Abstract
Toll like receptors (TLRs) use Toll–IL-1 receptor (TIR) domain-containing adapters, such as myeloid differentiation primary response gene 88 (MyD88) and TIR domain-containing adapter inducing IFN-β (TRIF), to induce activation of transcription factors, including NF-κB, MAP kinases, and IFN regulatory factors. TLR signaling also leads to activation of PI3K, but the molecular mechanism is not understood. Here we have discovered a unique role for B-cell adapter for PI3K (BCAP) in the TLR-signaling pathway. We find that BCAP has a functional N-terminal TIR homology domain and links TLR signaling to activation of PI3K. In addition, BCAP negatively regulates proinflammatory cytokine secretion upon TLR stimulation. In vivo, the absence of BCAP leads to exaggerated recruitment of inflammatory myeloid cells following infections and enhanced susceptibility to dextran sulfate sodium-induced colitis. Our results demonstrate that BCAP is a unique TIR domain-containing TLR signaling adapter crucial for linking TLRs to PI3K activation and regulating the inflammatory response.
Keywords: inflammation, negative regulator, macrophage, innate immunity, pattern recognition receptors
Toll-like receptors (TLRs) are a family of transmembrane receptors that sense the presence of evolutionarily conserved microbial motifs, such as lipopolysaccharide, lipoproteins, and nucleic acids (1). Ligation of TLRs by their cognate ligands initiates a signaling cascade culminating in the production of proinflammatory mediators (1). Intracellular adapters for TLRs critically rely on homotypic Toll–IL-1 receptor (TIR) module interactions for signal propagation (2). All known signaling adapters for TLRs use TIR domains for engaging their respective receptors. Upon recognition of ligands, TLRs recruit their adapter myeloid differentiation primary response gene 88 (MyD88) or TIR domain-containing adapter inducing INF-β (TRIF), which then activate downstream components of the signaling pathway, ultimately leading to activation of transcription factors such as NF-κB, AP-1, IFN regulatory factor (IRF)-3, IRF-7, and so forth (1). TLR activation in cells of the innate immune system plays a major role in host defense by contributing to enhanced phagocytosis (3) and increased oxidative burst (4). TLR activation also leads to synthesis and secretion of proinflammatory cytokines and chemokines (5). These cytokines and chemokines play a major role in recruiting additional cells to the site of infection, as well as in shaping the nature of adaptive immune responses.
Uncontrolled activation of the TLR signaling pathway leads to damaging inflammation, as seen in sepsis and chronic autoimmune diseases. Furthermore, chronic exposure to endogenous TLR ligands is implicated in the pathogenesis of atherosclerosis (6) and susceptibility to tumor metastasis (7, 8). Many regulatory checks have evolved to counter-balance the potentially damaging consequences of TLR ligation. Regulation of TLRs occurs at several levels in the signaling pathway and is mediated by numerous proteins, including: SIGIRR (single immunoglobulin IL-1R-related molecule), A20, IRAK (IL-1 receptor-associated kinase)-M, and TANK (TRAF family member-associated NF-κB activator) (9–13). Furthermore, TLRs can be counter-regulated by secreted factors, including IL-10, agonists of TAM (Tyro3, Axl and Mer) receptors, and type I IFN, all of which can be induced by TLR signaling (14–16).
PI3K are a family of serine/threonine kinases that phosphorylate variants of PIP2, creating variants of PIP3, ultimately leading to activation of the downstream kinases, PDK1 and AKT (also called PKB) (17). The PI3K pathway plays a major role in the immune system, including promoting cell survival, proliferation, and protein synthesis (17). TLR signaling, in addition to promoting activation of transcription factors including NF-κB, MAP kinases, and IRFs, also leads to activation of PI3K (18, 19); however, its role is less well understood (18, 19). Examinations of the role of the PI3K pathway in TLR signaling have used chemical inhibitors of PI3K, as well as genetic modification and mRNA silencing. Studies using chemical inhibitors (i.e., wortmannin and LY294.002) have inconclusively demonstrated that TLR-mediated PI3K activation can be either pro- or anti-inflammatory, potentially because of differences in cell-types or off-target affects of the inhibitor. Growing genetic evidence, however, suggests that the PI3K pathway is involved in limiting the inflammatory response by TLRs (18–21).
We explored the possibility that uncharacterized TIR domains existed within the human genome and undertook a comprehensive computational screen to “predict” the existence of novel TIR-domain–containing proteins. This approach suggested the existence of conserved TIR folds at the amino terminal ends of the B-cell adapter for PI3K (BCAP, also known as PIK3AP1) and its paralog B-cell scaffold protein with ankyrin repeats 1 (BANK1). BCAP has previously been demonstrated to serve as a signaling adapter linking the B-cell receptor (BCR) and CD19 to activation of PI3K (22, 23). Here, we ascribe a previously undescribed role to BCAP in linking TLRs to PI3K through a previously unknown TIR domain. We show that BCAP regulates activation of NF-κB dependent on its TIR domain and associates with the TLR signaling adapters MyD88 and TIRAP (TIR-domain–containing adapter protein). In addition, BCAP is critically required for TLR-mediated activation of PI3K/Akt. Thus, we have identified a unique role for BCAP in regulation of inflammatory responses through its role as a proximal TIR-domain–containing adapter in the TLR signaling pathway.
