Upon detection of microbe-associated molecular patterns (MAMPs), plants usually shift cellular activities from normal growth to defense responses called microbe- or pathogen-induced immunity (MTI or PTI) (1, 2). Effective regulation of the tradeoffs between growth and immunity according to physiological and pathological conditions is critical for plant fitness in nature. The leucine-rich repeat receptor kinase (LRR-RK) BAK1 has been considered a candidate for mediating such a tradeoff, because it serves as a coreceptor for ligand-binding LRR-RKs for both steroid hormones and MAMPs, including BRI1, which binds brassinosteroids (BRs), and FLS2, the receptor for bacterial flagellin peptide (flg22) eliciting MTI (3, 4). However, it has remained unclear whether sharing BAK1 provides the benefit of signal cross-talk, or is simply because BAK1 does not determine signaling specificity and thus needs no diversification. Experimental evaluation of BAK1's role in the interplay between BR and flagellin, as described in two studies in PNAS (5, 6), however, discover complex actions of BR on immune responses, including positive and negative as well as BAK1-dependent and BAK1-independent effects.
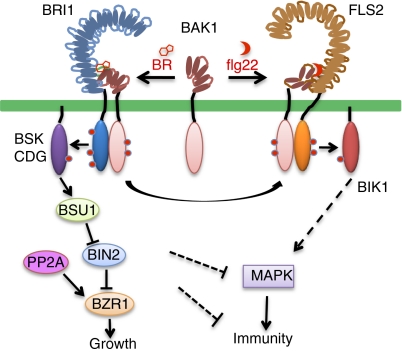
The steroid hormone BR binds to the extracellular domain of the LRR-RK BRI1 to initiate a phosphorylation cascade leading to BR responsive gene expression, cell elongation, and plant growth (7–9). Ligand-induced BRI1 kinase activation involves association and sequential transphosphorylation with BAK1 (BRI1-associated kinase 1) or its homologs (SERK1, -2, and -4) (10, 11). Activated BRI1 phosphorylates the BSK1 and CDG1 receptor-like cytoplasmic kinases, which activate the BSU1 phosphatase (12); BSU1 dephosphorylates and inactivates the GSK3-like kinase BIN2, which otherwise phosphorylates transcription factors that promote BR-regulated gene expression and plant growth (9) (Fig. 1). BR treatment has been shown to enhance disease resistance in rice and tobacco, but the molecular mechanism has remained unknown (13).
Fig. 1.
BR regulation of immunity at multiple levels. BR induces BRI1 binding to BAK1, which negatively affects FLS2 signaling by decreasing available BAK1, but some BRI1-activated BAK1 can activate FLS2. BR also suppresses MTI through an unknown mechanism downstream of BIK1.
Innate immune responses in plants are triggered through recognition of conserved MAMPs at the host cell surface by the pattern-recognition receptors, which are mostly LRR-RKs (2, 14). Well-characterized MAMPs include flagellin (flg22), an 18-aa peptide from the elongation factor Tu (elf18), and chitin from fungal cell wall (2). These MAMPs act through specific pattern-recognition receptors to activate the MAPKs, leading to metabolic changes, such as oxidative burst, that halt microbe proliferation (14). Analogous to BR-induced BRI1 signaling, flg22 binding to FLS2 induces rapid association and transphosphorylation with BAK1 (3), and activated FLS2 phosphorylates the receptor-like cytoplasmic kinase BIK1 to transduce the signal (15, 16) (Fig. 1). Sharing BAK1 as coreceptor, the BRI1 and FLS2 pathways may antagonize each other by competing for BAK1 or enhance each other by increasing the cellular pool of active BAK1 (4).
To test whether BR and MAMP signaling modulate each other through BAK1, Albrecht et al. treat wild-type plants with BR and flg22 and analyze the responses at biochemical and physiological levels (6). When applied separately to wild-type plants, BR and flg22 each induced distinct biochemical and gene expression responses, without detectable overlap. When plants were cotreated with BR and flg22, flg22 had no effect on BR-induced responses, but BR significantly decreased flg22-induced MTI responses, including oxidative burst and defense gene expression. The results suggest a unidirectional BR inhibition of PTI responses, whereas flg22 has no effect on BR-induced responses (6).
Although consistent with the idea that BRI1–BAK1 complex formation reduces the amount of BAK1 available for FLS2, the observed BR inhibition of flg22 responses surprisingly turned out to be independent of BAK1. Measurement of the FLS2–BAK1 complex using coimmunoprecipitation showed no decrease of BAK1 association with FLS2 by BR treatment. Only a very small fraction of BAK1 was recruited to BRI1, and BR treatment also had little effect on flg22-induced phosphorylation of FLS2 or its substrate BIK1. Furthermore, BR treatment also inhibited chitin-induced MTI responses, even though chitin signaling is independent of BAK1, and BR inhibited MTI in the null bak1-4 mutant. These results indicate that, instead of BRI1 recruiting BAK1 away from FLS2, BR inhibits PTI through an unknown signaling step downstream of the cell surface receptors (6).
