Abstract
BACKGROUND AND PURPOSE
ATP is released in response to cellular damage, and P2X7 receptors have an essential role in the onset and maintenance of pathological changes. Haemorrhagic cystitis (HC) is a well-known adverse effect of therapy with cyclophosphamide used for the treatment of many solid tumours and autoimmune conditions. Here we have evaluated the role of P2X7 receptors in a model of HC induced by cyclophosphamide.
EXPERIMENTAL APPROACH
Effects of pharmacological antagonism or genetic deletion of P2X7 receptor on cyclophosphamide-induced HC in mice was assessed by nociceptive and inflammatory measures. In addition, the presence of immunoreactive P2X7 receptors was assessed by immunohistochemistry.
KEY RESULTS
Pretreatment with the selective P2X7 receptor antagonist A-438079 or genetic ablation of P2X7 receptors reduced nociceptive behaviour scores in the HC model. The same strategies decreased both oedema and haemorrhage indices, on macroscopic or histological evaluation. Treatment with A-438079 decreased the staining for c-Fos in the lumbar spinal cord and brain cortical areas. Treatment with A-438079 also prevented the increase of urinary bladder myeloperoxidase activity and macrophage migration induced by cyclophosphamide and reduced the tissue levels of IL-1β and TNF-α. Finally, P2X7 receptors were markedly up-regulated in the bladders of mice with cyclophosphamide-induced HC.
CONCLUSIONS AND IMPLICATIONS
P2X7 receptors were significantly involved in a model of HC induced by cyclophosphamide. Pharmacological inhibition of these receptors might represent a new therapeutic option for this pathological condition.
Keywords: P2X7 receptor, haemorrhagic cystitis, cyclophosphamide, A-438079, genetic ablation, mice
Introduction
Haemorrhagic cystitis (HC) is an inflammatory condition of the urinary bladder, represented by haematuria, symptoms of local irritability (dysuria, frequency and urgency of urination) and pelvic pain. HC can be initiated by radiation or by chemical agents, such as busulfan, oxazaphosphorines (such as cyclophosphamide and ifosfamide), and occasionally by penicillins and non-steroidal anti-inflammatory drugs. Many cases of mild HC are spontaneously resolved; nevertheless, in some cases, moderate to severe HC can result in significant morbidity, or even mortality (Traxer et al., 2001; Nickel, 2002; Neheman et al., 2005; Giraud et al., 2006; Cheuk et al., 2007; Manikandan et al., 2010).
The alkylating agent cyclophosphamide acts in a non-specific manner on the cell cycle and it is commonly used for the treatment of many solid tumours, B cell malignancies, and as an immunosuppressive agent for autoimmune diseases, such as lupus and rheumatoid arthritis. It is a prodrug, metabolized by hepatic microsomal enzymes, generating its active metabolite phosphoramide mustard, and the toxic agent acrolein. Acrolein is highly toxic to the urinary bladder, promoting the release of inflammatory mediators, which ultimately lead to HC (Crocitto et al., 1996; Wong et al., 2000; Traxer et al., 2001; Batista et al., 2006; Manikandan et al., 2010).
Patient hydration, bladder instillation and the use of diuretics lead to increased urinary flux, favouring the elimination of acrolein. In addition, some agents able to neutralize the toxic metabolite, such as sodium 2-mercaptoethane sulfonate (Mesna), have also been widely used to prevent cyclophosphamide-induced HC (Traxer et al., 2001). Nevertheless, once HC is present, these methods have only minor beneficial effects. Other strategies have been described, such as antifibrinolytic drugs, potassium alum, silver nitrate, phenol, prostaglandins, formalin, arterial embolization, hyperbaric oxygen therapy, to treat HC, but all of them display limited efficacy (Traxer et al., 2001; Neheman et al., 2005; Chow et al., 2006; 2007; Manikandan et al., 2010).
The purinergic system is involved in several pathophysiological events. Purines exert their main effects by interacting with two general classes of purinergic receptors, P1 and P2 (Ralevic and Burnstock, 1998; Burnstock, 2011). The P1 class consists of the adenosine GPCRs (A1, A2A, A2B, A3) preferentially binding adenosine, whereas the P2 receptors recognize the nucleotides ADP, ATP, UTP and UDP. The P2 receptors are divided into two families; the P2Y GPCRs (metabotropic) with eight subtypes (P2Y1,4,6,11–14), and the P2X receptors (ligand–gated ion channels) with seven subtypes (P2X1-7) (Burnstock, 2007; 2009; 2011; Surprenant and North, 2009; receptor nomenclature follows Alexander et al., 2011). Several P2 receptor subtypes are expressed in the urinary bladder and are likely to be involved in functions such as sensory and motor transmission under normal conditions. Understanding the involvement of these receptors in the pathophysiology of micturition, urinary dysfunction, and mainly in disease states might identify new therapeutic targets to treat bladder dysfunction (Burnstock, 2009; 2011;).
Among the P2X receptor family, the P2X7 subtype presents a series of peculiar features. First, its activation requires near-millimolar concentrations of ATP (up to 300 µM), while the other P2X receptors display a very high sensitivity for ATP. Next, P2X7 receptors are highly expressed in immune and inflammatory cells, throughout the central or peripheral nervous systems. Activation of P2X7 receptors results in Ca2+ and Na+ influx and efflux of K+, accompanied by the release of the pro-inflammatory cytokine IL-1β (Ferrari et al., 1997; MacKenzie et al., 2001; Chen and Brosnan, 2006; Donnelly-Roberts and Jarvis, 2007; Dubyak, 2007; Skaper et al., 2010; Burnstock, 2011). This receptor has been implicated in activation of peripheral macrophages and glia, neutrophil infiltration and prostaglandin production (Labasi et al., 2002; Chessell et al., 2005; Burnstock, 2006; Di Virgilio, 2007; Donnelly-Roberts and Jarvis, 2007; King, 2007; Yoon et al., 2007; Burnstock, 2011). A number of recent studies have demonstrated the relevance of P2X7 receptors in a wide range of experimental models of disease, such as depression, epilepsy, Parkinson's disease, arthritis, cancer and chronic pain, by using either selective receptor antagonists or mice with genetic deletion of P2X7 receptors (KO mice) (Chessell et al., 2005; Honore et al., 2006; Li et al., 2006; Donnelly-Roberts and Jarvis, 2007; Basso et al., 2009; Marcellino et al., 2010; Teixeira et al., 2010).
We therefore designed the present study to evaluate whether P2X7 receptors are implicated in inflammatory and nociceptive changes in a mouse model of HC, induced by cyclophosphamide. In this study, we have evaluated the effects of the selective P2X7 receptor antagonist A-438079 and when possible, we have also assessed the inflammatory and nociceptive responses related to cyclophosphamide-induced HC in mice with genetic deletion of P2X7 receptors (KO mice).
Methods
Animals
All animal care and the experimental procedures were in accordance with the Guidelines for the Use and Care with Laboratorial Animals from National Institute of Health and ethical guidelines for investigations of experimental pain in conscious animals, and were approved by the Local Animal Ethics Committee (protocol number 08/00074). The number of animals and the intensity of noxious stimuli were the minimum necessary to demonstrate the consistent effects of the drug treatments.
Male Swiss, C57/BL6 and P2X7 receptor deleted (KO) mice (25–30 g) were used throughout this study. Swiss and C57/BL6 mice were obtained from Universidade Federal de Pelotas (UFPEL; Pelotas, RS, Brazil), and P2X7 receptor KO mice were donated by Dr Robson Coutinho, Federal University of Rio de Janeiro (UFRJ, Rio de Janeiro, Brazil). The KO mice were generated by the method developed by Dr James Mobley (PGRD, Pfizer Inc, Groton, CT, USA). The KO mice used in the present study were C57/BL6 inbred. The animals were housed in groups of five per cage and maintained in controlled temperature (22 ± 2°C) and humidity (60–70%), under a 12 h light–dark cycle, with food and water ad libitum.