Results
BCAP Contains a Unique Amino-Terminal TIR Domain.
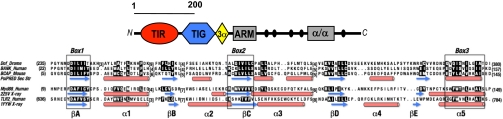
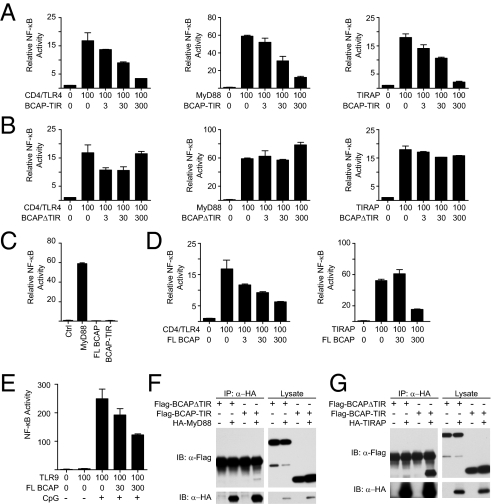
Using sensitive fold-recognition methods (24), we screened the human genome for novel TIR-domain proteins and discovered that BCAP, a protein previously reported to be downstream of B-cell receptor signaling (23, 25), has a cryptic TIR domain in its N-terminal region (Fig. 1, Fig. S1, and SI Results). To functionally confirm the presence of a previously undescribed TIR domain in BCAP, we cloned mutants by truncating the C terminus (referred to here as BCAP-TIR) or by truncating the N terminus (referred to as BCAPΔTIR) (Fig. S2). In reporter assays, TLR adapter proteins, such as MyD88 and TIRAP, minimally truncated to only their respective TIR modules and lacking their relevant activation domains, act as dominant-negative repressors of NF-κB reporters downstream of TLRs and TLR signaling adapters (26–28). Using NF-κB reporter assays, we confirmed that the TIR domain of BCAP, similar to the TIR domains of MyD88 and TIRAP (27, 29), represses activation of a NF-κB reporter downstream of a constitutively active TLR4 (CD4/TLR4) (30), MyD88, and TIRAP (Fig. 2A). Importantly, in contrast to the other known TLR signaling adapters, overexpression of BCAP did not lead to activation of NF-κB (Fig. 2C). Therefore, we tested whether full-length BCAP synergizes with other TLR signaling adapters to induce NF-κB activation but surprisingly found that full-length BCAP represses CD4/TLR4- and TIRAP-mediated activation of NF-κB (Fig. 2D). Similarly, full-length BCAP represses activation of NF-κB through CpG-dependent activation of TLR9 (Fig. 2E). BCAPΔTIR does not have the ability to inhibit NF-κB activation induced by TLR signaling adapters, suggesting that the inhibitory activity of BCAP is dependent on the presence of its previously uncharacterized TIR domain (Fig. 2B). Because activation of TLRs depends on homotypic interaction of the cytosolic TIR domains of TLRs and the TIR domains of TLR signaling adapters, we tested whether epitope-tagged BCAP mutants can associate with components of the TLR signaling pathway. These experiments demonstrate that BCAP-TIR, but not BCAPΔTIR, associates with MyD88 (Fig. 2F) and TIRAP (Fig. 2G), suggesting that the TIR domain of BCAP can associate with TLR signaling adapters. Similarly, overexpression studies also suggest that full-length BCAP, both endogenous and transfected, can associate with the TLR signaling adapters MyD88 and TIRAP (Fig. S3).
Fig. 1.
BCAP contains a cryptic amino-terminal TIR domain. Modular architecture of BCAP and TIR domain relationships. The N-terminal TIR domain is closely tied to a transcription factor-Ig (TIG) and 3-α-helix (3α) structural unit, a short set of armadillo repeats (ARM), and a C-terminal helical (α/α) module bracket a region of known phosphotyrosine sites (black circles). The mouse BCAP TIR domain is aligned to a second human paralog, BANK, and its Drosophila ortholog, Dof (43), and in turn, superposed using HHPRED (42) on the human MyD88 and TLR2 TIR domain structures (PDB IDs 2Z5V and 1FYW, respectively), highlighting the register of PsiPRED (44) and X-ray–defined α-helices (red cylinders) and β-strands (blue arrows) that comprise the conserved TIR fold (45). Conserved residues are noted by reverse lettering and Box 1–3 regions are diagnostic of TIR domains (45).
