tripartite vaccine composed of a glycopeptide derived from MUC1, a promiscuous Thelper peptide, and a TLR2 agonist can elicit in wild-type mice exceptionally high titers of IgG antibodies (45). Here, we report a detailed mechanistic study using a humanized mouse model of mammary cancer that demonstrates that the tripartite vaccine can elicit IgG antibodies that can lyse MUC1-expressing cancer cells, stimulate cytotoxicity of T lymphocytes, and activate innate immune responses, thereby reversing tolerance and generating a therapeutic response. The tumor model was selected because it is convenient for screening a relatively large number of compounds and resembles a model for treatment of a minimal residual disease in which cancer patients (breast cancer patients in particular) are apparently cancer free after surgery, radiation, and/or chemotherapy but are in danger of relapse due to the presence of micrometastatic tumors. It is the expectation that a cancer vaccine can destroy the remaining cancer cells, thereby improving long-term survival.
Analysis of control compounds revealed that reduction in tumor burden mediated by the tripartite vaccine was caused by specific immunity against MUC1 and by nonspecific adjuvant effects mediated by the TLR2 agonist. Evidence is emerging that TLRs are widely expressed by tumor cells and their activation can result in inhibition or promotion of tumorigenicity (46). Furthermore, cytokines and chemokines, which are produced following the activation of the TLRs, can stimulate the expression of a number of costimulatory proteins for optimum interactions between helper T, B, and antigen-presenting cells. A recent study indicates that TLR1/2 agonists have a unique ability to reduce the suppressive function of Foxp3+ regulatory T cells (Tregs) and enhance the cytotoxicity of tumor-specific CTL in vitro and in vivo and potentially have more favorable antitumor effects than other TLR agonists (47).
The studies presented here also demonstrated that covalent attachment of the TLR2 agonist to the glycolipoptide epitope is critical for eliciting antibodies and optimal CTL function. Lipidation with the TLR2 agonist makes it possible to formulate the candidate vaccine in a liposomal preparation, which probably will enhance its circulation time. Furthermore, a liposomal preparation presents the glycopeptide epitopes in a multivalent manner, thereby providing an opportunity for efficient clustering of Ig receptors of B-cells, which is required to initiate B cell signaling and antibody production. Furthermore our previous studies have shown that covalent attachment of the TLR2 agonist Pam3CysSK4 facilitates selective internalization by TLR2-expressing immune cells such B cells and APCs (45). Uptake and processing of antigen and subsequent presentation of the Thelper epitope as a complex with MHC class I or II on the cell surface of APCs, is critical for eliciting IgG antibodies. Over the past decade, numerous studies have shown that selective targeting of antigens to APCs will result in improved immune responses (48, 49). For example, oxidized mannan, heat shock proteins, bacterial toxins, and antibodies targeting cell surface receptors of DCs have been attached to antigens to increase uptake by DCs. Although these uptake strategies are attractive, they have as a disadvantage that the targeting device is antigenic, which may result in immune suppression of tumor-associated carbohydrates. The attractiveness of Pam3CysSK4 for facilitating uptake by APCs lies in its low intrinsic immunity. Thus, the three-component vaccine will facilitate uptake without suffering immune suppression.
Finally, we found that glycosylation of the MUC1 epitope was critical for optimal reduction in tumor burden. The mechanistic studies provided a rationale for these observations, and immunization with compound 1 led to somewhat higher titers of antibodies that were significantly more lytic compared with the use of compound 2, which lacks the Tn-antigen. Conformational studies by NMR complemented by light scattering measurements have indicated that deglycosylation of MUC1 results in a less extended and more globular structure (50). Similar studies using MUC1-related O-glycopeptides have shown that the carbohydrate moieties exert conformational effects (10–12), which may provide a rationale for differences in immune responses. Also, the use of glycosylated 1 led to the efficient activation of CTLs, which were able to recognize glycosylated and unglycosylated structures, with the former ones being preferred. On the other hand, immunizations with unglycosylated compound 2 led to CTLs that mainly recognize unglycosylated structures. It is known that short O-linked glycans such as the Tn and STn on MUC1 tandem repeats remain intact during DC processing in the MHC class I and II pathways (14–17, 51, 52) and thus it is possible to elicit glycopeptide selective CTL responses. Moreover, there is evidence that MUC1 glycopeptides can bind more strongly to the MHC class I mouse allele H-2Kb compared with the corresponding unglycosylated peptide (38). The progression of carcinomas is not only associated with the modification of MUC1 with truncated saccharides such as the Tn antigen but these structures are present at much higher densities and thus effective immunotherapy needs to elicit responses that are directed to such structures.
