Abstract
The receptor-like tyrosine phosphatase CD45 positively regulates antigen receptor signaling by dephosphorylating the inhibitory tyrosine of the src family kinases. CD45-deficient mice fail to fully unmask the role of CD45 in B cells because of the expression of a partially redundant tyrosine phosphatase, CD148. However, mice that are doubly deficient in CD45 and CD148 exhibit a very early block in B-cell development, thereby obscuring later roles for CD45. To overcome these limitations, here we take advantage of an allelic series of mice in which CD45 expression is titrated broadly (0–180%). Although high expression of CD45 inhibits T-cell receptor (TCR) signaling, we show that CD45 plays a purely positive regulatory role during B-cell receptor (BCR) signaling. In concert with exaggerated BCR signaling, increasing CD45 expression drives enhanced receptor editing in the bone marrow and profound loss of follicular and marginal zone B cells in the spleen. In the context of the IgHEL/sHEL model of B-cell tolerance, such high CD45 expression transforms anergy into deletion. Unexpectedly, elimination of the autoantigen sHEL in this model system in order to block clonal deletion fails to rescue survival of mature B cells. Rather, high CD45 expression reduces B-cell activating factor receptor (BAFFR) expression and inhibits B-cell activating factor (BAFF)–induced B-cell survival in a cell-intrinsic manner. Taken together, our findings reveal how CD45 function diverges in T cells and B cells, as well as how autoreactive B cells are censored as they transit development.
Keywords: antigen receptor signaling, Lyn
Autoreactive B cells continuously arise in the bone marrow (BM) as a byproduct of somatic cell recombination of heavy and light chains. Critical tolerance checkpoints throughout B-cell development normally account for editing, deletion, or unrespon-siveness of such autoreactive clones within the mature repertoire (1). Autoreactive B-cell clones are progressively selected out of the developing B-cell pool in a manner that depends on the magnitude and duration of B-cell receptor (BCR) and B-cell activating factor receptor (BAFFR) signals that serve to enforce these checkpoints (2–4). Each pathway is independently required for normal B-cell development and survival, and dysregulation of either signaling pathway can cause devastating autoimmune disease (5–10).
The TNF superfamily member BAFF and its receptor BAFFR define a pathway that is absolutely required for B-cell survival beyond the early transitional (T1) stage of splenic B-cell development, when BAFFR expression is normally up-regulated (11). Mice deficient for either BAFF or BAFFR lack late transitional (T2) and mature follicular and marginal zone (MZ) B cells (9, 10). BAFFR delivers critical cell growth and survival signals to B cells through the phosphoinositide-3 kinase (PI3K) and noncanonical NF-κB pathways (2, 3, 11). In addition, through an as-yet unclear mechanism, BAFF promotes survival by suppressing protein kinase C-δ function (12). How these varied pathways are connected to one another and in turn to BCR signals is not yet fully understood.
BCR signaling requires the sequential action of the Src family kinases (SFKs) and Syk kinase (13). SFKs phosphorylate immunoreceptor tyrosine-based activation motif domains of the BCR Igα/β chains, in turn recruiting and activating the Syk kinase. Together, these kinases proceed to trigger downstream signaling events and considerable signal diversification. During B-cell development, PI3K and NF-κB signals are required for cell survival and metabolism, and MAPK signaling is required for positive selection (14–16). Furthermore, tonic and inducible BCR signals play distinct roles during development, such that antigen-driven signals are thought to mediate negative selection, whereas tonic signals are critical for cell survival and positive selection (14, 15, 17).
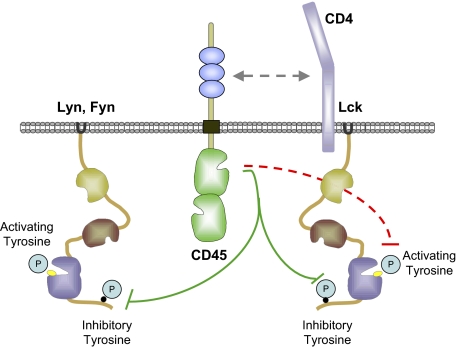
SFKs are situated at the top of these diverse signaling cascades, and their activity is tightly regulated (18). Phosphorylation of the c-terminal tyrosine of the SFKs stabilizes an autoinhibited conformation, whereas phosphorylation of the activation loop tyrosine of the SFKs is required for full enzyme activity. Reciprocal regulation of the inhibitory tyrosine of the SFKs is imposed by the kinase/phosphatase pair Csk and CD45, setting a threshold on antigen receptor (AgR) signals (18, 19).
CD45 is a receptor-like tyrosine phosphatase expressed at high levels on the surface of all nucleated hematopoietic cells (19). Consistent with CD45's role as a critical regulator of SFKs and AgR signaling, CD45-deficient mice exhibit defects in both T cells and B cells (20–22). TCR signaling is profoundly impaired in CD45-deficient mice, whereas BCR signaling is much less strongly affected (23, 24). Because CD45-deficient mice exhibit a severe block in thymic-positive selection, mature T cells are not produced (23). However, in contrast to the profound T-cell phenotype, B-cell number is unexpectedly preserved in CD45-deficient mice, and abnormalities in B-cell development are limited to the spleen (20, 24, 25). Our laboratory recently demonstrated that this is due in part to the expression of a partially redundant phosphatase, CD148 (24). Indeed, mice doubly deficient for CD45 and CD148 unmask a critical early role for CD45 during the pre–B-cell stage in the BM. However, these mice lack peripheral B cells, such that subsequent roles for CD45 during B-cell development remain obscured. Profoundly impaired B-cell development and B-cell number is also unmasked when the BCR repertoire of CD45-deficient mice is restricted in the absence of ligand through the addition of a BCR transgene (26). Reintroduction of autoantigen into this system can rescue B-cell maturation in CD45-deficient mice, compensating for impaired BCR signaling. Thus, we can conclude that CD45-deficient mice exhibit a B-cell phenotype that is incompletely revealed due to both phosphatase redundancy and compensation by a BCR repertoire shift.
Unexpectedly, studies of mice with subphysiological expression of CD45 single isoforms demonstrated that low levels of CD45 were sufficient to rescue T-cell development, but not to normalize B-cell maturation (27–29). We and others have shown that CD45 plays a negative regulatory role as well as a positive regulatory role during TCR signaling by dephosphorylating the activation loop as well as the inhibitory tyrosine of Lck (29, 30). Whether an analogous negative regulatory role exists in B cells is unclear, however (31).
To unmask unique roles for CD45 during BCR signaling and B-cell development, we took advantage of an allelic series of mice in which CD45 expression is titrated across a broad range, but regulated isoform splicing is unperturbed. In contrast to T cells, CD45 plays a purely positive regulatory role during BCR signaling that is correlated with distinct regulation of the activation loop tyrosines of the SFKs Lck and Lyn. In concert with enhanced BCR signaling, mice with supraphysiological CD45 expression exhibit enhanced receptor editing in the BM and marked loss of follicular and MZ B cells at the late transitional and mature stages of splenic development. We found that, unexpectedly, this cell loss is not rescued by restricting the BCR repertoire to eliminate negative selection. Rather, high CD45 expression reduces BAFFR expression and inhibits BAFF-induced B-cell survival in a cell-intrinsic manner. This represents a unique mechanism by which B cells exhibiting hyperresponsive AgR signaling are kept in check during late transitional and mature stages of development.
Results
Differential Regulation of BCR and TCR Signaling by CD45.
