Abstract
Wolbachia inherited bacteria are able to invade insect populations using cytoplasmic incompatibility and provide new strategies for controlling mosquito-borne tropical diseases, such as dengue. The overreplicating wMelPop strain was recently shown to strongly inhibit the replication of dengue virus when introduced into Aedes aegypti mosquitoes, as well as to stimulate chronic immune up-regulation. Here we show that stable introduction of the wMel strain of Drosophila melanogaster into Aedes albopictus, a vector of dengue and other arboviruses, abolished the transmission capacity of dengue virus-challenged mosquitoes. Immune up-regulation was observed in the transinfected line, but at a much lower level than that previously found for transinfected Ae. aegypti. Transient infection experiments suggest that this difference is related to Ae. albopictus immunotolerance of Wolbachia, rather than to the Wolbachia strain used. This study provides an example of strong pathogen inhibition in a naturally Wolbachia-infected mosquito species, demonstrating that this inhibition is not limited to naturally naïve species, and suggests that the Wolbachia strain is more important than host background for viral inhibition. Complete bidirectional cytoplasmic incompatibility was observed with WT strains infected with the naturally occurring Ae. albopictus Wolbachia, and this provides a mechanism for introducing wMel into natural populations of this species.
The Asian tiger mosquito Aedes albopictus, a native of southeast Asia that in recent decades has invaded Africa, the Americas, and southern Europe, is now an important rural/semiurban vector of dengue virus across the tropics (1). It likely is involved in maintaining sylvatic cycles of transmission and acting as a bridge vector from these to urban epidemic cycles; it also transmits other Flaviviruses, such as yellow fever and West Nile, and the Alphavirus chikungunya. Like the primary urban dengue vector Aedes aegypti, Ae. albopictus is a day-biting species and thus is not amenable to control/prevention using insecticide-treated bed nets. This factor, along with the absence of a vaccine for dengue and the expanding disease range, calls for new methods of control.
All known wild populations of Ae. albopictus are naturally infected with two strains of the maternally inherited bacterium Wolbachia pipientis, known as wAlbA and wAlbB (2, 3); Ae. aegypti is naturally uninfected with the bacterium. Recent work has shown that when an overreplicating strain of Wolbachia from Drosophila melanogaster, wMelPop, was transferred into Ae. aegypti (4), the dissemination of dengue virus was strongly inhibited, as was the dissemination of chikungunya virus (5). In addition, transfer of the wAlbB strain from Ae. albopictus into Ae. aegypti (6) led to reduced susceptibility to dengue (7). Both Wolbachia strains also induced cytoplasmic incompatibility (CI) in Ae. aegypti, whereby uninfected females mated with infected males produce embryos that die shortly after fertilization. This mechanism is used by Wolbachia to spread through insect populations because in contrast, infected females can mate successfully with either infected or uninfected males, giving them a frequency-dependent reproductive advantage (8–10). Thus, the combination of viral inhibition and a built-in self-spreading mechanism provides attractive prospects for the control of dengue transmission by Ae. aegypti (11).
In addition to life shortening, the wMelPop strain also causes chronic immune up-regulation in Ae. aegypti (5, 12). The Toll pathway, some components of which are up-regulated in Ae. aegypti in the presence of wMelPop (5, 12), has been shown to play a role in the control of dengue dissemination in Ae. aegypti (13, 14). A general role of immune up-regulation in pathogen inhibition is also supported by the knockdown of the major immune gene TEP1, which partially rescues the inhibitory effect of the presence of wMelPop on Plasmodium berghei development in transiently infected Anopheles gambiae (15). The fact that the wAlbB transinfection caused dengue inhibition in Ae. aegypti (7) even through the original host of this Wolbachia strain Ae. albopictus is a fairly efficient dengue vector suggests a significant contribution of host background to the dengue inhibition phenotype, which possibly could be mediated by the increased immune response to Wolbachia found in a novel insect host. Thus, it is unclear whether any Wolbachia strain can produce strong dengue inhibition in a naturally Wolbachia-infected mosquito such as Ae. albopictus, which would be expected to have acquired a degree of immune tolerance to Wolbachia over time.
