Abstract
BACKGROUND AND PURPOSE
This study evaluated gene expression differences between two mouse strains, characterized by opposite impulsivity-like traits and the involvement of the cannabinoid CB2 receptor in the modulation of impulsivity.
EXPERIMENTAL APPROACH
Behavioural tests were conducted to compare motor activity, exploration and novelty seeking, attention and cognitive and motor impulsivity (delayed reinforcement task: session duration 30 min; timeout 30 s) between A/J and DBA/2 mice. Expression of genes for dopamine D2 receptors, CB1 and CB2 receptors were measured in the cingulate cortex (CgCtx), caudate-putamen (CPu), accumbens (Acc), amygdala (Amy) and hippocampus (Hipp). Involvement of CB2 receptors in impulsivity was evaluated in DBA/2 mice with a CB2 receptor agonist (JWH133) and an antagonist (AM630).
KEY RESULTS
DBA/2 mice presented higher motor and exploratory activity, pre-pulse inhibition impairment and higher cognitive and motor impulsivity level than A/J mice. In addition, DBA/2 mice showed lower (CgCtx, Acc, CPu) D2 receptor, lower (Amy) and higher (CgCtx, Acc, CPu, Hipp) CB1 receptor and higher (CgCtx, Acc, Amy) and similar (CPu, Hipp) CB2 receptor gene expressions. Treatment with JWH133 (0.5, 1, 3 mg·kg−1, i.p.) reduced cognitive and motor impulsivity level, accompanied by CB2 receptor down-regulation (CgCtx, Acc, Amy) but did not modify other behaviours. In contrast, AM630 (1, 2, 3 mg·kg−1, i.p.) improved pre-pulse inhibition and reduced novelty seeking behaviour in DBA/2 mice.
CONCLUSIONS AND IMPLICATIONS
CB2 receptors might play an important role in regulating impulsive behaviours and should be considered a promising therapeutic target in the treatment of impulsivity-related disorders.
Keywords: impulsivity, cannabinoid, dopamine, gene expression, DBA/2, A/J
Introduction
Impulsive behaviour is characterized by the inclination to act prematurely without forethought and, as such, has an important role in normal behaviour, allowing individuals to adapt to uncertainty, complexity and rapidly changing environments (Pattij and Vanderschuren, 2008). On the other hand, impulsivity is present in many psychiatric disorders such as bipolar disorder, personality disorders, attention-deficit hyperactivity disorder (ADHD) and substance use disorders (Swann et al., 2004; Winstanley et al., 2006; Malloy-Diniz et al., 2007). There is increasing consensus that impulsivity is a heterogeneous and multi-factorial concept consisting of several distinct behavioural phenomena that are dissociable at the neuroanatomical and neuropharmacological levels. In the present study, different varieties of impulsivity such as hyperactivity (open field), high exploration (head dipping on the hole board), novelty seeking (object preference on the hole board), attention deficit (pre-pulse inhibition) and high delay discounting or low behavioural inhibition (delayed reinforcement task) have been assessed.
Several studies have considered the isogenic nature of inbred strains of mice to evaluate the contribution of genetics to different behavioural phenotypes (Crawley et al., 1997). The use of different strains of mice with a homogeneous and stable genetic background has made it possible to postulate that impulsive behaviour is probably affected by genetic factors (Isles et al., 2004; Otobe and Makino, 2004). In this study, two inbred strains of mice (A/J and DBA/2) were selected on the basis of previous studies showing clear motor activity and anxiety differences (Bolivar et al., 2000; Moy et al., 2007). We employed such a two-strain comparison to study motor and exploratory activity, anxiety, learning and memory, stress reactivity and wildness (http://phenome.jax.org/ of the Mouse Phenome Database, The Jackson Laboratory, Bar Harbor, ME).