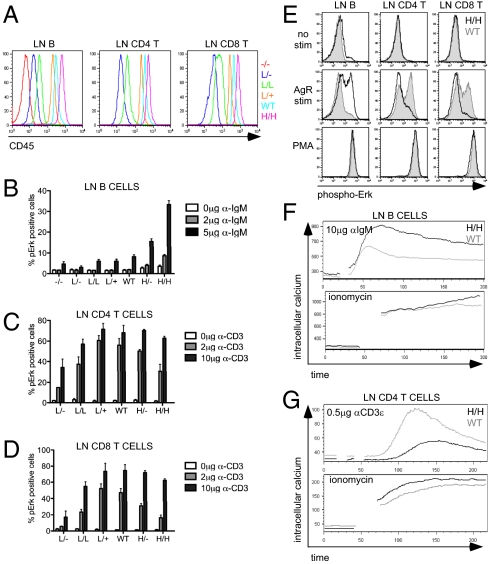
We previously described the characterization of the CD45 lightning (L) allele in which a single point mutation in the extracellular domain of CD45 results in low surface expression but does not alter tightly regulated isoform splicing (30). By combining the CD45 L allele with CD45 WT and null alleles, we generated an allelic series of mice expressing a range of CD45 on the surface of hematopoietic cells (30). L/−, L/L, and L/+ lymphocytes express 7%, 15%, and 55% of WT surface CD45, respectively (Fig. 1A). In addition, we have taken advantage of a line of mice in which a normally splicing CD45 transgene (H) is expressed in the context of two copies of endogenous CD45 to produce H/− and H/H mice carrying one copy and two copies of the transgene, respectively (32). Peripheral B cells from H/− and H/H mice express higher amounts of CD45 relative to T cells, nearly 140% and 180% WT, respectively (Fig. 1A). We include these transgenic mice with supraphysiological CD45 expression in our allelic series. Importantly, CD45 expression is titrated across this allelic series of mice during all stages of B-cell development with no effect on regulated splicing (SI Appendix, Fig. S1).
Fig. 1.
Titration of CD45 expression differentially regulates AgR signaling in T and B cells. (A) CD19+, CD4+, and CD8+ LN cells from an allelic series of mice generated by crossing CD45 WT, lightning (L), and null alleles, and CD45 ‘H’ Tg were stained for surface CD45 expression and assessed by flow cytometry. Representative histograms are plotted. (Genotypes in this and all subsequent figures: −/− = CD45−/−; WT or +/+ = CD45+/+; L/L = two lightning alleles; L/− = one lightning allele and one CD45 null allele; H/H = two H Tg in a CD45+/+ background; H/− = one H Tg in a CD45+/+ background.) CD45−/− mice are omitted from peripheral T-cell histograms and in B and C, because T-cell development is completely blocked in the thymus. (B) Allelic series LN cells were stimulated with α-IgM for 2.5 min and then fixed, permeabilized, and stained for phospho-Erk. Cells were costained for CD23 and B220 to identify mature follicular B cells. Data were collected by flow cytometry. The graph quantifies phospho-Erk+ CD23+ LN B cells (as depicted in E). Values are mean ± SEM of three independent experiments. (C and D) Allelic series LN cells were stimulated with α-CD3ε for 2.5 min and then fixed, permeabilized, and stained for phospho-Erk. Cells were costained for CD44, CD8, and CD4 to identify naïve T-cell subsets. Data were collected by flow cytometry. The graph quantifies phospho-Erk+ naïve CD44lo CD4 (C) or CD44lo CD8 (D) LN T cells (as depicted in E). Values are mean ± SEM of three independent experiments. (E) Lymphocytes were stimulated and stained as described in B–D. Histograms depict intracellular phospho-Erk expression in CD23+ B, CD4 T, or CD8 T LN cells from WT mice (gray-shaded histogram) and H/H mice (black line). (F and G) Intracellular calcium increase was assessed by flow cytometry in Fluo-3-loaded LN B cells (F) or LN CD4 T cells (G) from WT mice (gray) or H/H mice (black) after either AgR or ionomycin stimulation. Data in A, E, F, and G are representative of at least three independent experiments.
We interrogated AgR signaling in T and B cells from allelic series mice to determine how CD45 expression influences these pathways. CD45−/− mice contain virtually no mature T cells because of an absolute block in thymic positive selection (23). Peripheral T cells with low CD45 expression (L/−) exhibited impaired Erk phosphorylation in response to TCR ligation, whereas intermediate levels of CD45 were sufficient to rescue TCR signaling in both CD4 and CD8 naive T cells (Fig. 1 C and D). Increasing CD45 expression beyond intermediate levels led to progressive inhibition of TCR signaling, suggesting that CD45 has a negative regulatory role, in addition to its well-recognized positive regulatory role (Fig. 1 C and D). This is consistent with our previous findings in allelic series thymocytes as well as independent reports with other transgenic lines (29, 30).
Studies of BCR signaling in the CD45 allelic series mice have not yet been reported. In striking contrast to T cells, increasing expression of CD45 exerted purely positive regulation on BCR signaling, such that the induction of Erk phosphorylation was markedly enhanced and prolonged by supraphysiological expression of CD45 (Fig. 1 B and E and SI Appendix, Fig. S2A). Other signaling events downstream of the BCR, such as the PI3K pathway, exhibited analogous responses to CD45 expression; increasing CD45 expression was associated with prolonged S6 kinase phosphorylation in allelic series B cells (SI Appendix, Fig. S2B).
Finally, we assessed intracellular calcium increase in allelic series lymph node (LN) B and T cells in response to AgR ligation (Fig. 1 F and G and SI Appendix, Fig. S2C). Consistent with previous studies, BCR-induced calcium increase was relatively well preserved in mice with low CD45 expression (SI Appendix, Fig. S2C), attributed to the expression of a partially redundant phosphatase CD148 (24). Increasing CD45 expression to supraphysi-ological levels produced enhanced calcium in response to BCR ligation (Fig. 1F and SI Appendix, Fig. S2C). In contrast, T cells with supraphysiological CD45 expression exhibited impaired calcium increase relative to WT in response to TCR ligation (Fig. 1G).
These findings indicate that high levels of CD45 expression promote BCR signaling but inhibit TCR signaling, whereas the opposite occurs with low levels of CD45. Along with its obligatory positive regulatory role, a negative regulatory role for CD45 is thus evident during TCR signaling, but not during BCR signaling.
Differential Regulation of SFK Phosphorylation by CD45 in T and B Cells.
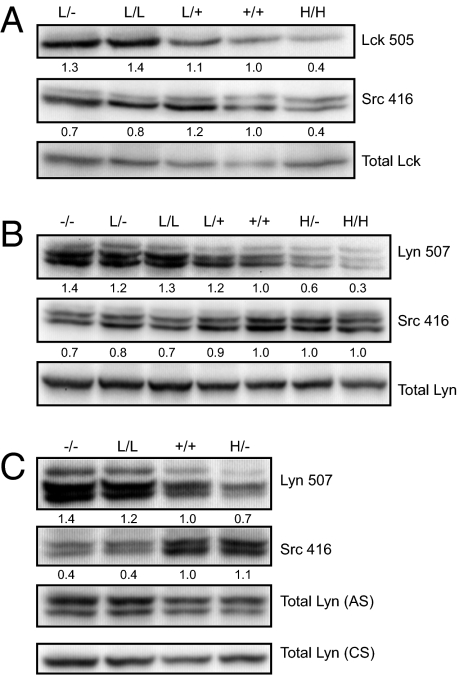
CD45 constitutively regulates SFK tyrosine phosphorylation. To understand why CD45 differentially regulates TCR and BCR signaling, we evaluated the phosphorylation state of the activation loop and inhibitory tyrosines of the SFKs in unstimulated T and B cells from the allelic series. We found that phosphorylation of the inhibitory tyrosine of Lck (Y505) in peripheral CD4 T cells was inversely proportional to the expression of CD45 in allelic series mice (Fig. 2A). Phosphorylation of the activation loop tyrosine of Lck (Y394) was also reduced with high expression of CD45 (Fig. 2A). These data are consistent with our previous biochemistry results in thymocytes from allelic series mice, as well as with similar studies by an independent group, and suggest that the positive and negative regulatory roles of CD45 in T cells may be mediated by dephosphorylation of these two distinct tyrosines with opposing functions (29, 30).