The wMelPop strain overreplicates and can approximately halve the lifespan of both its D. melanogaster (16) and Ae. aegypti (4) hosts. However, a wMelPop transinfection into Ae. albopictus also produced a greatly reduced egg hatch from intrastrain matings, and this appeared to preclude its application to disease control in Ae. albopictus (17). The wMel strain, which is phylogenetically close to the wMelPop variant (18), does not produce the life-shortening phenotype of the latter in its native D. melanogaster host (16). However, wMel can significantly delay the accumulation of RNA viruses, such as Drosophila C virus, in D. melanogaster (19–21). Thus, we selected wMel for experimental transfer into Ae. albopictus to examine whether this strain is capable of producing dengue inhibition and CI in this new host background.
Results
Generation of the Uju.wMel Line and Crossing Experiments.
A stable infection of wMel in a previously tetracycline-cured Ae. albopictus strain (UjuT) was generated. Cytoplasm from D. melanogaster was microinjected into UjuT, resulting in four G0 females, one of which was positive and produced sufficient progeny to establish an isofemale line. This line was backcrossed with UjuT males each generation to minimize bottlenecking and was selected for maximal maternal inheritance; proportions confirmed as positive for Wolbachia by PCR were 70% (n = 10) for G1, 81% (n = 16) for G2, 89% (n = 9) for G3, and 100% (n = 10) for G4. The infection remained at 100% after G4 (n = 152; tested up to G9). The Wolbachia strain present was confirmed to be wMel by sequencing of the wsp (Wolbachia surface protein) gene, which demonstrated 100% identity with published wMel wsp sequence.
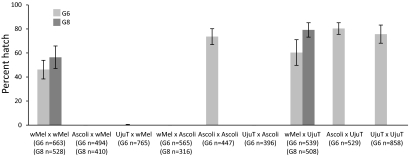
The transinfected strain initially showed reduced hatch rates compared with WT females, a 46.1 ± 7.8% hatch for Uju.wMel in G6 compared with 65.1 ± 10.7% for UjuT. After two successive generations of selection for high hatch rates, the Uju.wMel × Uju.wMel hatch rate had risen to 56.4 ± 9.4% by G8 (Fig. 1). A similar effect was previously reported in newly transinfected Drosophila (22) and Ae. aegypti (6). The effects of wMel on fecundity of Ae. albopictus remain to be characterized in detail; preliminary data suggest that wMel has no major negative effect on fecundity, with an average number of eggs laid per female of 60.3 ± 7.0 (n = 11) for Uju.wMel and 61.2 ± 5.0 (n = 13) for UjuT (P = 0.9716; Wilcoxon test).
Fig. 1.
Uju.wMel crossing type. Experiments to characterize the crossing type of the Uju.wMel line at G6 were performed using UjuT and Ascoli strains. Error bars represent the SEM of hatch rates between females; 15 adult males and 15 females were used for each mass cross. A second round of crossing experiments was performed two generations later (G8) using Uju.wMel × Uju.wMel, Ascoli × Uju.wMel, Uju.wMel × Ascoli, and Uju.wMel × UjuT following to the same procedure as in the previous experiment. No statistically significant difference was found between G6 and G8 of the same cross using Wilcoxon tests. The number of eggs counted for each generation is shown under the x-axis.
Crossing experiments were performed to examine whether the wMel strain was able to produce CI in this background, by crossing the Uju.wMel line in both directions with the uninfected UjuT and WT Ascoli strain (infected with Wolbachia strains wAlbA and wAlbB) (Fig. 1). As expected, UjuT males were compatible with all females. Males of the Uju.wMel line produced strong CI when mated to UjuT females (0.26% hatch). The Uju.wMel and Ascoli lines showed complete bidirectional incompatibility, with 0% hatch when females of either strain were mated with males of the other strain.
Dengue Infection.