In conclusion, a tripartite vaccine engineered to emulate glycosylated MUC1 was unique in its capacity to generate CTL and ADCC-mediating antibodies, which recognized tumor-associated MUC1. This was associated with a significantly superior therapeutic antitumor effect. We hypothesize that a tumor-specific anti-MUC1 response is attainable, but only when the MUC1 component of the vaccine contains the conformational elements of aberrant glycosylation.
Materials and Methods
General Methods for Automated Synthesis of Solid-Phase (Glyco)(lipo)peptides 1–11.
(Glyco)(lipo)peptides and were synthesized on RinkAmide AM resin (0.1 mmol) by established protocols on an Applied Biosystems, ABI 433A peptide synthesizer equipped with a UV detector using Nα-Fmoc-protected amino acids and the following side chain protection was used: N-α-Fmoc-Asp-Thr(ΨMe,Me pro)-OH, N-α-Fmoc-Ile-Thr(ΨMe,Me pro)-OH, N-α-Fmoc-N-ε-tert-Boc-l-lysine, N-α-Fmoc-O-tert-butyl-l-serine, N-α-Fmoc-O-tert-butyl-l-threonine, N-α-Fmoc-O-tert-butyl-l-tyrosine. The lipid moiety was installed using N-α-Fmoc-R-(2,3-bis (palmitoyloxy)-(2R-propyl)-(R)-cysteine. The activating reagent was 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/1-hydroxybenzotriazole (HOBt). Single coupling steps were performed with conditional capping. The Tn moiety was installed manually using Nα-Fmoc-Thr-(AcO3-α-d-GalNAc) (134 mg, 0.2 mmol) in DMF (2 mL), 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) (76 mg, 0.2 mmol), and DIPEA (67 μL, 0.4 mmol) as the activating reagent. The manual coupling was monitored by standard Kaiser test. The resulting glycopeptides were purified by reversed-phase high performance liquid chromatography (RP HPLC) on an Agilent 1100 series system equipped with an autosampler, UV detector and fraction collector using a Zorbax Eclipse semipreparative C-18 column using a linear gradient of 0→100% B (acetonitrile 95%, water 5%, TFA 0.1%) in A (water 95%, acetonitrile 5%, TFA 0.1%) over 40 min. Compound 1 was prepared by liposome-mediated NCL using appropriate glyco and lipopeptides as detailed in SI Text. Compounds 1–3 were purified by RP-HPLC on a Phenomenex Jupiter analytical C-4 reversed phase column using a gradient of 0–100% B in A over 40 min.
Liposome Preparation for Immunizations.
Each glycolipopeptide was incorporated into phospholipid-based small unilamellar vesicles (SUVs) by hydration of a thin film of the synthetic compounds, egg phosphatidylcholine, phosphatidylglycerol, and cholesterol in a Hepes buffer (10 mM, pH 7.4) containing NaCl (145 mM) followed by extrusion through a 0.1-μm Nucleopore polycarbonate membrane.
Immunizations and Tumor Palpation.
Eight- to 12-wk-old MUC1.Tg mice (C57BL/6; H-2b) that express human MUC1 at physiological levels were immunized three times at biweekly intervals at the base of the tail intradermally with liposomal preparations of three-component vaccine constructs (25 μg containing 3 μg of carbohydrate) and the respective controls which lack the tumor-associated MUC1 epitope. After 35 d, the mice were challenged with MMT mammary tumor cells (1 × 106 cells), which express MUC1 and Tn. On day 42, one more immunization was given. Palpable tumors were measured by calipers, and tumor weight was calculated according to the formula: grams = [(length) × (width)2]/2, where length and width are measured in centimeters. On day 49, the mice were killed, the tumors were surgically removed, and tumor wet weight was determined.
