Abstract
BACKGROUND AND PURPOSE
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is an active component of many herb-based laxatives. However, its mechanism of action is unclear. The aim of the present study was to investigate the role of mast cells and enteric neurons in emodin-induced ion secretion in the rat colon.
EXPERIMENTAL APPROACH
Short-circuit current (ISC) recording was used to measure epithelial ion transport. A scanning ion-selective electrode technique was used to directly measure Cl- flux (JCl−) across the epithelium. RIA was used to measure emodin-induced histamine release.
KEY RESULTS
Basolateral addition of emodin induced a concentration-dependent increase in ISC in colonic mucosa/submucosa preparations, EC50 75 µM. The effect of emodin was blocked by apically applied glibenclamide, a Cl- channel blocker, and by basolateral application of bumetanide, an inhibitor of the Na+-K+-2Cl- cotransporter. Emodin-evoked JCl− in mucosa/submucosa preparations was measured by scanning ion-selective electrode technique, which correlated to the increase in ISC and was significantly suppressed by glibenclamide and bumetanide. Pretreatment with tetrodotoxin and the muscarinic receptor antagonist atropine had no effect on emodin-induced ΔISC in mucosa-only preparations, but significantly reduced emodin-induced ΔISC and JCl− in mucosa/submucosa preparations. The COX inhibitor indomethacin, the mast cell stabilizer ketotifen and H1 receptor antagonist pyrilamine significantly reduced emodin-induced ΔISC in mucosa and mucosa/submucosa preparations. The H2 receptor antagonist cimetidine inhibited emodin-induced ΔISC and JCl− only in the mucosa/submucosa preparations. Furthermore, emodin increased histamine release from the colonic mucosa/submucosa tissues.
CONCLUSIONS AND IMPLICATIONS
The results suggest that emodin-induced colonic Cl- secretion involves mast cell degranulation and activation of cholinergic and non-cholinergic submucosal neurons.
Keywords: emodin, ion secretion, colon, submucosal plexus, short-circuit current, Cl- flux, scanning ion-selective electrode technique, mast cell, histamine, PGs
Introduction
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a naturally occurring anthraquinone present in rhubarb and numerous other plants. Besides being used as a laxative, emodin has many other biological effects, including antibacterial (Hatano et al., 1999), antiviral (Sydiskis et al., 1991), anti-inflammatory (Goel et al., 1991) and anticancer (Lee et al., 2005). Emodin also plays an important role in brain protection against severe cerebral injury (Gu et al., 2000; Huang et al., 2005). In the gastrointestinal tract, emodin stimulates smooth muscle contraction (Ma et al., 2004) and enhances peristalsis activity (Zhang et al., 2005). Emodin stimulates intestinal smooth muscle contraction by evoking ACh release, which increases intracellular Ca2+ concentration (Zhang et al., 2006; Zheyu et al., 2006) by activating muscarinic receptors (Huang et al., 1991; Ali et al., 2004). Aloe-emodin anthrone, a decomposition product of barbaloin by bacteria in the large intestine, has been found to cause an increase in intestinal water content and mucus secretion, which may contribute to barbaloin-induced diarrhoea (Sydiskis et al., 1991). Furthermore, aloe-emodin anthrone and rhein anthrone exert synergistic purgative effects in mice, as a result of synergistic stimulation of large intestinal transit and water secretion (Sydiskis et al., 1991). The ethanol extract of the rhubarb root, which contains emodin, has been shown to augment ion secretion in the rat ileal epithelia (Ali et al., 2004). We have previously reported that high purity emodin (>98.1%) stimulates rat colonic epithelial ion secretion, which is predominantly mediated by endogenous PG release (Xu et al., 2007). In addition, aloe-emodin anthrone enhances intestinal epithelial permeability (Kai et al., 2002).
The gastrointestinal mucosa contain mast cells that account for 2–3% of lamina propria cells. Mast cells play an important role in the regulation of normal gastrointestinal functions, such as gastric acid secretion, smooth muscle contraction, peristalsis, epithelial ion and mucus secretion. In addition, mast cells are an important defense line of the intestine against foreign invasion at a vulnerable interface between the body and the outside environment (Wood, 2004). Upon stimulation, mast cells release preformed and newly synthesized mediators into the extracellular space surrounding the enteric neurons and activate the defence system stored in the enteric nervous system designed to eliminate the threat from the intestinal lumen (Wood, 2004). This system involves large quantities of fluid being secreted to power the propulsion of the luminal contents from the body (Wood, 2004). Histamine, a major inflammatory mediator derived from mast cells, increases intestinal ion secretion by activating H1 receptors on epithelial cells (Wang et al., 1990b; Schultheiss et al., 2006) and the H2 receptors on submucosal neurons (Wang and Cooke, 1990; Frieling et al., 1994; Cooke et al., 1995). Emodin has been reported to stimulate histamine release from rat isolated peritoneal mast cells (Kai et al., 2002) and increase histamine levels in intestinal mucosa in rats with intestinal obstruction (Lin et al., 1992). In the present study, we investigated the role of mast cells and the enteric nervous system in emodin-induced colonic ion secretion.
Methods
Emodin extraction and identification
The polygonum multiflorum rhubarb roots were purchased from Beijing-tongrentang, Beijing, China. As described previously (Xu et al., 2007), the air-dried roots were powdered and extracted with 80% ethanol. The combined solution was concentrated to afford a residue. After dissolving the residue in water, the aqueous solution was extracted with n-butanol. The extract was concentrated and chromatographed on a silica gel column using ether acetate (EtOAc) (10:1–0:10), to give nine fractions. Fraction 3 was re-chromatographed on a silica gel column using EtOAc (7:1) as eluent to yield the product as a yellow needle crystal, which was identified by comparing the data with those of an authentic sample. The purity of the emodin extract was 98.1% as determined by HPLC. The HPLC conditions include an YMC C18 column, a mixture of methanol and 0.1% phosphoric acid (85:15) as the mobile phase, and uv detection at 254 nm.
Reagents
Amiloride hydrochloride, tetrodotoxin (TTX), glibenclamide, bumetanide, atropine, cimetidine, ranitidine, pyrilamine and ketotifen were from Sigma (St. Louis, MO, USA). The stock solutions of some chemicals were prepared in dimethylsulphoxide (DMSO). Final concentrations of DMSO were less than 0.1% (v/v). Preliminary experiments indicated that the vehicle did not alter any baseline electrophysiological parameters.
Solutions
Krebs-Henseit solution (K-HS) had the following composition (mM): NaCl, 117; KCl, 4.5; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 24.8; KH2PO4, 1.2; glucose, 11.1; pH 7.4. In Cl--free solution, NaCl, KCl and CaCl2 were replaced by sodium gluconate, potassium gluconate and calcium gluconate, respectively.