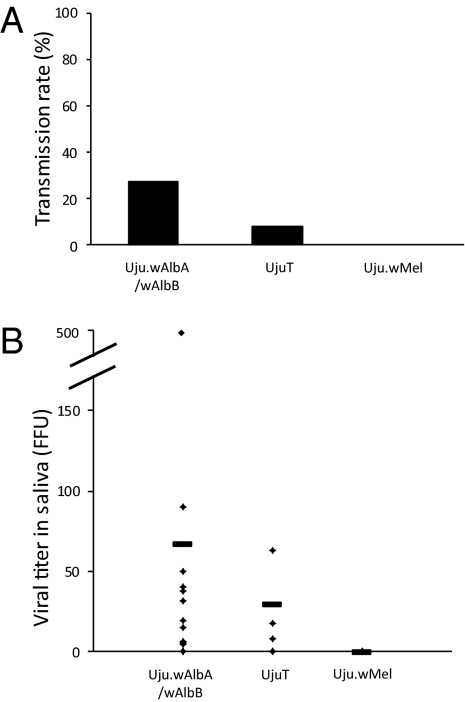
At 14 d after challenge with dengue 2 virus provided in an artificial blood meal, the transinfected Uju.wMel strain showed complete inhibition of dengue transmission capacity, with no infectious viral particles detected in the saliva of any tested mosquito. In contrast, the superinfected Uju.wAlbA/wAlbB strain (generated by the introgression of Wolbachia from Ascoli into UjuT to minimize any effect of host genetic background on dengue virus), and the uninfected UjuT strain were both able to transmit dengue 2 virus at day 14 postinfection (infection rate, 27.3% for the Uju.wAlbA/wAlbB strain and 8.3% for the UjuT strain) (Fig. 2). Mosquito saliva contained numbers of viral particles in the expected range for Ae. albopictus infected with dengue virus (23) (Fig. 2), with an average of 67 ± 139 viral particles for the Uju.wAlbA/wAlbB strain and 29 ± 29 viral particles for the UjuT strain.
Fig. 2.
Transmission capacity (A) and titer of dengue virus in mosquito saliva (B). Three Ae. albopictus strains (Uju.wAlbA/wAlbB, UjuT, and Uju.wMel) were orally infected with dengue 2 virus using glass feeders covered with a chicken skin membrane containing the infectious blood meal at a titer of 107 FFU/mL. After blood feeding, mosquitoes were transferred into cardboard containers and maintained in BSL3 insectaries at 28 °C. After 14 d, surviving mosquitoes were tested for the presence of viral particles in saliva collected using the forced salivation technique. The number of fluorescent foci in the saliva of each mosquito was estimated on C6/36 Aedes albopictus cell culture. The transmission capacity representing the percentage of mosquitoes with infectious saliva among tested mosquitoes was also calculated. The number of fed and assayed females was 44 for Uju.wAlbA/wAlbB, 26 for UjuT, and 44 for Uju.wMel.
Immune Gene Expression in Uju.wMel.
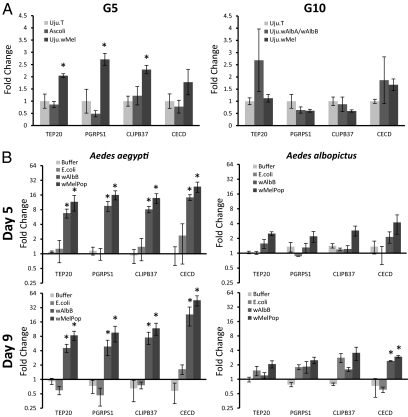
Because the wMelPop strain transinfection in Ae. aegypti has been shown to produce up-regulation of a number of immune genes, and given the possibility that this could be responsible for or contribute to the viral inhibition phenotypes, the effects of wMel transinfection on transcription levels were investigated for four Aedes albopictus immune genes. These four genes were selected to represent a range of immune gene categories including important antimicrobial effectors [a cecropin, a peptoglycan recognition protein (PGRP), and a thioester-containing protein (TEP) (15, 24–27)], and also because their orthologs were previously shown to be up-regulated in the presence of wMelPop (12). The transcription of immune genes was measured by qRT-PCR using G5 Uju.wMel, UjuT, and Ascoli females, as well as G10 Uju.wMel, UjuT, and Uju.wAlbA/wAlbB females, at 11 d after eclosion (Fig. 3A). There was no significant difference in immune gene transcription between the cured UjuT strain and the wAlbA/wAlbB superinfected line. Significant immune up-regulation was observed in Uju.wMel compared with the other two strains for three of the four genes assayed in the G5 experiment, but no significant immune up-regulation was observed at G10.
Fig. 3.