Fig. 2.
CD45 differentially regulates Src family kinases in T and B cells. (A) Whole-cell lysates of resting allelic series LN CD4 T cells were blotted with Ab to the inhibitory and activating tyrosines of Lck (Lck505/Src416). Src416 Ab binds activating tyrosines of all SFKs. The lower band represents p56 Lck; the upper band, p59 Fyn. Total Lck is detected as a loading control. Quantification was performed by densitometry on single Lck505 bands and lower Src416 bands (representing Lck). Values are normalized to total Lck and adjusted relative to WT values of 1. Data are representative of two independent experiments. (B and C) Whole-cell lysates of resting allelic series splenic (B) or LN (C) B cells were blotted with Ab to the inhibitory tyrosine of Lyn (Lyn507) and activating tyrosines of the SFKs (Src416). Total Lyn was detected as a loading control using an Ab from cell signaling (CS) that recognizes one Lyn isoform or anti-serum from Clifford Lowell's laboratory (AS) that recognizes both Lyn isoforms. Quantification was performed by densitometry on two lower Lyn507 and Src416 bands (representing Lyn). Values are normalized to total Lyn and adjusted relative to WT values of 1. Data in B are representative of three independent experiments; data in C, of two independent experiments.
Phosphorylation of the inhibitory tyrosine of Lyn (Y507) in purified splenic and LN B cells was progressively reduced with increasing CD45 expression, analogous to the observations in T cells (Fig. 2 B and C). However, the activation loop tyrosine of the SFKs was differentially regulated in B and T cells (Fig. 2 A–C). B-cell SFKs did not exhibit reduced phosphorylation of the activation loop tyrosine with higher levels of CD45 expression as was seen in T cells. We suggest that these observations provide a biochemical mechanism for the differential regulation of TCR and BCR signaling by CD45.
In both B cells and T cells, the inhibitory tyrosine of the SFKs is dephosphorylated by CD45, mediating the positive regulatory role of CD45. However, only in T cells is the activation loop tyrosine dephosphorylated with increasing CD45 expression, providing a mechanism for the unique negative regulatory role of CD45 in T cells, but not in B cells.
Differential Regulation of Proximal AgR Signaling Pathways by CD45 in T Cells and B Cells.
We next probed intermediate signaling events in allelic series T and B cells to understand how proximal regulation of SFKs produces downstream alterations in MAPK and calcium signals. We found that phosphorylation of Zap70, Lat, and PLCγ1 in allelic series CD4 T cells mirrored downstream events, such that maximal phosphorylation of these signaling molecules was seen at intermediate levels of CD45 expression rather than at low or supraphysiological levels (Fig. 1C and SI Appendix, Fig. S3A). In contrast, we found no profound alterations in the amplitude of proximal signals, such as Syk phosphorlation, in allelic series B cells in response to BCR stimulation (SI Appendix, Fig. S3 B and C); rather, allelic series B cells demonstrated more apparent differences in the duration of signaling and in downstream events such as MAPK and S6 kinase phosphorylation (SI Appendix, Figs. S2 A and B and S3C). We speculate that this profound difference between allelic series T cells and B cells may be related to the unique dual positive and negative regulatory roles of the B-cell SFK, Lyn (33). In addition, these biochemical studies reveal a subtle but consistent increase in basal Erk phosphorylation in B cells expressing supraphysiological CD45, suggesting enhanced tonic as well as inducible BCR signaling in those cells (SI Appendix, Fig. S3 B–D).
High CD45 Expression Inhibits B2 Cell Development.
Although T-cell development is completely blocked at the CD4/CD8 DP stage in CD45−/− mice, B-cell development in these mice is only mildly affected because of the expression of a partially redundant phosphatase, CD148 (20–22, 24). Indeed, total B-cell number was relatively preserved in mice with complete or partial deficiency in CD45 expression (Fig. 3 A and B and SI Appendix, Fig. S4A). However, B-cell number in both spleen and LN was strikingly reduced in mice with supraphysiological expression of CD45 (Fig. 3 A and B and SI Appendix, Fig. S4A).
Fig. 3.
High CD45 expression negatively regulates follicular B-cell development. (A) Graph showing total splenic cells from allelic series mice. Values are mean ± SEM of between four and six biological replicates. (B) Graph presenting total splenic CD19+ B-cells from allelic series mice. Values are mean +/− SEM of 5–12 biological replicates. (C) Representative plots of CD19+ BM B cells from allelic series mice stained for IgM and IgD to identify developmental subsets as gated in SI Appendix, Fig. S1A. (D) Representative plots of CD19+ splenic B cells from allelic series mice stained for AA4.1 and CD23 to identify developmental subsets as gated in SI Appendix, Fig. S1C. Data in C and D are representative of at least five independent experiments. (E) Graph quantifying T1, T2, and FM splenic B-cells from allelic series mice as gated in D. Values are mean ± SEM of between five and seven biological replicates. (F) Competitive chimeras were generated with a 1:1 mix of H/H and WT donor BM. BM and splenic B cells from chimeras were stained to identify B-cell developmental subsets (as gated in SI Appendix, Fig. S1 A and C). The bar graph represents relative contribution of 45.1+ H/H and 45.2+ WT B cells to each developmental compartment (gating as in SI Appendix, Fig. S3 F–I). Data are normalized to a 1:1 ratio of 45.1:45.2 cells at the IgM− BM B-cell stage of development. Values are mean ± SEM of three biological replicates. (G) BM cells from allelic series mice were surface stained for CD19, IgM, and IgD expression to identify immature BM B cells (IgM+IgD−), and then fixed, permeabilized, and stained with antibodies to either Igκ or Igλ. The graph quantifes the percentage of λ+ cells in the immature BM B-cell compartment (gating as in SI Appendix, Fig. S1A). Values are mean ± SEM of three biological replicates. In this and subsequent figures, pairwise comparisons of data as in A, B, and F were performed using the unpaired t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (i.e, P > 0.05). Linear regression analysis was performed as described in Materials and Methods. In all figures where linear regression is performed (as in A, B, E, and G), r2 represents goodness of fit, and P values were determined using Fisher's exact test. In E, each B-cell compartment (T1, T2, and FM) was independently subjected to linear regression analysis.
We assessed sequential stages of conventional B2 cell development in the BM and spleen of allelic series mice to determine whether high CD45 expression drives B-cell loss during development or after B-cell maturation (16, 34) (SI Appendix, Fig. S1). BM B-cell subsets in allelic series mice were identified via IgM and IgD surface expression and showed no marked abnormalities during early developmental stages (Fig. 3C). However, the numbers of mature recirculating BM B cells in H/H mice were profoundly reduced, suggesting that B-cell loss occurs after BM development (Fig. 3C). We assessed early (T1) and late (T2) transitional as well as follicular mature (FM) B-cell subsets in the spleen using the well-defined markers CD23, AA4.1, IgM, and IgD (Figs. 3D and SI Appendix, Fig. S4 B and C). Absolute numbers of T1 B cells in allelic series mice were only modestly affected by CD45 expression; however, T2 and FM B-cell numbers were markedly reduced in mice with supraphysiological CD45 expression (Fig. 3E).