Dopaminergic brain circuits have been related to the neuropharmacology of impulsivity (van Gaalen et al., 2006; Winstanley et al., 2006). The dopaminergic system has an important role in the regulation of impulsivity since previous studies reported that activation of dopamine receptors decreased delay aversion, one of the multiple traits that characterize impulsive behaviour (van Gaalen et al., 2006). A study with methamphetamine-dependent subjects, displaying high impulsivity scores, showed a negative relationship between impulsiveness and striatal dopamine D2/D3 receptor availability in the caudate-putamen (CPu) and accumbens (Acc) (Lee et al., 2009; receptor nomenclature follows Alexander et al., 2011). Furthermore, an opposite modulation of impulsive behaviour by the dopamine D2/D3 receptors in core or shell parts of the Acc has been recently proposed (Besson et al., 2009). Another recent study related the A1 allele of the TaqIA polymorphism in the gene for the D2 receptor with impulsive behaviour, concluding that this association is a vulnerability factor for the development of problematic drug use (Esposito-Smythers et al., 2009).
Recently, it has been suggested that other brain systems, such as the noradrenergic, glutamatergic or cannabinoid systems, may also play a relevant role in the regulation of impulsive-like behaviours (Pattij and Vanderschuren, 2008). The endocannabinoid system has garnered much interest in the regulation of impulsivity in recent years, supported by the following studies: (i) the administration of Δ9-tetrahydrocannabinol increased some behavioural measures of impulsivity, suggesting that impulsivity is an assemblage of distinct components rather than a unitary process (McDonald et al., 2003); (ii) impulsive subpopulations of the spontaneously hypertensive rat (SHR) strain present low densities of cannabinoid CB1 receptor in the prefrontal cortex, and the administration of the cannabinoid agonist WIN-55212 normalizes the impulsive behavioural profile (Adriani et al., 2003); and (iii) the administration of the CB1 receptor antagonist rimonabant (SR141716A) improved inhibitory control, and this effect was blocked by WIN-55212 without affecting other impulse measures, providing further support for distinct forms of impulsivity that can be pharmacologically dissociated (Pattij et al., 2007). In addition, an association between several polymorphisms in or near the CB1 receptor gene with impulsivity has been reported in Southwest Californian Indians (Ehlers et al., 2007), as well as with the risk of both drug and alcohol dependence (Zuo et al., 2007).
The cannabinoid CB2 receptor, thought to be restricted to immune cells and peripheral tissues, has been identified in the brain of certain rodents (Van Sickle et al., 2005). Although immunohistochemical studies in rat brain reported CB2 receptors at levels much lower than CB1 receptors, the CB2 receptors have been found in abundance in hippocampus (Hipp) and amygdala (Amy) (Gong et al., 2006), specifically on postsynaptic elements (Brusco et al., 2008). A recent study of our group demonstrated the distribution of CB2 receptors in the brain of Swiss ICR and CB2 receptor transgenic mice and pointed out the involvement of this receptor in depressive-related disorders (Garcia-Gutierrez et al., 2010). A few more reports have evaluated the involvement of CB2 receptors in psychiatric disorders, such as depression (Onaivi et al., 2008; Hu et al., 2009), drug abuse (Ishiguro et al., 2007; Onaivi et al., 2008) or schizophrenia (Ishiguro et al., 2010), suggesting a role of this brain neuronal cannabinoid receptor in cognition. However, the role of this promising therapeutic target in impulsivity was still unexplored.
In the present study, motor activity, exploration, novelty seeking, attention and impulsivity were analysed in A/J and DBA/2 strains of mice, to identify the strain displaying the higher level of impulsive-like behaviours. Expression of the genes for D2 receptors and CB1 receptors was evaluated to identify differences that could be related with the opposing impulsivity trait between both strains, taking into account that these targets have been already related to the regulation of impulsivity. Furthermore, analysis of the expression of the gene for CB2 receptors and pharmacological studies with a highly selective CB2 receptor agonist (JWH133) and antagonist (AM630) were carried out to evaluate the involvement of this receptor in the regulation of certain aspects of the heightened impulsive-like behaviours displayed by DBA/2 mice.