Fig. 2.
BCAP has a functional TIR domain and associates with TLR signaling adapters MyD88 and TIRAP. (A) NF-κB reporter assay using 293T cells transfected with constitutively active TLR4(30) (CD4-TLR4), MyD88, or TIRAP as indicated, and increasing amounts of BCAP-TIR. Numerical values represent the mass of plasmid in nanograms transfected. (B) As in A, with increasing amounts of BCAPΔTIR. (C) Assay for NF-κB reporter activity in 293T cells transfected with full-length BCAP (FL BCAP) alone or BCAP-TIR alone. (D) As in A, with increasing amounts of FL BCAP. (E) NF-κB reporter assay in 293T cells transfected overnight with TLR9 and increasing amounts of FL BCAP. Cells were stimulated as indicated with CpG for 6 h before assaying for reporter activity. Data are presented as mean ± SD and represent three or more independent experiments (A–D) or one experiment (E). (F) 293T cells cotransfected as indicated with Flag-BCAPΔTIR, Flag-BCAP-TIR, and/or HA-MyD88 were lysed and subjected to immunoprecipitation with anti-HA antibodies. Precipitates were split and assayed for precipitation of HA-MyD88 or coprecipitation of Flag tagged BCAP mutants by immunoblotting. (G) As in F, with cells transfected with HA-TIRAP.
BCAP Negatively Regulates TLR Responses in Macrophages.
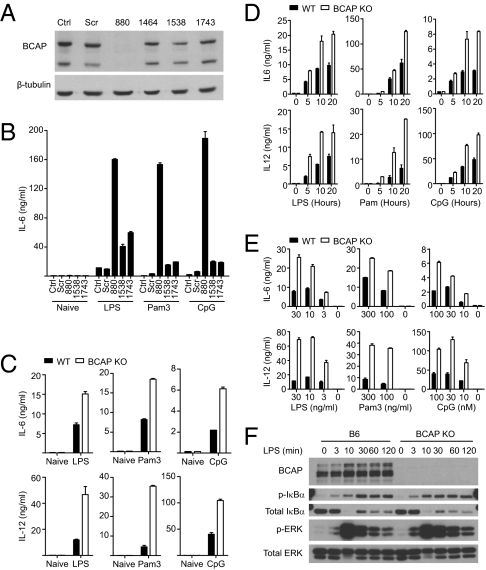
BCAP is expressed in B cells, macrophages, and natural killer cells (23, 31). In B cells, BCAP undergoes tyrosine phosphorylation upon stimulation of the BCR or CD19 (22, 23). To investigate whether BCAP is phosphorylated following TLR activation, we assayed the phosphorylation status of BCAP in TLR-stimulated B cells and macrophages. We found that BCAP undergoes a rapid and transient phosphorylation in both RAW264.7 macrophages and primary murine B cells upon stimulation with TLR ligands. To further examine the function of BCAP in TLR signaling, we created BCAP-silenced RAW264.7 macrophage cells by using shRNA-mediated gene silencing. Several shRNA clones induced varying amounts of BCAP silencing and one particular clone (880) had no detectable expression of BCAP protein (Fig. 3A). We stimulated the BCAP-silenced RAW264.7 cells and the relevant controls using ligands for TLR4, TLR2, and TLR9. These experiments revealed that BCAP-silenced RAW macrophages, in comparison with control RAW macrophages, secreted higher quantities of IL-6 in response to TLR ligands (Fig. 3B). BCAP-silenced RAW macrophages are sensitive to activation by TLR ligands at doses where control RAW macrophages fail to secrete detectable IL-6 (Fig. S4A). Consistent with BCAP-silenced cells, bone marrow-derived macrophages (BMDM) derived from BCAP-deficient mice made significantly higher quantities of the proinflammatory cytokines IL-6, IL-12 (Fig. 3 C–E), and TNF-α (Fig. S5) in response to TLR ligands. Thioglycollate induced peritoneal macrophages from BCAP-deficient mice, compared with cells from WT mice, also secreted significantly higher levels of IL-6 in response to TLR stimulation. In contrast, when BMDMs from BCAP-deficient mice were tested for a response to secondary stimulation by TLR ligands, they were tolerized, as were WT BMDMs, suggesting that BCAP is dispensable for the induction of TLR tolerance in macrophages (Fig. S6A). Similarly, BCAP-deficient BMDMs had an equivalent capacity to induce message levels of Irak3 and Tnfaip3, the genes encoding IRAK-M and A20 (Fig. S6B). Examination of NF-κB and MAP kinase activation upon TLR stimulation of BCAP-silenced macrophages or BCAP-deficient BMDMs demonstrated early modest enhancements in phosphorylation of IκBα and sustained activation of, JNK and ERK, compared with control cells (Fig. 3F and Fig. S4B). Taken together, these data suggest that BCAP is important for regulating the initial burst of proinflammatory cytokine production, in a manner independent of A20, and induction of other negative regulators, including IRAK-M, might then contribute to tolerance toward secondary stimulation by TLR ligands.