Tissue preparation
Animal protocols were approved by Animal Care and Use Committee of Capital Medical University and conformed to NIH guidelines. Adult male Sprague-Dawley rats (Laboratory Animal Services Center, Capital Medical University) ranging in weight from 200 to 300 g had free access to standard rodent laboratory food and water until the day of the experiments. The animals were killed by CO2 inhalation followed by cervical dislocation. The distal colonic segment about 7 cm proximal to the lymph node (typically situated 3 cm away from the anus) was quickly removed and placed in K-HS. The distal colon was divided into four segments, which were cut along the mesenteric border into a flat sheet and flushed with ice-cold K-HS. The tissue was pinned flat with the mucosal side down in a Sylgard-lined Petri dish containing ice-cold oxygenated K-HS solution. The serosa and muscularis externa were carefully stripped away by blunt dissection to obtain a mucosa/submucosa preparation. In some tissues, the submucosa layer was also stripped away to obtain the mucosa-only preparations.
Short-circuit current measurement
The short-circuit current (ISC) was measured in vitro in Ussing chambers. Flat sheets of colonic mucosa-only or mucosa/submucosa preparations were mounted between the two halves of modified Ussing chambers, in which the total cross-sectional area was 0.5 cm2. The mucosal and serosal surfaces of the preparation were bathed with 5 mL K-HS by recirculating from a reservoir maintained at 37°C during the experiments. The K-HS was continuously oxygenated with 95% O2 and 5% CO2. Drugs were added either to the apical or basolateral side of the mucosa. Responses were continuously recorded using the Acquire & Analyze data acquisition system (Physiologic Instruments, San Diego, CA, USA). Transepithelial potential difference for each preparation was measured by the Ag/AgCl reference electrodes (Physiologic Instruments, P2020S) connected to a preamplifier that was in turn connected to a voltage-clamp amplifier VCC MC6 (Physiologic Instruments). The changes in ISC were calculated on the basis of the values before and after drug application and were normalized as current per unit area of epithelium (µA·cm−2). The current change in response to applied potential was used to calculate the transmural resistance of the preparation by Ohm's Law. Experiments were repeated in different batches of tissues to ensure that the data were reproducible. Positive ISC corresponds to the movement of anion from the serosal to mucosal compartments or movement of cation from the mucosal to serosal compartments or a combination of both.
Measurement of Cl- flux
Measurement of Cl- flux (JCl−) was performed using the scanning ion-selective electrode technique (SIET) (BIO-IM, Younger USA Sci. & Tech. Co., Amherst, MA, USA; Applicable Electronics Inc., Forestdale, MA, USA; and ScienceWares Inc., East Falmouth, MA, USA). The Cl- microelectrode was placed to the basolateral side of the preparation and was controlled to move with an excursion of 10 µm at a programmable frequency in the range of 0.3–0.5 Hz. This minimized the mixture of microelectrode fluid with the bathing solution. To construct the microelectrodes, silanized borosilicate micropipettes (2–4 µm aperture, XY-Cl-08, Xuyue Science and Technology Co., Ltd, Beijing, China) were back-filled with 100 mM KCl and front-filled with a 30 mm column of chloride ionophore I–cocktail A (Fluka 24902, Younger USA Sci. & Tech. Corp.). A Ag/AgCl wire electrode holder (XYEH01-1; Xuyue Sci. & Tech. Co., Ltd) was inserted in the back of the electrode to make electrical contact with the electrolyte solution. Only electrodes with Nernstian slopes >56 mV were used. JCl− was calculated by Fick's Law of diffusion: J0=−[D × (dC/dX)]; where J0 represents the net JCl− (in nmol·cm−2·s−1), D is the self-diffusion coefficient for Cl- (in cm2·s−1), dC is the difference of Cl- concentrations between the two positions and dX is the 10 µm excursion for the tissue over which the electrode moved in the experiments. Data and image acquisition, preliminary processing, control of the three-dimensional electrode position and stepper-motor-controlled fine focus of the microscope stage were performed with the Automated Scanning Electrode Technique software (Younger USA Science & Technology Company, USA).
Quantification of histamine levels
The amount of histamine released by the distal colonic segments in vitro was evaluated as previously described (Eutamene et al., 1998). Segments of distal colon (about 150 mg each) were equilibrated in 1 mL oxygenated Kreb's solution at 37°C for 30 min. The tissues were then incubated with vehicle (0.9% NaCl) or emodin (10 and 100 µM) for another 30 min. At the end of the incubation, the tubes were vortex mixed and were immediately put on ice to minimize the breakdown of histamine. The supernatant was aliquoted and frozen at −20°C for the analysis of histamine content as a measure of histamine release. The tissue pieces at the bottom of each tube were blot dried, weighed and frozen in liquid nitrogen. The tissues were then homogenized in saline (0.9% NaCl) on ice and centrifuged (10 000× g, 5 min). Histamine levels in the supernatant and the tissues were measured through a commercial RIA kit (Beijing Sinouk Institute of Biological Technology, Beijing, China). Histamine level was expressed in ng·mg−1 of tissue.
Statistical analysis
All values are expressed as mean and SEM; n is the number of animals in each experiment. EC50 and IC50 were calculated using the GraphPad Prism software 4.0 package. The increase in ISC was quantified by subtracting the peak of an ISC response from its respective baseline value before drug administration. The statistical differences between control and treatment means were analysed using Student's paired or unpaired t-test when appropriate. The differences among groups were analysed using a one-way anova followed by Dunnett's multiple comparison. A P-value less than 0.05 was considered statistically significant.
Results
Emodin-induced ISC responses
Emodin applied to the basolateral side of the colonic mucosa/submucosa preparations evoked an increase in ISC (Figure 1A), which was similar to that observed in the colonic mucosa-only preparations (Xu et al., 2007). Mucosal addition of emodin produced only a small increase in ISC (Figure 1A). Thus, in the following experiments, emodin was applied to the basolateral side of the preparations. The response to emodin was concentration-dependent, with an EC50 of 75.0 µM (n= 6) (Figure 1B). When added at 100 µM, emodin produced an increase in ISC from 21.20 ± 3.90 µA·cm−2 to 81.86 ± 12.91 µA·cm−2 (n= 20, P < 0.01), which lasted about 25 min. The emodin (100 µM)-induced ISC response was accompanied by a 16.2% decrease in transmural resistance, from 55.06 ± 5.65 Ω.cm2 (n= 15) to 46.12 ± 5.40 Ω.cm2 (n= 16, P < 0.05).
Figure 1.