51Chromium (Cr) Release Assay.
Cytolytic activity was determined by a standard 51Cr release method using CD8+ T cells from tumor-draining lymph nodes without any in vitro stimulation as effector cells and 51Cr labeled DCs pulsed with respective peptide as target cells at a 100:1 ratio for 6 h. Target cells were loaded with 100 μCi of 51Cr (Amersham Biosciences) per 106 target cells for 2 h before incubation with effectors. Radioactive 51Cr release was determined using the Topcount Microscintillation Counter (Packard Biosciences) and specific lysis was calculated: (experimental cpms – spontaneous cpms/complete cpms – spontaneous cpms) × 100. Spontaneous lysis was <15% of total lysis.
Determination of ADCC.
Tumor cells (Yac-1.MUC1 or C57mg.MUC1; see SI Appendix for cell maintenance and transfection procedures) were labeled with 100 μCi 51Cr for 2 h at 37 °C, washed, and incubated with serum (1 in 25 dilutions) obtained from the vaccinated mice for 30 min at 37 °C. NK cells, which have high expression of CD16 receptor, were used as effectors. These cells were stimulated with IL-2 (200 units/mL) for 24 h before assay. Effector cells were seeded with the antibody-labeled tumor cells in 96-well culture plates (Costar high binding plates) at an effector-to-target cell ratio of 50:1 for 4 h. The release of 51Cr was determined by the Top Count. Spontaneous and maximum release of 51Cr was determined. The percentage of specific release was determined: (release – spontaneous release/maximal release – spontaneous release) × 100.
IFN-γ ELISPOT Assay.
At time of sacrifice, MAC sorted CD8+ T cells from tumor-draining lymph nodes were isolated from treated MUC1.Tg mice and used as responders in an IFN-γ ELISPOT assay as described (34). Spot numbers were determined using computer-assisted video image analysis by ZellNet Consulting. Splenocytes from C57BL/6 mice stimulated with Concavalin A were used as a positive control.
Serologic Assays.
Anti-MUC1 IgG, IgG1, IgG2a, IgG2b, IgG3, and IgM antibody titers were determined by ELISA as described (53). Briefly, ELISA plates (Thermo Electron) were coated with a conjugate of the MUC1 glycopeptide conjugated to BSA through a maleimide linker [BSA-MI-CTSAPDT(αGalNAc)RPAP]. Serial dilutions of the sera were allowed to bind to immobilized MUC1. Detection was accomplished by the addition of phosphate-conjugated anti-mouse antibodies and p-nitrophenyl phosphate (Sigma). To determine antibody titers against the Thelper (polio) epitope, Reacti-bind NeutrAvidin-coated and preblocked plates (Pierce) were incubated with biotin-labeled Thelper (10 μg/mL; 100 μL per well) for 2 h. Next, serial dilutions of the sera were allowed to bind to immobilized Thelper epitope. Detection was accomplished as described above.
Inhibition ELISAs.
Serum samples were diluted in diluent buffer to give without-inhibitor expected final optical density values of ∼1. The diluted serum samples (60 μL) were mixed in an uncoated microtiter plate with diluent buffer, glycopeptide SAPDT(αGalNAc)RPAP (6), peptide SAPDTRPAP (7), or (α-O-GalNAc-Thr (Tn-antigen) in diluent buffer (60 μL) with a final concentration of 0–500 μM. After incubation at room temperature for 30 min, the mixtures (100 μL) were transferred to a plate coated with BSA-MI-CTSAPDT(αGalNAc)RPAP. The microtiter plates were incubated and developed as described above using an alkaline phosphatase-conjugated detection antibody for IgG total. Optical density values were normalized for the optical density values obtained with monoclonal antibody alone (no inhibitor, 100%).
Cytokine Assays.