Fig. 3.
BCAP controls the TLR-induced inflammatory response. (A) BCAP was silenced in the RAW264.7 macrophage cell line using shRNA and efficiency was monitored by immunoblotting. Ctrl, untransduced control cells; Scr, scrambled sequence; 880, 1464, 1538, and 1743, shRNA silencing at the corresponding nucleic acid positions of BCAP. (B) BCAP-silenced RAW264.7 cells were stimulated for 20 h with 100 ng/mL LPS, 100 ng/mL Pam3CSK4 (Pam3), or 1 μM CpG and secreted IL-6 was quantified by ELISA. Data represent four independent experiments. (C) BMDMs from WT or BCAP KO mice were stimulated for 20 h with 100 ng/mL LPS, 100 ng/mL Pam3CSK4 (Pam3), or 1 μM CpG and secreted IL-6 or IL-12 p40/p70 was analyzed by ELISA. (D and E) As in C, with BMDM stimulated with the LPS, Pam3CKS4, or CpG (D) for the indicated times or (E) at the indicated concentrations. For C–E, data represent three or more independent experiments and are presented as the mean ± SD. (F) Lysates from B6 or BCAP KO BMDMs stimulated with 100 ng/mL LPS as indicated and immunoblotted for phosphorylation of IκBα or ERK.
BCAP Links TLRs to PI3K/Akt.
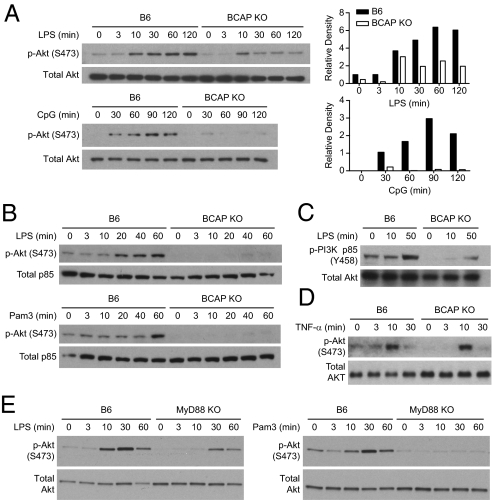
Because BCAP has previously been demonstrated to play a role in activating PI3K downstream of the BCR (23), we hypothesized that BCAP may also play a role in PI3K activation upon TLR stimulation. There are contradictory reports regarding the role of PI3K in activation of NF-κB following TLR activation (18, 19). Pharmacological drugs that inhibit PI3K implicate the PI3K pathway in both enhancing and inhibiting TLR-mediated NF-κB and MAP kinase activation (18, 19). Genetic evidence, however, suggests that PI3K activation is involved in dampening the proinflammatory profile of both macrophages and dendritic cells (20, 21, 32). To investigate if BCAP links TLR signaling and PI3K activation, we examined the phosphorylation status of the downstream kinase, Akt, following activation of TLR2, TLR4, and TLR9. TLR activation induced phosphorylation of Akt at serine 473 in WT cells, (Fig. 4 A and B); however, BCAP-deficient cells had decreased phosphorylation of Akt upon activation of TLR4 and TLR9 (Fig. 4A). The defect in Akt phosphorylation by TLR-stimulated BCAP-deficient cells was further enhanced during serum deprivation (Fig. 4B). Serum-deprived BCAP-deficient macrophages, but not WT macrophages, also secreted more IL-12 than cells cultured under serum-replete condition (Fig. S7), further supporting a role for PI3K in regulating TLRs. Furthermore, BCAP-deficient cells had a reduced capacity to phosphorylate the p85 subunit of PI3K upon TLR stimulation (Fig. 4C). Analysis of MyD88-deficient macrophages demonstrated that activation of Akt also depends on MyD88 (Fig. 4E). Activation of BMDMs using TNF-α led to comparable phosphorylation of Akt in both WT and BCAP-deficient cells, suggesting that BCAP plays a specific role in TLR-mediated activation of Akt (Fig. 4D). These data clearly demonstrate that BCAP participates in TLR signaling, potentially downstream of MyD88, and is a critical link between TLR signaling and activation of the PI3K-Akt pathway.
Fig. 4.