AA4.1 (CD93)+ CD23+ splenic B cells have been further subdivided into T2 IgMhi and T3/AN1 IgMlo B-cell subsets (35). The latter population was recently suggested to represent a naturally occurring pool of anergic B cells (35). We assessed the relative proportion of these subsets across the allelic series and found a threshold requirement of CD45 expression necessary for the development of this compartment (SI Appendix, Fig. S4 C and D). Mice with subphysiological levels of CD45 expression demonstrated a complete loss of this population, whereas those with WT or higher CD45 expression levels had a normal relative distribution of T2 and T3/AN1 splenic B cells (SI Appendix, Fig. S4D). Interestingly, a progressive down-regulation of IgM surface expression with increasing CD45 expression was seen in CD23+AA4.1− follicular mature splenic B cells (SI Appendix, Fig. S4E). Generation of competitive repopulation chimeras in which H/H origin hematopoietic cells constituted a minority of total donor cells demonstrated that these developmental phenotypes were cell-intrinsic and failed to unmask an earlier fall-off at the BM negative selection checkpoint (Fig. 3F and SI Appendix, Fig. S5 A–F).
Increasing Expression of CD45 Promotes Receptor Editing.
Although significant loss of H/− and H/H B cells during splenic development was observed, no deletion of H/H B cells was evident during BM stages of B-cell development even in the stringent context of a competitive chimera (Fig. 3 E and F). In response to strong BCR signaling, developing B cells can undergo receptor editing early during BM development and switch light chains from κ to λ (36). Normally inbred C57BL/6 mice express a BCR repertoire comprising mostly κ+ cells. We assessed allelic series mice for κ and λ light chain expression in the immature BM B-cell compartment (IgM+ IgD−), and found a progressive increase in λ+ B cells with increasing CD45 expression (Fig. 3G). We conclude that titration of BCR signal strength by CD45 expression triggers receptor editing rather than deletion in developing B cells in the BM.
Increasing Doses of CD45 Down-Regulate the Expression of B-Cell Coreceptors.
It has been previously shown that CD45−/− B cells exhibit a block in “maturation” between the T2 and FM stages of B-cell development characterized by impaired IgM down-regulation (20, 24, 25). Importantly, subphysiological expression of single CD45 isoforms failed to rescue this phenotype (28). To further characterize the phenotype of mature follicular B cells in allelic series mice, we quantified the expression of the CD21, CD1d, and CD19 coreceptors, as well as the IgM BCR, on the surface of both splenic and LN B cells across the series. We found that these surface receptors were progressively down-regulated with increasing CD45 expression (SI Appendix, Figs. S4E and S6 A and B). Receptor down-regulation occurred even on B cells with supraphysiological CD45 expression and on mature follicular B cells (AA4.1− CD23+), suggesting that this phenotype does not represent a pure developmental arrest (SI Appendix, Figs. S4 C–E and S6 A and B). Importantly, other surface markers, such as IgD and CD23, were not down-regulated with increasing CD45 expression, suggesting some specificity to this process. Furthermore, mixed BM chimeras from H/H and WT donors revealed that coreceptor dysregulation in these genotypes is B-cell intrinsic (SI Appendix, Fig. S6C).
Titration of CD45 Expression has Opposing Effects on MZ and B1 B-Cell Lineage Commitment.
MZ and B1 lineages contain innate-like rapidly responding B cells capable of mounting an antibody response independent of T-cell help (37). Commitment to these lineages has been previously correlated with BCR signal strength in several distinct mutant mouse strains characterized by perturbed BCR signaling (37). We assessed B-cell lineage commitment in allelic series mice to define how titration of CD45 expression regulates these processes. MZ B cells normally reside in the spleen and are characterized by high CD21 and CD1d surface expression. As reported previously, (24) we found that CD45 expression is dispensable for MZ B-cell production (SI Appendix, Fig. S7A). We observed progressive reduction in both relative and absolute MZ B-cell numbers with increasing CD45 expression, and nearly complete loss of this population in H/− and H/H mice with supraphysiological CD45 expression (SI Appendix, Fig. S7 A and C). It is notable that titration of BCR signal intensity along the allelic series revealed continuous rather than threshold requirements for MZ lineage commitment. Our observations also suggest a greater sensitivity to BCR signaling intensity in MZ B-cell development than in follicular B-cell development. Moreover, competitive chimeras generated from H/H and WT donor BM demonstrated that altered MZ B-cell commitment in the allelic series is cell-intrinsic (SI Appendix, Fig. S7E).
Although MZ B-cell fate is inversely correlated with BCR signal strength in our system, B1 lineage commitment requires high BCR signal strength (38). B1 lineage B cells in mice are found predominantly in the peritoneal cavity and can be identified by CD5 and CD23 surface markers to distinguish B1a (CD5int CD23lo) and B2 (CD5lo CD23hi) lineages respectively. Allelic series mice demonstrated an inverse relationship between MZ and B1 B-cell production (SI Appendix, Fig. S7 B–D). Consistent with previous reports, B1 B-cell production was impaired at low levels of CD45 expression (SI Appendix, Fig. S7 B and D). Increasing CD45 expression beyond 50% of WT levels was associated with a relative, but not an absolute, expansion of B1 B cells at the expense of B2 B cells (SI Appendix, Fig. S7D). This suggests that B1 B cells, unlike MZ and follicular B cells, require moderate BCR signal strength and tolerate strong BCR signaling, but do not expand beyond a maximal compartment size.
Restricting the BCR Repertoire to Eliminate Clonal Deletion Fails to Rescue B-Cell Survival in H/H Mice.
Next, we wanted to understand whether restricting the BCR repertoire to eliminate the effects of ligand-driven BCR signaling events modified the development of CD45 allelic series B cells. In particular, we hoped to determine whether B-cell loss in mice with supraphysiological CD45 expression and enhanced BCR signaling was driven by autoantigen-mediated clonal deletion. To do this, we used the IgHEL BCR transgenic system, in which ligand-dependent events, such as clonal deletion and anergy, can be regulated by modulating the expression of HEL ligand (39, 40). We generated CD45−/−, CD45+/+, and H/H IgHEL mice that lacked expression of HEL antigen, and assessed B-cell development in the absence of strong ligand and compensatory repertoire shifts. We found that restricting the BCR repertoire in the absence of ligand unmasked a severe block in CD45−/− B-cell development, as reported by Cyster et al. (26). However, B-cell number was not rescued in H/H IgHEL mice, suggesting that B-cell loss in these mice is not driven primarily by antigen-mediated clonal deletion (Fig. 4A).
Fig. 4.
Restricting the BCR repertoire to eliminate clonal deletion does not rescue B-cell survival in H/H mice. (A) Graph quantifying splenic B-cell number in CD45−/−, +/+, and H/H mice expressing the IgHEL Tg in the absence of sHEL ligand. Values are mean ± SEM of between three and six biological replicates. (B) Representative plots of CD19+ splenocytes from mice in A stained for IgM and IgD expression to identify T1 (IgMhiIgDint) and T2/FM (IgDhi) subsets. (C) Representative plots of CD19+ splenocytes from mice in A stained for CD23 and AA4.1 to identify T1, T2, and FM subsets (as gated in SI Appendix, Fig. S1C). (D and E) Representative plots of CD19+ splenocytes from mice in A stained for CD21 and CD23 (D) or IgM and CD1d (E) to identify CD21hi, CD1dhi, and IgMhi MZ B cells. Data in B–E are representative of at least three mice per genotype.