Immune gene expression in Wolbachia-infected and uninfected mosquitoes. (A) RNA was extracted from adult females of G5 Uju.wMel, UjuT, and Ascoli, and separately from G10 Uju.wMel, UjuT, and Uju.wAlbA/wAlbB, at 11 d posteclosion. The expression of four Ae. albopictus orthologs for Ae. aegypti immune genes was analyzed by qRT-PCR: a peptidoglycan recognition protein (PGRPS1), cecropin D (CECD), CLIP-domain serine protease (CLIPB37), and a thioester-containing protein (TEP20). Expression was normalized to that of the UjuT adult females. Error bars show the SEM of three biological replicates, each containing four adult females for the G5 experiment and six adults for the G10 experiment (a total of 12 and 18 mosquitoes per condition). *P < 0.05 compared with UjuT, Wilcoxon test. (B) Adult females were transiently infected with Wolbachia using intrathoracic injections with either wMelPop or wAlbB, controls of either heat-killed E. coli, or the buffer alone, approximately 3 d after eclosion. RNA was extracted from half of the females at 5 d postinjection and from the other half at 9 d postinjection. The expression of four Ae. aegypti immune genes was analyzed by qRT-PCR: a peptidoglycan recognition protein (PGRPS1) cecropin D (CECD), CLIP-domain serine protease (CLIPB37), and a thioester-containing protein (TEP20). Orthologs for these genes in Ae. albopictus were also analyzed by qRT-PCR. Expression was normalized to noninjected adult females of the same age from the same colony. Error bars show the SEM of three biological replicates, each containing five adult females (a total of 30 mosquitoes per condition). *P < 0.05 compared with noninjected control, Wilcoxon test.
To compare the contribution of Wolbachia strain type with host species background, transient somatic infections of wMelPop and wAlbB were also created in Ae. aegypti and Ae. albopictus using intrathoracic inoculation as described previously (15, 28). Adult females were injected with suspensions of Wolbachia purified from Ae. albopictus cell lines (Aa23) approximately 3 d after eclosion, and the transcription of immune genes was measured by qRT-PCR at 5 d after injection (Fig. 3B) (15, 29). Strong immune up-regulation was observed in Ae. aegypti with both wMelPop and wAlbB strains compared with noninjected, buffer-injected, and heat-killed E. coli-injected controls. However, no significant immune up-regulation was observed in Ae. albopictus injected with either Wolbachia strain, with the exception of CECD at day 9. Live Wolbachia was confirmed in wMelPop- and wAlbB-injected mosquitoes at 5 d and 9 d after injection by RT-PCR on the cDNA generated for this experiment.
Concentration of Wolbachia in Uju.wMel.
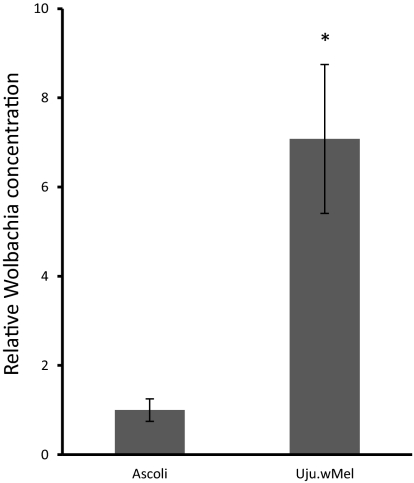
The concentration of wMel in Uju.wMel was compared with the combined concentration of both wAlbA and wAlbB in the Ascoli strain in adults at 11 d after eclosion. The ratio of Wolbachia wsp DNA to host S17 DNA was used to estimate the Wolbachia concentration The concentration of wMel was found to be approximately 7 times greater than the total concentration of Wolbachia in the superinfected Ascoli strain (Fig. 4).
Fig. 4.
Concentration of Wolbachia in Ascoli and Uju.wMel lines. DNA was extracted from adult females of Uju.wMel and Ascoli at 11 d after eclosion. The ratio between Wolbachia wsp DNA and host S17 DNA was used to estimate the concentration of Wolbachia. The concentration of wAlbA/wAlbB in the Ascoli strain was arbitrarily designated “1,” and the concentration of wMel in Uju.wMel was plotted relative to this. Error bars show the SEM of three biological replicates, each containing four adult females (a total of 12 mosquitoes per condition). *P < 0.05, Wilcoxon test.