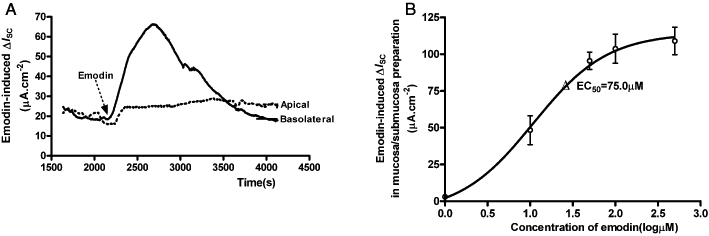
Emodin-evoked increase in ISC of the mucosa/submucosa preparations of the rat distal colon. (A) Addition of emodin (100 µM) to the basolateral side of the mucosa/submucosa preparation evoked an increase in ISC, while addition to the apical side evoked only a small increase in ISC. Arrowhead indicates the time of emodin addition. (B) Concentration–response relationship for emodin-evoked ΔISC in the mucosa/submucosa preparations. The EC50 was 75.0 µM.
Apically applied glibenclamide (1 mM), a Cl- channel blocker, reduced the emodin-induced ΔISC by 58.3% (Figure 2), from 81.86 ± 12.91 µA·cm−2 (n= 20) to 34.11 ± 7.09 µA·cm−2 (P < 0.05, n= 3). Basolateral application of bumetanide (10 µM), an inhibitor of the Na+-K+-2Cl- cotransporter, resulted in 78.3% reduction in the emodin-induced ΔISC (Figure 2), from 81.86 ± 12.91 µA·cm−2 (n= 20) to 17.75 ± 6.91 µA·cm−2 (n= 5, P < 0.001). These results suggest that emodin-induced ΔISC is due to an increase in Cl- secretion.
Figure 2.
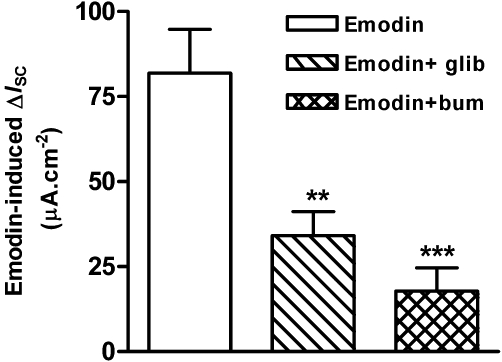
Effects of glibenclamide and bumetanide on emodin-evoked ΔISC in the mucosa/submucosa preparations of the rat distal colon. Application of glibenclamide (1 mM), a Cl- channel blocker, to the apical side of the preparations reduced emodin-evoked ΔISC by 58.3% (P < 0.05, n= 3). Application of bumetanide (10 µM), an inhibitor of the Na+-K+-2Cl- cotransporter, to the basolateral side of the preparations resulted in 78.3% reduction of emodin-evoked ΔISC (**P < 0.01; ***P < 0.001; n= 5).
Emodin-induced JCl−
While the ISC technique provides a direct measurement of net charge transport across a flat sheet preparation, it lacks chemical selectivity (Nair et al., 2008). In order to directly identify the ion species involved in emodin-induced ISC, a non-invasive SIET was used to detect changes in the direction and magnitude of Cl- flux (JCl−) from the basolateral side of the distal colonic epithelium as a function of external Cl- concentration. The spontaneously generated baseline JCl− was about 1.70 ± 0.05 nmol·cm−2·s−1 (n= 4). Emodin (100 µM), when added to the basolateral side of the preparation, evoked a sharp increase in JCl− to 11.90 ± 0.92 nmol·cm−2·s−1 (n= 4, P < 0.001) (Figure 3A and B). Basolateral application of bumetanide (10 µM) suppressed emodin-induced increase in JCl− by 51.9%, from 16.68 ± 1.31 nmol·cm−2·s−1 to 8.03 ± 0.42 nmol·cm−2·s−1 (n= 3, P < 0.001; Figure 3C and D), suggesting that the Na+-K+-2Cl- cotransporter is involved in the emodin-induced increase in JCl-.
Figure 3.
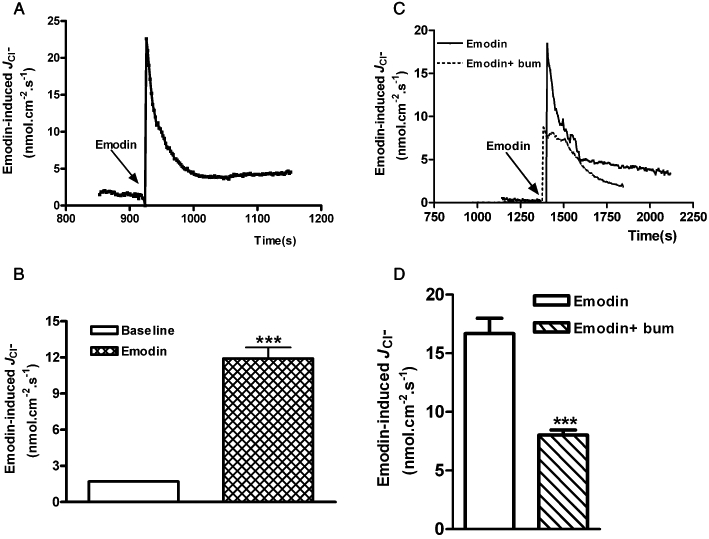
Effects of emodin on transepithelial JCl− in the mucosa/submucosa preparations of the rat distal colon. (A) Representative trace of emodin-evoked JCl−. (B) Emodin (100 µM)-induced increase in JCl− compared with baseline JCl−. (C) Representative trace of emodin-evoked JCl− in the presence and absence of bumetanide (10 µM). (D) Emodin (100 µM)-induced increase in JCl− was significantly reduced by bumetanide (10 µM). Data represents mean ± SEM (***P < 0.001).
Role of submucosal neurons
To determine whether submucosal neurons are involved in emodin-induced chloride secretion in the rat colon, TTX, a neuronal Na+ channel blocker, was administered to the basolateral side of the preparations. As shown in Figure 4A, pretreatment with TTX (1 µM) had no effect on emodin (100 µM)-induced ΔISC in mucosa-only preparations (without submucosal plexus). However, TTX (1 µM) significantly inhibited emodin (100 µM)-induced ISC response by 66.7% in the mucosa/submucosa preparations, from 61.77 ± 8.50 µA·cm−2 (n= 5) to 20.56 ± 4.60 µA·cm−2 (n= 6, P < 0.01) (Figure 4B). Similarly, basolateral pretreatment with TTX (1 µM) significantly reduced emodin-evoked JCl− in mucosa/submucosa preparations by 74.3%, from 14.34 ± 1.19 nmol·cm−2·s−1 to 3.69 ± 0.6 nmol·cm−2·s−1 (n= 11 P < 0.001) (Figure 5A and B).
Figure 4.