Methods
Animals
All animal care and experimental procedures were in accordance with guidelines established by the European Council Directive (86/609/EEC) and were approved by the Institutional Animal Care Committee. A/J and DBA/2 strains were purchased from Harlan (Barcelona, Spain). Male mice of 8–10 weeks of age and 25 ± 2 g of weight were housed in groups of 8–10 per cage (40 × 25 × 22 cm) under controlled conditions (temperature, 23 ± 2°C; relative humidity, 60 ± 10%; 12 h light/dark cycle, lights on from 8:00 to 20:00 h.). Behavioural analyses, initiated after 1 week of acclimatization in the animal room, were performed after placing the home cage in the operant-task room for 1 h before starting experiments. Standard laboratory chow and water were available ad libitum in all procedures, except for the delayed reinforcement task in which standard chow was restricted to only 60 min access per day. This regimen was applied from 3 days before start and during the operant task (after the end of each daily session) to guarantee the response of the mice to reinforcers. Food restriction schedule produced a weight loss in mice of around 15% from their free-feeding weight (see Table 1).
Table 1.
Mean (±SEM) percentage of body weight reduction of A/J and DBA/2 strains (n= 12) and DBA/2-treated mice (n= 8) during the delayed reinforcement task
| Group | Mean (±SEM) % of body weight reduction |
|---|---|
| A/J | 15.77 ± 4.19 |
| DBA/2 | 16.95 ± 2.04 |
| DBA/2 Vehicle | 14.35 ± 3.46 |
| DBA/2 JWH133 (0.5 mg·kg−1) | 13.62 ± 0.59 |
| DBA/2 JWH133 (1 mg·kg−1) | 16.35 ± 4.04 |
| DBA/2 JWH133 (3 mg·kg−1) | 15.62 ± 2.51 |
| DBA/2 AM630 (1 mg·kg−1) | 15.15 ± 3.27 |
| DBA/2 AM630 (2 mg·kg−1) | 10.97 ± 0.50 |
| DBA/2 AM630 (3 mg·kg−1) | 14.56 ± 4.27 |
| Total mean | 14.82 ± 0.59 |
Motor activity – open field
The open field consists of a transparent square cage 25 × 25 × 25 cm with a white Plexiglas floor. Mice were individually placed in the centre to initiate a 30 min test that was recorded with a video camera and analysed with the SMART (Spontaneous Motor Activity Recording and Tracking) v2.5.3 software system (Panlab, Barcelona, Spain). The distance travelled and the mean speed divided into three 10 min intervals were analysed.
Exploration and novelty seeking – hole board
The exploratory level and novelty seeking behaviour were measured using an apparatus that consisted of a 40 × 40 × 40 cm transparent acrylic square box with a black acrylic board with four equidistant holes placed in each corner and equipped with infrared photocells to detect introductions. Animals were tested in two similar tasks. First, the number of head dips into each hole was recorded (head dipping on the hole board). The day after, a small object was introduced in two of the holes at opposite corners to measure preference as the amount of time spent head dipping in holes that had objects divided by total time spent head dipping (object preference on the hole board). Mice were placed individually in the centre to initiate a 10 min test that was video recorded and analysed with the SMART programme.