Critical requirement of BCAP for TLR-mediated activation of PI3K. (A) Lysates from B6 or BCAP KO BMDMs stimulated with 100 ng/mL LPS or 1 μM CpG, as indicated, were analyzed for S473 phosphorylation of Akt by immunoblotting. The relative density was calculated using densitometry (Right). (B) BMDMs from B6 or BCAP KO BMDMs were serum-deprived for 4 h with media containing 1% FCS. BMDM were then stimulated using 100 ng/mL LPS or 100 ng/mL Pam3CSK4 as indicated, and lysates subjected to immunoblotting to detect phosphorylation of Akt. Data represent three independent experiments. (C) BMDM were serum-deprived for 16 h with 1% FCS then stimulated as indicated with 100 ng/mL LPS. Lysates were analyzed for Y458 phosphorylation of PI3K-p85. (D) B6 or BCAP KO BMDMs were stimulated using 10 ng/mL of mTNF-α for the indicated periods of time. Lysates were analyzed for phosphorylation of Akt. As in other experiments with TLR ligands, WT BMDMs have constitutively phosophorylated Akt at the 0- and 3-min time points. Data represent two independent experiments. (E) B6 or MyD88 KO BMDMs were stimulated using 100 ng/mL LPS or 100 ng/mL Pam3CSK4 as indicated and lysates subjected to immunoblotting. Data represent three independent experiments.
BCAP Regulates Inflammatory Response in Vivo.
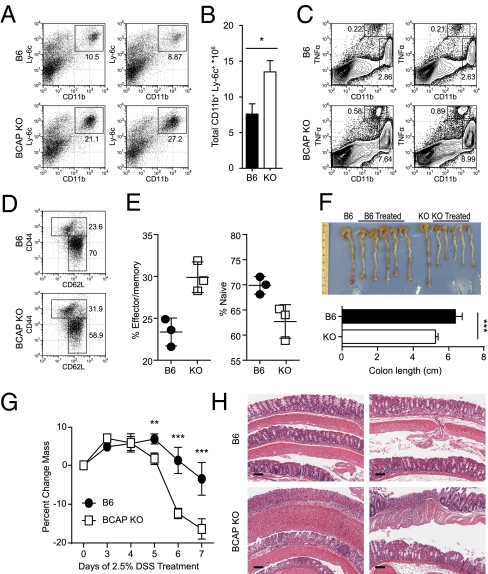
To understand the significance of BCAP in regulating proinflammatory responses in vivo, we challenged cohorts of WT and BCAP-deficient mice with live Salmonella typhimurium by the intraperitoneal route. Examination of these mice 72 h after infection revealed significantly enhanced recruitment of inflammatory myeloid cells in the spleens of BCAP-deficient mice compared with WT mice (Fig. 5 A and B). BCAP-deficient mice also recruited higher proportions of TNF-α producing dendritic cells (DCs) (33) and CD11b+ myeloid cells into their spleens following intraperitoneal infection by S. typhimurium (Fig. 5C). Because these data demonstrate increased inflammation in BCAP-deficient mice, we investigated if such profound activation of the innate immune system influences the activation status of CD4 T cells. Examination of proportions of naïve and memory CD4 T cells in the spleens of resting mice demonstrated that BCAP-deficient mice had higher proportions of CD44Hi CD62LLo effector/memory phenotype cells compared with age-matched control mice (Fig. 5 D and E). In vitro priming assays using WT and BCAP-deficient splenic DCs and OT-II CD4 T cells demonstrated that BCAP-deficient DCs induced enhanced priming of T cells compared with control DCs (Fig. S8A), leading to increased commitment of OT-II T cells toward both Th1 and Th17 phenotypes (Fig. S8 B and C). Taken together, these results demonstrate that BCAP plays a critical role in regulating inflammation following early activation of TLRs, and the absence of BCAP causes exaggerated responses in the innate immune system, consequently leading to higher activation of CD4 T cells.
Fig. 5.
BCAP regulates in vivo inflammatory responses. (A and B) B6 or BCAP KO mice were infected with 1 × 106 CFU S. typhimurium intraperitoneally and 3 d later splenocytes were analyzed by flow cytometry for recruitment and expansion of CD11b+ Ly-6c+ inflammatory myeloid cells. (A) Data from two independent mice are shown. (B) Quantification of cell number from A. *P < 0.05 as determined by t test. Error bars denote SD. (C) Splenocytes from two independent mice infected as in A were stimulated ex vivo with heat-killed S. typhimurium for 4 h in the presence of brefeldin A and TNF-α production by CD11bInt DCs and CD11bHi cells was monitored by intracellular staining. (D and E) Proportions of naïve (CD62LHi CD44Lo) and effector/memory (CD62LLo CD44Hi) CD4+ TCRβ+ T cells were analyzed in the spleens of age-matched naïve B6 or BCAP KO mice. Representative flow cytometry plots are shown in D and data from three independent mice are shown in E. Error bars denote SD. (F–H) Cohorts of B6 or BCAP KO mice were restricted to 2.5% DSS in the drinking water for 7 d. (F) Following treatment, mice were euthanized and colons harvested, photographed, and lengths measured (Lower). ***P < 0.001 as determined by t test. Error bars denote SD. (G) DSS-treated mice were monitored daily for weight loss. Data represent mean and SD of five mice. **P < 0.01 and ***P < 0.001 as determined by two-way repeated-measures ANOVA and subsequent Bonferonni posttest. (H) Tissue sections from DSS-treated mice (G) were stained with H&E to monitor colon damage histologically and images are from two independent DSS-treated mice. (Scale bars, 100 μm.) Data represent two independent experiments (A–C) or three independent experiments (D–H).