Discussion
Our results show that wMel infection can block dengue virus transmission in the increasingly widespread vector species Ae. albopictus. RNA viral inhibition by wMel has been previously demonstrated in Drosophila (20), and we have shown that this viral interference is also produced when it is transferred into Ae. albopictus. The inhibition appears to be limited to specific strains of Wolbachia, given that Ae. albopictus is naturally infected with two strains of Wolbachia, wAlbA and wAlbB, which seem to have no inhibitory effect on the virus. This is the first time that Wolbachia-mediated dengue virus inhibition has been demonstrated in Ae. albopictus, and this result has significant implications for dengue control.
Uju.wMel produces complete bidirectional CI with the wild-type Ascoli line (containing a wAlbA and wAlbB superinfection, like all known wild populations of this species). The wMel crossing type differs from the crossing type of a wMelPop transinfection into Ae. albopictus (17), unexpectedly given the phylogenetic similarity of wMel and wMelPop (18). Bidirectional CI provides a method for stably introducing Wolbachia into populations, because bidirectionally incompatible crossing types cannot stably coexist (30); whichever strain is at a local majority has a reproductive advantage, because its females will more frequently encounter and mate with males with which they are compatible. Assuming that complete bidirectional CI also will be produced under field conditions, once the wMel infection reaches a population majority, it would then be expected to go to local fixation and be stable to further immigration of wAlbA/wAlbB-infected (WT) individuals with which they are incompatible. Large-scale female releases would not be essential for this strategy to be successful; because Aedes pupae are readily separated by sex, heavily male-biased releases could be done, which would suppress the female population (31). Given that most populations are seasonal, appropriately timed releases at the start of the rainy season could achieve local replacement with wMel.
Future experiments are needed to examine whether the creation of a stable wMel/wAlbA/wAlbB triple infection is possible. Based on previous work (32), we expect the crossing type of this triple infection to produce unidirectional incompatibility with the parental lines and thereby have the potential to spread wMel more efficiently through field populations. In any event, at the initial field trial stage, using the bidirectionally incompatible line would be preferable, to maintain better control over the geographical extent to which population replacement occurs.
There was 7 times more Wolbachia in the Uju.wMel strain compared with the superinfected (wAlbA and wAlbB) Ascoli strain. This result is quite surprising given the relatively high concentrations found in the natural superinfection. High levels of Wolbachia are expected in recently transinfected species (33) and may decrease over time as coadaptation of strain and host occur. It is possible that the increased density of Wolbachia had a negative effect on egg hatch rates from intrastrain matings in the early generations.
The immune gene up-regulation caused by wMel in the stably infected Uju.wMel line (Fig. 3A) was statistically significant for three of the four genes in one experiment, but on a much lower scale than that observed in a stably wMelPop-infected line of Ae. aegypti (12) or transiently wMelPop-transinfected Anopheles gambiae (15). By G10, no significant up-regulation was observed, possibly due to coadaption between the host and the transinfecting strain. Immune up-regulation was observed when Ae. aegypti was transiently infected with wAlbB (Fig. 3B), and there was no significant difference between the immune up-regulation caused by wMelPop and wAlbB in Ae. aegypti, demonstrating that this effect is not limited to the overreplicating wMelPop strain. The lack of significant immune up-regulation observed in Ae. albopictus when transiently infected with wAlbB, one of its natural Wolbachia, or with wMelPop for three of the four genes assayed, together with our previous findings (15), suggests that whether or not the host species is naturally infected with Wolbachia is a more important factor in the level of immune response than the Wolbachia strain used.
The fact that Ae. aegypti stably transinfected with wAlbB showed reduced transmission of dengue (7) suggests a host component, possibly immune-related, to the viral inhibition in that transinfection. However, the relatively modest immune up-regulation observed in the transinfected Ae. albopictus Uju.wMel line, together with the fact that no dengue virus was detected in saliva after the challenge, suggests that priming of the host immune system might not be the most important factor in this case of viral inhibition. The Wolbachia strain used seems to be the critical consideration here. Possible mechanisms for direct viral inhibition by Wolbachia include the production of reactive oxygen species by the bacterium (34) and resource competition, such as for cholesterol (5).