Treatments
In our model, HC was induced by a single administration of cyclophosphamide (300 mg·kg−1) (Olivar and Laird, 1999; Wantuch et al., 2007). The selective P2X7 receptor antagonist A-438079 (50, 100 and 200 µmol·kg−1) or with the reference compound Mesna (60 mg·kg−1) was given in two doses; the first one was given 30 min before cyclophosphamide, and the second dose 4 h after the injection of cyclophosphamide, except in the experiments for assessing cytokines, in which the drugs were given as a single i.p. dose, 30 min before cyclophosphamide. The doses of A-438079 or Mesna were chosen on the basis of published data (Batista et al., 2006; McGaraughty et al., 2007).
In separate groups of experiments, the relevance of P2X7 receptors was assessed by using animals with genetic deletion this receptor (KO mice). C57/BL6 mice were used as the control group for this series of experiments. HC was induced as described above.
Behavioural studies (nociception)
The method used in the present study was similar to that described by Olivar and Laird (1999), with minor modifications. Experiments were performed between 8:00 and 12:00 AM to minimize the potential circadian variations in the behavioural responses. Immediately after the i.p. injection of cyclophosphamide, mice were housed in individual plastic cages, without sawdust bedding, and the spontaneous behaviour was measured for 2 min, every 30 min, over a total period of 4 h. The following behavioural changes were evaluated: (i) activity (walking, rearing, climbing, grooming, etc.); (ii) immobility; and (iii) behaviours indicative of visceral pain (‘crises’). In addition, the behavioural alterations were scored according to the following scale: 0 = normal; 1 = piloerection; 2 = strong piloerection; 3 = laboured breathing; 4 = licking of the abdomen; or 5 = stretching and contractions of the abdomen. At the end of the 4 h observation period, an open-field test was carried out. The animals were placed individually in a box divided in nine squares, for 5 min. The time spent in the following behavioural categories was recorded: (i) rearing; (ii) walking; and (iii) not exploring (grooming, immobility). The number of squares crossed with the four paws was also registered, and taken as an index of locomotor activity (Olivar and Laird, 1999; Wantuch et al., 2007).
For behavioural experiments, we have used 6–10 Swiss mice, or 3–4 C57/BL6 or KO mice per group. The same number of animals was employed for gross evaluation and histological analysis.
Gross evaluation
For this series of experiments, the animals were killed 6 h following cyclophosphamide administration. The gross evaluation was based on criteria established by Gray et al. (1986). All bladders were dissected free from connecting tissues, and transected at the bladder neck. Each bladder was macroscopically evaluated, by an examiner unaware of the treatment groups. The oedema formation was categorized as severe (3), moderate (2), mild (1) or absent (0). Oedema was considered severe when fluid was seen externally in the walls of the bladder, as well as internally. When oedema was confined to the internal mucosa, it was reported as moderate; when it was between normal and moderate, the oedema was defined as mild. Upon examination, the bladders were also surveyed for bleeding in the walls and categorized into four classes, depending on the presence of intravesical clots (3), mucosal hematomas (2), telangiectasia or dilatation of the bladder vessels (1), or normal aspect (0). As an additional measure of bladder oedema, the wet weight of each bladder was recorded and expressed as mg per 100 g of animal, (Gray et al., 1986).
Histological analysis
Following the gross evaluation, the bladders were fixed in buffered formalin solution (10%) for 24 h. After this period, the samples were embedded in paraffin, and stained with haematoxylin and eosin. A pathologist, who was unaware of the treatment, reviewed each specimen, considering the following parameters, as proposed by Gray et al. (1986), with some modifications: normal (normal epithelium, no inflammatory cell infiltrate or ulcers); mild (diminished epithelial cells, flattening with submucosal oedema, mild haemorrhage, few ulcerations); moderate (mucosal erosion, inflammatory cell infiltrate, fibrin deposition, haemorrhage and multiple ulcerations); severe (mucosal erosion, inflammatory cell infiltrate, fibrin deposition, multiple ulcerations and transmural haemorrhage with severe oedema).
Immunohistochemistry for c-Fos
The expression of c-Fos, a known biochemical marker of nociception, was measured by immunohistochemistry, as previously described by Labrousse et al. (2009). The lumbar spinal cords and the brains were rapidly excised (6 h after cyclophosphamide application), and fixed in buffered neutral formalin. Sections were mounted onto gelatine-coated slides. Rabbit polyclonal antiserum raised against c-Fos (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted in Tris buffered saline containing 0.3% Triton X-100, 2% donkey serum and 1% BSA, and the sections were incubated overnight at room temperature, before being incubated for 2 h with biotinylated donkey antirabbit antibody (1:1000; Amersham Pharmacia Biotech Europe, Freiburg, Germany), for 2 h with avidin-biotin peroxidase complex (1:1000; Vectastain ABC kit, Vector laboratories, Burlingame, CA, USA), and finally revealed with diaminobenzidine via the nickel-enhanced glucose-oxidase method. The procedure also included negative controls with omission of the primary antibody, which did not show any immunoreaction. The images were captured by a digital camera (DS-5 M-L1, Nikon, NY, USA), connected to an optical microscope (Nikon Eclipse 50i) and analysed through the Image NIH Image J 1.36b Software. The number of c-Fos-positive cells was quantified and expressed as the positive area per field (Labrousse et al., 2009). For this series of experiments, we have used five Swiss mice per group.
Immunohistochemistry for F4/80 and P2X7 receptors
Expression of P2X7 purinergic receptors and macrophage migration in bladder tissue were assessed by immunohistochemical analysis. For these experiments, the bladders were collected 6 h after HC induction. Macrophages were quantified in the bladder tissues using the F4/80 rat antimouse macrophage monoclonal antibody (1:100; Serotec Ltd, Oxford, UK), while the expression of P2X7 receptors was determined, using the rabbit anti-P2X7 receptor-purified polyclonal antibody (1:200; Alomone, Jerusalem, Israel, catalogue number APR-004). For the immunostaining study, the general procedures described in the previous section were adopted. For immunohistochemistry, we have used 4–10 Swiss mice depending on the experimental protocol, or 3–4 C57/BL6 or KO mice per group.
Myeloperoxidase (MPO) activity
Neutrophil recruitment to the mouse bladder was measured by means of tissue MPO activity, according to the method described by Passos et al., (2004). The bladders of 5–7 Swiss mice were removed at different time points (1, 2, 4, 6, 8 and 12 h) after cyclophosphamide injection. The tissues were homogenized at 5% (w·v−1) in EDTA/NaCl buffer (pH 4.7) and centrifuged at 4000× g for 15 min, at 4°C. The pellet was resuspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (pH 5.4), and the samples were re-centrifuged (4000× g, 15 min, 4°C). Twenty-five microlitres of the supernatant was used for the MPO assay. The enzymatic reaction was assessed with tetramethylbenzidine 1.6 mM, NaPO4 80 mM and hydrogen peroxide 0.3 mM. The absorbance was measured at 595 nm, and the results are expressed in optical density per milligram of tissue. As the increase in MPO activity peaked at 6 h following cyclophosphamide administration, this time interval was used for additional experiments on MPO activity determination.
Determination of cytokine production
The levels of IL-1β and TNF-α were measured according to the protocol described by Fernandes et al. (2005), with minor adaptations (Fernandes et al., 2005). The animals were treated with cyclophosphamide (300 mg·kg−1, i.p.), and the bladders of six Swiss mice were collected at 4 h. Tissues were placed on PBS containing Tween-20 0.05%, phenylmethylsulphonyl fluoride 0.1 mM, benzethonium chloride 0.1 mM, EDTA 10 mM and aprotinin A 20 KIU, homogenized, centrifuged at 3000×g for 10 min and stored at −70°C until further analysis. Cytokine levels were evaluated using specific elisa kits, according to the manufacturer's recommendations (R&D Systems).