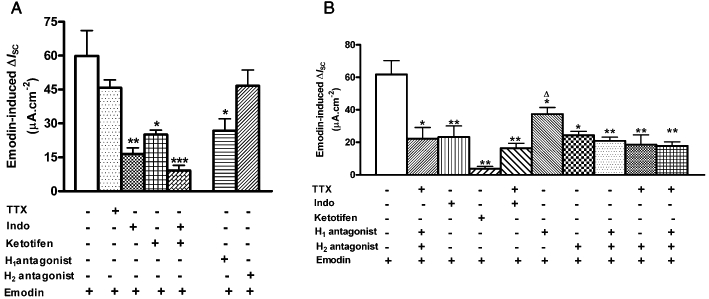
Pharmacology of emodin-induced ΔISC in mucosa-only and mucosa/submucosa preparations. (A) Emodin-induced ΔISC in mucosa-only preparations were significantly reduced by the COX inhibitor indomethacin (10 µM), the mast cell membrane stabilizer ketotifen (100 µM) and the H1 receptor antagonist pyrilamine (10 µM). A combination of indomethacin and ketotifen caused a further reduction in ISC. TTX (1 µM) and the H2 receptor antagonist cimetidine (100 µM) had no effect on emodin-evoked-ΔISC. (B) Emodin-induced ΔISC in mucosa/submucosa preparations were significantly reduced by TTX, indomethacin, ketotifen, pyrilamine and cimetidine. A combination of pyrilamine and cimetidine caused a further reduction in ISC. (*P < 0.05; **P < 0.01; ***P < 0.001).
Figure 5.
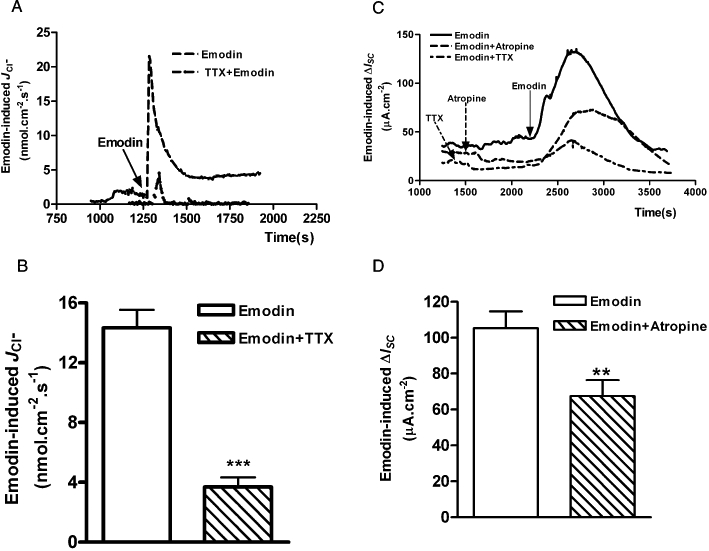
Effects of TTX and atropine on emodin-evoked JCl− and ΔISC in the mucosa/submucosa preparations of the rat distal colon. (A) Pretreatment with TTX (1 µM) significantly decreased emodin-evoked JCl−. (B) The average maximal increases in JCl− produced by emodin alone and after pretreatment with TTX. (C) Application of TTX (1 µM) and atropine (10 µM) to the basolateral side of the preparations significantly reduced emodin (100 µM)-evoked ΔISC. (D) The average maximal increases in ISC produced by emodin in the absence or presence of atropine (10 µM). (**P < 0.01; ***P < 0.001).
ACh is an important neurotransmitter in the submucosal plexus that mediates intestinal secretion (Cooke, 1984). To determine whether submucosal cholinergic neurons are involved in emodin-induced Cl- secretion, the muscarinic receptor antagonist, atropine, was applied to the basolateral side of the mucosa/submucosa preparations. Atropine (10 µM) significantly reduced emodin (100 µM)-induced ΔISC by 36.0%, from 105.30 ± 9.40 µA·cm−2 to 67.40 ± 8.90 µA·cm−2 (n= 6, P < 0.05. Figure 5C and D), suggesting that cholinergic secretomotor neurons are involved in this process. These results indicate that emodin-induced ΔISC or JCl− is largely due to an increase in neuronal activity in the submucosal plexus and is partially mediated by the cholinergic secretomotor neurons.
Role of mast cells
Emodin has been reported to degranulate mast cells and increase histamine release in the intestine (Lin et al., 1992; Ishii et al., 1994; Liu et al., 2007; Xu et al., 2007), which could affect colonic transepithelial ion transport by acting on the epithelial H1 (Keely et al., 1995a) and neuronal H2 receptors (Wang and Cooke, 1990; Frieling et al., 1994; Cooke et al., 1995). Basolateral pretreatment with ketotifen (100 µM), a mast cell membrane stabilizer, decreased emodin (100 µM)-induced ΔISC by 58.2%, from 59.88 ± 11.28 µA·cm−2 (n= 9) to 25.01 ± 2.04 µA·cm−2 (n= 4, P < 0.05; Figure 4A) in mucosa-only preparations. In the mucosa/submucosa preparations, ketotifen (100 µM) caused a stronger reduction of emodin (100 µM)-induced ΔISC by 94.6%, from 61.77 ± 8.57 µA·cm−2 (n= 9) to 3.34 ± 0.77 µA·cm−2 (n= 4, P < 0.01; Figure 4B). These results suggest that emodin-induced increase in ISC is largely mediated by mast cell degranulation.
Role of histamine
The major product of mast cells is histamine. The involvement of histamine in emodin-induced ΔISC was investigated by basolateral application of the H1 receptor antagonist pyrilamine and the H2 receptor antagonist cimetidine. In mucosa-only preparations, pretreatment with the H1 receptor antagonist, pyrilamine (10 µM), reduced emodin (100 µM)-induced ΔISC by 58.2%, from 59.88 ± 11.28 µA·cm−2 (n= 9) to 26.70 ± 5.35 µA·cm−2 (n= 4, P < 0.05), while the H2 receptor antagonist, cimetidine (100 µM), had no effect (Figure 4A). In mucosa/submucosa preparations, both pyrilamine and cimetidine reduced emodin-induced ΔISC. Pyrilamine (10 µM) reduced emodin (100 µM)-induced ΔISC by 44.1%, from 61.77 ± 8.50 µA·cm−2 (n= 9) to 34.50 ± 2.66 µA·cm−2 (n= 7, P < 0.05), while cimetidine (100 µM) reduced the response by 60.6% from 61.77 ± 8.50 µA·cm−2 to 24.32 ± 2.45 µA·cm−2 (n= 4, P < 0.05) (Figure 4B). A combination of pyrilamine and cimetidine caused a further reduction of emodin response by 66.2%, from 61.77 ± 8.50 µA·cm−2 to 20.87 ± 2.33 µA·cm−2 (n= 7, P < 0.01; Figure 4B).
The effect of cimetidine on emodin-induced ΔISC in mucosa/submucosa preparations was concentration- dependent with an IC50 of 46.0 µM (Figure 6A and B). Similarly, pretreatment with cimetidine (25 µM) resulted in a 69.2% reduction of emodin (100 µM)-induced JCl− in the mucosa/submucosa preparations from 13.39 ± 0.79 nmol·cm−2·s−1 to 5.15 ± 0.30 nmol·cm−2·s−1 (n= 3, P < 0.001) (Figure 6C and D).