Attention – acoustic pre-pulse inhibition
Pre-pulse inhibition is the phenomenon by which a weak pre-pulse stimulus suppresses or diminishes the response to a startling stimulus showing the individual's ability to inhibit or gate the incoming inputs by adapting the amplitude of startle reflects. Animals were placed in a soundproof chamber equipped with loudspeakers (Panlab). Animals were inside a Plexiglas cylinder contacting a piezoelectric accelerometer in order to transform mice movements into digital signals. Animals were tested in the pre-pulse inhibition paradigm, using a protocol described previously (Paylor and Crawley, 1997). Each test began with a habituation phase by placing a subject undisturbed for 10 min with a constant 65 dB background noise. After that, each subject was given 80 trials over the 37 min test interval, including eight trial types in pseudorandom order: 1 × 40 ms, 120 dB acoustic startle stimulus; 3 × 20 ms pre-pulse stimulus (74, 82 and 90 dB); and 3 × 20 ms pre-pulse (100 ms before the onset) plus startle stimulus trials. Finally, trials where no stimulus was presented were used to measure baseline movements. The average inter-trial interval was 15 s. The maximum startle amplitude recorded during the 100 ms sampling window was used as the dependent variable. The following formula was used to calculate % pre-pulse inhibition of a startle response: 100 −[(startle response on acoustic pre-pulse plus startle stimulus trials / startle response alone trials) × 100].
Impulsivity – delayed reinforcement task
The evaluation of the delay discounting was carried out in eight modular operant chambers (Panlab) placed inside eight noise-isolation boxes (which have a fan and a light) and equipped with a chamber light, two levers, one feeder device with a magazine to drop food pellets (20 mg Dustless precision rodent pellets, Bio-Serv, Frenchtown, NJ), one stimulus light and a buzzer. In the training phase, each session began with the chamber light on, and mice lever press switched it off. One lever press delivered one food pellet (immediate lever), whereas the other lever delivered three food pellets combined with 0.5 s stimulus light and 0.5 s, 2850 Hz, 85 dB buzzer beep (delayed lever). Following food delivery, a 30 s timeout (signalled with the chamber light off) was established during which additional lever presses in either lever were recorded but without consequence. After the 30 s timeout, the chamber light was turned on indicating the start of the inter-trial interval (ITI) in which the next trial is initiated depending on each subject's spontaneous waiting before lever press. Each session lasted 30 min, and all mice performed one session per day. With to the 30 s timeout period, the maximal number of trials that an animal could theoretically complete (if responding immediately after end of the timeout) during the training phase (without delays) was 60. The length of the training phase depended on the time to achieve learning task criteria consisting of (i) reaching >75% of preference for the delayed lever, (ii) >10 reinforced trials by session and (iii) <20% deviation of number of reinforced trials, all during 3 days. Once these criteria were reached, mice continued to the test phase where a time delay was introduced between lever pressing in the delayed lever and the delivery of the three pellets. In this period, the stimulus light (not the 0.5 s buzzer beep) was turned on, and additional lever presses in either lever were recorded but without consequence. Delay was fixed for a given daily session and increased progressively over subsequent days (0, 6, 12, 18, 24, 30, 42, 54, 66, 78, 90 s). Change in the percentage of preference for the delayed lever in relation to different delays (cognitive impulsivity) and the number of immediate lever presses during the delay time (motor impulsivity) were analysed. For pharmacological studies in DBA/2 mice, the eight mice of each treatment group were tested at the same time, and the order of treatment groups was changed day by day, by placing the treatment group that first initiated the daily session, at the end of the session on the following day. In addition, a detailed analysis of the mean number of trials that mice completed and the mean response time after the end of timeout, through the whole task, was carried out. During the last 3 days of training, mice stabilize to around 50–60 s of response time, allowing them to complete around 20 trials out of 60 (theoretical). Details of the analysis method used for each parameter are described in the Supplementary Information.