Data analysis
Results are expressed as the mean ± SEM. The percentages of inhibition were determined for each individual experiment. Statistical analysis was performed by one or two way analysis of variance (anova), depending on the experimental protocol, followed by Bonferroni's post hoc test. P-values < 0.05 were considered as significant. All tests were performed using the GraphPad 4 Software (USA).
Materials
The following drugs were used: cyclophosphamide [Genuxa1@200 and Mesna (Mitexan) were from Baxter Oncology Gmbh (Frankfurt, Germany), A-438079 (3-((5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine; Tocris, Bristol, UK]. Hexadecyltrimethyl ammonium bromide and tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO, USA). NaPO4, hydrogen peroxide, NaCl and Tween 20 (all from Merck, Haar, Germany). All the dilutions were made in NaCl 0.9% (saline solution).
Results
Antagonism or absence of P2X7 purinergic receptor decrease nociceptive-related behaviour associated to HC
The i.p. injection of cyclophosphamide (300 mg·kg−1) induced a marked and time-related increase in the nociceptive behaviour score, in either Swiss or C57/BL6 mice, scores that were clearly reduced by pretreating animals with the reference compound Mesna (Figure 1).
Figure 1.
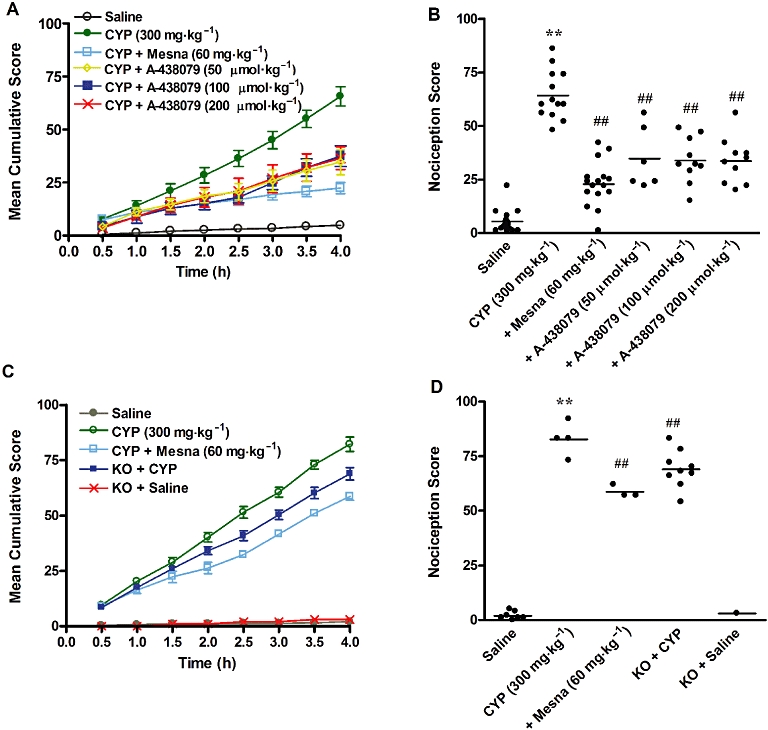
Behavioural scores, assigned at 30 min intervals, over 4 h after injection of cyclophosphamide (CYP). (A) Time course of behavioural scores plotted as cumulative score over the 4 h observation period. (B) Effect of treatment with Mesna (60 mg·kg−1, i.p.) and A-438079 (50, 100 or 200 µmol·kg−1, i.p.) on the nociceptive responses in the model of HC induced by cyclophosphamide in Swiss mice. (C) Time course of behavioural scores plotted as cumulative score over the 4 h observation period. (D) Effect of treatment with Mesna (60 mg·kg−1, i.p.) or effect of P2X7 receptor genetic deletion on the nociceptive responses in cyclophosphamide-induced HC in C57/BL6 mice. Each column represents the mean of 3–10 animals, and the vertical lines show the SEM **P < 0.01 significantly different from saline values; ##P < 0.01 significantly different from control (CYP) values.
Interestingly, systemic administration of the selective P2X7 receptor antagonist A-438079 (50 µmol·kg−1; i.p.) resulted in a significant inhibition of the nociceptive behaviour evoked by cyclophosphamide, as assessed in Swiss mice (Figure 1A 1B). Treatment with higher doses of A-438079, 100 and 200 µmol·kg−1, also significantly reduced the nociceptive response, to the same level as the dose of 50 µmol·kg−1 (Figure 1B). Additionally, P2X7 receptor KO mice displayed a partial reduction of the nociceptive behaviour score (Figure 1D), compared with C57/BL6 mice. Note that either the pharmacological antagonist or the genetic deletion of P2X7 receptors produced significant reductions of cyclophosphamide-induced nociceptive changes between 2.5 and 4 h of evaluation (Figure 1A and C). Furthermore, none of the treatments (A-438079 and Mesna), or genetic deletion of P2X7 receptors, produced significant changes of rearing, walking or general exploring, as assessed by the open-field arena (data not shown).
Antagonism of P2X7 receptors is able to modulate the increased c-Fos expression in central structures of cyclophosphamide-treated mice
As described above, the nociception associated with cyclophosphamide-evoked HC was significantly inhibited by blocking P2X7 receptors. In an attempt to determine whether the effects of A-438079 might involve the modulation of central pathways of pain transmission, we have tested the effects of this antagonist on c-Fos expression in the lumbar spinal cord and the brain cortical areas of mice treated with cyclophosphamide.
The administration of cyclophosphamide increased the levels of c-Fos, in both the spinal cord and the brain, compared with the saline control groups. Interestingly, treatment with A-438079 (100 µmol·kg−1 i.p.) produced a striking decrease of c-Fos levels, in both brain and spinal cord as did treatment with Mesna (60 mg·kg−1) (Figure 2A and B). Representative images of immunostaining for c-Fos are provided in the Figure 2C–J. Regarding the brain, the c-Fos immunoreaction was predominantly located in the cortical areas (panels C to F); for this reason only this area has been considered for the analysis. In the lumbar spinal cord, the immunoreactive c-Fos protein was found mainly in the lateral and the medial dorsal horn, as well as in the sacral parasympathetic nucleus (panels G to J).
Figure 2.
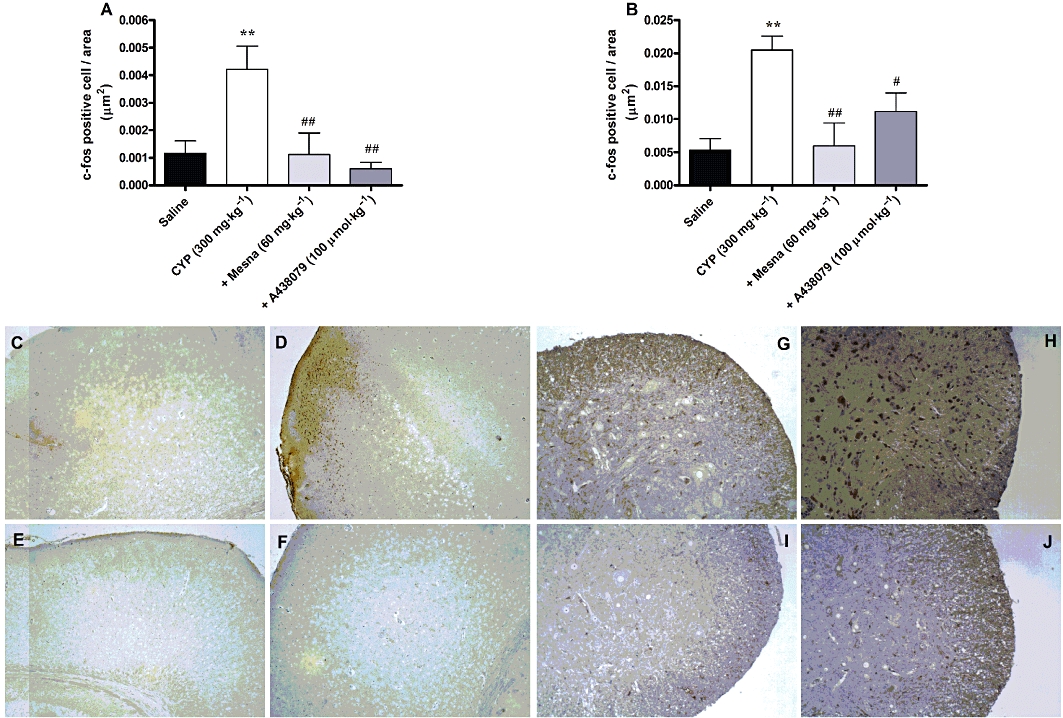
Effect of treatment with Mesna (60 mg·kg−1, i.p.) and A-438079 (100 µmol·kg−1, i.p.) on c-Fos expression in the brain cortical areas (A) and the lumbar spinal cord (B) in the model of HC induced by cyclophosphamide (CYP). Each column represents the mean of 5–8 animals, and the vertical lines show the SEM. **P < 0.01 significantly different from saline values; #P < 0.05 and ##P < 0.01 significantly different from control (CYP) values. (C to F) Representative images of immunostaining for c-Fos in the brain cortical areas into the following groups: (C) saline (D) cyclophosphamide (E) cyclophosphamide plus Mesna (60 mg·kg−1) and (F) cyclophosphamide plus A-438079 (100 µmol·kg−1). (G to J) Representative images of immunostaining for c-Fos in the lumbar spinal cord into the following groups: (G) saline (H) cyclophosphamide (I) cyclophosphamide plus Mesna (60 mg·kg−1) and (J) cyclophosphamide plus A-438079 (100 µmol·kg−1).