Figure 6.
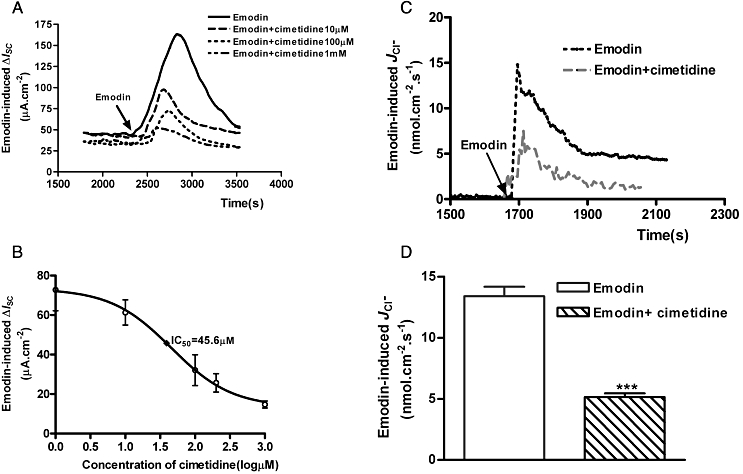
Effects of cimetidine on emodin-evoked ΔISC and JCl− in the mucosa/submucosa preparations of the rat distal colon. (A) Pretreatment with cimetidine (10, 100 and 1000 µM) caused concentration-dependent suppression of emodin (100 µM)-evoked ΔISC. (B) The concentration–response curve of cimetidine suppression of emodin-evoked ΔISC. The IC50 of cimetidin was 18.5 µM. Each data point was obtained from four preparations. (C) Pretreatment with cimetidine (25 µM) reduced emodin-evoked JCl− in the mucosa/submucosa preparations of the rat distal colon. Arrowhead indicates the time of emodin addition. (D) The average maximal increases in JCl− produced by emodin alone or after pretreatment with cimetidine (25 µM). Peak values are expressed as means ± SEM. (***P < 0.001).
To further test the hypothesis that mast cell-derived histamine is involved in emodin-induced colonic ion secretion, RIA was used to measure histamine release from the rat colonic tissues following emodin treatment. In the basal condition, histamine accumulation in the supernatant of the colonic tissues was 0.13 ± 0.03 ng·mg−1·mL−1 (n= 8). Pretreatment of the tissues with emodin significantly increased histamine release to the supernatant to 0.35 ± 0.01 ng·mg−1·mL−1 (emodin 10 µM; P < 0.01, n= 8) and 0.49 ± 0.04 ng·mg−1·mL−1 (emodin 100 µM; P < 0.01, n= 8) (Figure 7A), respectively. Pretreatment of the tissues with ketotifen (100 µM) 10 min before emodin (100 µM) significantly reduced histamine concentration in the supernatant (0.16 ± 0.19 ng·mg−1·mL−1; P > 0.05, n= 8, Figure 7A).
Figure 7.
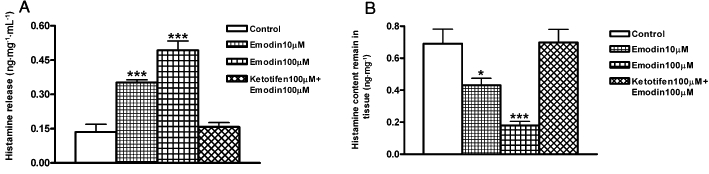
Emodin-induced histamine release from the rat distal colon. (A) Pretreatment of segments of rat distal colon with emodin (10 µM or 100 µM) significantly increased histamine release into the supernatant, while pretreatment of the tissues with ketotifen (100 µM) prevented emodin-induced histamine release into the supernatant. (B) The histamine content in the colonic tissues was decreased after pretreatment with emodin, reflecting the release of histamine to the supernatant and pretreatment with ketotifen prevented emodin-induced decrease of tissue histamine content. Data represents mean ± SEM (*P < 0.05; **P < 0.01; ***P < 0.001).
In the meantime, the histamine content that remains in the colonic tissues was also measured. In the basal condition, histamine content in the colonic tissues was 0.69 ± 0.09 ng·mg−1 (n= 10). Emodin significantly reduced the histamine content of the tissues to 0.43 ± 0.04 ng·mg−1 (emodin 10 µM; P < 0.05, n= 11) and 0.18 ± 0.02 ng·mg−1 (emodin 100 µM; P < 0.01, n= 13), respectively, which reflects the release of histamine to the incubation media. Pretreatment with ketotifen (100 µM) 10 min before emodin (100 µM) prevented the decrease in the histamine content of the tissues (0.70 ± 0.08 ng·mg−1; P > 0.05, n= 8, Figure 7B).
Role of PGs
Several previous studies have shown that histamine-induced intestinal ion secretion is mediated by PGs (Hardcastle and Hardcastle, 1987; Wang et al., 1990a; Keely et al., 1995b). We also reported that in mucosa-only preparations, emodin increases chloride secretion via PG release and directly acting at the epithelial level (Xu et al., 2007). To determine the extent that PGs contribute to the effect of emodin in the mucosa-only and mucosa/submucosa preparations, indomethacin, a COX inhibitor, was added to the serosal bath of the flux chamber. Pretreatment with indomethacin (10 µM) inhibited the ISC response to emodin (100 µM) by 72.3% in the mucosa-only preparations, from 59.88 ± 11.28 µA·cm−2 (n= 9) to 16.56 ± 2.60 µA·cm−2 (n= 6, P < 0.01) (Figure 4A). In the mucosa/submucosa preparations, emodin-induced ΔISC was reduced by 72.3%, from 61.77 ± 8.50 µA·cm−2 (n= 9) to 17.14 ± 2.87 µA·cm−2 (n= 6, P < 0.01) (Figure 4B). Pretreatment with indomethacin and ketotifen together caused a further reduction in emodin-induced ΔISC in the mucosa preparations by 84.7%, from 59.88 ± 11.3 µA·cm−2 (n= 9) to 9.19 ± 2.3 µA·cm−2 (n= 5, P < 0.01) (Figure 4A).
Discussion
Rhubarb root extracts have been used to treat constipation since ancient times. They are currently still used in the preparation of herbal laxatives, even though the actual mechanism of action is not fully understood. Emodin is the principle biologically active component in rhubarb root extracts. We previously reported that emodin increases Cl- secretion in the colonic mucosa (Xu et al., 2007), which would contribute to the laxative action. In this study, we aimed to investigate the mechanisms underlying emodin-induced Cl- secretion using colonic mucosa/submucosa preparations.
