Abstract
The calcium-sensing receptor (CaR) is the key controller of extracellular calcium (Ca2+o) homeostasis via its regulation of parathyroid hormone (PTH) secretion and renal Ca2+ reabsorption. The CaR-selective calcimimetic drug Cinacalcet stimulates the CaR to suppress PTH secretion in chronic kidney disease and represents the world's first clinically available receptor positive allosteric modulator (PAM). Negative CaR allosteric modulators (NAMs), known as calcilytics, can increase PTH secretion and are being investigated as possible bone anabolic treatments against age-related osteoporosis. Here we address the current state of development and clinical use of a series of positive and negative CaR modulators. In addition, clinical CaR mutations and transgenic mice carrying tissue-specific CaR deletions have provided a novel understanding of the relative functional importance of CaR in both calciotropic tissues and those elsewhere in the body. The development of CaR-selective modulators and signalling reagents have provided us with a more detailed appreciation of how the CaR signals in vivo. Thus, both of these areas of CaR research will be reviewed.
Keywords: extracellular calcium, calcimimetics, calcilytics, allosteric modulators, Class C G protein-coupled receptors, cell signalling, receptor phosphorylation
Introduction
The principal physiological action of the CaR has been well defined in vivo, both in clinical studies of human CaR mutations (Thakker, 2004), as well as in transgenic animal studies in which CaR is underexpressed in particular tissues (Ho et al., 1995; Chang et al., 2008). The CaR (also commonly referred to as CaSR) has been given the provisional nomenclature CaS by NC-IUPHAR, but this should not be confused with the plant calcium receptor, CaS (Han et al., 2003; Tang et al., 2007; Vainonen et al., 2008), with which the animal receptor bears no sequence or structural relation. CaR is a class C GPCR located on chromosome 3 in humans (Aida et al., 1995) and, with the exceptions described below, it is expressed on the cell membrane as a disulphide-linked constitutive homodimer (Bai et al., 1998a; Ward et al., 1998).
Clinical and transgenic evidence for the central role of CaR in calcium homeostasis
The two tissues in which CaR expression is highest are the parathyroid glands, where CaR activation suppresses parathyroid hormone (PTH) secretion (Brown et al., 1993), and the kidneys (Riccardi et al., 1998), where CaR limits Ca2+ reabsorption to protect against hypercalcaemia (Kantham et al., 2009). Heterozygous, loss-of-function CaR mutation results in mild-to-moderate increases in PTH secretion (familial hypocalciuric hypercalcaemia, FHH), whereas homozygous loss-of-function CaR mutations can cause life-threatening elevations in PTH secretion and blood calcium levels (neonatal severe hyperparathyroidism, NSHPT) (reviewed by Thakker, 2004; Egbuna and Brown, 2008). An FHH-like condition can also result from CaR autoantibodies (Kifor et al., 2003; Pallais et al., 2004; Brown, 2009) directed against the N-terminal region of the receptor's extracellular domain (ECD) as demonstrated using a phage-display library of CaR peptides (Kemp et al., 2009). The original CaR (−/−) knockout mouse (produced by deleting CaR exon 5) displayed an NSHPT-like phenotype with greatly elevated neonatal PTH levels causing growth retardation and early death (Ho et al., 1995). The heterozygous CaR knockout (+/−) more closely resembled FHH in humans with a more moderate increase in PTH secretion. The lethal rise in PTH secretion and blood calcium levels in these mice can be overcome in double knockout mice in which the CaR knockout is combined with either a PTH (−/−) knockout (Kos et al., 2003), or, a Glial cell missing-2 (Gcm-2) (−/−) knockout in which the parathyroid glands fail to develop (Tu et al., 2003). Again, this replicates the situation in humans whereby early parathyroidectomy in NSHPT saves the life of the infant.
In fact, the Exon-5 CaR knockout is a ‘hypomorph’ as it retains some function in cartilage (Rodriguez et al., 2005), in keratinocyte (Oda et al., 2000) and lung (Finney et al., 2011). Thus, an alternative murine model has been produced by deleting Exon-7, which knocks out the entire transmembrane region and intracellular domain (ICD), allowing for the transcription and translation of a non-functional secreted fragment corresponding to amino acid 1–577 of the ECD (Chang et al., 2008). Indeed, a parathyroid-specific Exon-7 CaR knockout mouse exhibits even greater elevations in PTH levels (Chang et al., 2008), that is an even more severe phenotype, than the partial Exon-5 CaR knockout (Ho et al., 1995). Furthermore, in mice where the Exon-7 was selectively deleted from the osteoblasts, severe bone defects were observed thus demonstrating the specific role of CaR in bone, independent of its effects in parathyroid gland and kidney (Chang et al., 2008).
Together, these in vivo observations demonstrate consistently the crucial role of CaR in maintaining stable blood calcium levels. This is further supported by clinical and transgenic studies in which CaR activity is elevated resulting in hypoparathyroid conditions and hypocalcaemia (Thakker, 2004; Egbuna and Brown, 2008). Gain-of-function CaR mutations result in autosomal dominant hypocalcaemia (ADH) and many such gain-of-function (and indeed loss-of-function) CaR mutations have been collated at http://www.casrdb.mcgill.ca/. These gain-of-function mutations are generally, although not exclusively, clustered either close to the two cysteine residues responsible for homodimerization, namely Cys-129 and 131 (Zhang et al., 2001), or, in the transmembrane domain. Again, this condition can be mimicked by autoantibodies that bind the CaR except that here they elicit a gain-of-function effect by stabilizing activating conformations of the receptor (Brown, 2009). One such condition is autoimmune polyglandular syndrome type 1 (APS1) in which autoantibodies bind the N-terminal region of the CaR ECD leading to elevated CaR activity and thus hypoparathyroidism with characteristic hypocalcaemia and hyperphosphataemia (Brown, 2009; Kemp et al., 2009). Furthermore, there are two mouse models of CaR gain-of-function; the first is the Nuf mouse, which expresses a CaR mutation (L723Q) resulting from a chemical mutagenesis study (Hough et al., 2004). This mouse exhibits ectopic calcification, hypocalcaemia, hyperphosphataemia and inappropriately reduced levels of plasma PTH consistent with ADH in humans. In the second model, 5-point mutations were introduced to the CaR ECD but targeted to mature osteoblasts using the 3.5 kb osteocalcin promoter (Dvorak et al., 2007). The resulting mouse displayed normal PTH and calcium levels but with enhanced bone resorption, further supporting a tissue-specific role for CaR in bone.
Calcium-sensing receptor pharmacology
Most GPCRs respond to modified amino acids or polypeptides making it possible to predict and define their agonist binding sites. However, for elemental calcium and CaR this has proven very difficult. The Hill coefficient for CaR indicates the cooperative binding of three to five Ca2+ ions to the receptor (Bai, 2004). It has long been recognized that clusters of negatively charged extracellular amino acids most likely account for CaR agonist binding; however, the lack of a solved 3D structure for CaR and the low affinity of Ca2+o binding have seriously hindered further investigation of this. Recent modelling work, employing the crystal structure for mGluR, has identified at least three (Huang et al., 2007) and as many as five potential Ca2+o binding sites in CaR (Huang et al., 2009b). Two such regions, Gly222–Ile235 and Gly383–Ile408 (each containing five to six glutamate or aspartate residues) were individually inserted into a scaffold protein that exhibited no prior cation-binding capacity. Introduction of either of the two D/E-rich sequences gave the new protein the ability to bind calcium (Huang et al., 2007). Furthermore, selective substitution of either Glu-224, Glu-228/229 or Glu398/399 for isoleucine each resulted in loss of CaR function supporting these observations. Indeed, the Glu398/399Ile mutation had perhaps the greatest effect at reducing CaR Ca2+o sensitivity and maximal response (Huang et al., 2007). Such a steep agonist concentration/effect relationship is a critical feature of CaR function given the physiological necessity to maintain ionized serum Ca2+ concentration between 1.1 and 1.3 mM. Even moderate hypocalcaemia or hypercalcaemia can cause altered neuromuscular activity leading to tetany or arrhythmias respectively. Therefore, further studies are necessary to elucidate the mechanistic basis for such cooperativity.