Blocking P2X7 purinergic receptors decreases oedema and haemorrhage caused by cyclophosphamide
The indices of oedema and haemorrhage in the cyclophosphamide group were significantly higher than in the saline groups. As well, the inflammatory changes associated with cyclophosphamide-induced HC were similar in Swiss and C57/BL6 mice. From the gross evaluation of the bladders, the positive control drug Mesna (60 mg·kg−1) decreased haemorrhage and the oedema induced by cyclophosphamide, in either the Swiss or the C57/BL6 mouse strains (Figure 3A–F). Treatment with the selective P2X7 receptor antagonist A-438079 (50, 100 and 200 µmol·kg−1) dose-dependently inhibited oedema formation, induced by cyclophosphamide (Figure 3A). However, haemorrhage scores were significantly reduced by A-438079, at 50 µmol·kg−1 and 100 µmol·kg−1, but not by the highest dose (200 µmol·kg−1) (Figure 3B). Oedema in response to cyclophosphamide was significantly reduced in P2X7 receptor KO mice (Figure 3D) but not the haemorrhage score (Figure 3E), although, visually, haemorrhage appeared to be less in KO mice, compared with that in the C57/BL6 mice.
Figure 3.
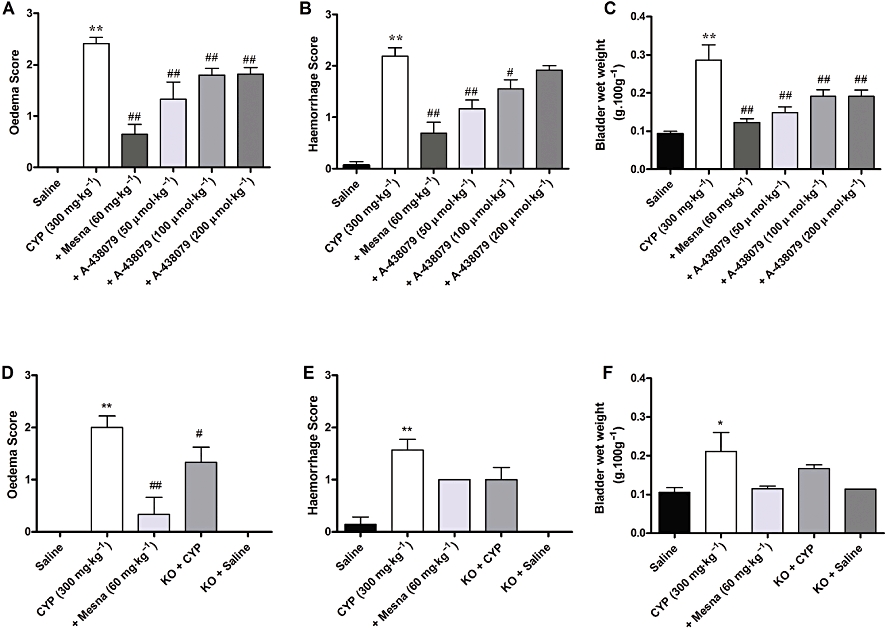
Effect of treatment with Mesna (60 mg·kg−1, i.p.) and A-438079 (50, 100 or 200 µmol·kg−1, i.p.) on macroscopic oedema (A) and haemorrhage (B) evaluation, and on wet weight bladder (C) in the model of HC induced by cyclophosphamide (CYP) in Swiss mice. Effect of treatment with Mesna (60 mg·kg−1, i.p.) or use of knockout P2X7 receptor mice on macroscopic oedema (D) and haemorrhage (E) evaluation, and on wet weight bladder (F) in C57/BL6 mice. Each column represents the mean of 3–10 animals, and the vertical lines show the SEM. *P < 0.05 and **P < 0.01 significantly different from saline values; #P < 0.05 and ##P < 0.01 significantly different from control (CYP) values.
Oedema induced by cyclophosphamide was also assessed by determining the mean empty bladder wet weight, expressed per 100 g of body weight. The treatment with A-438079 (50, 100 and 200 µmol·kg−1) produced a potent reduction of the increased wet bladder weights (Figure 3C). The KO mice showed an apparent reduction of the wet bladder weight, compared with the C57/BL mouse strain, but this effect was not significant (Figure 3F).
Histological changes following cyclophosphamide administration and the effects of P2X7 receptor inhibition
A brief description of the main histological findings is presented below and representative images to each group are provided in Figure 4. Overall, the treatment with A-438079 and genetic deletion of P2X7 receptors partly blocked inflammatory changes in the model of HC. Histologically, the bladder wall appeared thickened in cyclophosphamide-treated mice compared with controls and was associated with partial loss of the urothelium. The remaining urothelial cells in cyclophosphamide-treated mice were abnormally large, when compared with saline controls (Figure 4A and E). Furthermore, histological evaluation showed that submucosal layer was enlarged and presented severe oedema, fibrosis, haemorrhage and inflammatory cell infiltrate in both Swiss and C57/BL6 mice (Figure 4B and F). The animals pretreated with Mesna (60 mg·kg−1) displayed histology close to normal (Figure 4D). The animals treated with A-438079 (Figure 4C), as well as the P2X7 KO mice (Figure 4G) presented moderate mucosal ulceration, and mild to moderate oedema and haemorrhage. The smooth muscle had a normal appearance in all groups.
Figure 4.