To further investigate the role of BCAP in regulating inflammatory responses in vivo, we subjected cohorts of WT and BCAP-deficient mice to dextran sulfate sodium (DSS)-mediated colitis. These experiments revealed a more severe form of colitis in BCAP-deficient mice as demonstrated by increased colon shortening (Fig. 5F) and increased weight loss (Fig. 5G) following 7 d of DSS treatment in the drinking water. Similarly, histological examination of colons from DSS-treated mice showed that BCAP-deficient mice had higher crypt ablation and effacement of epithelial cells (Fig. 5H) compared with WT controls. There was also increased neutrophil recruitment into the tissues of BCAP-deficient mice. These data demonstrate a very important role for BCAP in regulating inflammation in vivo.
Discussion
Ligation of TLRs with their cognate ligand leads to a cytosolic signaling cascade beginning with the recruitment of the proximal adapters MyD88 and TRIF. MyD88 and TRIF's recruitment to TLRs depends on homotypic interactions of the respective TIR domains found within the TLRs as well as the adapters (2, 16). Although the molecules and events that connect TLRs to NF-κB and MAP kinases are well understood, the mechanism of how TLRs activate PI3K is not known. We demonstrate here that an adapter molecule shown to participate in BCR signaling and B-cell survival (23, 25, 34) contains a cryptic but functional TIR domain and plays a major role in connecting the TLR signaling pathway to PI3K/Akt activation. The interaction of BCAP with TLR signaling machinery not only interferes with NF-κB and MAP kinase activation, by potentially disrupting the interaction between TLRs and known signaling adapters such as MyD88 and TIRAP, but also leads to TLR-mediated activation of the PI3K-Akt pathway. As a consequence, BCAP-mediated regulation of TLR signaling serves to limit production of the proinflammatory cytokines IL-6, IL-12, and TNF-α, as well as limit inflammatory responses in vivo. Regulation of TLR signaling is critical for limiting inflammation in response to microbial stimuli, as well as limiting adverse affects associated with signaling in response to endogenous TLR ligands, such as DNA complexes (35) and fatty acids (36), that could potentially exacerbate inflammatory conditions such as lupus and atherosclerosis.
Many regulatory steps limiting the outcome of TLR ligation have been defined, including the regulation of posttranslational modifications, signaling intermediates, chromatin modifications, transcriptional control, RNA stability, and secreted inhibitory factors. An additional player in the control of TLR-mediated inflammation is PI3K (18, 19). However, contradictory results addressing its role have led to confusion in the field. In experiments using chemical inhibitors, both positive and negative roles have been attributed to PI3K activity upon TLR stimulation (18, 19). Recently, genetic tools have helped clarify this confusion and suggest that PI3K is involved in the negative regulation of TLR signaling outcomes. Using mice deficient for the p85 subunit of PI3K, it was demonstrated that PI3K regulates IL-12 production by DCs (21). Akt1-deficient mice have defects in regulating several miRNAs that subsequently regulate repression of inflammation (20). Conversely, cells deficient for the tumor suppressor phosphatase and tensin homolog (PTEN), which regulates PI3K by converting PIP3 back into PIP2, and subsequently have increased activity of effectors downstream of PI3K including Akt, produce less TNF-α and keratinocyte-derived chemokine (32). Clearly, PI3K is functionally important for shaping the cellular response to TLR ligation.