Future research using later generations of the line will more clearly identify whether wMel has any effects on the fitness or fecundity of Ae. albopictus similar to those demonstrated by the wAlbA/wAlbB superinfection (35, 36). Our results so far show no major effect on fecundity, unlike the significant fecundity reduction previously observed with wPip infection of Ae. albopictus (37). Furthermore, our wMel-transinfected line had a much higher hatch rate than that previously observed for a wMelPop strain transinfection in Ae. albopictus, which averaged only in the 10–20% range (17). This study has yielded an Ae. albopictus line that may provide the basis for a viable new option for dengue control in this species. A pair of studies published while this article was under review reported the generation of a wMel-transinfected Ae. aegypti line, dengue inhibition in this line (38), and successful field trials in Queensland, Australia (39), demonstrating the feasibility of field implementation of a Wolbachia population replacement strategy. Our study demonstrates that both of the two main vectors of dengue globally are amenable to such a strategy.
Materials and Methods
Mosquito Strains.
The Wolbachia-uninfected Ae. albopictus strain UjuT was generated by tetracycline treatment (40). The Ascoli strain of Ae. albopictus was colonized from San Benedetto del Tronto, Italy in 2006 by G. Favia and colleagues, and the Ae. aegypti Rockefeller strain originated in the Caribbean in the 1930s. All colonies and lines were maintained at 27 °C and 70% relative humidity on a 12-h light/dark cycle.
The wAlbA and wAlbB Wolbachia strains were introgressed into the UjuT background for four generations by removing all male pupae from one colony of the Ascoli strain and providing an approximately equal number of UjuT males. The resulting line was ∼94% UjuT nuclear background and contained both wAlbA and wAlbB. This line was generated to partially control for any effects of host background.
Embryo Microinjection and Line Establishment.
The wMel strain of Wolbachia was transferred from D. melanogaster yw67c23 embryos into Ae. albopictus (UjuT) by the transfer of cytoplasm. Adult Drosophila were encouraged to oviposit using apple juice agar plates and yeast paste. Eggs were collected at ∼30 min after oviposition. Ae. albopictus were encouraged to lay eggs by placing ∼15 females, blood-fed 7 d earlier, into a small (3 cm diameter, 10 cm tall) plastic vial with moist filter paper on the bottom. Eggs were collected at ∼30 min after oviposition. Ae. albopictus eggs were allowed to desiccate for 15–30 min. Both donor and recipient eggs were aligned on a nitrocellulose membrane and transferred to a glass slide using double-sided tape. The eggs were then covered with Voltalef oil ready for injection. Cytoplasm was aspirated from the posterior of the donor eggs using a FemtoJet microinjector (Eppendorf) and injected into the posterior of recipient eggs. After a short incubation time, eggs were transferred onto wet filter paper, stored at 100% humidity at 27 °C for 5 d, and then hatched in deoxygenated water. G0 larvae were reared under standard conditions. Females were separated 1–2 d after blood feeding into small plastic vials with moist filter paper on the bottom. Once females laid eggs, they underwent PCR analysis for the presence of Wolbachia using universal wsp primers 81F and 691R (3). These primers also were used for sequencing the wsp gene to confirm that the Wolbachia was the wMel strain. After initial optimization trials, the experiment from which the line was established involved microinjection of ∼100 embryos.
While the transinfected line was being established, only eggs from PCR-positive females were hatched. After G6, batches also were selected for high egg hatch. Eggs from individual Wolbachia-positive females were counted and hatched (with deoxygenated water in the small plastic vials), and then second instar larvae were counted. Approximately three-quarters of the broods with the highest hatch rates were pooled to form the next generation.
qRT-PCR and qPCR.
Gene transcription levels were tested by quantitative RT-PCR (qRT-PCR). RNA was extracted from adult mosquitoes using TRIzol reagent. cDNA was generated from 1 μg of this RNA using SuperScript Vilo (Invitrogen). cDNA was diluted to 1:20. The dsDNA dye SYBR Green (Invitrogen) was used for amplicon detection in a DNA Engine thermocycler (MJ Research) with a Chromo4 real-time PCR detection system (Bio-Rad). The following cycling conditions were used: 95 °C for 15 min, followed by 45 cycles of 95 °C for 10 s, 59 °C for 10 s, and 72 °C for 20 s, with fluorescence acquisition at the end of each cycle and a melting curve analysis.