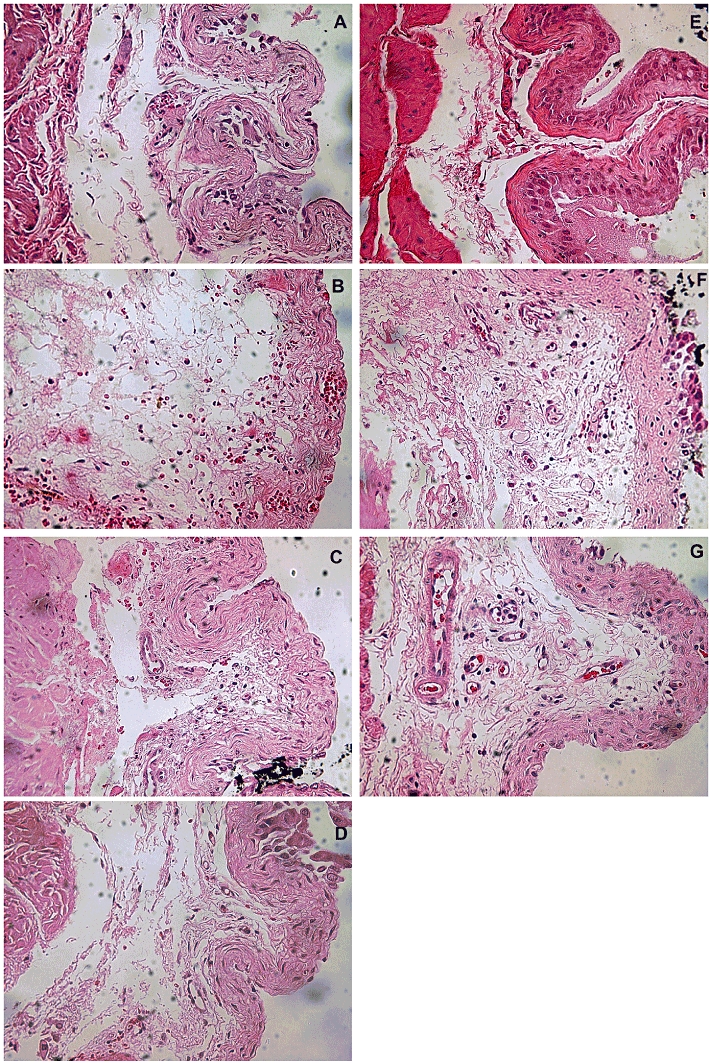
Representative images of histological evaluation in slides stained with haematoxylin and eosin. Saline groups in (A) Swiss and (E) C57/BL6 mouse lineages; cyclophosphamide groups in (B) Swiss and (F) C57/BL6 mouse lineages; animals treated with A-438079 (C), Mesna (D) or P2X7 receptor KO mice (G).
P2X7 receptors and cyclophosphamide-induced macrophage migration
From the immunohistochemical analysis with the macrophage marker F4/80, HC induced by cyclophosphamide was accompanied by the recruitment of macrophages to the bladder tissue, in either Swiss (Figure 5) or C57/BL6 (results not shown) mice. This macrophage migration was visibly reduced by pretreating Swiss mice with the reference compound Mesna (60 mg·kg−1) (Figure 5). Notably, immunoreactive F4/80 was significantly diminished by pretreating Swiss mice with the selective P2X7 receptor antagonist A-438079, at 200 µmol·kg−1 but not by a lower dose 100 µmol·kg−1 (Figure 5). In addition, cyclophosphamide-induced macrophage migration to the bladder was virtually abolished in P2X7 receptor KO mice, although this effect was not statistically significant (results not shown).
Figure 5.
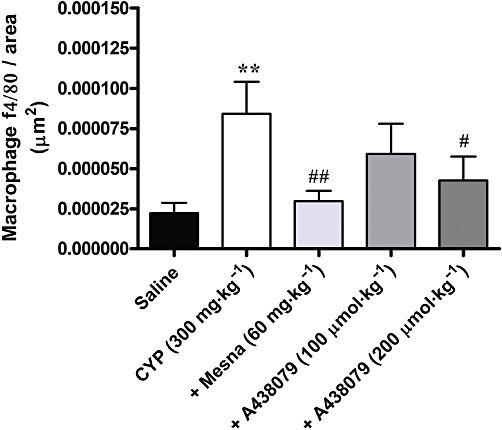
Effect of treatment with Mesna (60 mg·kg−1, i.p.) and A-438079 (100 or 200 µmol·kg−1, i.p.) on macrophage migration in bladder tissue, as determined by immunohistochemistry, in the model of HC induced by cyclophosphamide (CYP) in Swiss mice. Each column represents the mean of 4–10 animals, and the vertical lines show the SEM. **P < 0.01 significantly different from saline values; #P < 0.05 and ##P < 0.01 significantly different from control (CYP) values.
Representative images of data described above are given in the Figure 6, indicating a marked immunoreaction for F4/80 in the bladder submucosal layer of animals in the cyclophosphamide groups (panels B and F), in comparison with saline groups (panels A and E). The immunostaining for F4/80 was visibly diminished in either A-438079 and Mesna-treated animals (panels C and D) or in P2X7 receptor KO mice (panel G).
Figure 6.
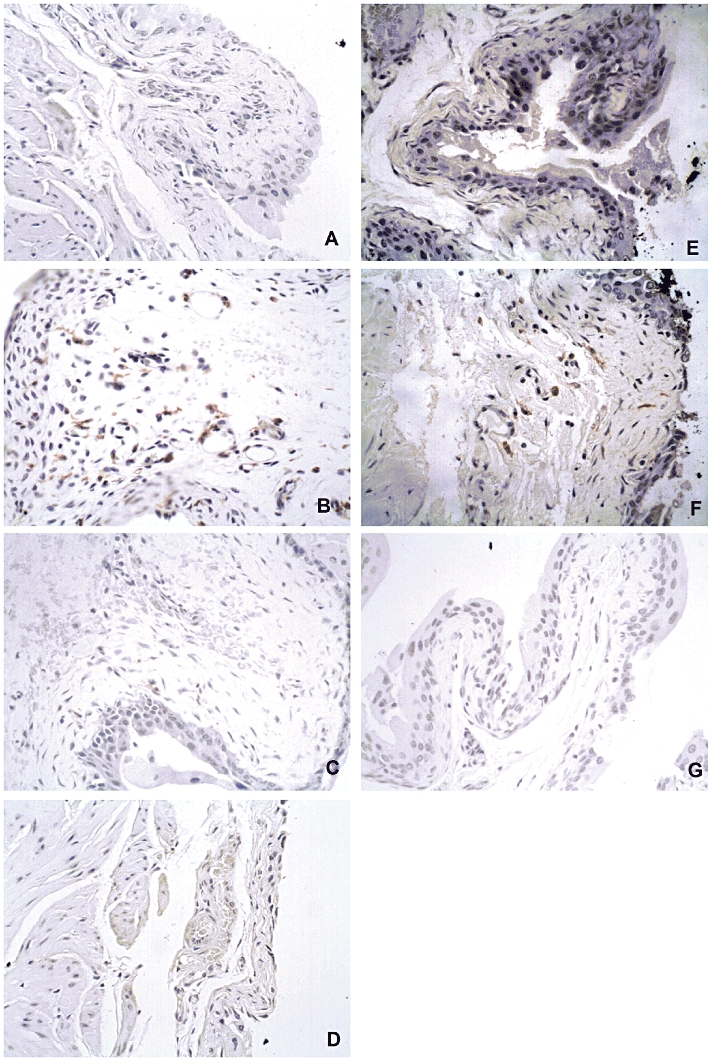
Representative images of immunostaining for the macrophage marker protein F4/80 in the bladder submucosal layer. Saline groups in (A) Swiss and (E) C57/BL6 mouse lineages; cyclophosphamide groups in (B) Swiss and (F) C57/BL6 mouse lineages indicating a marked positive staining for F4/80; animals treated with A-438079 (C), Mesna (D) or P2X7 receptor KO mice (G) displaying diminished immunostaining for F4/80.
Immunoreactive P2X7 receptors are increased following cyclophosphamide treatment
A single i.p. dose of cyclophosphamide (300 mg·kg−1) increased expression of immunoreactive P2X7 receptors in the Swiss mouse bladder, compared with the levels in saline-treated mice (Figure 7A). Representative images demonstrate low immunoreaction for the P2X7 receptor in saline-treated animals (Figure 7B), whereas immunoreactive receptors were markedly increased in the bladder submucosal layer of cyclophosphamide-treated mice (Figure 7C). As expected, no specific immunoreaction was observed in P2X7 receptor KO mice (Figure 7D).
Figure 7.