Studies of GPCR oligomerization challenge the notion that receptors always exhibit a simple linear relationship between receptor and ligand with a 1:1 stoichiometry (Park and Palczewski, 2005). For instance, serotonin/glutamate receptor complexes respond to hallucinogenic drugs and are new potential drug targets for psychosis (Gonzalez-Maeso et al., 2008). Furthermore, in cerebellar Purkinje cells, type B γ-aminobutyric acid receptors (GABABR) and type 1 metabotropic glutamate receptor (mGluR1) colocalize in the dendritic spines of excitatory synapses. Here, conformational changes evoked by elevated Ca2+o concentration onto GABABR lead to a constitutive increase in the glutamate sensitivity of mGluR1 (Tabata et al., 2004). The evidence that heterodimerization within group C GPCRs produces new types of receptors with entirely diverse pharmacological profiles has spurred a number of studies concerning CaR oligomerization. It has long been known that the CaR exists in the plasma membrane as a functional homodimer (Ward et al., 1998), but there is more recent evidence that CaR may also oligomerize with other family C GPCR members. For instance, in hippocampal and cerebellar neurons, the CaR heterodimerizes not only with mGluR1α and mGluR5 (Gama et al., 2001), but also with R1 and R2 GABAB receptors (Chang et al., 2007). In both circumstances, formation of the heteromeric complex leads to profound changes in CaR expression levels, agonist sensitivity and cell signalling. Furthermore, CaR heterodimerization with other Group C GPCRs involved in nutrient sensing has been reported, which would allow for differential sensing of food intake (Wellendorph et al., 2010).
Although Ca2+o-sensing is a crucial aspect of CaR function, many other divalent (Mg2+), trivalent (lanthanides such as Gd3+), polyvalent cations [e.g. poly-L-lysine, poly-arginine, protamine (Nemeth, 2002)] and the aminoglycoside antibiotics (Ward et al., 2002; 2005; Gibbons et al., 2008), are all agonists of the CaR (reviewed by Brown and MacLeod, 2001). These are orthosteric, type I agonists, in that they can activate the CaR in the absence of Ca2+o. In addition, compounds that modify the endogenous affinity of the receptor for Ca2+o, or allosteric modulators, have been identified. Some of these modulators are present in our body under physiological conditions including L-aromatic amino acids, glutathione, ionic strength and alkalinization (Bandyopadhyay et al., 2010). For example, such type II agonists have been shown to produce biologically relevant effects such as the modulation of PTH secretion by dietary protein intake (Conigrave et al., 2004; Broadhead et al., 2010), regulation of gastric function (Cheng et al., 1999), cholecystokinin secretion (Liou et al., 2011) and alterations in urinary Ca2+o excretion (Riccardi and Brown, 2010).
PTH plays an essential role in Ca2+o and inorganic phosphate (Pi) homeostasis and deviations in plasma PTH have a profound impact on mineral ion metabolism. For instance, prolonged increases in circulating PTH levels, such as those seen during primary hyperparathyroidism or hyperparathyroidism secondary to kidney failure, are associated with increase risks of cardiovascular morbidity and mortality (Block et al., 2004a) and activate bone-resorbing cells, the osteoclasts, causing net bone loss (Antonsen et al., 1998). In contrast, short-lived bursts in plasma PTH activate bone-forming cells, the osteoblasts, with an induction of bone formation markers and an improvement in bone mineral density in post-menopausal women (Dempster et al., 1993). Therefore, in an ageing population, where the incidence of chronic kidney disease and osteoporosis are on the rise, manoeuvres aimed at modifying circulating plasma PTH represent an area of great therapeutic potential. As it was well established that the parathyroid CaR represents the master regulator of PTH secretion, the preclinical development of compounds that could modulate its secretion by the parathyroid glands was already ongoing even before the receptor was molecularly identified in 1993 (Nemeth, 2002; 2006;). Knowledge of the molecular structure of the CaR confirmed what was already known, that because of the low affinity of Ca2+o and other inorganic cations at the CaR (Brown et al., 1993), it was unlikely that orthosteric ligands could provide the desired specificity and potency. Therefore, Nemeth and colleagues at NPS Pharmaceuticals, Inc., set out to identify compounds that could modify the concentration–response curves of the CaR for Ca2+o. This approach proved to be successful, and led to the identification of two small organic molecules that can mimic Ca2+o or potentiate its receptor affinity, but that are not polycations. These ligands are termed ‘type II calcimimetics’, while orthosteric agonists are considered ‘type I calcimimetics’ (Nemeth et al., 1998). On the other hand, compounds that reduce the CaR affinity for Ca2+o have also been identified and are named ‘calcilytics’ (Nemeth et al., 2001). As for all positive allosteric modulators (PAM) and negative allosteric modulators (NAM), calcimimetics and calcilytics offer the advantage that: (i) they do not alter the plasma concentrations of the endogenous ligands, nor do they compete with them and bind to different sites from the orthosteric agonists; (ii) they enhance or inhibit endogenous physiological responses, that is, they do not evoke supra-maximal ones or activate de novo signalling pathways; and (iii) their effects are saturable, that is, they are maximally active at EC50 values for Ca2+o and are ineffective at minimal or maximal concentrations of the physiological ligand (Christopoulos and Kenakin, 2002). A list of the main CaR allosteric modulators with their EC50 or IC50 values is presented in Table 1.
Table 1.
The table includes the calcimimetics and calcilytics discussed in the review, with EC50 (calcimimetics) or IC50 (calcilytics) values reported in heterologous expression systems (i.e. cells overexpressing the human CaR) or in cells endogenously expressing the CaR (generally, although not exclusively, parathyroid cells). The table also includes information concerning drug testing in vivo (n.a.: information not available)
| EC50 or IC50 (in vitro, recombinant) (nM) | EC50 or IC50 (in vitro, native) (nM) | PTH modulationin vivo | References | |
|---|---|---|---|---|
| Calcimimetics | ||||
| NPS 467 (1a) | 60 | 60 | yes | Nemeth et al., 1998 |
| NPS R 568 (1b) | 30 | 27 | yes | Nemeth et al., 1998 |
| Sensipar (Cinacalcet HCl) | 51 | 28 | yes | Nemeth et al., 2004 |
| Calindol | 310 | n.a. | n.a. | Petrel et al., 2004 |
| AC-265347 | 10 | n.a. | yes | Ma et al., 2011 |
| Calcilytics | ||||
| NPS 2143 (SB-262470) | 43 | 41 | yes | Nemeth et al., 2001 |
| NPS 53574 (quinazolin-4-ones) | 3500 | 97 | yes | Shcherbakova et al., 2005 |
| Ronacaleret (1b, SB-751689) | 320 | ✓ | yes | Fitzpatrick et al., 2011; Balan et al., 2009 |
| Calhex 231 | 330 | n.a. | n.a. | Petrel et al., 2004; Kessler et al., 2006 |
Calcimimetics: positive CaR modulation and chronic kidney disease
Most current calcimimetics are phenylalkylamines and are derived from Ca2+ channel blockers, such as verapamil or TMB-8 (Nemeth, 2002). An initial screening of this class of drug demonstrated that the calcium channel blocker fendiline could evoke a substantial mobilization of Ca2+i in isolated bovine parathyroid cells. Two of these compounds, NPS-467 and its chlorinated derivative, NPS-568, were identified and the racemic mixture separated into the R- and S-enantiomers for further pharmacological studies in bovine parathyroid cells. The R-enantiomer was shown to be 10-fold (NPS-568) or 100-fold (NPS-467) more potent than the S-enantiomer. Selectivity studies showed that, at concentrations below 10 µM, these two compounds did not activate nor affect the agonist responses of a variety of other GPCRs, including mGluRs. However, at high concentrations they did induce β adrenergic receptor-dependent second messenger production, an effect which is also mimicked by high (>5 mM) Ca2+o (Chen et al., 1989). Therefore, calcimimetics can evoke significant off-target pharmacology at high concentrations (>1 µM). However, specific effects on the CaR can be investigated by: (i) using drug concentrations at or below 1 µM; (ii) performing stereoselectivity experiments (the R-enantiomer being 10- to 100-fold more potent than the S-enantiomer); and (iii) using Ca2+o concentrations close to its EC50 value for the biological function to be assessed (as allosteric modulators, calcimimetics are ineffective at minimal and maximal Ca2+o concentrations – see above). It should be noted that when the calcimimetics are used outside of these experimental conditions, the resulting responses cannot be assumed to be true CaR-mediated events.
Calcimimetics are the first example of receptor PAMs to have received regulatory approval. Because extensive knowledge of the pharmacology of orthosteric and allosteric agonists in native parathyroid cells preceded the cloning of the CaR (Brown et al., 1993), the calcimimetic Cinacalcet® received Food and Drug Administration (FDA) approval 11 years after the CaR was cloned. Marketed as Sensipar® in the USA, Mimpara in the EU and Regpara in Japan, the calcimimetics have been approved for the treatment of hyperparathyroidism secondary to chronic kidney disease (Block et al., 2004b) and for parathyroid carcinoma (Silverberg et al., 2007). In addition, several other ‘off label’ applications have been reported in which Cinacalcet treatment aims at rectifying hypercalcaemic/hypophosphataemic states of various nature. These include, but are not limited to, hypercalcaemia after renal transplantation (Kruse et al., 2005), lithium-induced hyperparathyroidism (Sloand and Shelly, 2006), X-linked hypophosphataemia (Alon et al., 2008) and oncogenic osteomalacia (Geller et al., 2007). In addition, recent studies suggest the possibility of using calcimimetics to rescue CaR mutants, which result in protein misfolding (Huang et al., 2007).