The mechanism of PI3K activation by TLRs is not well understood. An initial study suggested that YXXM motifs in TLRs mediate the recruitment of PI3K to the signaling complex, which is in turn activated by RAC-1 (Ras-related C3 botulinum toxin substrate 1), but these assays were performed in the context of overexpression systems in cell lines and have yet to be confirmed genetically (37). Another study suggests that the TLR signaling adapter TIRAP (or MAL) links TLR2 signaling to PI3K activation (38). We have shown here that the adapter BCAP is specifically required for TLR-mediated PI3K activation. It is important to note here that MyD88-deficient cells, as shown here as well as by a previous study (39), are also defective in activation of Akt, suggesting that BCAP functions downstream of MyD88. Importantly, BCAP-deficient cells still maintain equivalent activation of Akt upon stimulation with TNF-α, an early secreted effector produced as a consequence of TLR stimulation. This finding, along with the early kinetic differences of Akt activation in BCAP-deficient cells compared with control cells, supports the hypothesis that BCAP acts as a proximal adapter in the TLR pathway and the resulting phenotype is not because of an off-target secondary effect. Taken together, our data suggest a complex including known TLR adapters and BCAP is required for subsequent TLR-mediated activation of PI3K. It is also possible that BCAP directly interacts with TLRs and the nature of endogenous association of BCAP with either MyD88, TIRAP, or TLRs, especially following TLR stimulation, requires further investigation. Functionally, BCAP could also be working by competitively inhibiting recruitment of MyD88 and TIRAP to the TLRs, as evidenced by our studies in 293T cells, where overexpression of full-length BCAP inhibits TLR4- and TLR9-mediated activation of NF-κB.
Improperly controlled signaling through TLRs contributes to aspects of several inflammatory diseases. Mice genetically deficient for A20 develop severe systemic inflammation, cachexia, and succumb to an early death, a process alleviated in the absence of MyD88 or upon deletion of the intestinal flora with broad-spectrum antibiotics (11). Another TLR regulator, SIGIRR, regulates intestinal inflammation and susceptibility to DSS-induced colitis (40), as well as regulating susceptibility to lupus (41). Control of inflammatory cytokines is critical for regulation of systemic inflammation, as well as shaping the adaptive immune response. As a result, priming of CD4 T cells by BCAP-deficient DCs results in increased proliferation and enhanced priming of these T cells toward the Th1 and Th17 lineages. Similarly, BCAP-deficient mice have enhanced proportions of effector/memory CD4 T cells, concurrent with our in vitro observation. Consequently, in mice deficient for BCAP, we find a substantial increase in numbers of inflammatory myeloid cells following a systemic infection. BCAP-deficient mice also display an enhanced susceptibility to DSS-induced colitis, corresponding with enhanced weight loss, as well as enhanced intestinal damage. This sentence is duplicated. It is probable that the increased susceptibility of BCAP-deficient mice to DSS colitis is because of a greater inflammatory response to intestinal bacteria breaching the intestinal barrier following DSS treatment. Although the in vivo data of greater numbers of inflammatory cells and greater inflammation of the colon are consistent with our finding that BCAP is a negative regulator of the TLR signaling pathway, it is plausible that there might be additional mechanisms by which BCAP could be regulating these in vivo outcomes.
Finally, our studies show that BCAP is a unique TIR-domain containing adapter molecule that forms a critical link between TLRs and activation of PI3K. Early in signal transduction, BCAP may engage MyD88 via its TIR domain and potentially reduce the availability of MyD88 for activation of NF-κB, thereby affecting the canonical pathway of TLR signaling. BCAP also mediates activation of PI3K downstream of TLRs and, as has been demonstrated before, PI3K activation has regulatory effects on the outcome of TLR signaling, including limiting cytokine secretion and inflammation. As the classic MyD88-dependent pathway is still functional in BCAP-deficient cells, utilization of cells deficient in BCAP will enable further genetic studies addressing the role of PI3K specifically through the TLR pathway. Future studies using BCAP-deficient mice or mice with cell-type specific BCAP deletion will further expand our understanding of the biological processes regulated by BCAP.
Materials and Methods
Computational Screens.
Using methods described previously (24), BCAP, BANK, and Dof alignments were structurally aligned against proteins in the PDB using HHPRED (42), resulting in a series of top matches to TIR domain structures (z scores of 43.2–26, with high probabilities ranging from 94.8 to 58), strongly suggestive of common ancestry and function.
Mice.
BCAP KO mice were previously generated (25) and were bred and maintained on C57BL/6 background (31) at the animal facility of the University of Texas Southwestern Medical Center. Control C57BL/6 mice were obtained from the University of Texas Southwestern Mouse Breeding Core Facility. Mice were maintained in specific pathogen-free conditions. Mice were used between 6 and 12 wk of age and all mouse experiments were done as per protocols approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Infections.
For infection experiments, one million CFU of S. typhimurium χ 4550 were injected into mice by the intraperitoneal route. Three days after infection, mice were killed and tissues harvested for analysis.
NF-κB Reporter Assay.
293T cells were transfected using linear polyethyleneimine (Polysciences). Cells were transfected with the NF-κB reporter vector pBIIX-luc and pCMV-Renilla-luc. Cells were cotransfected as described with CD4/TLR4 (30), Flag-MyD88, HA-TIRAP, or HA-TLR9 and the indicated BCAP constructs. Empty cloning vectors were used to equalize total plasmid mass transfected. Twenty hours later, cells were lysed in TNT lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100) and lysates were assayed for luciferase activity using the Dual-Glo luciferase kit (Promega). Data are reported relative to Renilla luciferase counts and normalized to cells transfected with only the empty vector.