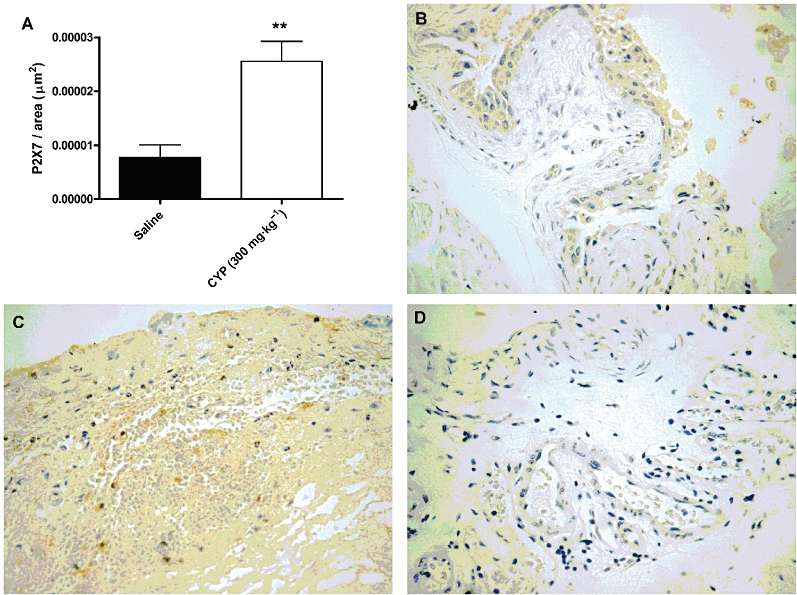
Immunostaining for P2X7 receptors in the model of HC induced by cyclophosphamide. (A) Effect of treatment with cyclophosphamide (CYP) (300 mg·kg−1, i.p.) on P2X7 receptor expression in the submucosal layer of bladder from Swiss mice. Each column represents the mean of six animals, and the vertical lines show the SEM. **P < 0.01 significantly different from saline values. Representative images demonstrate low immunostaining for the P2X7 receptor in saline-treated animals (B), whereas the immunoreaction for this receptor was markedly enhanced in the bladder submucosal layer of cyclophosphamide-treated mice (C), and it was absent in P2X7 receptor KO mice (D).
Inhibition of MPO activity by P2X7 receptor antagonism
Cyclophosphamide administration was also associated with a marked migration of neutrophils, as indicated by the increase of MPO activity in the bladder tissue, compared with the saline group. The increase of MPO activity induced by cyclophosphamide was similarly reduced by the treatment with Mesna (60 mg·kg−1) and A-438079 (100 µmol·kg−1) (Figure 8A). No significant differences were observed between treatments with Mesna and A-438079.
Figure 8.
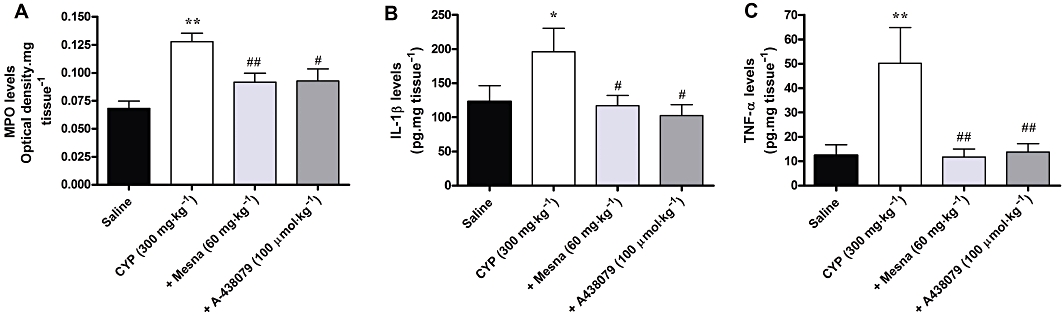
Effect of treatment with Mesna (60 mg·kg−1, i.p.) and A-438079 (100 µmol·kg−1, i.p.) on increased MPO activity (A) and on generation of IL-1β (B) and TNF-α (C) in the model of HC induced by cyclophosphamide in Swiss mice. Each column represents the mean of 5–7 animals, and the vertical lines show the SEM. *P < 0.05 and **P < 0.01 significantly different from saline values; #P < 0.05 and ##P < 0.01 significantly different from control (CYP) values.
Evaluation of cytokine levels in the bladder
TNF-α and IL-1β production was assessed in the bladder tissue 4 h after cyclophosphamide and disclosed a marked increase of both IL-1β (Figure 8B) and TNF-α (Figure 8C) levels. Interestingly, treatment with Mesna (60 mg·kg−1) or A430879 (100 µmol·kg−1) reversed the increased production of IL-1β and TNF-α, to the basal levels (Figure 8). Again, no significant differences were observed between treatments with Mesna and A-438079.
Discussion
The toxic metabolite of cyclophosphamide, acrolein, induces HC by extended contact with the bladder epithelium, causing cellular damage by promoting the release of several inflammatory mediators, including purine nucleotides. Increased release of ATP from the bladder urothelial cells has been associated with interstitial cystitis, and also occurs in response to bladder stretch or distension (Namasivayam et al., 1999; Sun and Chai, 2006). P2X7 purinergic receptors, known to be activated by elevated ATP concentrations, are constitutively expressed in the bladder tissue under normal conditions (O'Reilly et al., 2001a,b; Birder et al., 2004) and are up-regulated in the urinary bladder of patients with symptomatic outlet obstruction (O'Reilly et al., 2001a,b;). However, further studies are still required in order to determine whether these receptors might be involved in other pathological conditions of the bladder, such as cyclophosphamide-induced HC.
The present study brings new evidence on the pathological role of P2X7 receptors, by demonstrating, for the first time, that either pharmacological blockade or the genetic deletion of this receptor prevented the nociceptive and inflammatory events associated with HC evoked by cyclophosphamide treatment in mice. Furthermore, our data revealed that cyclophosphamide-induced HC was likely to be accompanied by increased expression of P2X7 receptors, supporting the relevance of this receptor in HC.
Our experimental model of HC induced by cyclophosphamide can be used to evaluate visceral pain in both rats and mice (Gray et al., 1986; Olivar and Laird, 1999; Bon et al., 2003; Batista et al., 2006; Wantuch et al., 2007). Pain is the principal symptom in cystitis and could be associated with purinergic signalling changes, for example, stretching-induced ATP release, as well as the up-regulation of purinergic receptors (Tempest et al., 2004). Relevantly, P2X7 receptors have been investigated as a target of new analgesic drugs and these receptors play a pivotal role in the onset and persistence of certain types of inflammatory and neuropathic pain in rodents (Honore et al., 2006; Donnelly-Roberts and Jarvis, 2007; King, 2007; McGaraughty et al., 2007). Our results revealed that systemic treatment of mice with the selective P2X7 receptor antagonist A-438079 clearly prevented the nociceptive changes related to cyclophosphamide-evoked HC, with efficacy comparable to that of the reference compound Mesna. Furthermore, the nociceptive behavioural changes elicited by cyclophosphamide administration were also diminished in P2X7 receptor KO mice. Neither the pharmacological antagonism nor the genetic deletion of P2X7 receptors, affected the general locomotor activity of mice in the open-field test, discounting possible non-specific central effects of P2X7 receptor inhibition. A similar result was obtained from earlier experiments showing that P2X7 KO mice displayed antidepressant behaviour in the tail suspension and forced swimming tests, without changing spontaneous locomotor activity (Basso et al., 2009). Therefore, it is feasible to suggest that P2X7 receptors play a relevant role in HC-related painful alterations, and selective antagonists for this receptor might be attractive options for treating clinical pain in cystitis.