Quantitative PCR (qPCR) was used to determine Wolbachia copy number. DNA was extracted from adult mosquitoes using the Livak method (41). DNA was diluted to 100 ng/μL using a NanoDrop spectrophotometer. Thermocycler conditions and reaction chemistry followed the same protocol as for qRT-PCR. Primer pairs used for Ae. albopictus qRT-PCR and qPCR are listed in Table 1. Ae. albopictus primers were designed using sequence data generated using degenerate primers based on Ae. aegypti Vectorbase sequences or from Ae. albopictus EST data from the National Center for Biotechnology Information. Primers for Ae. aegypti PGRPS1 (AAEL009474), CECD (AAEL000598), CLIPB37 (AAEL005093), and TEP 20 (AAEL001794) were as listed by Kambris et al. (12), and those for Actin 5c (AAEL011197) were as described by Kambris (15). Concentrations of all Wolbachia strains were measured using wsp primers (42).
Table 1.
Oligonucleotides designed for amplification from Ae. albopictus
| Gene | Oligonucleotide: forward; reverse |
| PGRPS1 | GCAACTTACTGGCCGCTCGC; CGTTGGAGCGCATACCCGTG |
| CECD | TTCACGAAGTTGTTCGCAAT; GGCATTGAAGACTCGTTTGC |
| CLIPB37 | ACCCGAACCAGGTTGTGTAGCG; GGATGCAACCAGTACGCCGTCC |
| TEP20 | TGCCCAGCGGATTTGTAGCAGAAG; AAACAGTCTGATTCGGGTCCCATGT |
| S17 | AAGCCCCTGCGTAACAAGAT; GTTATCTCTGCGCTCACGTTC |
CI Crosses.
Crossing experiments designed to characterize the crossing type of wMel were performed using UjuT, Ascoli, and Uju.wMel lines. All individuals were sexed as pupae. Adults were blood-fed at age 6 d, and the females were separated out into plastic vials for individual laying. Eggs were dried and allowed to mature at 27 °C and ∼70% relative humidity for 5 d, counted, and hatched in deoxygenated water containing algae and yeast. Larvae were fed with dried liver powder. Second instar larvae from each female were counted to give hatch rates. Females with no egg hatch were dissected to check for successful mating; egg hatch rates from unmated females were disregarded. Adults were given a constant supply of water and sucrose.
Dengue Infection.
One-wk-old Uju.wMel, UjuT and Uju.wAlbA/wAlbB females were deprived of sucrose solution for 24 h before exposure to the infectious blood meal containing 107 FFU (foci fluorescent units)/mL of virus. The dengue serotype 2 virus strain (provided by Leon Rosen) was isolated in 1974 from a human sera from Bangkok, Thailand. The artificial blood meal provided in glass feeders covered with a chicken skin membrane and maintained at 37 °C, consisted of a virus suspension (1/3 vol/vol), washed rabbit erythrocytes (2/3 vol/vol), and 5 mM ATP as a phagostimulant. Engorged mosquitoes were transferred into cardboard containers, provided with sucrose solution, and maintained in BSL-3 insectaries at 28 °C for 14 d. Saliva was collected using the forced salivation technique, which consists of inserting a capillary tube containing FCS into the proboscis of females whose legs and wings had been removed. After 45 min, saliva was collected and titrated by focus fluorescent assay on C6/36 Ae. albopictus cell culture. The transmission capacity was estimated as the percentage of mosquitoes with infectious saliva among tested mosquitoes.
Wolbachia Purification and Intrathoracic Inoculation.
Wolbachia were maintained in the Ae. albopictus cell line Aa23 (43). Cells were grown in 75-cm2 culture flasks to ∼50% confluence in Schneider's media containing 10% FBS, 140 U/mL of penicillin, and 140 μg/mL of streptomycin. Cells were passaged every 3–5 d. Wolbachia was extracted from cells and purified as described previously (44) at 3 d after the previous passage. The Wolbachia pellet was resuspended in Schneider's media with 10% FBS (without antibiotics) to an optical density of OD = 0.06 at a wavelength of 400 nm. For Escherichia coli controls, an OD of 0.01 at 400 nm was used. Then 69 nL of Wolbachia suspension (or 69 nL of Schneider's/E. coli suspension for the controls) was microinjected into the thorax of ∼3-d-old Ae. aegypti Rockefeller/Ae. albopictus UjuT strains using a Nanoject microinjector (Drummond). The mosquitoes were supplied with water and sucrose and left for 5 d before the qRT-PCR experiments.