We also found that CD45−/− IgHEL B cells in the absence of ligand failed to mature beyond the T1 stage of development into the follicular compartment, but instead adopted the MZ B-cell phenotype (Fig. 4 B–E). Conversely, no MZ B cells developed in IgHEL mice with supraphysiological CD45 expression despite elimination of strong ligand signaling in the context of a permissive BCR specificity (Fig. 4 D–E and SI Appendix, Fig. S8A). We also assessed the effect of restricting the BCR repertoire on the B1a population in the peritoneal lavage. We found a profound decrease in this population in the absence of sHEL antigen irrespective of CD45 expression level (SI Appendix, Fig. S8E). This result suggests that titration of tonic BCR signaling is not sufficient in the absence of selecting antigen to drive differentiation into this B-cell compartment. Surface expression of coreceptors on H/H and H/− B cells also was not rescued by a restricted BCR repertoire (SI Appendix, Fig. S8B). Taken together, these data suggest that either a ligand-independent signal through the BCR or a non-BCR signal drives the loss of FM and MZ B cells and coreceptor down-regulation in H/H mice.
High CD45 Expression Transforms Anergy into Deletion in the Context of the IgHEL/sHEL Double-Transgenic Model System.
Monoclonal IgHEL B cells that develop in the context of soluble ligand (sHEL) are not deleted, but instead exhibit an “anergic” phenotype characterized by impaired responses to restimulation through the BCR (39). In contrast, sHEL ligand can rescue development of CD45−/− IgHEL B cells, presumably by compensating for impaired BCR signaling (26). We examined the effect of sHEL ligand on the development of IgHEL B cells expressing supraphysiological CD45. We observed that in the context of soluble ligand, H/− IgHEL B cells underwent marked deletion (Fig. 5A).
Fig. 5.
Supraphysiological CD45 expression transforms anergy into deletion in the context of a restricted BCR repertoire. (A) Graph quantifying splenic B-cell number in CD45+/+ and H/− mice expressing IgHEL Tg with or without sHEL expression. Mice were generated genetically rather than through chimeras. Values are mean ± SEM of between three and six biological replicates. (B) Graph representing MFI of surface IgM in mice described in A. Values are mean ± SEM of three biological replicates. (C) Representative plots of total splenocytes from CD45+/+ and H/− mice with or without restricted BCR repertoire (IgHEL) in the presence or absence of autoantigen (sHEL) stained for IgM and IgD to identify phenotypic distribution of B cells. (D) Intracellular calcium increase upon α-IgM stimulation assessed by flow cytometry in fluo-3–loaded B lymphocytes from IgHEL+ CD45+/+ and IgHEL H/− mice with or without sHEL antigen. Data are representative of three biological replicates. (E) Graph representing MFI of CD86 expression on B lymphocytes from IgHEL+ CD45+/+ and IgHEL+ H/− mice with or without sHEL antigen expression stimulated overnight with either PMA/ionomycin or α-IgM (μg/mL). Values are mean ± SEM of three biological replicates. The unpaired t-test was used to compare genotypes stimulated with 1 μg, 4 μg, and PMA/ionomycin stimuli independently. The lowest P value of each independent pairwise comparison is plotted for ease of interpretation. (F) Graph representing MFI of CD86 expression on LN B cells from CD45+/+ and H/H mice with an unrestricted repertoire stimulated as in E. Values are mean ± SEM of three biological replicates.
IgHEL B cells chronically exposed to sHEL ligand exhibit marked down-regulation of IgM surface expression, which is thought to contribute to the anergic phenotype of these cells (39). Both H/− and CD45+/+ IgHEL B cells that developed in the context of sHEL exhibited this marked IgM down-regulation (Fig. 5 B and C). Surprisingly, H/− as well as H/H IgHEL B cells developing in the absence of sHEL also exhibited IgM down-regulation (Figs. 4B and 5 B and C). This down-regulation was more dramatic than the relatively modest change observed in the context of an unrestricted repertoire, but not as severe as the sHEL-induced down-regulation (Fig. 5 B and C and SI Appendix, Figs. S4 B and E and S6). We suggest that either a weak endogenous ligand or a ligand-independent tonic BCR signal may drive IgM down-regulation in H/− and H/H IgHEL mice in the absence of sHEL antigen.
Anergy or functional unresponsiveness to BCR signaling is an important characteristic of IgHEL sHEL B cells. Both CD45+/+ and H/− IgHEL B cells from mice expressing sHEL exhibited severely impaired calcium increase in response to BCR ligation, suggesting that surviving B cells in these mice are anergic (Fig. 5D). Unexpectedly, H/− IgHEL B cells from mice that do not express sHEL showed modestly reduced calcium responses to BCR ligation as well (Fig. 5D). In contrast, in the context of an unrestricted repertoire, supraphysiological CD45 expression promoted early biochemical events, such as calcium increase, in response to BCR ligation (Fig. 1 B, E, and F and SI Appendix, Fig. S2). We propose that the dramatic down-regulation of surface IgM expression in H/− and H/H IgHEL B cells may contribute to this difference in signaling between restricted and unrestricted repertoire B cells.
Impaired up-regulation of activation markers in response to prolonged BCR stimulation is another feature of anergic IgHEL sHEL B cells. In the context of sHEL, both CD45+/+ and H/− IgHEL B cells exhibited severely reduced CD86 up-regulation across a broad dose–response curve (Fig. 5E). Even in the absence of sHEL ligand expression, H/− IgHEL B cells exhibited decreased CD86 up-regulation relative to CD45+/+ IgHEL B cells. Importantly, phorbol myristate acetate (PMA) and ionomycin treatment of cells to bypass proximal BCR signaling also produced impaired activation marker up-regulation (Fig. 5E), demonstrating that functional unresponsiveness in B cells with supraphysiological CD45 expression is at least partially driven by mechanisms independent of surface IgM down-regulation.
H/H B Cells with an Unrestricted Repertoire Are Partially Unresponsive to BCR Ligation.
In the context of a restricted BCR repertoire, H/− B cells exhibit a state of diminished responsiveness even in the absence of chronic autoantigen exposure (Fig. 5 D and E). We next sought to determine whether this is exclusively a feature of restricting the BCR repertoire of mice with supraphysiological CD45 expression. We thus assessed activation marker up-regulation in response to BCR ligation in allelic series B cells from mice with an unrestricted repertoire. Indeed, despite hyperresponsive proximal BCR signaling events (Fig. 1 B, E, and F and SI Appendix, Fig. S2), up-regulation of CD86 on H/H B cells was significantly impaired relative to CD45+/+ B cells (Fig. 5F). Consistent with observations in the context of a restricted repertoire, H/H B cells with an unrestricted repertoire also exhibited impaired activation marker up-regulation in response to PMA and ionomycin treatment in which proximal signaling events were bypassed (Fig. 5F). Taken together, these data suggest that H/H B cells are in a partially unresponsive state in which distal, but not proximal, signaling events are dampened. Given that a similar state of partial unresponsiveness is exhibited by H/− and H/H IgHEL B cells even in the absence of ligand (Fig. 5E), we speculate that ligand-independent tonic BCR signals also may drive this phenotype in mice with supraphysiological CD45 expression in the context of an unrestricted BCR repertoire.
High CD45 Expression Renders B Cells Refractory to BAFF due to Reduced BAFFR.