The analgesic effects of P2X7 receptor inhibition could reflect the modulation of such receptors expressed in the tissue (O'Reilly et al., 2001a,b; Birder et al., 2004) or at peripheral sensory nerve endings, in the bladder. Alternatively, analgesia mediated by P2X7 receptors could involve the modulation of central pathways of pain transmission, as these receptors are highly expressed in the spinal cord (Cotrina and Nedergaard, 2009). Hence, we tested the effects of A-438079 on c-Fos expression in the lumbar spinal cord and the brain cortical areas in our HC model. Immunohistochemical analysis demonstrated that c-Fos staining was markedly increased in either the lumbar spinal cord or the brain cortex of mice that received cyclophosphamide and this immunolabelling for c-Fos, in both anatomical structures, was markedly inhibited by A-438079, given i.p., indicating that systemic administration of this antagonist modulated pain processing at central levels. Several earlier studies have used the expression of the immediate early gene c-Fos as a marker for postsynaptic activation of spinal cord neurons receiving afferent inputs from the bladder. The number of c-Fos immunoreactive neurons increased following injury of the lower urinary tract (Morais et al., 1999; Vizzard, 2000a,b; Nickel, 2002). Of interest, antagonism of the P2X7 receptor, via selective ligands, modulated central sensitization and produced anti-nociception in animal models of nociception (McGaraughty et al., 2007). Our data from c-Fos immunostaining reinforce the potential of P2X7 receptor antagonists as treatments for pain related to cystitis.
Damage to bladder tissue following chemotherapy with cyclophosphamide is related to exacerbated inflammatory alterations induced by acrolein (Namasivayam et al., 1999; Sun and Chai, 2006). In the present study, we have also assessed to what extent the interference with P2X7 receptors might affect the inflammatory changes evoked by cyclophosphamide administration. As reported earlier (Olivar and Laird, 1999; Santos et al., 2010), a single treatment with cyclophosphamide increased oedema and haemorrhage, measured by either gross or histological evaluation. Notably, the grade of oedema and bleeding was markedly reduced in mice pretreated with the selective P2X7 receptor antagonist A-438079, or even in animals with genetic P2X7 receptor deletion, to an extent comparable to that of the clinically used compound, Mesna. We could therefore conclude that the nociceptive and inflammatory changes associated with cyclophosphamide-induced HC involved the activation of P2X7 receptors. Additionally, we can also hypothesize that analgesic effects observed for A-438079 are mediated, at least partially, by modulation of the inflammatory responses. Indeed, P2X7 receptors are well-recognized regulators of inflammation, as they are involved in the production of pro-inflammatory cytokines, such as IL-1β, leading to the up-regulation of cyclooxygenase-2, metalloproteinases, inducible NO synthase and superoxide production (Labasi et al., 2002; Chessell et al., 2005; Di Virgilio, 2007; Donnelly-Roberts and Jarvis, 2007; Grassi, 2010). Furthermore, supporting our data, a recent study conducted by Riteau et al. (2010) demonstrated that P2X7 receptor KO mice displayed a marked reduction of lung inflammation in a murine model of lung fibrosis.
The P2X7 receptor has been implicated in peripheral macrophage and glial activation and in neutrophil infiltration (Labasi et al., 2002; Chessell et al., 2005; Burnstock, 2006; 2011; Di Virgilio, 2007; Donnelly-Roberts and Jarvis, 2007; King, 2007; Yoon et al., 2007). It was therefore likely that inhibition of P2X7 receptors would modulate inflammatory cells in cyclophosphamide-induced HC. Our results clearly demonstrate that cyclophosphamide markedly increased macrophage infiltration to the urinary bladder, as assessed by immunohistochemical evaluation of F4/80 staining. This protein F4/80 has been considered one of the best macrophage markers to date, as this monoclonal antibody was found to detect antigens present exclusively on mononuclear phagocytes (Khazen et al., 2005; Maus et al., 2006). Interestingly, this parameter was markedly reduced by A-438079 pretreatment or in P2X7 receptor KO mice. Allied to our data, Gonçalves and colleagues (2006) demonstrated that P2X7 receptor KO mice presented a notable reduction of interstitial macrophage migration, in an experimental model of unilateral urethral obstruction. Thus, it is possible to suggest that P2X7 receptors are likely to be implicated in the mechanisms of macrophage influx and/or proliferation in inflammatory diseases of the urinary tract.
Myeloperoxidase is an enzyme contained within the primary granules of neutrophils and the augmented activity of this enzyme represents an indirect measure of neutrophil migration (Passos et al., 2004). It has been previously shown that purinergic signalling is essential for neutrophil migration (Chen et al., 2010). Our work extends this evidence by demonstrating that increased MPO activity following cyclophosphamide administration was prevented by pretreating animals with the selective P2X7 receptor antagonist A-438079. These results clearly indicate that both macrophage and neutrophil migration in our experimental model of bladder inflammation were broadly dependent on the activation of P2X7 receptors.
It is well known that P2X7 receptors are involved in the maturation and the release of IL-1β in peripheral macrophages and microglia, via pannexin-1 hemichannels (Labasi et al., 2002; Chessell et al., 2005; Di Virgilio, 2007; Donnelly-Roberts and Jarvis, 2007; Iglesias et al., 2008; Grassi, 2010). Activation of P2X7 receptors by the selective agonist BzATP stimulated production of IL-1β in macrophages obtained from wild type, but not from P2X7 receptor KO mice (Basso et al., (2009)). As well, A-438079 inhibited LPS-induced IL-1β release from human acute monocytic leukaemia cell line macrophages (Nelson et al., 2006). A-438079 decreased carrageenan-induced mechanical hyperalgesia in rats, by interfering with the release of IL-6, TNF-α and the chemokine cytokine-induced neutrophil chemoattractant-1, although the production of IL-1β was not affected(Teixeira et al. (2010)) In the experimental model of HC elicited by cyclophosphamide, treatment of mice with the selective P2X7 receptor A-438079 virtually brought the production of the pro-inflammatory cytokines IL-1β and TNF-α to the basal levels. It is tempting to surmise that A-438079 was able to interfere with cytokine production by indirectly modulating leukocyte migration/activation, or even by blocking cytokine release from endothelial or bladder smooth muscle cells. Furthermore, we might also infer that part of the antinociceptive actions observed for A-438079 are likely to be mediated by decreased IL-1β and TNF-α generation, as both cytokines are strongly implicated in sensitization of primary afferent nociceptors. Supporting this idea, the antinociceptive effects displayed by the selective P2X7 receptor antagonist A-839977 were diminished in IL-1αβ KO mice, in a model of chronic inflammation (Honore et al., 2009).
Our present data, obtained from pharmacological antagonism or the genetic deletion of P2X7 receptors, clearly show the relevance of these receptors to cyclophosphamide-induced cystitis. The antagonist we have used is highly potent and selective in blocking the activation of human, rat and mouse P2X7 receptors (Donnelly-Roberts et al., 2009). Additionally, although genetically modified animals might exhibit compensatory changes in signalling transduction, KO mice (including the P2X7) generally represent a good tool to confirm and extend functional data using pharmacological approaches (Chessell et al., 2005). To gain further insight on the relevance of P2X7 receptors in our experimental model, we have also investigated whether a single dose of cyclophosphamide would modulate the expression of P2X7 receptors in the bladder tissue. Immunohistochemical analysis showed about threefold up-regulation of P2X7 receptors in the urinary bladder of mice with cyclophosphamide-induced HC. The immunostaining observed in our study is likely to be specific, as P2X7 receptor KO mice did not exhibit immunoreaction. Relevantly, in our model, the immunoreactive P2X7 receptors were mainly in the submucosal layer of bladders, suggesting that receptor up-regulation probably takes place in immunological cells, for instance macrophages and neutrophils. An increased neuronal expression of P2X7 receptors was recently described in an experimental model of multiple sclerosis, at the peak of neurological symptoms, which is in accord with our results (Grygorowicz et al., 2010).
The results of the present study extend earlier data, showing for the first time that P2X7 purinergic receptors are implicated in the inflammatory process of cyclophosphamide-induced HC, probably by increasing macrophage and neutrophil migration, and generating pro-inflammatory cytokines, which in turn might directly activate peripheral and central nociceptive pathways, or facilitate nociceptive processing. Hence, P2X7 receptors would represent attractive targets for treating the symptoms related to severe HC in patients under chemotherapy with cyclophosphamide. However, the relevance of other P2 receptor subtypes in this model remains to be investigated.