Epithelial Cl- secretion is the driving force of intestinal fluid secretion. Cl- enters the intestinal epithelial cells via the Na+-K+-2Cl- cotransporters in the basolateral membrane and exits to the intestinal lumen through the Cl- channels located in the apical membrane of the epithelial cells (Barrett and Keely, 2000). The secretion of Cl- leads to Na+ secretion and together NaCl secretion drags water across the epithelium by osmosis. Emodin increases the short-circuit current in the colonic mucosa/submucosa preparations. The increased short-circuit current reflects a net C1- secretion from basal to apical epithelial surfaces because apical application of the Cl- channel blocker, glibenclamide and basolateral application of the Na+-K+-2Cl- cotransporters inhibitor, bumetanide, significantly inhibited emodin-induced increase of ISC. This was confirmed by simultaneous measurement of JCl− with the SIET (Kuhtreiber and Jaffe, 1990; Wang et al., 1993; Duthie et al., 1994; Smith et al., 1994; 1999; Cardenas et al., 1999). Using this technique we can obtain a variety of information about ion activity with ion-selective electrodes without access into the tested tissues and, therefore, without damaging these tissues. Also, it overcomes the shortcomings of ISC recording, such as lacking chemical selectivity and being only able to measure electrogenic ion transport. In addition, the technique can carry out three-dimensional and real-time measurement of ion concentration, velocity and direction of motion through specific ion-sensitive electrodes, such as Cl--sensitive electrodes. In the present study, we combined the technique of ISC recording of epithelial ion transport with the novel SIET measuring colonic JCl− in fresh isolated colonic mucosa/submucosa preparations and demonstrated for the first time the stimulating effect of emodin on colonic Cl- secretion. The results with SIET showed an increase in net Cl- secretion by emodin from the basolateral side to the apical side of the colonic mucosa/submucosa preparations. The emodin-induced JCl− correlated with the increase of short-circuit current. In addition, emodin-induced JCl− was also blocked by bumetanide, suggesting that Cl- secretion was responsible for the observed electrical changes.
Chloride secretion by intestinal epithelial cells is controlled by secretomotor neurons in the submucosal plexus (Cooke, 1998). Any effect that increases the excitability of secretomotor neurons is expected to lead to neurogenic secretory diarrhoea. Similarly, any action that suppresses excitability of the secretomotor neurons is expected to lead to reduce liquidity of the intestinal contents and constipation. Secretomotor neurons of submucosal origin use ACh or vasoactive intestinal peptide as their main neurotransmitter along with other chemicals whose functions are unknown (Furness, 2006). Emodin-induced increase of ISC and JCl− were inhibited by TTX, indicating the involvement of submucosal neurons in its action. In addition, the muscarinic receptor antagonist, atropine, partially inhibited the emodin-induced increase in ISC, suggesting that cholinergic secretomotor neurons are activated by emodin. These data suggest that emodin causes its effect on rat colon partially through activating secretomotor neurons, which would increase the release of neurotransmitters including ACh, and these in turn increase Cl- secretion in the epithelial cells.
The regulation of chloride secretion is a complex interplay between epithelium, enteric neurons and immune cells. Emodin has been demonstrated to stimulate mast cells within the colonic mucosa to degranulate and release histamine (Kai et al., 2002). Mucosal mast cells are important for the detection of foreign antigens as they are located at the interface between the external environment and the subepithelium. Degranulation of mucosal mast cells in the gut triggers a type I hypersensitivity response, which is characterized by powerful propulsive muscular contractions and hypersecretion that underlie a diarrhoea state (Cooke, 1994). Mast cells are closely associated with nerve fibres in the intestine (Stead et al., 1987; Ottaway, 1991). Most of the nerve fibres arise from enteric neurons, but sympathetic axons or extrinsic afferent fibres are present as well (Stead et al., 1987; Blennerhassett and Bienenstock, 1990; Ottaway, 1991; Stead, 1992). Because of the proximity of mast cells and neural fibres, spontaneous release of mast cell mediators modulates neural reflex systems, which regulate epithelial function (Castro, 1990; Frieling et al., 1994). Degranulation of mast cells causes the release of mast cell mediators, which stimulate intestinal ionic secretion by directly acting on epithelial cells and also indirectly via the enteric nervous system (Wang et al., 1991; Frieling et al., 1994). The results obtained in the present study suggest that mast cells play a role in emodin-induced Cl- secretion because the mast cell stabilizer ketotifen significantly suppressed the response to emodin in both mucosa-only and mucosa/submucosa preparations.
Histamine and PGs are key chemical messengers released from mast cells. Histamine plays an important role in promoting colonic secretion by activating the H1 receptors in enterocytes (Wang et al., 1990b; Schultheiss et al., 2006) and the H2 receptors in submucosal neurons (Ahrens et al., 2003). Emodin stimulated histamine release in the colonic mucosa/submucosa preparations. In addition, the ISC response to emodin was sensitive to histamine receptor antagonists. In mucosa-only preparations, emodin-induced ISC was reduced by the H1 receptor antagonist pyrilamine but was unaffected by the H2 receptor antagonist cimetidine, which is consistent with previous reports (Wang et al., 1990b; Schultheiss et al., 2006). However, in the mucosa/submucosa preparations, the H2 receptor antagonist caused a greater reduction in emodin-induced ΔISC and JCl− than did the H1 receptor antagonist. As the H2 receptor antagonist had no effect in the mucosa-only preparations, we deduced that the H2 receptors are located in the submucosal neuronal plexus. Previous studies with intracellular recording showed functional expression of H2 receptors in the submucosal neurons, which mediate the excitatory histamine response (Tokimasa and Akasu, 1989; Frieling et al., 1994). In the present study, cimetidine and TTX reduced the emodin-induced ΔISC to a similar extent, which suggests that the H2 receptors are located on submucosal neurons.
We previously showed that PGs are involved in emodin-induced increase in ISC in the mucosa-only preparations (Xu et al., 2007). The present study further demonstrated the participation of PGs in the emodin response in the mucosa/submucosa preparations. Mast cells are the major source of PGs in the intestine. In addition to mast cells, smooth muscle cells, enteric neurons, endothelial cells of arterioles are other potential sources of PGs (Ishimura et al., 1993). Emodin could directly release PGs through mast cell degranulation. Besides, histamine stimulates PG production by the intestine (Hardcastle and Hardcastle, 1987; Wang et al., 1990a; Keely et al., 1995b). Release of PGs further enhances intestinal ion secretion.
In summary, the results of this study support our hypothesis (Figure 8) that emodin evokes colonic ion secretion via mast cell degranulation, which increases histamine and PG levels in the intestine. Histamine acts both directly on the enterocytes and via submucosal neurons to stimulate colonic Cl- secretion. Cholinergic secretomotor neurons mediate part of the neurogenic response. However, non-cholinergic neuronal pathway(s) might also be involved as atropine only suppressed 37% of the emodin-induced ISC responses. PGs also participate in the emodin-induced ISC responses; they either work in parallel with histamine or as downstream chemical mediators of histamine.