DCs were prepared from mouse bone marrow cultures as described (54, 55). On the day of the exposure assay, mature DCs were plated as 4 × 106 cells per well in 1.8 mL in 24-well tissue culture plates. Cells were then incubated with different stimuli (200 μL, 10×) for 24 h in a final volume of 2 mL per well. Stimuli were given at a wide concentration range (corresponding to final concentrations of 0.1 ng/mL to 100 μg/mL PAM3CysSK4 for 1, 5, or 6 in liposomes and 0.001 ng/mL to 10 μg/mL for E. coli LPS). Supernatants were collected. For estimation of the effect of ATP on IL-1β secretion, DCs were reincubated for 30 min in the same volume of medium containing ATP (5 mM; Sigma), after which supernatants were harvested. Cytokine quantification of mouse TNF-α, RANTES, IL-6, IL-1β, IL-10, IP-10, IL-12 p70, IL-12/23 p40, and IFN-β was performed by ELISA as described (56).
Statistical Analysis.
Multiple comparisons were performed using one-way ANOVA with Bonferroni's multiple comparison test. Differences were considered significant when P < 0.05. Asterisks in figures indicate statistically significant difference (*P < 0.05, **P < 0.01, and ***P < 0.001) and ns indicates no significant difference.
Supplementary Material
Acknowledgments
We thank Dr. W. M. Yokoyama for the murine NK cell clone KY-1 and the Mayo Clinic Natalie Schafer Animal Care attendants for excellent animal care. This research was supported by the National Cancer Institute of the US National Institutes of Health Grant R01 CA88986 (to G.-J.B.), Mayo Breast Specialized Programs of Research Excellence (SPORE) Grant P50 CA116201 (to S.J.G.), and Mayo Pancreas SPORE Grant P50 CA102701 (to P.A.C. and S.J.G.).
Footnotes
Conflict of interest statement: G.-J.B. has established the company Viamune to commercialize the tripartite vaccine.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115166109/-/DCSupplemental.
References
- 1.Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy. 2010;2:305–327. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch FG, Ninkovic T. Immunology of O-glycosylated proteins: Approaches to the design of a MUC1 glycopeptide-based tumor vaccine. Curr Protein Pept Sci. 2006;7:307–315. doi: 10.2174/138920306778018034. [DOI] [PubMed] [Google Scholar]
- 4.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheever MA, et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Mensdorff-Pouilly S, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 7.Blixt O, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13:R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Mensdorff-Pouilly S, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. Int J Cancer. 2000;86:702–712. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Graves CR, Robertson JF, Murray A, Price MR, Chapman CJ. Malignancy-induced autoimmunity to MUC1: Initial antibody characterization. J Pept Res. 2005;66:357–363. doi: 10.1111/j.1399-3011.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Coltart DM, et al. Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J Am Chem Soc. 2002;124:9833–9844. doi: 10.1021/ja020208f. [DOI] [PubMed] [Google Scholar]
- 11.Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S. Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1) Glycobiology. 2004;14:681–692. doi: 10.1093/glycob/cwh090. [DOI] [PubMed] [Google Scholar]
- 12.Dziadek S, Griesinger C, Kunz H, Reinscheid UM. Synthesis and structural model of an alpha(2,6)-sialyl-t glycosylated MUC1 eicosapeptide under physiological conditions. Chemistry. 2006;12:4981–4993. doi: 10.1002/chem.200600144. [DOI] [PubMed] [Google Scholar]
- 13.Doménech N, Henderson RA, Finn OJ. Identification of an HLA-A11-restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol. 1995;155:4766–4774. [PubMed] [Google Scholar]
- 14.Haurum JS, et al. Presentation of cytosolic glycosylated peptides by human class I major histocompatibility complex molecules in vivo. J Exp Med. 1999;190:145–150. doi: 10.1084/jem.190.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlad AM, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: Processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepensky D, Tzehoval E, Vadai E, Eisenbach L. O-glycosylated versus non-glycosylated MUC1-derived peptides as potential targets for cytotoxic immunotherapy of carcinoma. Clin Exp Immunol. 2006;143:139–149. doi: 10.1111/j.1365-2249.2005.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninkovic T, Hanisch FG. O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J Immunol. 2007;179:2380–2388. doi: 10.4049/jimmunol.179.4.2380. [DOI] [PubMed] [Google Scholar]