Calcimimetics bind to the transmembrane region of the CaR (Miedlich et al., 2002; 2004; Petrel et al., 2004), and are believed to act by stabilizing CaR in an active state. In both rodent models and human subjects with end-stage chronic kidney disease, treatment with the marketed calcimimetic, Cinacalcet HCl, lowers PTH with the nadir after approximately 2–4 h, before PTH levels rise slowly again until ∼12 h post-dosing (Nemeth et al., 2004). Studies in patients indicate that Cinacalcet treatment not only helps achieve the stringent targets set for an effective management of advanced chronic kidney disease, but it also provides a better control of plasma Pi and the calcium x inorganic phosphate (CaxPi) product, disturbances of which are associated with an increase in cardiovascular morbidity and mortality (Block et al., 1998; 2004a;). A recent prospective study carried out on a large cohort of dialysis patients over 26 months has demonstrated that calcimimetic treatment improves all-cause and cardiovascular survival (Block et al., 2010).
The blood pressure-lowering effects of a high calcium diet in spontaneously hypertensive rats and humans has been known for more than 40 years (Ayachi, 1979). However, it is only 14 years since the late Dr Bukoski first hypothesized that a CaR in the perivascular nerve network might provide the molecular transducer for the effects of hypercalcaemia on blood pressure (Bukoski et al., 1997). These studies spurred a number of laboratories to elaborate on this initial idea, and thus identify the potential importance of the CaR in the vasculature. Thus, the existence of a functional CaR has been demonstrated in intact arteries (Ohanian et al., 2005), endothelial (Weston et al., 2005) and smooth muscle cells (Wonneberger et al., 2000; Smajilovic et al., 2006) and in intact human arteries (Molostvov et al., 2007; Alam et al., 2009). As some patients with advanced chronic kidney disease also manifest with profound cardiovascular complications and most of them take Cinacalcet, we set out to investigate the role of the CaR and potential clinical utility of the calcimimetics in the vasculature. Our observations have shown that loss of CaR expression is associated with an increase in vascular calcification both in vitro and in vivo (Alam et al., 2009), and that the calcimimetics delay smooth muscle cell calcification in vitro (Alam et al., 2009). In addition, other investigators have shown that the calcimimetics appear to protect against the vascular remodelling, which occurs during advanced chronic kidney disease (Koleganova et al., 2009) and to delay calcification and atherosclerosis, which occur in apolipoprotein E-deficient mice (Ivanovski et al., 2009), either alone or in combination with vitamin D (Rodriguez et al., 2008). The first evidence of an effect of calcimimetics on the cardiovascular system in humans derives from a recent publication by Block et al. (2010). In a prospective study involving a large cohort of patients with advanced chronic kidney disease which required haemodialysis, the authors have demonstrated that 2 year calcimimetic treatment reduces all-cause cardiovascular mortality. It remains to be determined whether these beneficial effects of calcimimetics on patient survival are mediated directly through the vascular CaR. Alternatively, they could be ascribed to calcium channel inhibition by these compounds (see above) or, as the recent literature seems to suggest, through inhibition of renin secretion by the juxtaglomerular apparatus of the kidney (Beierwaltes, 2010), which would result in blood pressure reduction. While hypotensive effects of calcimimetics have been demonstrated in normal rats in vivo, they are not equally present in all blood vessel types (i.e. they are more prominent in the mesenteric, rather than the renal artery), tend to be detected at concentrations of the drug well above those used to inhibit PTH secretion and do not appear to be stereoselective. These studies suggest that the effect of the calcimimetics may be mediated through an inhibition of calcium channels, rather than to an activation of the CaR (Nakagawa et al., 2009). The latter conclusion is supported by a recent study by Thakore and Ho (2011), who also used a calcimimetic other than Cinacalcet, namely calindol, and that ascribed its vasorelaxant properties to L-type Ca2+ channel blockade. Whatever their molecular target (i.e. whether Ca2+ channel inhibition or CaR activation), the improvement of all-cause mortality by calcimimetics in patients with advanced chronic kidney disease reported in the Block et al. (2010) study remains a significant positive side-effect of these drugs. A randomized clinical trial in haemodialysis patients taking Cinacalcet is now due and it will no doubt shed light on the potential vasorelaxant effects of this drug.
The relative therapeutic success of Cinacalcet has lead to the development of other calcimimetic compounds, for example the structurally novel benzothiazole compounds reported recently by Acadia Pharmaceuticals (Ma et al., 2011). These compounds reportedly exhibit greater CaSR potency than the phenylalkylamine calcimimetics, at least in vitro, and may have a different binding site (Ma et al., 2011).
Calcilytics: negative modulation and osteoporosis
PTH is a potent regulator of skeletal metabolism owing to its unique pharmacokinetic profile, which allows this hormone to evoke both anabolic and catabolic effects (Rizzoli et al., 1992). It is the osteoanabolic action of this hormone, which has led to the testing of intact PTH (1–84, Preos/Preotact) or one of its biologically active fragments (1–34, Forteo) in preclinical models of hormone-deficient bone loss and, subsequently, in post-menopausal women. On the basis of the ability to reduce fracture risk and to increase bone mineral density (Rittmaster et al., 2000; Neer et al., 2001; Greenspan et al., 2007), PTH (1–34) and intact PTH (1–84) were granted FDA and European Medicines Agency (EMEA) approval to treat hormone-deficient osteoporosis in 2002 and 2006 respectively. However, PTH treatment carries a ‘black box’ label and has only been approved for 2 years based on the evidence that it increased the risk of osteosarcoma in rats (Vahle et al., 2002). These findings, together with an inconvenient route of administration (daily subcutaneous injections) and the elevated costs, suggested that identification of specific, potent CaR antagonists capable of evoking endogenous bursts in plasma PTH could overcome some of the issues associated with exogenous PTH treatment. If successful, this strategy has the additional advantage that CaR modulation can only evoke changes in plasma PTH within the physiological range, rather than supra-maximal effects potentially achieved with exogenous PTH administration. At the same time as the calcimimetic studies were carried out, NPS Pharmaceuticals, Inc., together with the then SmithKline Beecham (now GlaxoSmithKline) had already initiated a drug discovery programme for the identification of pharmacological inhibitors of the CaR (Nemeth, 2002), which could evoke bone anabolic effects without inducing sustained PTH-dependent bone loss. That is, the ideal drug had to achieve rapid plasma PTH CMAX, followed by a rapid return to baseline levels shortly after administration of the compound (Nemeth, 2002).
A first screen identified a weak CaR antagonist, which had off-target pharmacology at the β-adrenoceptors, the cardiac human ether-a-go-go related gene (hHERG) ion channel and cytochrome P450 2D6 (CYP2D6) (Nemeth et al., 2001; Marquis et al., 2009b). Subsequent modifications of this compound yielded the first calcilytic, NPS-2143, an orally active compound with greater potency and specificity for the CaR (Nemeth et al., 2001; Marquis et al., 2009a).
Mutagenesis and homology modelling studies have subsequently demonstrated that the calcilytic binding site is located within the transmembrane domain of the CaR molecule, where it partly overlaps with the calcimimetic (NPS-R568) binding site (Miedlich et al., 2004). Initial functional studies carried out in recombinant systems demonstrated that NPS-2143 suppressed CaR activity. In vivo, NPS-2143 altered plasma PTH levels with a good overlap with its in vitro potency, with EC50 values of ∼40 nM (Nemeth et al., 2001). Treating rats with NPS-2143 evoked an increase in plasma PTH which was comparable to that observed following exogenous PTH administration (Nemeth et al., 2001). These in vivo effects on plasma PTH have never been tested for other calcilytics, including Calhex 231 (Petrel et al., 2003; Kessler et al., 2006), for which the therapeutic benefits are unknown, as thus far efficacy, potency and safety studies in vivo are unavailable.