Figure 8.
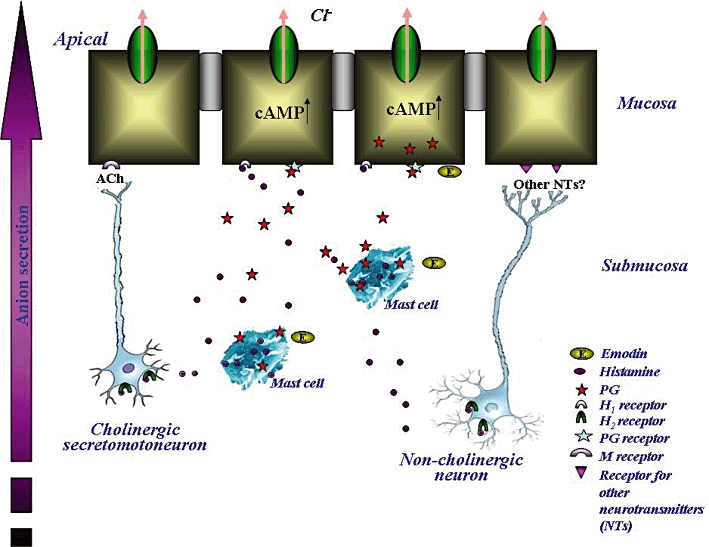
An illustration of the working hypothesis of emodin in the rat colon. Emodin stimulates mast cells to release histamine, which binds to the H1 receptors on the colonic epithelium and leads to an increase in chloride secretion in the rat colon. Histamine also binds to the H2 receptor on cholinergic neurons in the submucosal plexus and causes the release of ACh. ACh acts on the muscarinic (M) receptors on the colonic epithelium to stimulate Cl- secretion. Histamine may also stimulate non-cholinergic secretomotor neuron activity, which leads to an enhancement of ion secretion. Emodin also causes PG release, which further increases colonic ion secretion.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30971076, JXZ), Scientific Research Key Program of Beijing Municipal Commission of Education (KZ200910025003, JXZ), Beijing Municipal Project for Developing Advanced Human Resources for Higher Education (PHR201007110, JXZ; PHR201008384, JDX), National Science and Technology Major Project ‘Created a Major New Drug’‘11–5’ Program Foundation (2009ZX09103-366, WW) and Beijing Education Committee Foundation Grant (KM201110025004, JDX). We express our thanks to Dr Yue Xu, Wenjun Wang and Jin Song for their kindly help on the Cl- flux measurement with SIET in this study.
Glossary
Abbreviations
- EtOAc
ether acetate
- emodin
1,3,8-trihydroxy-6-methylanthraquinone
- JCl−
Cl- flux
- K-HS
Krebs-Henseit solution
- SIET
scanning ion-selective electrode technique
- TTX
tetrodotoxin
Conflict of interest
The authors have no financial, consultant, institutional or other relationships that might lead to bias or conflict of interest.
References
- Ahrens F, Gabel G, Garz B, Aschenbach JR. Histamine-induced chloride secretion is mediated via H2-receptors in the pig proximal colon. Inflamm Res. 2003;52:79–85. doi: 10.1007/s000110300005. [DOI] [PubMed] [Google Scholar]
- Ali S, Watson MS, Osborne RH. The stimulant cathartic, emodin, contracts the rat isolated ileum by triggering release of endogenous acetylcholine. Auton Autacoid Pharmacol. 2004;24:103–105. doi: 10.1111/j.1474-8673.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Blennerhassett MG, Bienenstock J. Apparent innervation of rat basophilic leukaemia (RBL-2H3) cells by sympathetic neurons in vitro. Neurosci Lett. 1990;120:50–54. doi: 10.1016/0304-3940(90)90165-6. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Feijo JA, Kunkel JG, Sanchez F, Holdaway-Clarke T, Hepler PK, et al. Rhizobium nod factors induce increases in intracellular free calcium and extracellular calcium influxes in bean root hairs. Plant J. 1999;19:347–352. doi: 10.1046/j.1365-313x.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- Castro GA. Immunological regulation of electrolyte transport. In: Duffey ELAM, editor. Textbook of Secretory Diarrhea. New York: Raven; 1990. pp. 31–58. [Google Scholar]
- Cooke HJ. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol. 1984;246:G263–G267. doi: 10.1152/ajpgi.1984.246.3.G263. [DOI] [PubMed] [Google Scholar]
- Cooke HJ. Neuroimmune signaling in regulation of intestinal ion transport. Am J Physiol. 1994;266:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- Cooke HJ. ‘Enteric Tears’: chloride secretion and its neural regulation. News Physiol Sci. 1998;13:269–274. [PubMed] [Google Scholar]
- Cooke HJ, Wang YZ, Reddix R, Javed N. Cholinergic and VIP-ergic pathways mediate histamine H2 receptor-induced cyclical secretion in the guinea pig colon. Am J Physiol. 1995;268:G465–G470. doi: 10.1152/ajpgi.1995.268.3.G465. [DOI] [PubMed] [Google Scholar]
- Duthie GG, Shipley A, Smith PJ. Use of a vibrating electrode to measure changes in calcium fluxes across the cell membranes of oxidatively challenged Aplysia nerve cells. Free Radic Res. 1994;20:307–313. doi: 10.3109/10715769409145630. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Theodorou V, Vergnolle N, Comera C, Fioramonti J, Bueno L. Involvement of interleukin-1, prostaglandins and mast cells in rectal distension-induced colonic water secretion in rats. J Physiol. 1998;506:245–252. doi: 10.1111/j.1469-7793.1998.245bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology. 1994;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Carlton, Victoria, Australia: Blackwell Publishing Asia Pty Ltd; 2006. [Google Scholar]
- Goel RK, Das Gupta G, Ram SN, Pandey VB. Antiulcerogenic and anti-inflammatory effects of emodin, isolated from Rhamnus triquerta wall. Indian J Exp Biol. 1991;29:230–232. [PubMed] [Google Scholar]
- Gu J, Zhang X, Fei Z, Wen A, Qin S, Yi S, et al. Rhubarb extracts in treating complications of severe cerebral injury. Chin Med J (Engl) 2000;113:529–531. [PubMed] [Google Scholar]
- Hardcastle J, Hardcastle PT. The Secretory actions of histamine in rat small-intestine. J Physiol Lond. 1987;388:521–532. doi: 10.1113/jphysiol.1987.sp016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull (Tokyo) 1999;47:1121–1127. doi: 10.1248/cpb.47.1121. [DOI] [PubMed] [Google Scholar]
- Huang HC, Lee CR, Chao PD, Chen CC, Chu SH. Vasorelaxant effect of emodin, an anthraquinone from a Chinese herb. Eur J Pharmacol. 1991;205:289–294. doi: 10.1016/0014-2999(91)90912-a. [DOI] [PubMed] [Google Scholar]
- Huang LY, Hu JD, Chen XJ, Zhu F, Hu HL. Effects of emodin on the proliferation inhibition and apoptosis induction in HL-60 cells and the involvement of c-myc gene. Zhonghua Xue Ye Xue Za Zhi. 2005;26:348–351. [PubMed] [Google Scholar]
- Ishii Y, Tanizawa H, Takino Y. Studies of aloe 5. Mechanism of cathartic effect. 4. Chem Pharm Bull (Tokyo) 1994;17:651–653. doi: 10.1248/bpb.17.651. [DOI] [PubMed] [Google Scholar]