- 18.Brossart P, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 19.Ninkovic T, et al. Identification of O-glycosylated decapeptides within the MUC1 repeat domain as potential MHC class I (A2) binding epitopes. Mol Immunol. 2009;47:131–140. doi: 10.1016/j.molimm.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 21.Karanikas V, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 23.Adluri S, et al. Specificity analysis of sera from breast cancer patients vaccinated with MUC1-KLH plus QS-21. Br J Cancer. 1999;79:1806–1812. doi: 10.1038/sj.bjc.6990288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acres B, et al. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–594. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilewski T, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 26.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 27.Butts C, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 28.Longenecker BM, Reddish M, Koganty R, MacLean GD. Specificity of the IgG response in mice and human breast cancer patients following immunization against synthetic sialyl-Tn, an epitope with possible functional significance in metastasis. Adv Exp Med Biol. 1994;353:105–124. doi: 10.1007/978-1-4615-2443-4_11. [DOI] [PubMed] [Google Scholar]
- 29.Ragupathi G, et al. Vaccines prepared with sialyl-Tn and sialyl-Tn trimers using the 4-(4-maleimidomethyl)cyclohexane-1-carboxyl hydrazide linker group result in optimal antibody titers against ovine submaxillary mucin and sialyl-Tn-positive tumor cells. Cancer Immunol Immunother. 1999;48:1–8. doi: 10.1007/s002620050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagan E, et al. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julien S, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen AL, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199:707–716. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee P, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee P, et al. Mucin 1-specific immunotherapy in a mouse model of spontaneous breast cancer. J Immunother. 2003;26:47–62. doi: 10.1097/00002371-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc C, Deriaud E, Mimic V, van der Werf S. Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J Virol. 1991;65:711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spohn R, et al. Synthetic lipopeptide adjuvants and Toll-like receptor 2—structure-activity relationships. Vaccine. 2004;22:2494–2499. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 38.Apostolopoulos V, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci USA. 2003;100:15029–15034. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingale S, Buskas T, Boons GJ. Synthesis of glyco(lipo)peptides by liposome-mediated native chemical ligation. Org Lett. 2006;8:5785–5788. doi: 10.1021/ol062423x. [DOI] [PubMed] [Google Scholar]
- 40.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 41.Van Amersfoort ES, Van Berkel TJC, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen PA, et al. STAT3- and STAT5-dependent pathways competitively regulate the pan-differentiation of CD34pos cells into tumor-competent dendritic cells. Blood. 2008;112:1832–1843. doi: 10.1182/blood-2007-12-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirotani T, et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem Biophys Res Commun. 2005;328:383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 44.Morse MA, Whelan M. A year of successful cancer vaccines points to a path forward. Curr Opin Mol Ther. 2010;12:11–13. [PubMed] [Google Scholar]
- 45.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 2011;186:1963–1969. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
- 48.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. 2004;4:1953–1962. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- 49.Tacken PJ, Torensma R, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology. 2006;211:599–608. doi: 10.1016/j.imbio.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Braun P, et al. Effects of glycosylation on fragments of tumour associated human epithelial mucin MUC1. Bioorg Med Chem. 1998;6:1531–1545. doi: 10.1016/s0968-0896(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 51.Deck MB, Sjölin P, Unanue ER, Kihlberg J. MHC-restricted, glycopeptide-specific T cells show specificity for both carbohydrate and peptide residues. J Immunol. 1999;162:4740–4744. [PubMed] [Google Scholar]
- 52.Bäcklund J, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263-270) in humanized transgenic mice and in rheumatoid arthritis. Proc Natl Acad Sci USA. 2002;99:9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buskas T, Li YH, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 54.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee P, et al. MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J. 2001;18:931–942. doi: 10.1023/a:1022260711583. [DOI] [PubMed] [Google Scholar]
- 56.Gaekwad J, et al. Differential induction of innate immune responses by synthetic lipid a derivatives. J Biol Chem. 2010;285:29375–29386. doi: 10.1074/jbc.M110.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.