Acknowledgments
We thank H. K. Phuc for assisting with the embryo microinjection trial experiments and P. Kittayapong and G. Favia for kindly providing the Ae. albopictus eggs. This study was supported by the Biotechnology and Biological Sciences Research Council, the European Commission Seventh Framework Program (INFRAvec), the Wellcome Trust, and Institut Pasteur (ACIP A-10-2009).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 2.Sinkins SP, Braig HR, O'Neill SL. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc Biol Sci. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- 3.Zhou W, Rousset F, O'Neil S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 5.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 7.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 9.Turelli M, Hoffmann AA. Cytoplasmic incompatibility in Drosophila simulans: Dynamics and parameter estimates from natural populations. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann AA, Turelli M. In: In Influential Passengers. O'Neill RV, Hoffmann AA, Werren JH, editors. Oxford: Oxford Univ Press; 1997. pp. 42–80. [Google Scholar]
- 11.Iturbe-Ormaetxe I, Walker T, O’ Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12:508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min KT, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA. 1997;94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh E, Mercer DR, Fu Y, Dobson SL. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Environ Microbiol. 2009;75:7783–7788. doi: 10.1128/AEM.01331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun LV, Riegler M, O'Neill SL. Development of a physical and genetic map of the virulent Wolbachia strain wMelPop. J Bacteriol. 2003;185:7077–7084. doi: 10.1128/JB.185.24.7077-7084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 20.Osborne SE, Leong YS, O'Neill SL, Johnson KN. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009;5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGraw EA, Merritt DJ, Droller JN, O'Neill SL. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci USA. 2002;99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazeille M, Mousson L, Martin E, Failloux AB. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis. 2010;4:e706. doi: 10.1371/journal.pntd.0000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalk R, Townson H, Ham PJ. Brugia pahangi: The effects of cecropins on microfilariae in vitro and in Aedes aegypti. Exp Parasitol. 1995;80:401–406. doi: 10.1006/expr.1995.1052. [DOI] [PubMed] [Google Scholar]
- 25.Gwadz RW, et al. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect Immun. 1989;57:2628–2633. doi: 10.1128/iai.57.9.2628-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meister S, et al. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 2009;5:e1000542. doi: 10.1371/journal.ppat.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blandin S, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 28.Jin C, Ren X, Rasgon JL. The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl Environ Microbiol. 2009;75:3373–3376. doi: 10.1128/AEM.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meister S, et al. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laven H. In: in Genetics of Insect Vectors of Disease. Wright R, Pal R, editors. Amsterdam: Elsevier; 1967. pp. 251–275. [Google Scholar]
- 31.Hancock PA, Sinkins SP, Godfray HC. Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl Trop Dis. 2011;5:e1024. doi: 10.1371/journal.pntd.0001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Gavotte L, Mercer DR, Dobson SL. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl Environ Microbiol. 2010;76:5887–5891. doi: 10.1128/AEM.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook PE, McGraw EA. Wolbachia pipientis: An expanding bag of tricks to explore for disease control. Trends Parasitol. 2010;26:373–375. doi: 10.1016/j.pt.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Cirimotich CM, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobson SL, Marsland EJ, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: Accelerating cytoplasmic drive. Genetics. 2002;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobson SL, Rattanadechakul W, Marsland EJ. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity. 2004;93:135–142. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- 37.Calvitti M, Moretti R, Lampazzi E, Bellini R, Dobson SL. Characterization of a new Aedes albopictus (Diptera: Culicidae)–Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae) J Med Entomol. 2010;47:179–187. doi: 10.1603/me09140. [DOI] [PubMed] [Google Scholar]
- 38.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 40.Otsuka Y, Takoaka H. Elimination of Wolbachia pipientis from Aedes albopictus. Medical Entomology and Zoology. 1997;48:257–260. [Google Scholar]
- 41.Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voronin D, Tran-Van V, Potier P, Mavingui P. Transinfection and growth discrepancy of Drosophila Wolbachia strain wMel in cell lines of the mosquito Aedes albopictus. J Appl Microbiol. 2010;108:2133–2141. doi: 10.1111/j.1365-2672.2009.04621.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill SL, et al. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 44.Rasgon JL, Gamston CE, Ren X. Survival of Wolbachia pipientis in cell-free medium. Appl Environ Microbiol. 2006;72:6934–6937. doi: 10.1128/AEM.01673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]