Analysis of the expression of the genes for D2 receptors CB1 receptors and CB2 receptors – real-time PCR
Mice were killed 24 h after the last delayed reinforcement task session (mice were under food restriction), and brains were removed from the skull and frozen over dry ice. Coronal brain sections (500 µm), which were obtained in a cryostat (−10°C), contained the regions of interest according to Paxinos and Franklin (2001) beginning at plates 19–20 (distance from bregma: 1.42 and 1.34 mm, respectively). The cingulate cortex (CgCtx), CPu, Acc, Amy and Hipp were microdissected according to the Palkovits method (Palkovits, 1983). Total RNA was isolated from brain tissue micropunches using Biozol reagent (Inilab, Madrid, Spain) and subsequently retrotranscribed to cDNA. Quantitative analysis of the relative abundance of D2 receptor (Mm00438541_m1), CB1 receptor (Mm00432621_s1) and CB2 receptor (Mm00438286_m1) gene expressions was performed on the ABI PRISM 7700 Sequence Detector System (Applied Biosystems, Foster City, CA) on the A/J and DBA/2 strains. All reagents were obtained from Applied Biosystems, and the manufacturer's protocols were followed. The reference gene used was 18S rRNA (Hs99999901_s1), detected using Taqman ribosomal RNA control reagents. All primer–probe combinations were optimized and validated for relative quantification of gene expression. Briefly, data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene mRNA abundance was determined using the 2−ΔΔCt method (Schmittgen and Zakrajsek, 2000), so that DBA/2 or DBA/2 JWH133-treated levels were expressed relative to A/J or DBA/2 vehicle-treated levels respectively.
Data analyses
Statistical analyses were performed using the Student's t-test to compare two groups and one or two-way anova followed by the Holm–Sidak test to compare groups affected by one or two factors respectively. Two-way anova with repeated measures followed by the Holm–Sidak test was used to compare two or more groups at different time points. Differences were considered significant if P < 0.05. SigmaStat v3.11 software (Systat software Inc., Chicago, IL, USA) was used for all statistical analyses.
Materials
JWH133 (3-(1,1-dimethylbutyl)-1-deoxy-Δ8-tetrahydrocannabinol) and AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl]-(4-methoxyphenyl)-methanone) were purchased from Tocris (Biogen, Madrid, Spain). Both were dissolved in DMSO, Tween 80 and distilled water (ratio 1:1:8). In all experiments performed with DBA/2 mice, JWH133 (0.5, 1 and 3 mg·kg−1, i.p.) and AM630 (1, 2 and 3 mg·kg−1, i.p.) were injected 30 min before testing in a volume of 10 ml kg−1. In the delayed reinforcement task, mice received the corresponding dose on the different days of the test phase in which a delay was imposed (a total of 10 injections). Drugs were freshly prepared each day before testing. A total of seven groups of mice (eight mice per group) were employed for pharmacological studies, and therefore, we housed eight mice, corresponding to each dose of drug, per cage.
Results
Behavioural comparisons between A/J and DBA/2 mice
Motor activity
In order to assess the differences in motor activity of both strains, a 30 min open-field session divided into 10 min periods was carried out. A two-way repeated-measures anova (factor 1: strain – two levels: A/J and DBA/2 and factor 2: period – three levels: 0–10, 10–20 and 20–30 min) followed by the Holm–Sidak test revealed that the travelled distance was significantly different between strains (Figures 1A-1) (strain: F1,59= 46.340, P < 0.001; period: F2,59= 13.536, P < 0.001; strain × period interaction: F2,59= 11.948, P < 0.001) and that the mean speed values were also significantly different between A/J and DBA/2 mice (Figure 1A, A2) (strain: F1,53= 15.522, P= 0.004; period: F2,53= 5.008, P= 0.020; strain × period interaction: F2,53= 2.086, P= 0.157). These results clearly indicate that DBA/2 mice presented higher locomotor activity than A/J mice.
Figure 1.