Immunoblotting.
RAW264.7 cells lines or BMDM were stimulated with 100 ng/mL LPS, 100 ng/mL Pam3CSK4, or 1 μM CpG, as indicated, washed twice with ice-cold PBS, and lysed with TNT lysis buffer containing Complete Protease Inhibitor Mixture (Roche), 1 mM sodium orthovandate, and 20 mM glycerol 2-phosphate. For the TNF-α stimulation experiment, cells were stimulated with 10 ng/mL mTNF-α (R&D Systems) and lysed directly in SDS-sample buffer. Lysates were resolved on SDS-PAGE gels followed by transfer to PVDF membranes (Millipore). Membranes were analyzed by immunoblotting using specific antibodies. Stained membranes were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed to film (Kodak).
Immunoprecipitations.
For coimmunoprecipitation experiments, 293T cells were transiently transfected with the indicated combinations of Flag-BCAP mutants and HA-MyD88 or HA-TIRAP, or empty vectors. Thirty-six hours later, cells were lysed with TNT lysis buffer containing protease inhibitors. Lysates were quantified by BCA assay and 1 mg was incubated with anti-HA (Covance; clone 16B12) overnight and complexes were pulled down using protein G Sepharose beads (GE Healthcare). Precipitates were resolved on SDS-Page gels and transferred to PVDF membranes. Membranes were analyzed by immunoblotting for anti-Flag (Sigma Aldrich; clone M2) or anti-HA for detection of coprecipitating proteins.
DSS Colitis Model.
Age-matched B6 or BCAP KO mice were treated for 7 d with 2.5% 36–50 kDa DSS (MP Biomedicals) ad libitum in their drinking water. Mass was monitored by weighing daily. Following 7 d of treatment, mice were killed and colons was removed from the cecum to the anus, photographed, fixed with 4% paraformaldehyde, and submitted to the University of Texas Southwestern Molecular Pathology Core for paraffin embedding, sectioning, and H&E staining.
Supplementary Material
Acknowledgments
We thank Dr. K. Campbell for his help in acquiring the B-cell adapter for PI3K-deficient mice; Dr. J. Richardson, J. Shelton, and the University of Texas Southwestern Molecular Pathology Core facility for help with histology; and Drs. J. Forman, G. Barton, A. Unni, and members of the C.P. laboratory for helpful discussions and critical reading of the manuscript. T.D.T. is supported by National Institutes of Health Grant T32-AI005284-33.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118579109/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 3.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 4.West AP, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Karin M. Role of TLR2-dependent inflammation in metastatic progression. Ann N Y Acad Sci. 2011;1217:191–206. doi: 10.1111/j.1749-6632.2010.05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawagoe T, et al. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat Immunol. 2009;10:965–972. doi: 10.1038/ni.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald D, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 11.Turer EE, et al. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 17.Katso R, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 18.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 19.Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol Res. 2006;34:33–48. doi: 10.1385/IR:34:1:33. [DOI] [PubMed] [Google Scholar]
- 20.Androulidaki A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukao T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 22.Inabe K, Kurosaki T. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood. 2002;99:584–589. doi: 10.1182/blood.v99.2.584. [DOI] [PubMed] [Google Scholar]
- 23.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 24.Bazan JF, de Sauvage FJ. Structural ties between cholesterol transport and morphogen signaling. Cell. 2009;138:1055–1056. doi: 10.1016/j.cell.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T, et al. Essential immunoregulatory role for BCAP in B cell development and function. J Exp Med. 2002;195:535–545. doi: 10.1084/jem.20011751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald KA, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 27.Horng T, Medzhitov R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc Natl Acad Sci USA. 2001;98:12654–12658. doi: 10.1073/pnas.231471798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto M, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 29.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 31.MacFarlane AW, 4th, et al. Enhanced NK-cell development and function in BCAP-deficient mice. Blood. 2008;112:131–140. doi: 10.1182/blood-2007-08-107847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schabbauer G, et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 33.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki T, Kurosaki T. Contribution of BCAP to maintenance of mature B cells through c-Rel. Nat Immunol. 2003;4:780–786. doi: 10.1038/ni949. [DOI] [PubMed] [Google Scholar]
- 35.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbibe L, et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Sierra S, et al. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 2009;28:2018–2027. doi: 10.1038/emboj.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laird MH, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao H, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Lech M, et al. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Söding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 43.Battersby A, Csiszár A, Leptin M, Wilson R. Isolation of proteins that interact with the signal transduction molecule Dof and identification of a functional domain conserved between Dof and vertebrate BCAP. J Mol Biol. 2003;329:479–493. doi: 10.1016/s0022-2836(03)00489-3. [DOI] [PubMed] [Google Scholar]
- 44.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 45.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.