Following NPS-2143 treatment bone mineral density did not increase, due to the long half-life of NPS-2143, which resulted in an increase in plasma PTH, a bone catabolic stimulus. In support of this hypothesis, blocking the sustained, PTH osteocatabolic effects with estrogens resulted in formation of new bone (Gowen et al., 2000). Thus, these observations suggested feasibility in producing short bursts in plasma PTH as an anti-osteoporotic treatment, an effect that could be achieved by rapid and transient inhibition of the parathyroid CaR. Efforts to improve the pharmacokinetics of this amino alcohol chemotype led to the identification of compounds with better PK, but with poor oral bioavailability. To obviate this problem, a ‘pro-drug’ approach was used. An esteric form of NPS-2143 was produced (SB-423557) which, following a rapid absorption, was quickly cleaved into the free acid form (SB-423562) before entering the systemic circulation (Nemeth, 2008). In a rat model of osteoporosis, treatment with SB-423557 caused a transient increase (two- to threefold) in plasma PTH concentration, which was maximal after 20 min and that had returned to baseline 2 h post-treatment (Kumar et al., 2010). These effects were accompanied by an increase in bone mineral density and an activation of markers of bone formation. Early clinical studies in healthy volunteers infused with the pro-drug or free acid showed a dose-dependent increase in plasma PTH of twofold or greater for up to 8 h, and that SB-423557 was safe and well tolerated (Kumar et al., 2010).
Further developments have led to the synthesis of another orally bioavailable amino alcohol chemotype, ronacaleret hydrochloride (SB-751689) (Nemeth, 2008), with improved specificity and pharmacokinetic profiles. Although ronacaleret reached phase 2 and treatment in postmenopausal women did activate bone turnover markers, its development was terminated in 2008 due to lack of efficacy (Balan et al., 2009).
Subsequent screening of an internal compound library at Novartis led to the identification of compound ‘2a’, which, following a series of optimization steps to improve its PK, led to 1H-quinazolin-2-ones derivatives which were found to act as calcilytics. Of these, the orally bioavailable compounds ‘7h’ and ‘11m’ evoked a very rapid (within minutes) and short-lasting (an hour or so) peak in plasma PTH at sub-nanomolar concentrations (Widler et al., 2010). While these observations sound promising, clinical studies are necessary to assess the efficacy, potency and safety profiles of these compounds.
Recently Japan Tobacco Inc. has synthesized a novel calcilytic, JTT-305 (now licensed to Merck, Inc., as MK-5442) (Balan et al., 2009). It is a similar chemotype of ronacaleret and, in phase 2 clinical trials, it shows a significant improvement of bone mineral density (Fukumoto, 2011).
Calcium-sensing receptor-mediated intracellular signalling
The CaR exhibits pleiotropic interaction with Gαq/11, Gαi/o and Gα12/13 in a variety of cell types (reviewed in Ward, 2004; Khan and Conigrave, 2009; Magno et al., 2010) and even to Gαs in mammary epithelial tumour cells (Mamillapalli et al., 2008; Magno et al., 2010) and murine pituitary corticotroph-derived, AtT-20 cells (Mamillapalli and Wysolmerski, 2010). Such apparent promiscuity may be necessary to provide selective regulation of the wide array of cellular effects associated with the CaR both within and without the calcium homeostatic system. Such interactions may be cell- and context-dependent; however, it is nevertheless possible to identify particular pathways as being responsible for mediating certain CaR functions. For example, in mice lacking either Gαq or Gα11 in their parathyroid glands, there is a modest increase in PTH secretion; however, where both Gαq and Gα11 are missing, PTH levels are greatly elevated (Wettschureck et al., 2007). Therefore, it would appear that, for PTH secretion control at least, CaR-induced Gαq/11 activation contributes significantly to the response.
Calcium-sensing receptor-induced Ca2+i oscillations
To understand the mechanism of CaR-mediated signalling we must look at the activity of the receptor in individual cells. Ca2+i imaging methods that measure only total fluorescence from multiple cells (e.g. the cuvette system) tend to show a standard, if steep, sigmoidal agonist concentration – effect relationship for CaR. However, single cell Ca2+i imaging reveals significant cell-to-cell variability in the dynamics of the Ca2+i mobilization ranging from transient and oscillatory responses to sustained responses. It is essential therefore to understand how these signals are shaped in order to understand both the mechanism of CaR signalling per se but also the reason for the steepness of the CaR agonist concentration–effect curve in particular. This cell-to-cell variability does mean though that significant care must be taken when reporting single cell studies to determine precisely what constitutes a ‘representative cell’. This means categorizing the various CaR responses where possible (Bruce et al., 1999; Davies et al., 2007) as well as developing mathematical descriptions of the oscillations (Szekely et al., 2009).
Stimulation of CaR expressed in HEK-293 cells elicits Ca2+i mobilization from intracellular stores (reviewed in Breitwieser, 2006) rather than Ca2+o influx per se, although it should be noted that modest increases in Ca2+i concentration can follow Ca2+o elevation even in non-transfected HEK-293 cells, especially following exposure to low Ca2+o or Ca2+o-free buffers (Nemeth et al., 2004). CaR-induced Ca2+i mobilization has also been reported in parathyroid cells (Ridefelt et al., 1995) and other cell types where CaR is expressed endogenously (Shorte et al., 1995; Adebanjo et al., 1998; Ward et al., 2002) and thus a number of studies have explored the mechanistic basis and functional consequences of CaR-induced Ca2+i oscillations.
Phosphorylation of the CaR intracellular tail regulates receptor activity
Most CaR-HEK cell studies employ baseline Ca2+o concentrations of 0.5 or 1 mM in the experimental buffer and at these concentrations no cells are activated. However, Ca2+i mobilization is observed at ∼2 mM Ca2+o mostly in the form of transient responses or low frequency oscillations. At ∼3 mM Ca2+o most responses are high frequency oscillations, while at 5 mM Ca2+o most responses are sustained (Breitwieser and Gama, 2001; Davies et al., 2006; 2007;) (Figure 1). It should be noted that the actual concentrations at which these ‘transitions’ occur are subject to multiple factors such as temperature, receptor expression levels and buffer ingredients but the trend is broadly true under most conditions. What is interesting is that the linear part of the Ca2+o concentration–effect curve comprises each of these response types and thus any signal or condition that may independently modulate oscillation frequency will tend to alter the EC50 accordingly without necessarily affecting agonist binding. This is interesting both from a molecular perspective and also as a means of altering CaR efficacy. The single most important CaR residue for controlling these oscillations is threonine-888 (in humans) and this serves as a highly conserved protein kinase C phosphorylation site. Cloning and sequencing of the bovine CaR and then human CaR, revealed at least five conserved PKC consensus sequences in the intracellular loops (T646 and S794) and intracellular tail (T888, S895 and S915) of the receptor. Mutating each putative PKC site in turn to a non-phosphorylatable residue, Bai et al. (1998b) found that the two intracellular loop residues do not contribute to regulation of CaR Ca2+o sensitivity and that mutation of residues S895 and S915 elicit only modest effects on the EC50 for Ca2+o or on responsiveness to the phorbol ester phorbol-12-myristate-13-acetate (PMA). In contrast, mutation of T888 (to valine) elicited a substantial leftward shift in the Ca2+o concentration–effect curve for Ca2+i mobilization as well as substantially reducing the inhibitory effect of PMA on the CaR response. Furthermore, CaRT888D and CaRT888E, whose mutations are phosphomimetic, revealed a significant rightward-shift in Ca2+o concentration dependence (Jiang et al., 2002). Together, these data suggested that CaRT888 is central to the control of CaR signalling. This idea was advanced by single cell imaging which revealed that CaRT888A tends to elicit sustained rather than oscillatory Ca2+i mobilization (Young et al., 2002) which can be mimicked in cells expressing wild-type CaR by inhibiting PKC. This suggested that for each Ca2+i oscillation, PKC-mediated phosphorylation on CaRT888 terminated the Ca2+i mobilization caused by receptor activation (Figure 2) and a phospho-specific antibody for CaRT888 did indeed reveal that CaR can be phosphorylated by PKC in that way (Davies et al., 2007). The concept of dynamic phosphorylation as a mechanism of class C GPCR regulation was first proposed by Nash et al. (2001; 2002;) with specific regard to the metabotropic glutamate receptor mGluR5. The residue phosphorylated dynamically in this case is mGluR5S839 (Kim et al., 2005), this residue being 13 amino acids closer to the transmembrane region than CaRT888. Indeed, there are both interesting parallels as well as specific differences between the dynamic phosphorylation of the two receptors.
Figure 1.