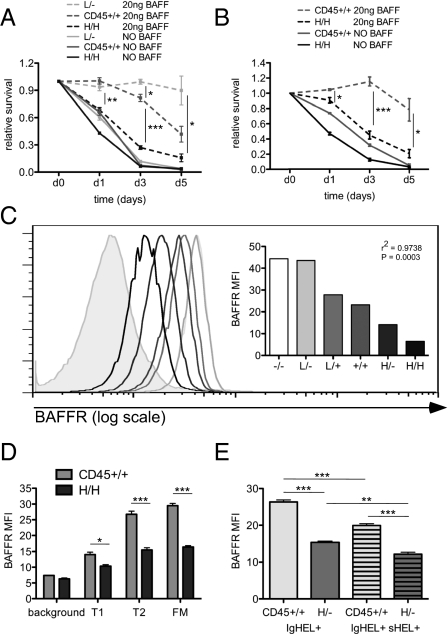
Restricting the BCR repertoire in H/H mice to eliminate antigen-driven clonal deletion did not rescue B-cell loss (Fig. 4A); thus, we postulated that another mechanism might account for this phenotype. H/H mice are characterized by preferential loss of T2, FM, and MZ B cells. This phenotype resembles the effects of BAFF or BAFFR deficiency (9, 10). Consequently, we explored this pathway in the allelic series to establish whether a BAFF survival defect could contribute to the loss of B cells in H/H mice. To do so, we assessed the survival of allelic series B cells in vitro in the presence or absence of BAFF. H/H B cells with supraphysiological CD45 expression exhibited impaired survival in response to BAFF relative to WT B cells, whereas low CD45 expression (L/−) promoted survival in response to BAFF (Fig. 6A), suggesting that CD45 titration negatively regulates the B-cell response to BAFF. In contrast, increasing CD45 expression promoted T-cell survival in vitro (SI Appendix, Fig. S9A), suggesting that CD45 does not drive apoptosis directly, but does so rather by regulating B-cell–specific pathways.
Fig. 6.
High CD45 expression renders B cells refractory to BAFF. (A) L/−, CD45+/+, and H/H LN B cells were incubated in media with or without 20 ng/mL of BAFF for 5 d. Viability of allotype-marked CD23+ follicular LN B cells was assessed at d0, d1, d3, and d5 by flow cytometry. The graph represents viable B cells of each genotype normalized to starting viability. Values are mean ± SEM of three biological replicates. (B) CD45+/+ and H/H LN B cells from mixed chimeric mice were assayed for survival as described in A to isolate cell intrinsic contributions to this assay. Values are mean ± SEM of three biological replicates. (C) Histograms representing BAFFR expression on CD23+ (T2 and FM) splenic B cells from allelic series mice. Gray shading represents background staining of non–B-cell splenocytes. BAFFR MFI of distinct histograms is plotted on the right. Data are representative of three independent experiments. (D) CD45.1 (H/H) and CD45.2 (WT) splenic B cells from mixed chimeras stained for allotype markers and expression of BAFFR, CD23, and AA4.1 to identify the cell intrinsic contribution of each genotype to BAFFR expression at sequential B-cell developmental stages. The graph shows MFI of BAFFR surface expression on T1, T2, and FM subsets (gated as in SI Appendix, Fig. S1C) in H/H (45.1) and WT (45.2) splenocytes from mixed chimeras. Background staining is defined by BAFFR staining of non–B-cell splenocytes and is plotted for reference. Values are mean ± SEM of three biological replicates. (E) Graph representing MFI of BAFFR surface expression in CD23+ splenocytes from IgHEL+ CD45+/+ and IgHEL+ H/− mice with or without autoantigen (sHEL) expression. Values are mean ± SEM of three biological replicates.
To determine whether this abnormality in B-cell survival was due to cell intrinsic factors or merely reflected the indirect or cell extrinsic consequences of B-cell lymphopenia, we repeated these assays with B cells from mixed chimeras, such that the competing genotypes were harvested from a common developmental milieu. This approach neutralized the effect of soluble BAFF levels on B-cell responses and allowed the isolation of B-cell intrinsic phenotypes. The survival defect exhibited by H/H B cells in response to BAFF was cell-intrinsic (Fig. 6B), suggesting that differences in the local BAFF environment do not account for these observations.
To understand why H/H B cells are refractory to BAFF survival signals, we assessed expression of the BAFF receptor on the surface of B cells in allelic series mice. BAFFR is normally expressed at low levels before the T2 stage of development, at which point it is up-regulated (11). We observed increased expression of BAFFR on the surface of T2 and FM B cells from mice with low CD45 expression, but markedly reduced expression of BAFFR on those subsets in H/H and H/− mice (Fig. 6C and SI Appendix, Fig. S9B). By assessing BAFFR expression in mixed chimeras, we demonstrated that reduced surface expression of BAFFR in H/H mice is not due to soluble BAFF concentration, but rather represents a cell-intrinsic phenotype (Fig. 6D).
BAFFR expression is normally up-regulated during B-cell development. One mechanism by which this may occur is a BCR-mediated increase in BAFFR expression (41). However, the effect of chronic antigen stimulation is often distinct from that of acute antigen stimulation. To better understand whether exposure to chronic antigen during development could impair BAFFR up-regulation, we evaluated BAFFR expression on the surface of anergic IgHEL/sHEL double-transgenic (DTg) B cells. We found reduced BAFFR expression relative to IgHEL B cells not exposed to sHEL (Fig. 6E), suggesting that chronic antigen stimulation during B-cell development can impair BAFFR up-regulation. IgHEL/sHEL DTg B cells are known to be more BAFF-dependent (42, 43). Reduced BAFFR expression may help account for this phenomenon. To determine whether reduced BAFFR expression on H/H B cells is driven by chronic antigen stimulation, we fixed the IgHEL BCR in the absence of sHEL. This did not rescue BAFFR expression on H/H B cells, suggesting that tonic rather than antigen-driven BCR signaling in H/H mice drives this phenotype (SI Appendix, Fig. S9C). Taken together, these data suggest that either chronic antigen stimulation or tonic BCR signaling, in contrast to acute BCR stimulation, reduces BAFFR expression.
Discussion
CD45 is critical for normal AgR signaling and lymphocyte development (20–22). However, CD45-deficient B-cell phenotypes are partially masked by both BCR repertoire compensation and expression of a partially redundant phosphatase, CD148 (24, 26). Although mice deficient for both CD45 and CD148 reveal an early requirement for CD45 at the pre-B stage of BM development, these mice develop few peripheral B cells (24). Our study of the CD45 allelic series of mice overcomes these limitations and reveals unique functions for CD45 later in B-cell development.
In this paper, we have identified distinct roles for CD45 during TCR and BCR signaling. We and others previously reported both positive and negative regulatory roles for CD45 during TCR signaling, raising the possibility of a similar negative regulatory role in B cells (29–31). Here we show that at high and supraphysiological levels of CD45, TCR signaling is inhibited, whereas BCR signaling is enhanced. Our findings definitively demonstrate that CD45 has a purely positive regulatory role during BCR signaling. This finding is reminiscent of the role of CD45 in thymic β-selection and basal signaling in preselection DP thymocytes (30).
We and others previously reported that low levels of CD45 are sufficient to reconstitute T-cell development (27–30). In contrast, subphysiological transgenic expression of single CD45 isoforms cannot rescue normal B-cell maturation (28). Our results suggest that the differential requirement for CD45 in T-cell and B-cell development is independent of isoform. Rather, we propose that this apparent paradox is explained by the unique negative regulatory role of CD45 in T cells, but not in B cells.