In contrast to the conclusions of Albrecht et al., the report by Belkhadir et al. (5) provides evidence for BR modulation of MTI responses through both BAK1-dependent and independent mechanisms. Both increase and decrease of endogenous BR level compromise flg22-induced responses, suggesting that an appropriate endogenous level of BR is required for optimal flg22 signaling. Overexpression of BRI1 greatly reduced the responses induced by MAMPs (flg22, elf18, and peptidoglycans) that require BAK1 for signaling but had little effect on the responses induced by MAMP (chitin) that acts through a BAK1-independent signaling mechanism. These effects of BRI1 overexpression on MAMP responses were cancelled by overexpression of BAK1, and conversely, a cell-death phenotype of plants expressing elevated level of BAK1 was suppressed by overexpression of BRI1. The results demonstrate that BRI1 can recruit BAK1 away from the MAMP receptors (5).
Can BRI1-activated BAK1 enhance FLS2 signaling? This was tested using a hyperactive allele of BRI1sud1. Plants expressing BRI1sud1 protein at wild-type levels show an increased level of FLS2 phosphorylation without flg22 treatment and enhanced flg22-induced responses and resistance to a hemibiotrophic bacterial pathogen (5). The activation of FLS2 signaling by BRI1sud1 requires BAK1, consistent with the notion that some of the BRI1-activated BAK1 molecules can activate FLS2 signaling.
Thus, BR activation of BRI1 seems to have two opposite effects on BAK1-mediated FLS2 signaling, and the outcome seems to depend on the relative levels of BR, BRI1, and BAK1. When the BRI1 level is high and the BAK1 level is relatively low, a likely situation in young growing tissues, titration of BAK1 may be the dominant effect of BR on MTI. When the BRI1 level is low and the BAK1 level is not rate-limiting, a likely situation in mature leaves, increased BR signaling would enhance MTI signaling by providing active BAK1; this may explain the BR promotion of disease resistance observed in rice and tobacco (13). Under certain conditions these opposite effects of BR on MTI may cancel each other, which may explain why Albrecht et al. did not detect a significant BAK1-dependent BR effect on MTI (10), although other differences, such as exogenous BR treatment vs. changing endogenous BR levels, may also contribute to the discrepancy between the two studies.
Together with recent studies (17), these two PNAS reports support a paradigm that developmental signals modulate immunity in plants. It was shown recently that
BR treatment has been shown to enhance disease resistance in rice and tobacco.
the CLV3 peptide, previously known as a key signal controlling the balance between cell division and differentiation in the shoot apical meristem, directly activates FLS2 to provide immunity in the meristem and young developing tissues (17). Because both BR and BRI1 levels are developmentally regulated, the multiple actions of BR on FLS2 signaling are likely to provide robust mechanisms for modulating the tradeoff between growth and immunity. A future challenge is to unravel how plants use these mechanisms in the context of development and physiology.
There are more than 600 receptor-like kinases (RLKs) in Arabidopsis and more than 1,000 in rice (18). Although many of them are involved in perceiving specific signals, BAK1 and other members of the SERK family are not directly involved in signal perception or transduction but rather serve as coreceptors that help activate the ligand-binding RLKs. Because coreceptors are not involved in signaling specificity, they can be shared by multiple ligand-binding RLKs; this not only is genomically efficient and convenient, but apparently can also provide additional benefits of cross-talk between different pathways, which increases plasticity and coordination in the regulatory system (5). Additional cross-talk mechanisms that link BR to components downstream of FLS2/BAK1/BIK1 kinases may also involve sharing of ancient downstream signaling components such as MAPKs, but this needs further study. It is intriguing that the cross-talk is unidirectional from BR/BRI1 to the flg22/FLS2 pathway. Although FLS2 signaling inhibits plant growth, it seems independent of BR signaling (6). Perhaps competition for BAK1 is not an effective mechanism to inhibit BR promotion of growth because BRI1 can also use BAK1 homologs as coreceptors, whereas FLS2 mainly associates with BAK1. How BR inhibits MTI independent of BAK1 and how flg22 inhibits growth are outstanding questions for fully understanding the tradeoffs between growth and immunity.
Acknowledgments
Work on brassinosteroid signal transduction mechanisms in my laboratory is supported by National Institutes of Health Grant R01GM066258.
Footnotes
References
- 1.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 3.Chinchilla D, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 4.Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: The receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–541. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkhadir Y, et al. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albrecht C, et al. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc Natl Acad Sci USA. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.She J, et al. Structural insight into brassinosteroid perception by BRI1. Nature. 2011;474:472–476. doi: 10.1038/nature10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hothorn M, et al. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature. 2011;474:467–471. doi: 10.1038/nature10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TW, Wang ZY. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- 10.Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol. 2008;148:611–619. doi: 10.1104/pp.108.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, et al. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim TW, Guan S, Burlingame AL, Wang Z-Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashita H, et al. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33:887–898. doi: 10.1046/j.1365-313x.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 14.Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Lu D, et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Chah OK, Sheen J. Stem-cell-triggered immunity through CLV3p-FLS2 signalling. Nature. 2011;473:376–379. doi: 10.1038/nature09958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiu SH, et al. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]