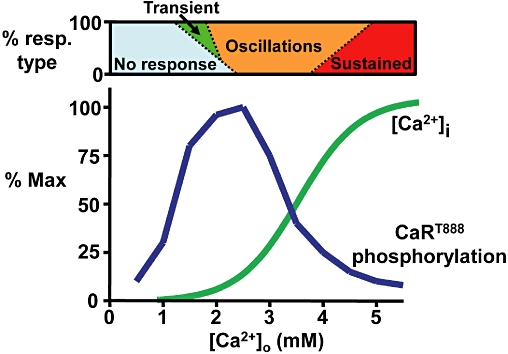
Schematic representation of the Ca2+o concentration dependence of CaRT888 phosphorylation and CaR-induced Ca2+i mobilization. The classic sigmoidal concentration dependence of CaR-induced Ca2+i mobilization is not matched by a sigmoidal relationship for CaRT888 phosphorylation but by a biphasic (bell-shaped) relationship. Therefore, at higher Ca2+o concentrations, CaRT888 phosphorylation is low or absent (at least after a brief initial rise), thus permitting the sustained responses seen for Ca2+i mobilization. The relative concentration dependence of the transient (green), oscillatory (orange) and sustained (red) responses normally observed represent approximations based on data from Davies et al. (2007) and McCormick et al. (2010). This bar is intended to indicate the heterogeneity of the cellular responses at each concentration (see also Nash et al., 2002).
Figure 2.
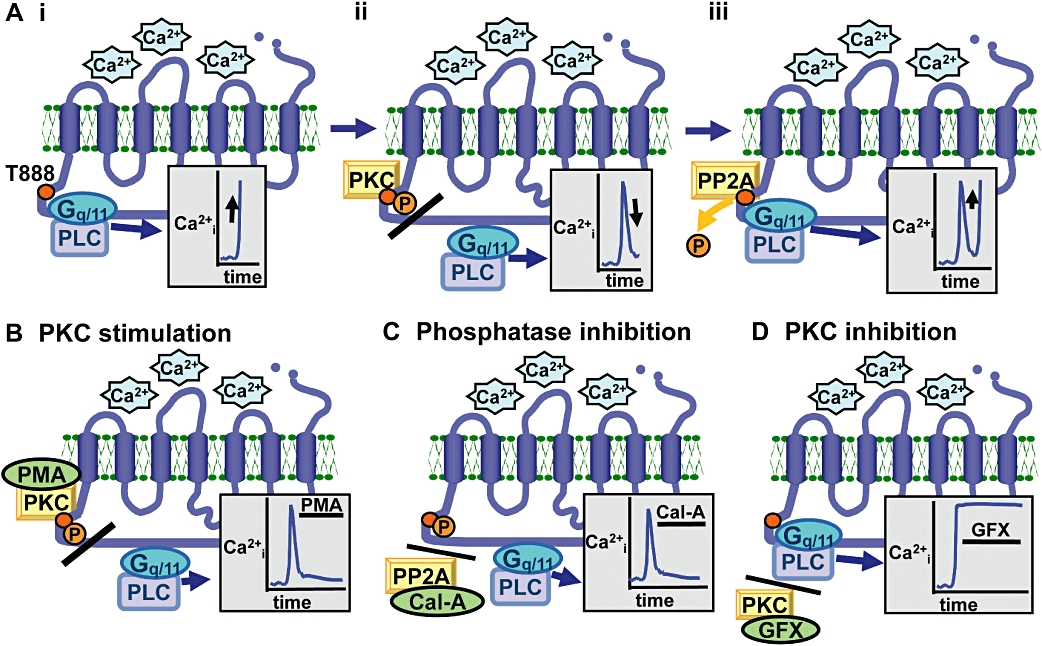
Schematic representation of the generation of CaR-induced Ca2+i oscillations based on dynamic phosphorylation of CaRT888. Panel A indicates the three components of each oscillatory cycle including (i) CaR activation eliciting Ca2+i mobilization, (ii) CaRT888 phosphorylation uncoupling the pathway components and (iii) CaRT888 dephosphorylation leading to further Ca2+i mobilization. This model therefore explains the suppressive effects of phorbol ester (PMA, Panel B) and calyculin-A (Cal-A, Panel C) and the stimulatory effect of GF109203X (GFX, Panel D) on CaR-induced Ca2+i mobilization.
CaRT888 dephosphorylation shapes downstream signalling
There is a significant problem, however, with the idea that CaR activation leads to CaRT888 phosphorylation and thus to receptor inhibition. The most striking problem is that at the top end of the Ca2+o concentration effect curve, the responses of single cells tend to change from oscillatory into sustained, whereas one might expect their CaRs to be maximally phosphorylated and thus maximally inhibited. To resolve this issue the actual concentration dependence of Ca2+o on CaRT888 phosphorylation was examined by immunoblotting (10 min agonist treatment) and instead of a sigmoidal curve, a biphasic, bell-shaped profile was observed. That is, at low to moderate Ca2+o concentrations, there is a concentration-dependent increase in CaRT888 phosphorylation, which peaks around the Ca2+o concentration at which oscillatory Ca2+i mobilization occurs. However, as Ca2+o concentrations are increased further, the amount of CaRT888 phosphorylation observed after 10 min declines consistent with the increased oscillation frequency seen at those concentrations. Finally, at ∼5 mM Ca2+o little CaRT888 phosphorylation is observed after 10 min consistent with the sustained, that is, uninhibited Ca2+i mobilization observed at those concentrations. This implies that, at moderate Ca2+o concentrations, PKC-mediated phosphorylation of CaRT888 matches its rate of dephosphorylation over time, albeit in an alternating manner so as to elicit stable oscillations. However, at higher Ca2+o concentrations, CaR preferentially elicits differentially greater levels of phosphatase activity rendering the receptor less phosphorylated and thus able to elicit either high frequency oscillations or even sustained Ca2+i mobilization (Figure 1). In support of this, the calcimimetic NPS-R467 (1 µM; a high potency PAM) elicited rapid but transient CaRT888 phosphorylation and thus sustained Ca2+i mobilization, in a manner similar to high Ca2+o concentration (McCormick et al., 2010). In contrast, the L-aromatic amino acids (10 mM; lower potency PAM) elicited sustained CaRT888 phosphorylation and oscillatory Ca2+i mobilization similarly to the effects of moderate Ca2+o concentration.
The identity of the phosphatase responsible remains unknown although some evidence points towards PP2A being involved. For example, the PP1/PP2A inhibitor calyculin-A prevents dephosphorylation of CaRT888 and inhibits CaR-induced Ca2+i mobilization in CaR-HEK and PT cells and abolishes the inhibitory effect of CaR on PTH secretion in human isolated PT cells. Furthermore, PP2A catalytic subunit colocalizes with phosphorylated CaR and PP2A activity is elevated following CaR activation in HEK-293 cells (Davies et al., 2007; McCormick et al., 2010).
Together, this model of differential CaRT888 phosphorylation explains both the suppressive effect of phorbol esters and calyculin-A and stimulatory effect of PKC inhibition on CaR-induced Ca2+i mobilization (Figure 2), and the transition from oscillatory to sustained CaR signalling (Figure 1).
Functional importance of CaRT888 phosphorylation
Whilst mutation of CaRT888 to non-phosphorylatable residues such as alanine and valine produces a leftward shift in Ca2+o concentration dependence in HEK-293 cells, that is, in vitro, it is necessary to determine whether this has any consequence in vivo. Indeed, we have recently identified a case of ADH resulting from the mutation CaRT888M (Lazarus et al., 2010). The index case and his father both exhibited hypocalcaemia, inappropriately normal PTH concentrations and relative hypercalciuria typical of ADH. When recapitulated in vitro, CaRT888M exhibited a leftward shift in Ca2+o concentration dependence and resistance to signalling inhibition by PMA (Lazarus et al., 2010). This would suggest that, under normal conditions, the human CaR is subject to feedback inhibition by phosphorylation on Thr-888 raising the possibility that PTH secretion and thus Ca2+o homeostasis may be controlled not only by pharmacological modulation of the ECD of the receptor but also by interfering with those intracellular signalling pathways responsible for the mediation of CaR signalling. Together, these molecular and clinical data explain functional observations first made ∼20 years ago showing that phorbol esters inhibit the suppressive effects of high Ca2+o concentration on PTH secretion (Nemeth et al., 1986; Kobayashi et al., 1988; Morrissey, 1988; Membreno et al., 1989).
Mitogen-activated protein kinase activation
CaR is known to activate an array of protein kinase pathways most notably the mitogen-activated protein (MAP) kinases ERK and p38MAPK (reviewed in Ward, 2004; Khan and Conigrave, 2009; Magno et al., 2010). The mechanism of CaR-induced ERK activation, at least in culture, involves triple-pass signalling whereby CaR stimulates an extracellular matrix metalloproteinase to elicit the extracellular release of an EGF-like peptide, which then stimulates EGF receptor (EGFR)-mediated signalling (MacLeod et al., 2004). In CaR-expressing HEK-293 cells, chronic receptor activation stimulates PTH-related protein (PTHrP) secretion into the medium (MacLeod et al., 2004), an effect also observed in PC-3 human prostate cancer cells (Yano et al., 2004) and H-500 rat Leydig cells (Sanders et al., 2000), and in each case the release of PTHrP is dependent on ERK activation resulting from transactivation of the EGFR (Yano et al., 2004). Similarly, matrix metalloproteinase and EGFR activation also result from CaR activation in human MCF-7 breast cancer cells (El Hiani et al., 2009). Indeed, this mechanism may contribute to the putative oncogenic effects of CaR in breast and prostate cancers – whereas CaR is associated with tumour suppression in colon and parathyroid tumours (Saidak et al., 2009).