Although the c-terminal inhibitory tyrosine of the SFKs is dephosphorylated by increasing CD45 expression in both T-cell and B-cell lineages, regulation of the activation loop tyrosine is distinct. We propose that dephosphorylation of the activation loop tyrosine of Lck, but not of Lyn, accounts for CD45's unique negative regulatory role in T cells. Interestingly, we previously showed that Fyn and Lck are differentially regulated by CD45 in T cells at this tyrosine residue (30). Phosphorylation of Fyn at the activation loop tyrosine resembles Lyn in the allelic series, suggesting that regulation of the Lck activation loop may be unique among the SFKs. We speculate that this may be related to the association of Lck with CD4/CD8 coreceptors, which in turn are preferentially associated with CD45 (44, 45). Local sequence variation also may contribute, although the region around the activation loop is highly conserved across SFKs. Of course, we cannot exclude the possibility of an indirect role of CD45 in regulating the Lck activation loop.
Studies of Lyn−/− mice have unmasked a nonredundant negative regulatory role for Lyn during BCR signaling (33). Mice harboring a constitutively active variant of Lyn (Lynup/up) in which the c-terminal inhibitory tyrosine is mutated to a phenylalanine (Y507F) exhibit hyperresponsive BCR signaling, suggesting that Lyn also plays a positive regulatory role (46). We found that Lyn Y507 is progressively dephosphorylated with increasing CD45 expression in allelic series B cells. Consistent with this observation, H/H mice partially phenocopy Lynup/up mice. Importantly, Lynup/up B cells exhibit constitutive phosphorylation of multiple Lyn substrates, but H/H B cells do not. This highlights a critical difference between these mutants and suggests that in H/H B cells, Lyn activity is still regulated, presumably by Csk, and not constitutively active as in Lynup/up B cells, on which Csk has no effect. Both mutants exhibit reduced FM and MZ B-cell numbers with relative expansion of B1 B cells, as well as down-regulation of numerous surface receptors, including CD19, CD21, and IgM. In response to BCR ligation, calcium signaling is enhanced, but B-cell activation is impaired in both mutants. H/H B cells show paradoxically impaired up-regulation of activation markers, whereas Lynup/up B cells exhibit impaired proliferative responses in vitro. These data suggest that both mutants produce a partially unresponsive functional state. This phenocopy argues that Lyn may mediate the major B-cell phenotypes driven by supraphysiological CD45 expression.
In vivo characterization of B-cell development in the CD45 allelic series reveals several previously unreported functions of CD45. Although the BM developmental stages are a site of negative selection, we did not find significant cell loss in H/− and H/H B cells during BM stages of development, even in the stringent setting of competitive repopulation experiments. Instead, we observed increasing λ light chain use in the immature BM B-cell compartment with increasing CD45 expression, suggesting that receptor editing rather than deletion is the predominant tolerance mechanism triggered by titrating CD45 expression during BM development.
In contrast to BM development, during splenic development increasing CD45 expression and BCR signaling drives B-cell loss. Restricting the BCR repertoire with the IgHEL transgene unmasks a severe developmental block in CD45−/− B cells (26), but unexpectedly fails to rescue B-cell loss in mice with supraphysiological CD45 expression. This suggests that antigen-induced clonal deletion does not drive B-cell loss in H/H mice.
The loss of T2, FM, and MZ B cells with relative preservation of B1 and T1 B cells in mice with supraphysiological CD45 expression resembles the phenotype of mice deficient for BAFF or BAFFR, suggesting that impairment of this pathway might help explain these phenotypes (9, 10). Indeed, we observed that titration of CD45 expression inversely regulates BAFFR expression and BAFF responsiveness in a cell-intrinsic manner. Because reduced BAFFR expression in H/H B cells is seen even in phenotypically mature AA4.1− CD23+ B cells, we propose that this phenotype is not merely a consequence of generally impaired B-cell development. We also note that restricting the BCR repertoire to limit ligand binding does not rescue BAFFR expression on H/H B cells at all, suggesting that tonic rather than antigen-mediated BCR signals may drive this phenotype in our system. Adding chronic antigen exposure to this system further drives BAFFR down-regulation in both CD45+/+ and H/− IgHEL/sHEL DTg B cells, suggesting that chronic ligand as well as tonic BCR signals can contribute to this phenotype.
It has been reported that overnight BCR ligation drives up-regulation of BAFFR in WT B cells in a manner that depends on Rac1/2 and new gene transcription (41, 47). Although this mechanism remains incompletely defined, it may play an important role during BCR-induced positive selection. We suggest that whereas acute BCR stimulation up-regulates BAFFR expression at positive selection checkpoints, chronic or tonic BCR signals during susceptible developmental stages have the opposite effect. Promotion of B-cell loss rather than survival of potentially autoreactive B cells may constitute an important B-cell tolerance mechanism. In the case of the allelic series, we speculate that BAFFR expression is reduced with high CD45 levels because of enhanced tonic BCR signaling. We cannot exclude the possibility of a more direct role for CD45 and SFKs in either regulation of BAFFR expression or response to BAFF stimulation.
Although a minimal amount of tonic BCR signaling is required for B-cell survival, chronic BCR ligation is thought to produce a state of functional unresponsiveness known as anergy (6, 39, 48). Unexpectedly, in the context of either a restricted or an unrestricted repertoire, B cells with supraphysiological CD45 expression exhibit certain characteristics of anergic B cells, including IgM down-regulation in the face of near-normal IgD expression, increased basal Erk phosphorylation, and impaired activation marker up-regulation. This is seen even in the absence of sHEL ligand, suggesting that weak or tonic BCR signals drive this phenotype. We speculate that H/H and H/− B cells respond to enhanced tonic BCR signaling as they might to chronic BCR stimulation driven by autoantigen in order to produce an anergic phenotype.
Although monoclonal IgHEL B cells that develop in the presence of sHEL ligand survive and become anergic, introduction of competitor B cells with an unrestricted repertoire has been shown to drive deletion of autoreactive IgHEL B cells (39, 49, 50). This cell loss in the context of competition is mediated by limiting the amount of BAFF (42, 43). “Self-reactive” IgHEL B cells are more dependent on BAFF for survival than WT B cells. This has been previously attributed to Bim up-regulation in response to chronic antigen stimulation (42). We suggest that reduced BAFFR expression in the context of chronic antigen stimulation also may contribute to this phenotype by rendering self-reactive B cells less responsive to BAFF. BAFFR down-regulation has been reported in the context of a distinct model of autoreactive B cells, suggesting that this may represent a general mechanism (51).
Through genetic titration of CD45 expression, we have identified different roles for CD45 during TCR and BCR signaling, and unmasked novel roles for CD45 during B-cell development. We propose that physiological expression of CD45 must strike a fine balance between impairing B-cell development at low levels of CD45 expression and triggering tolerance mechanisms such as editing, anergy, and cell loss at high levels of CD45 expression. Thus, the expression level of CD45 reflects a system that serves to optimize the developing naïve B-cell repertoire in the context of pressure to avoid autoreactivity.
Materials and Methods
Mice.
Lightning mice were generated directly on the C57BL/6 genetic background during N-ethyl-N-nitrosourea mutagenesis screen conducted at Australian National University (30). Lightning mice were backcrossed to C57BL/6 at least six generations. H/H (HE) mice and CD45−/− mice have been described previously (22, 32), as have IgHEL (MD4) and sHEL (ML5) mice (39). All knockout and transgenic strains were fully backcrossed to C57BL/6 genetic background. Mice were used for all functional and biochemical experiments at age 5–9 wk. All mice were housed in a specific pathogen-free facility at University of California San Francisco in accordance with the university's Animal Care Committee and National Institutes of Health guidelines.
Antibodies and Reagents.