- Ishimura K, Suzuki T, Fukui K, Yamamoto A, Omoto Y, Ueda N, et al. Immunocytochemical localization of prostaglandin endoperoxide synthase in the bovine intestine. Histochemistry. 1993;99:485–490. doi: 10.1007/BF00274102. [DOI] [PubMed] [Google Scholar]
- Kai M, Hayashi K, Kaida I, Aki H, Yamamoto M. Permeation-enhancing effect of aloe-emodin anthrone on water-soluble and poorly permeable compounds in rat colonic mucosa. Biol Pharm Bull. 2002;25:1608–1613. doi: 10.1248/bpb.25.1608. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Stack WA, O'Donoghue DP, Baird AW. Regulation of ion transport by histamine in human colon. Eur J Pharmacol. 1995a;279:203–209. doi: 10.1016/0014-2999(95)00156-f. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Stack WA, Odonoghue DP, Baird AW. Regulation of ion-transport by histamine in human colon. Eur J Pharmacol. 1995b;279:203–209. doi: 10.1016/0014-2999(95)00156-f. [DOI] [PubMed] [Google Scholar]
- Kuhtreiber WM, Jaffe LF. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HZ, Wu CH, Chang SP. Release of nucleophosmin from the nucleus: involvement in aloe-emodin-induced human lung non small carcinoma cell apoptosis. Int J Cancer. 2005;113:971–976. doi: 10.1002/ijc.20676. [DOI] [PubMed] [Google Scholar]
- Lin X, Guo S, Hou Q, Cui R, Kang Y. The effects of dachengqi decoction, shaogan decoction, emodin and sennoside on the histamine level of intestinal mucosa in intestinally obstructed rats. Zhongguo Zhong Yao Za Zhi. 1992;17:427–429. 447. [PubMed] [Google Scholar]
- Liu RH, Zhang JY, Liang MJ, Zhang WD, Yan SK, Lin M. Simultaneous analysis of eight bioactive compounds in Danning tablet by HPLC-ESI-MS and HPLC-UV. J Pharm Biomed Anal. 2007;43:1007–1012. doi: 10.1016/j.jpba.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Ma T, Qi QH, Xu J, Dong ZL, Yang WX. Signal pathways involved in emodin-induced contraction of smooth muscle cells from rat colon. World J Gastroenterol. 2004;10:1476–1479. doi: 10.3748/wjg.v10.i10.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Kashyap R, Laboisse CL, Hopfer U, Gratzl M. Time resolved secretion of chloride from a monolayer of mucin-secreting epithelial cells. Eur Biophys J. 2008;37:411–419. doi: 10.1007/s00249-007-0226-3. [DOI] [PubMed] [Google Scholar]
- Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am. 1991;20:511–529. [PubMed] [Google Scholar]
- Schultheiss G, Hennig B, Schunack W, Prinz G, Diener M. Histamine-induced ion secretion across rat distal colon: involvement of histamine H1 and H2 receptors. Eur J Pharmacol. 2006;546:161–170. doi: 10.1016/j.ejphar.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Sanger RH, Jaffe LF. The vibrating Ca2+ electrode: a new technique for detecting plasma membrane regions of Ca2+ influx and efflux. Methods Cell Biol. 1994;40:115–134. doi: 10.1016/s0091-679x(08)61112-7. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc Res Tech. 1999;46:398–417. doi: 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Stead RH. Nerve remodeling during intestinal inflammation. 1992. In Neuro-Immune-Physiology of the Gastrointestinal Mucosa: Implications for Inflammatory Diseases. pp. 443–455: Ann NY Acad Sci. [DOI] [PubMed]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis RJ, Owen DG, Lohr JL, Rosler KH, Blomster RN. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991;35:2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T, Akasu T. Histamine H2 receptor mediates postsynaptic excitation and presynaptic inhibition in submucous plexus neurons of the guinea-pig. Neuroscience. 1989;28:735–744. doi: 10.1016/0306-4522(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Cooke HJ. H2 receptors mediate cyclical chloride secretion in guinea pig distal colon. Am J Physiol. 1990;258:G887–G893. doi: 10.1152/ajpgi.1990.258.6.G887. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Cooke HJ, Su HC, Fertel R. Histamine augments colonic secretion in guinea-pig distal colon. Am J Physiol. 1990a;258:G432–G439. doi: 10.1152/ajpgi.1990.258.3.G432. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Cooke HJ, Su HC, Fertel R. Histamine augments colonic secretion in guinea pig distal colon. Am J Physiol. 1990b;258:G432–G439. doi: 10.1152/ajpgi.1990.258.3.G432. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Palmer JM, Cooke HJ. Neuroimmune regulation of colonic secretion in guinea pigs. Am J Physiol. 1991;260:G307–G314. doi: 10.1152/ajpgi.1991.260.2.G307. [DOI] [PubMed] [Google Scholar]
- Wang R, Pang PK, Wu L, Shipley A, Karpinski E, Harvey S, et al. Neural effects of parathyroid hormone: modulation of the calcium channel current and metabolism of monoamines in identified Helisoma snail neurons. Can J Physiol Pharmacol. 1993;71:582–591. doi: 10.1139/y93-082. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Xu JD, Wang W, Li LS, Chen X, Zhu JX. Involvement of endogenous prostaglandin in emodin-evoked rat colonic anion secretion. Biol Pharm Bull. 2007;30:2058–2062. doi: 10.1248/bpb.30.2058. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Zhou CH, Wu YQ. Effect of emodin on small intestinal peristalsis of mice and relevant mechanism. World J Gastroenterol. 2005;11:3147–3150. doi: 10.3748/wjg.v11.i20.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Tang ZY, Yang JX, Zhang Y, Li Y, Lin Y. Bi-directional regulation of emodin and quercetin on smooth muscle myosin of gizzard. FEBS Lett. 2006;580:469–473. doi: 10.1016/j.febslet.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Zheyu C, Qinghui QI, Lixin L, Tao MA, Xu J, Zhang L, et al. Effects of emodin on Ca2+ signal transduction of smooth muscle cells in multiple organ dysfunction syndrome. J Surg Res. 2006;131:80–85. doi: 10.1016/j.jss.2005.08.031. [DOI] [PubMed] [Google Scholar]