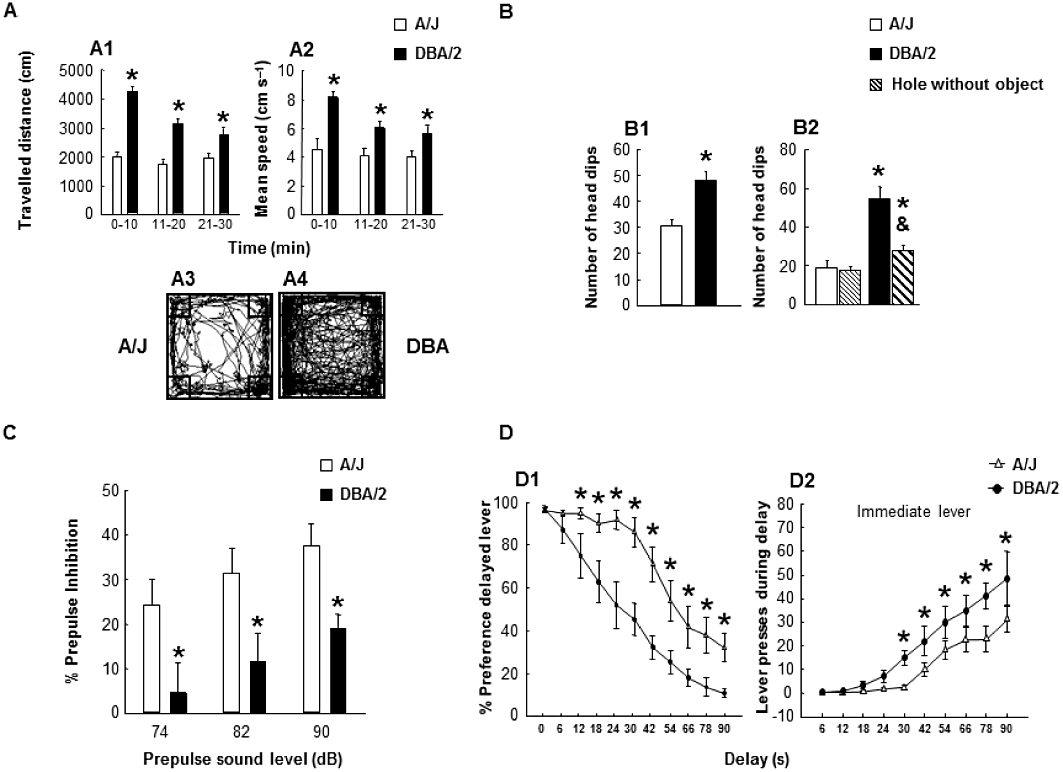
Behavioural assessment to distinguish opposing impulsivity patterns between A/J (n= 10–12) and DBA/2 mice (n= 10–12). (A) Spontaneous locomotor activity in the open field. (A1) Travelled distance and (A2) mean speed were measured in a 30 min session divided in 10 min periods. Data are expressed as mean ± SEM of travelled distance (cm) and mean speed (cm s−1). *P < 0.05, significant difference between DBA/2 mice and A/J mice. (A3 and A4) Representative images of the locomotor activity of the A/J and the DBA/2 mice (SMART tracking analysis). (B) Exploratory behaviour analysis and assessment of novelty seeking behaviour on the hole board task. Data are expressed as mean ± SEM of (B1) number of head dips and (B2) percentage of object preference. *P < 0.05, significant difference between DBA/2 mice and A/J mice; & P < 0.05, significantly different from hole with object. (C) Pre-pulse inhibition of the acoustic startle response using 74, 82 and 90 dB pre-pulse stimuli. Data are expressed as mean ± SEM of % pre-pulse inhibition. *P < 0.05, significant difference between DBA/2 mice and A/J mice. (D) Delay discounting (cognitive impulsivity) and inadequate responding (motor impulsivity) evaluation in the delayed reinforcement task. Data are expressed as mean ± SEM of (D1) % preference for delayed reinforcement and (D2) number of immediate lever presses during time delay. *P < 0.05, significant difference between DBA/2 mice and A/J mice.
Exploration and novelty seeking
Head dipping on the hole board
The numbers of head introductions performed by A/J and DBA/2 mice were evaluated to determine the difference in the level of exploratory behaviour. DBA/2 mice displayed a high exploratory phenotype associated with increased motor activity. This pattern of behaviour induced more head introductions in DBA/2 compared with A/J mice (Figures 1B-1) (Student's t-test: t=−4.087, P < 0.001, 18 d.f.).