The following antibodies were used: murine BAFFR, CD1d, CD3, CD4, CD5, CD8, CD19, CD21, CD22, CD23, CD44, panCD45, B220, CD45.1, CD45.2, CD62L, AA4.1, IgM, IgD, Igκ, Igλ, and TCRβ antibodies conjugated to FITC, PE, PerCP-Cy5.5, PE-Cy5.5, PE-Cy7, Pacific blue, APC, or Alexa 647 (eBiosciences or BD Biosciences); pErk 202/204 (197G2) antibody for both blotting and intracellular staining, phospho-S6 A-488 (2F9) antibody for intracellular staining, pSrc Y416, pLyn Y507, pPLCγ2 Y1217, pZap70 Y493, Syk, pSyk 525/526, and Lyn antibodies (Cell Signaling); Erk 1 and 2 antibodies (Santa Cruz Biotechnology); Lat and pLat Y171 antibodies (Abcam); pLck Y505 and CD45 antibodies (BD Transduction); PLCγ1 antibody (United Biomedical); pPLCγ1 Y783 antibody (Biosource); unconjugated CD3ε (2C11) antibody (Harlan); goat anti-Armenian hamster IgG(H+L) antibody, goat anti-mouse IgM, and goat anti-rabbit IgG antibody conjugated to either PE or APC (Jackson Immunoresearch). Lck (1F6) and Zap70 (1E7) antibodies were prepared in our laboratory, and Lyn antiserum was obtained from Clifford Lowell's laboratory at University of California San Francisco.
Flow Cytometry and Data Analysis.
Cells were stained with indicated antibodies and analyzed on a FACSCalibur (BD Biosciences), Fortessa (BD Biosciences), or CyAN ADP (DAKO) flow cytometer as described previously (25). Data analysis was performed using FlowJo v8.8.4 (Treestar). Statistical analysis and graphs were generated using Prism v4c (GraphPad Software). The unpaired t-test was used to determine P values for comparison of paired datasets. Linear regression analysis was performed to capture allelic series phenotypes that vary continuously with CD45 expression (Fig. 3 A, B, E, and G and SI Appendix, Figs. S4A, S6B, and S7 C and D). For the purpose of statistical linear regression analysis, relative CD45 expression was plotted in graphs as a quantitative continuous variable along the x-axis. Statistical significance was determined using Fisher's exact test, and r2 (goodness of fit) and P value (line of best fit for the data has a significantly nonzero slope) were plotted.
BM Chimeras.
Host mice (CD45.1) were lethally irradiated and reconstituted with H/H (H Tg = CD45.1) and WT (CD45.2) BM via tail vein injection. The mice were allowed to recover and reconstitute hematopoietic lineages before analysis at 6–10 wk postirradiation.
LN T-Cell and B-Cell Stimulation, Calcium Flux, and Intracellular Phospho-Erk and Phospho-S6 Staining.
These assays were performed as described previously (52). Phospho-S6 kinase staining was performed using the same protocol as for phospho-Erk, but with phospho-S6 kinase antibody directly conjugated to fluorophore.
Immunoprecipitation and Immunoblotting.
Immunoprecipitation and immunoblotting were performed as described previously (24). Purified cell populations of splenic B cells, LN B cells, and CD4+ T cells were obtained by sorting with MACS Kits (Miltenyi) in accordance with the manufacturer's instructions. Blot densitometry was performed using Kodak Imagestation software.
Intracellular Staining for κ/λ Light Chain Expression.
Single cell suspensions of BM and splenic B cells were stained for surface markers to define developmental subsets, and then fixed, permeabilized, and stained with antibodies to κ and λ light chains in accordance with BD Bioscience's Cytofix/Cytoperm protocol.
BAFF Survival Assay.
Total LN cells from either unmanipulated or mixed chimera mice were incubated in complete DMEM at 37 °C at a concentration of 500,000 cells/mL in the presence or absence of 20 ng/mL of BAFF (R&D Systems); the use of 200 ng/mL of BAFF yielded similar results. Cells from competitor genotypes or mixed chimeras were mixed for this assay and subsequently identified by CD45 surface expression and allotype markers (H Tg = CD45.1). At day 1, day 3, and day 5 time points, absolute and relative numbers of surviving lymphocytes were assessed by flow cytometry staining and size exclusion of dead cells.
Supplementary Material
Acknowledgments
We thank Al Roque for assisting with animal husbandry, Jason Cyster and Wasif Khan for critically reading the manuscript, and Michelle Hermiston and Jason Cyster for supplying the MD4 and ML5 mice. This work was supported by the Rosalind Russell Medical Research Foundation Bechtel Award (to J.Z.), an American College of Rheumatology REF Rheumatology Investigator Award (to J.Z.), an Arthritis National Research Foundation grant (to J.Z.), and National Institutes of Health Grants K08 AR059723 (to J.Z.), PO1 AI35297 (to A.W.), and 5RO1 AI066120-05.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 9.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117374108/-/DCSupplemental.
References
- 1.Miosge LA, Goodnow CC. Genes, pathways and checkpoints in lymphocyte development and homeostasis. Immunol Cell Biol. 2005;83:318–335. doi: 10.1111/j.1440-1711.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 3.Cancro MP. Signalling crosstalk in B cells: Managing worth and need. Nat Rev Immunol. 2009;9:657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 5.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Zouali M, Sarmay G. B lymphocyte signaling pathways in systemic autoimmunity: Implications for pathogenesis and treatment. Arthritis Rheum. 2004;50:2730–2741. doi: 10.1002/art.20487. [DOI] [PubMed] [Google Scholar]
- 8.Khare SD, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 10.Shulga-Morskaya S, et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 11.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 12.Mecklenbräuker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCδ-mediated nuclear signalling. Nature. 2004;431:456–461. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 14.Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. J Exp Med. 2010;207:607–621. doi: 10.1084/jem.20091673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas MD, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell Immunol. 2006;239:92–102. doi: 10.1016/j.cellimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Bannish G, Fuentes-Pananá EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583–1596. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–8006. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- 19.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: Critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byth KF, et al. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mee PJ, et al. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur J Immunol. 1999;29:2923–2933. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Kishihara K, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 23.Stone JD, et al. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ, and ZAP-70. J Immunol. 1997;158:5773–5782. [PubMed] [Google Scholar]
- 24.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28:183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23:635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Cyster JG, et al. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 27.Kozieradzki I, et al. T cell development in mice expressing splice variants of the protein tyrosine phosphatase CD45. J Immunol. 1997;158:3130–3139. [PubMed] [Google Scholar]
- 28.Ogilvy S, et al. Either of the CD45RB and CD45RO isoforms are effective in restoring T cell, but not B cell, development and function in CD45-null mice. J Immunol. 2003;171:1792–1800. doi: 10.4049/jimmunol.171.4.1792. [DOI] [PubMed] [Google Scholar]
- 29.McNeill L, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Zikherman J, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamoyska R. Why is there so much CD45 on T cells? Immunity. 2007;27:421–423. doi: 10.1016/j.immuni.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Virts EL, Diago O, Raschke WC. A CD45 minigene restores regulated isoform expression and immune function in CD45-deficient mice: Therapeutic implications for human CD45-null severe combined immunodeficiency. Blood. 2003;101:849–855. doi: 10.1182/blood-2002-07-1969. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: Accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr Opin Immunol. 2002;14:241–249. doi: 10.1016/s0952-7915(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 35.Merrell KT, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 37.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire: Marginal zone and B1 B cells as part of a “natural immune memory.”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 39.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 40.Hartley SB, et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 41.Smith SH, Cancro MP. Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J Immunol. 2003;170:5820–5823. doi: 10.4049/jimmunol.170.12.5820. [DOI] [PubMed] [Google Scholar]
- 42.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 43.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Dornan S, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 45.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 46.Hibbs ML, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002;196:1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walmsley MJ, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 48.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–119. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 49.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 50.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 51.Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol. 2010;185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zikherman J, et al. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]