Calcium-sensing receptor and the cytoskeleton
Another possible mechanism by which the CaR might limit PTH secretion is to attenuate the trafficking of secretory granules through the cytoskeletal network. The exocytotic barrier function of cortical actin has been described in endocrine cells such as adrenal chromaffin cells and pancreatic beta-cells (Burgoyne and Morgan, 2003) but may also exist in parathyroid cells (Quinn et al., 2007). Significantly, the cortical actin barrier is not simply a constitutive brake on secretion but can be regulated via extracellular signals causing actin assembly/disassembly (Burgoyne and Morgan, 2003). In dispersed bovine parathyroid cells, the filamentous actin-severing compounds, latrunculin and cytochalasin, permitted increased PTH secretion, whereas the actin polymerizing agent, jasplakinolide, substantially inhibited PTH secretion (Quinn et al., 2007). In addition, CaR activation elicits ρ-mediated actin filament formation in HEK-293 cells (Davies et al., 2006) and thus could be directly involved in cytoskeletal alteration as a mechanism for secretory modulation. This idea is further supported by the fact that CaR binds the cytoskeletal protein filamin-A, via the receptor's ICD and disruption of this interaction inhibits CaR-mediated ERK activation (Hjalm et al., 2001). Also, CaR-induced Gα12/13 and ρ activation has been reported in Madin-Darby canine kidney cells (Huang et al., 2004) and in breast cancer cells (Huang et al., 2009a). Together, Gα12/13 proteins have been implicated in a variety of cellular functions including regulation of platelet activation and smooth muscle contraction, as well as embryonic angiogenesis, cell migration in the immune system and metastatic spread in tumour cells (Worzfeld et al., 2008). Therefore, it will be interesting to determine the extent to which CaR-induced Gα12/13 activation may influence these processes in cells in which the CaR is expressed.
Conclusions
Because of their ability to modulate PTH secretion, CaR-based therapeutics constitute an important and novel toolkit for the treatment of disturbances in Ca2+o metabolism. Calcimimetics are the first class of receptor PAMs on the market and are being used increasingly for the treatment of hyperparathyroidism and of other hypercalcaemic disorders. Indeed, recent clinical observations suggest that they also ameliorate cardiovascular complications associated with advanced chronic kidney disease and might allow for a reduction in vascular calcification and an improvement in blood pressure control in patients suffering from this disease. As for calcilytics, they improve bone mineral density and induce markers of bone formation in ovariectomized rats and have reached phase III clinical trials for the treatment of post-menopausal osteoporosis, a disease which affects 1:3 women and 1:10 men above the age of 50, worldwide. Finally, basic science research has made significant advances in understanding CaR-mediated cell signalling in vivo. Further studies must go hand-in-hand with these drug discovery programmes to establish how best to achieve an inhibition of the CaR to mobilize the pool of PTH-containing vesicles, in order to evoke short-lived osteoanabolic bursts of this hormone without its undesired effects on bone breakdown.
Acknowledgments
We wish to thank members of our laboratories and of the Arthritis Research UK Biomechanics and Bioengineering Centre (DR) for helpful discussions.
Glossary
Abbreviations
- ADH
autosomal dominant hypocalcaemia
- CaR
calcium-sensing receptor
- ECD
extracellular domain
- FHH
familial hypocalciuric hypercalcaemia
- ICD
intracellular domain
- NAM
negative allosteric modulator
- NSHPT
neonatal severe hyperparathyroidism
- PAM
positive allosteric modulator
- PMA
phorbol-12-myristate-13-acetate
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone-related protein
Conflict of interest
Both authors declare no conflict of interest.
References
- Adebanjo OA, Igietseme J, Huang CL, Zaidi M. The effect of extracellularly applied divalent cations on cytosolic Ca2+ in murine leydig cells: evidence for a Ca2+-sensing receptor. J Physiol. 1998;513:399–410. doi: 10.1111/j.1469-7793.1998.399bb.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida K, Koishi S, Tawata M, Onaya T. Molecular cloning of a putative Ca(2+)-sensing receptor cDNA from human kidney. Biochem Biophys Res Commun. 1995;214:524–529. doi: 10.1006/bbrc.1995.2318. [DOI] [PubMed] [Google Scholar]
- Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsen JE, Sherrard DJ, Andress DL. A calcimimetic agent acutely suppresses parathyroid hormone levels in patients with chronic renal failure. Rapid communication. Kidney Int. 1998;53:223–227. doi: 10.1046/j.1523-1755.1998.00735.x. [DOI] [PubMed] [Google Scholar]
- Ayachi S. Increased dietary calcium lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1979;28:1234–1238. doi: 10.1016/0026-0495(79)90136-7. [DOI] [PubMed] [Google Scholar]
- Bai M. Structure-function relationship of the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:197–207. doi: 10.1016/j.ceca.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem. 1998a;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Lane CR, Yang YH, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca-0(2+)-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem. 1998b;273:21267–21275. doi: 10.1074/jbc.273.33.21267. [DOI] [PubMed] [Google Scholar]
- Balan G, Bauman J, Bhattacharya S, Castrodad M, Healy DR, Herr M, et al. The discovery of novel calcium sensing receptor negative allosteric modulators. Bioorg Med Chem Lett. 2009;19:3328–3332. doi: 10.1016/j.bmcl.2009.04.044. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Tfelt-Hansen J, Chattopadhyay N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J Neurosci Res. 2010;88:2073–2082. doi: 10.1002/jnr.22391. [DOI] [PubMed] [Google Scholar]
- Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004a;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004b;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78:578–589. doi: 10.1038/ki.2010.167. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. Calcium sensing receptors and calcium oscillations: calcium as a first messenger. Curr Top Dev Biol. 2006;73:85–114. doi: 10.1016/S0070-2153(05)73003-9. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, Gama L. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am J Physiol Cell Physiol. 2001;280:C1412–C1421. doi: 10.1152/ajpcell.2001.280.6.C1412. [DOI] [PubMed] [Google Scholar]
- Broadhead GK, Mun HC, Avlani VA, Jourdon O, Church WB, Christopoulos A, et al. Allosteric modulation of the calcium-sensing receptor by {gamma}-glutamyl peptides inhibition of PTH secretion, suppression of intracellular cAMP levels and a common mechanism of action with L-amino acids. J Biol Chem. 2010;286:8786–8797. doi: 10.1074/jbc.M110.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM. Anti-parathyroid and anti-calcium sensing receptor antibodies in autoimmune hypoparathyroidism. Endocrinol Metab Clin North Am. 2009;38:437–445. doi: 10.1016/j.ecl.2009.01.001. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Bruce JIE, Yang XS, Ferguson CJ, Elliott AC, Steward MC, Case RM, et al. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Bian K, Wang Y, Mupanomunda M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension. 1997;30:1431–1439. doi: 10.1161/01.hyp.30.6.1431. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, et al. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Barnett JV, Congo DA, Brown EM. Divalent cations suppress 3′,5′-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology. 1989;124:233–239. doi: 10.1210/endo-124-1-233. [DOI] [PubMed] [Google Scholar]
- Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, et al. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology. 1999;116:118–126. doi: 10.1016/s0016-5085(99)70235-0. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Mun HC, Delbridge L, Quinn SJ, Wilkinson M, Brown EM. L-amino acids regulate parathyroid hormone secretion. J Biol Chem. 2004;279:38151–38159. doi: 10.1074/jbc.M406373200. [DOI] [PubMed] [Google Scholar]
- Davies SL, Gibbons CE, Vizard T, Ward DT. Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am J Physiol Cell Physiol. 2006;290:C1543–C1551. doi: 10.1152/ajpcell.00482.2005. [DOI] [PubMed] [Google Scholar]
- Davies SL, Ozawa A, McCormick WD, Dvorak MM, Ward DT. Protein kinase C-mediated phosphorylation of the calcium-sensing receptor is stimulated by receptor activation and attenuated by calyculin-sensitive phosphatase activity. J Biol Chem. 2007;282:15048–15056. doi: 10.1074/jbc.M607469200. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- Dvorak MM, Chen T-H, Orwoll B, Garvey C, Chang W, Bikle DD, et al. Constitutive Activity of the Osteoblast Ca2+-Sensing Receptor Promotes Loss of Cancellous Bone. Endocrinology. 2007;148:3156–3163. doi: 10.1210/en.2007-0147. [DOI] [PubMed] [Google Scholar]
- Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol. 2008;22:129–148. doi: 10.1016/j.berh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hiani Y, Lehen'kyi V, Ouadid-Ahidouch H, Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys. 2009;486:58–63. doi: 10.1016/j.abb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Finney B, Wilkinson WJ, Searchfield L, Cole M, Bailey S, Kemp PJ, et al. An exon 5-less splice variant of the extracellular calcium-sensing receptor rescues absence of the full-length receptor in the developing mouse lung. Exp Lung Res. 2011;37:269–278. doi: 10.3109/01902148.2010.545471. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA, Dabrowski CE, Cicconettig, Gordon DN, Papapoulos S, Bone HG, III, et al. The effects of ronacaleret, a calcium-sensing receptor antagonist, on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mineral density. J Clin Endocrinol Meatab. 2011 doi: 10.1210/jc.2010-2855. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fukumoto S. [New therapeutics for disorders of bone and calcium metabolism. Antagonist for calcium-sensing receptor. – JTT-305/MK-5442 -] Clin Calcium. 2011;21:89–93. [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE. Heterodimerization of Calcium Sensing Receptors with Metabotropic Glutamate Receptors in Neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007;22:931–937. doi: 10.1359/jbmr.070304. [DOI] [PubMed] [Google Scholar]
- Gibbons CE, Maldonado-Perez D, Shah AN, Riccardi D, Ward DT. Calcium-sensing receptor antagonism or lithium treatment ameliorates aminoglycoside-induced cell death in renal epithelial cells. Biochim Biophys Acta. 2008;1782:188–195. doi: 10.1016/j.bbadis.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M, Stroup GB, Dodds RA, James IE, Votta BJ, Smith BR, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca(2+) sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- Hough TA, Bogani D, Cheeseman MT, Favor J, Nesbit MA, Thakker RV, et al. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci USA. 2004;101:13566–13571. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Hujer KM, Wu ZZ, Miller RT. The Ca2+-sensing receptor couples to G alpha(12/13) to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–C30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Yang W, Butters R, Lee HW, Li S, et al. Identification and dissection of Ca(2+)-binding sites in the extracellular domain of Ca(2+)-sensing receptor. J Biol Chem. 2007;282:19000–19010. doi: 10.1074/jbc.M701096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Hydo LM, Liu S, Miller RT. Activation of choline kinase by extracellular Ca2+ is Ca(2+)-sensing receptor, Galpha12 and Rho-dependent in breast cancer cells. Cell Signal. 2009a;21:1894–1900. doi: 10.1016/j.cellsig.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009b;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovski O, Nikolov IG, Joki N, Caudrillier A, Phan O, Mentaverri R, et al. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE−/−) mice. Atherosclerosis. 2009;205:55–62. doi: 10.1016/j.atherosclerosis.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Jiang YF, Zhang ZX, Kifor O, Lane CR, Quinn SJ, Bai M. Protein kinase C (PKC) phosphorylation of the Ca2+-sensing receptor (CaR) modulates functional interaction of g proteins with the CaR cytoplasmic tail. J Biol Chem. 2002;277:50543–50549. doi: 10.1074/jbc.M205798200. [DOI] [PubMed] [Google Scholar]
- Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang J, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independent of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297:E915–E923. doi: 10.1152/ajpendo.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp EH, Gavalas NG, Krohn KJ, Brown EM, Watson PF, Weetman AP. Activating autoantibodies against the calcium-sensing receptor detected in two patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2009;94:4749–4756. doi: 10.1210/jc.2009-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Rognan D, Cesario M, Ruat M, et al. N-1-benzoyl-N-2-[1-(1-naphthyl)ethyl]-trans-1,2-diaminocyclohexanes: development of 4-chlorophenylcarboxamide (Calhex 231) as a new calcium sensing receptor ligand demonstrating potent calcilytic activity. J Med Chem. 2006;49:5119–5128. doi: 10.1021/jm051233+. [DOI] [PubMed] [Google Scholar]
- Khan MA, Conigrave AD. Mechanisms of multimodal sensing by extracellular Ca(2+)-sensing receptors: a domain-based survey of requirements for binding and signalling. Br J Pharmacol. 2009;159:1039–1050. doi: 10.1111/j.1476-5381.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifor O, Moore FD, Delaney M, Garber J, Hendy GN, Butters R, et al. A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. J Clin Endocrinol Metab. 2003;88:60–72. doi: 10.1210/jc.2002-020249. [DOI] [PubMed] [Google Scholar]
- Kim CH, Braud S, Isaac JT, Roche KW. Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. J Biol Chem. 2005;280:25409–25415. doi: 10.1074/jbc.M502644200. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Russell J, Lettieri D, Sherwood LM. Regulation of protein kinase C by extracellular calcium in bovine parathyroid cells. Proc Natl Acad Sci USA. 1988;85:4857–4860. doi: 10.1073/pnas.85.13.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleganova N, Piecha G, Ritz E, Schmitt CP, Gross ML. A calcimimetic (R-568), but not calcitriol, prevents vascular remodeling in uremia. Kidney Int. 2009;75:60–71. doi: 10.1038/ki.2008.490. [DOI] [PubMed] [Google Scholar]
- Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AE, Eisenberger U, Frey FJ, Mohaupt MG. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1311–1314. doi: 10.1093/ndt/gfh924. [DOI] [PubMed] [Google Scholar]
- Kumar S, Matheny CJ, Hoffman SJ, Marquis RW, Schultz M, Liang X, et al. An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone. 2010;46:534–542. doi: 10.1016/j.bone.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Lazarus S, Pretorius CJ, Khafagi F, Campion KL, Brennan SC, Conigrave AD, et al. A novel mutation of the primary protein kinase C phosphorylation site in the calcium-sensing receptor causes autosomal dominant hypocalcemia. Eur J Endocrinol. 2010;164:429–435. doi: 10.1530/EJE-10-0907. [DOI] [PubMed] [Google Scholar]
- Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, et al. The extracellular calcium sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JN, Owens M, Gustafsson M, Jensen J, Tabatabaei A, Schmelzer K, et al. Characterization of Highly Efficacious Allosteric Agonists of the Human Calcium-Sensing Receptor. J Pharmacol Exp Ther. 2011;337:275–284. doi: 10.1124/jpet.110.178194. [DOI] [PubMed] [Google Scholar]
- MacLeod RJ, Yano S, Chattopadhyay N, Brown EM. Extracellular calcium-sensing receptor transactivates the epidermal growth factor receptor by a triple-membrane-spanning signaling mechanism. Biochem Biophys Res Commun. 2004;320:455–460. doi: 10.1016/j.bbrc.2004.05.198. [DOI] [PubMed] [Google Scholar]
- Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2010;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- Mamillapalli R, Wysolmerski J. The calcium-sensing receptor couples to Galpha(s) and regulates PTHrP and ACTH secretion in pituitary cells. J Endocrinol. 2010;204:287–297. doi: 10.1677/JOE-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium sensing receptor reverses its effect on PTHrP secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RW, Lago AM, Callahan JF, Rahman A, Dong X, Stroup GB, et al. Antagonists of the calcium receptor. 2. Amino alcohol-based parathyroid hormone secretagogues. J Med Chem. 2009a;52:6599–6605. doi: 10.1021/jm900563e. [DOI] [PubMed] [Google Scholar]
- Marquis RW, Lago AM, Callahan JF, Trout RE, Gowen M, DelMar EG, et al. Antagonists of the calcium receptor I. Amino alcohol-based parathyroid hormone secretagogues. J Med Chem. 2009b;52:3982–3993. doi: 10.1021/jm900364m. [DOI] [PubMed] [Google Scholar]
- McCormick WD, Atkinson-Dell R, Campion KL, Mun HC, Conigrave AD, Ward DT. Increased receptor stimulation elicits differential calcium-sensing receptor(T888) dephosphorylation. J Biol Chem. 2010;285:14170–14177. doi: 10.1074/jbc.M109.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membreno L, Chen TH, Woodley S, Gagucas R, Shoback D. The effects of protein kinase-C agonists on parathyroid hormone release and intracellular free Ca2+ in bovine parathyroid cells. Endocrinology. 1989;124:789–797. doi: 10.1210/endo-124-2-789. [DOI] [PubMed] [Google Scholar]
- Miedlich S, Gama L, Breitwieser GE. Calcium sensing receptor activation by a calcimimetic suggests a link between cooperativity and intracellular calcium oscillations. J Biol Chem. 2002;277:49691–49699. doi: 10.1074/jbc.M205578200. [DOI] [PubMed] [Google Scholar]
- Miedlich SU, Gama L, Seuwen K, Wolf RM, Breitwieser GE. Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J Biol Chem. 2004;279:7254–7263. doi: 10.1074/jbc.M307191200. [DOI] [PubMed] [Google Scholar]
- Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, et al. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293:F946–F955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- Morrissey JJ. Effect of phorbol myristate acetate on secretion of parathyroid hormone. Am J Physiol. 1988;254(1):E63–E70. doi: 10.1152/ajpendo.1988.254.1.E63. Pt 1. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Parekh N, Koleganova N, Ritz E, Schaefer F, Schmitt CP. Acute cardiovascular effects of the calcimimetic R-568 and its enantiomer S-568 in rats. Pediatr Nephrol. 2009;24:1385–1389. doi: 10.1007/s00467-009-1153-6. [DOI] [PubMed] [Google Scholar]
- Nash MS, Young KW, Challiss RA, Nahorski SR. Intracellular signalling. Receptor-specific messenger oscillations. Nature. 2001;413:381–382. doi: 10.1038/35096643. [DOI] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RA. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem. 2002;277:35947–35960. doi: 10.1074/jbc.M205622200. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. The search for calcium receptor antagonists (calcilytics) J Mol Endocrinol. 2002;29:15–21. doi: 10.1677/jme.0.0290015. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. Misconceptions about calcimimetics. Ann N Y Acad Sci. 2006;1068:471–476. doi: 10.1196/annals.1346.044. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. Anabolic therapy for osteoporosis: calcilytics. IBMS BoneKey. 2008;5:196–208. [Google Scholar]
- Nemeth EF, Wallace J, Scarpa A. Stimulus-secretion coupling in bovine parathyroid cells. Dissociation between secretion and net changes in cytosolic Ca2+ J Biol Chem. 1986;261:2668–2674. [PubMed] [Google Scholar]
- Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Van Wagenen BC, DelMar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth EF, Delmar EG, Heaton WL, Miller MA, Lambert LD, Conklin RL, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001;299:323–331. [PubMed] [Google Scholar]
- Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- Oda Y, Tu CL, Chang W, Crumrine D, Komuves L, Mauro T, et al. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J Biol Chem. 2000;275:1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1756–H1762. doi: 10.1152/ajpheart.00739.2004. [DOI] [PubMed] [Google Scholar]
- Pallais JC, Kifor O, Chen YB, Slovik D, Brown EM. Acquired hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor. N Engl J Med. 2004;351:362–369. doi: 10.1056/NEJMoa040008. [DOI] [PubMed] [Google Scholar]
- Park PS, Palczewski K. Diversifying the repertoire of G protein-coupled receptors through oligomerization. Proc Natl Acad Sci USA. 2005;102:8793–8794. doi: 10.1073/pnas.0504016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca2+-sensing receptor. J Biol Chem. 2003;278:49487–49494. doi: 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem. 2004;279:18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Kifor O, Kifor I, Butters RR, Brown EM. Role of the cytoskeleton in extracellular calcium-regulated PTH release. Biochem Biophys Res Commun. 2007;354:8–13. doi: 10.1016/j.bbrc.2006.12.160. [DOI] [PubMed] [Google Scholar]
- Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol. 2010;298:F485–F499. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+ polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol. 1998;43:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- Ridefelt P, Bjorklund E, Akerstrom G, Olsson MJ, Rastad J, Gylfe E. Ca(2+)-induced Ca2+ oscillations in parathyroid cells. Biochem Biophys Res Commun. 1995;215:903–909. doi: 10.1006/bbrc.1995.2549. [DOI] [PubMed] [Google Scholar]
- Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129–2134. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Ferrari SL, Pizurki L, Caverzasio J, Bonjour JP. Actions of parathyroid hormone and parathyroid hormone-related protein. J Endocrinol Invest. 1992;15(Suppl 6):51–56. [PubMed] [Google Scholar]
- Rodriguez L, Tu C, Cheng Z, Chen T-H, Bikle D, Shoback D, et al. Expression and Functional Assessment of an Alternatively Spliced Extracellular Ca2+-Sensing Receptor in Growth Plate Chondrocytes. Endocrinology. 2005;146:5294–5303. doi: 10.1210/en.2005-0256. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Aguilera-Tejero E, Mendoza FJ, Guerrero F, Lopez I. Effects of calcimimetics on extraskeletal calcifications in chronic kidney disease. Kidney Int Suppl. 2008:S50–S54. doi: 10.1038/ki.2008.546. 111. [DOI] [PubMed] [Google Scholar]
- Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Butters RR, Brown EM. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141:4357–4364. doi: 10.1210/endo.141.12.7849. [DOI] [PubMed] [Google Scholar]
- Shcherbakova I, Balandrin MF, Fox J, Ghatak A, Heaton WL, Conklin RL. 3H-quinazolin-4-ones as a new calcilytic template for the potential treatment of osteoporosis. Bioorg Med Chem Lett. 2005;15:1557–1560. doi: 10.1016/j.bmcl.2005.01.078. [DOI] [PubMed] [Google Scholar]
- Shorte SL, Stafford SJ, Collett VJ, Schofield JG. Simultaneous measurement of [Ca2+]i and secretion-coupled membrane turnover, by single cell fluorescence microscopy. Cell Calcium. 1995;18:440–454. doi: 10.1016/0143-4160(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
- Sloand JA, Shelly MA. Normalization of lithium-induced hypercalcemia and hyperparathyroidism with cinacalcet hydrochloride. Am J Kidney Dis. 2006;48:832–837. doi: 10.1053/j.ajkd.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- Szekely D, Brennan BS, Mun HC, Conigrave A, Kuchel PW. Effectors of the frequency of calcium oscillations in HEK-293 cells: wavelet analysis and a computer model. Eur Biophys J. 2009;39:149–165. doi: 10.1007/s00249-009-0469-2. [DOI] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, et al. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc Natl Acad Sci USA. 2004;101:16952–16957. doi: 10.1073/pnas.0405387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, et al. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science. 2007;315:1423–1426. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:275–282. doi: 10.1016/j.ceca.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Thakore P, Ho WS. Vascular actions of calcimimetics: role of Ca(2+) -sensing receptors versus Ca(2+) influx through L-type Ca(2+) channels. Br J Pharmacol. 2011;162:749–762. doi: 10.1111/j.1476-5381.2010.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- Vainonen JP, Sakuragi Y, Stael S, Tikkanen M, Allahverdiyeva Y, Paakkarinen V, et al. Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J. 2008;275:1767–1777. doi: 10.1111/j.1742-4658.2008.06335.x. [DOI] [PubMed] [Google Scholar]
- Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273:14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- Ward DT, McLarnon SJ, Riccardi D. Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J Am Soc Nephrol. 2002;13:1481–1489. doi: 10.1097/01.asn.0000015623.73739.b8. [DOI] [PubMed] [Google Scholar]
- Ward DT, Maldonado-Perez D, Hollins L, Riccardi D. Aminoglycosides induce acute cell signaling and chronic cell death in renal cells that express the calcium-sensing receptor. J Am Soc Nephrol. 2005;16:1236–1244. doi: 10.1681/ASN.2004080631. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Johansen LD, Brauner-Osborne H. The emerging role of promiscuous 7TM receptors as chemosensors for food intake. Vitam Horm. 2010;84:151–184. doi: 10.1016/B978-0-12-381517-0.00005-9. [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, et al. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells – studies with calindol and calhex 231. Circ Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 {alpha}-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+-sensing receptor. Mol Endocrinol. 2007;21:274–280. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- Widler L, Altmann E, Beerli R, Breitenstein W, Bouhelal R, Buhl T, et al. 1-Alkyl-4-phenyl-6-alkoxy-1H-quinazolin-2-ones: a novel series of potent calcium-sensing receptor antagonists. J Med Chem. 2010;53:2250–2263. doi: 10.1021/jm901811v. [DOI] [PubMed] [Google Scholar]
- Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol. 2000;175:203–212. doi: 10.1007/s00232001068. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Wettschureck N, Offermanns S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci. 2008;29:582–589. doi: 10.1016/j.tips.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yano S, Macleod RJ, Chattopadhyay N, Tfelt-Hansen J, Kifor O, Butters RR, et al. Calcium-sensing receptor activation stimulates parathyroid hormone-related protein secretion in prostate cancer cells: role of epidermal growth factor receptor transactivation. Bone. 2004;35:664–672. doi: 10.1016/j.bone.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem. 2002;277:46871–46876. doi: 10.1074/jbc.M207083200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun S, Quinn SJ, Brown EM, Bai M. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J Biol Chem. 2001;276:5316–5322. doi: 10.1074/jbc.M005958200. [DOI] [PubMed] [Google Scholar]