Acknowledgments
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and the Programa de Apoio aos Núcleos de Excelência (PRONEX), Brazil. JPM is a postgraduate student in Medicine and Health Sciences (PUCRS), supported by the Institution (PROBOLSAS Program/PUCRS). RBMS is an undergraduate Pharmacy student holding a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (PIBIC, CNPq, Brazil).
Glossary
Abbreviations
- HC
haemorrhagic cystitis
- KO
knockout
- Mesna
2-mercaptoethane sulfonate
- MPO
myeloperoxidase
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Batista CK, Brito GA, Souza ML, Leitao BT, Cunha FQ, Ribeiro RA. A model of hemorrhagic cystitis induced with acrolein in mice. Braz J Med Biol Res. 2006;39:1475–1481. doi: 10.1590/s0100-879x2006001100011. [DOI] [PubMed] [Google Scholar]
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Therapeutic potential of purinergic signalling for diseases of the urinary tract. BJU Int. 2011;107:192–204. doi: 10.1111/j.1464-410X.2010.09926.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol. 2006;26:499–513. doi: 10.1615/critrevimmunol.v26.i6.30. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cheuk DK, Lee TL, Chiang AK, Ha SY, Lau YL, Chan GC. Risk factors and treatment of hemorrhagic cystitis in children who underwent hematopoietic stem cell transplantation. Transpl Int. 2007;20:73–81. doi: 10.1111/j.1432-2277.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Chow YC, Yang S, Huang CJ, Tzen CY, Huang PL, Su YH, et al. Epinephrine promotes hemostasis in rats with cyclophosphamide-induced hemorrhagic cystitis. Urology. 2006;67:636–641. doi: 10.1016/j.urology.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chow YC, Yang S, Huang CJ, Tzen CY, Su YH, Wang PS. Prophylactic intravesical instillation of epinephrine prevents cyclophosphamide-induced hemorrhagic cystitis in rats. Exp Biol Med (Maywood) 2007;232:565–570. [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocitto LE, Simpson JF, Wilson TG. Bladder augmentation in the prevention of cyclophosphamide-induced haemorrhagic cystitis in the rat model. Br J Urol. 1996;78:530–533. doi: 10.1046/j.1464-410x.1996.01146.x. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR. Go it alone no more–P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol. 2007;72:1402–1405. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Passos GF, Campos MM, De Souza GE, Fittipaldi JF, Pesquero JL, et al. Cytokines and neutrophils as important mediators of platelet-activating factor-induced kinin B1 receptor expression. Br J Pharmacol. 2005;146:209–216. doi: 10.1038/sj.bjp.0706327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Giraud G, Bogdanovic G, Priftakis P, Remberger M, Svahn BM, Barkholt L, et al. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica. 2006;91:401–404. [PubMed] [Google Scholar]
- Gonçalves RG, Gabrich L, Rosario A, Takiya CM, Ferreira ML, Chiarini LB, et al. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006;70:1599–1606. doi: 10.1038/sj.ki.5001804. [DOI] [PubMed] [Google Scholar]
- Grassi F. Purinergic control of neutrophil activation. J Mol Cell Biol. 2010;2:176–177. doi: 10.1093/jmcb/mjq014. [DOI] [PubMed] [Google Scholar]
- Gray KJ, Engelmann UH, Johnson EH, Fishman IJ. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J Urol. 1986;136:497–500. doi: 10.1016/s0022-5347(17)44929-9. [DOI] [PubMed] [Google Scholar]
- Grygorowicz T, Struzynska L, Sulkowski G, Chalimoniuk M, Sulejczak D. Temporal expression of P2X7 purinergic receptor during the course of experimental autoimmune encephalomyelitis. Neurochem Int. 2010;57:823–829. doi: 10.1016/j.neuint.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, et al. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, et al. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazen W, M'bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, et al. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579:5631–5634. doi: 10.1016/j.febslet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- King BF. Novel P2X7 receptor antagonists ease the pain. Br J Pharmacol. 2007;151:565–567. doi: 10.1038/sj.bjp.0707266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, Mccurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, et al. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS ONE. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI. The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:1906–1913. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Manikandan R, Kumar S, Dorairajan LN. Hemorrhagic cystitis: a challenge to the urologist. Indian J Urol. 2010;26:159–166. doi: 10.4103/0970-1591.65380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Suarez-Boomgaard D, Sanchez-Reina MD, Aguirre JA, Yoshitake T, Yoshitake S, et al. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson's disease: studies with the P2X(7) receptor antagonist A-438079. J Neural Transm. 2010;117:681–687. doi: 10.1007/s00702-010-0400-0. [DOI] [PubMed] [Google Scholar]
- Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–235. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- Mcgaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, et al. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Morais MM, Belarmino-Filho JN, Brito GA, Ribeiro RA. Pharmacological and histopathological study of cyclophosphamide-induced hemorrhagic cystitis – comparison of the effects of dexamethasone and Mesna. Braz J Med Biol Res. 1999;32:1211–1215. doi: 10.1590/s0100-879x1999001000006. [DOI] [PubMed] [Google Scholar]
- Namasivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Neheman A, Nativ O, Moskovitz B, Melamed Y, Stein A. Hyperbaric oxygen therapy for radiation-induced haemorrhagic cystitis. BJU Int. 2005;96:107–109. doi: 10.1111/j.1464-410X.2005.05577.x. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, et al. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Interstitial cystitis: characterization and management of an enigmatic urologic syndrome. Rev Urol. 2002;4:112–121. [PMC free article] [PubMed] [Google Scholar]
- O'reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Mcmahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001a;87:617–622. doi: 10.1046/j.1464-410x.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- O'reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, et al. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001b;165:1730–1734. [PubMed] [Google Scholar]
- Olivar T, Laird JM. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. Eur J Pain. 1999;3:141–149. doi: 10.1053/eujp.1998.0105. [DOI] [PubMed] [Google Scholar]
- Passos GF, Fernandes ES, Campos MM, Araujo JG, Pesquero JL, Souza GE, et al. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol. 2004;172:1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- Santos AA, Jr, Leal PC, Edelweiss MI, Lopes TG, Calixto JB, Morrone FB, et al. Effects of the compounds MV8608 and MV8612 obtained from Mandevilla velutina in the model of hemorrhagic cystitis induced by cyclophosphamide in rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:399–407. doi: 10.1007/s00210-010-0555-0. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27–C34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Teixeira JM, Oliveira MC, Parada CA, Tambeli CH. Peripheral mechanisms underlying the essential role of P2X7 receptors in the development of inflammatory hyperalgesia. Eur J Pharmacol. 2010;644:55–60. doi: 10.1016/j.ejphar.2010.06.061. [DOI] [PubMed] [Google Scholar]
- Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344–1348. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- Traxer O, Desgrandchamps F, Sebe P, Haab F, Le Duc A, Gattegno B, et al. [Hemorrhagic cystitis: etiology and treatment] Prog Urol. 2001;11:591–601. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R1027–R1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000b;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Wantuch C, Piesla M, Leventhal L. Pharmacological validation of a model of cystitis pain in the mouse. Neurosci Lett. 2007;421:250–252. doi: 10.1016/j.neulet.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Wong TM, Yeo W, Chan LW, Mok TS. Hemorrhagic pyelitis, ureteritis, and cystitis secondary to cyclophosphamide: case report and review of the literature. Gynecol Oncol. 2000;76:223–225. doi: 10.1006/gyno.1999.5680. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Lee HJ, Lee YS, Kim JH, Park JK, Chang WK, et al. Extracellular ATP is involved in the induction of apoptosis in murine hematopoietic cells. Biol Pharm Bull. 2007;30:671–676. doi: 10.1248/bpb.30.671. [DOI] [PubMed] [Google Scholar]