Object preference on the hole board
To evaluate the novelty seeking behaviour between holes with or without an object, two objects (white stainless-steel clip and yellow plastic piece) were introduced in two of the four holes of the board, and the preference for the hole with an object over the hole without an object was assessed. A two-way anova (factor 1: strain – two levels: A/J and DBA/2 and factor 2: hole type – two levels: with and without object) revealed that the number of introductions in a hole type was significantly different between DBA/2 and A/J mice (Figure 1B, B2) (strain: F1,39= 33.013, P < 0.001; hole type: F1,39= 12.023, P= 0.001; strain × hole type interaction: F1,39= 9.872, P= 0.003). Post hoc comparisons showed that DBA/2 mice made significantly more head introductions in the holes with an object (69%), whereas A/J mice explored both holes in a similar manner (51%). The exploratory level of DBA/2 mice was higher than that displayed by A/J mice.
Attention
Mice were tested in a pre-pulse inhibition apparatus, and their startle response was analysed with or without a prior pre-pulse stimulus. The presentation of the 74, 82 and 90 dB pre-pulse stimuli revealed a significant difference between strains (Figure 1C): two-way anova (factor 1: strain – two levels: A/J and DBA/2 and factor 2: pre-pulse sound level – three levels: 74, 82 and 90 dB) followed by the Holm–Sidak test (strain: F1,59= 19 129, P < 0.001; pre-pulse sound level: F2,59= 3313, P= 0.044; strain × pre-pulse sound level interaction: F2,59= 0.0166, P= 0.984). These results suggest that the DBA/2 mice had a sensorimotor gating disturbance that probably reflected an attention deficit.
Impulsivity
The A/J and DBA/2 mice were evaluated in the delayed reinforcement task. The delayed discounting rate (change in the percentage of preference for the delayed lever) and inadequate responding (immediate lever presses during delay time) were assessed. Since no delay was imposed on test day 1, no differences were found between strains starting with a similar preference level. On subsequent days, DBA/2 mice discounted delayed rewards more markedly than A/J mice. The differences between the percent preference values in both strains reached statistical significance from the second up to the last delay (Figure 1D, D1): two-way repeated measures anova (factor 1: strain – two levels: A/J and DBA/2 and factor 2: delay – 10 levels: 6, 12, 18, 24, 30, 42, 54, 66, 78 and 90 s) following by the Holm–Sidak test (strain: F1,248= 12.640, P= 0.002; delay: F10,248= 66.366, P < 0.001; strain × delay interaction: F10,248= 3.752, P < 0.001). On the other hand, inadequate pressing of the immediate lever during the delay onset increased progressively, as expected, in both strains, rising significantly (P < 0.05) above the zero at 24 s for DBA/2 and at 42 s for A/J mice. DBA/2 mice performed significantly more inadequate lever presses compared with A/J mice, suggesting a higher motor impulsivity level (Figure 1D, D2): two-way repeated-measures anova, with the Holm–Sidak test (strain: F1,259= 5.383, P= 0.030; delay: F10,259= 30.654, P < 0.001; strain × delay interaction: F10,259= 1.643, P= 0.096). Results for the mean number of trials that mice completed and the mean response time after the end of timeout through the whole task are described in Supplementary Information (Figures S1, S2 and S3).
Expression of the genes for D2 receptors, CB1 receptors and CB2 receptors in selected brain regions
The statistical analyses revealed that D2 receptor gene expression was significantly reduced in the CgCtx (−69%) (Student's t-test: t= 7.866, P < 0.001, 9 d.f.), CPu (−61%) (Student's t-test: t= 3.087, P= 0.009, 13 d.f.) and Acc (−43%) (Student's t-test: t= 3.015, P= 0.013, 10 d.f.) of DBA/2 mice compared with A/J mice (Figure 2A).
Figure 2.
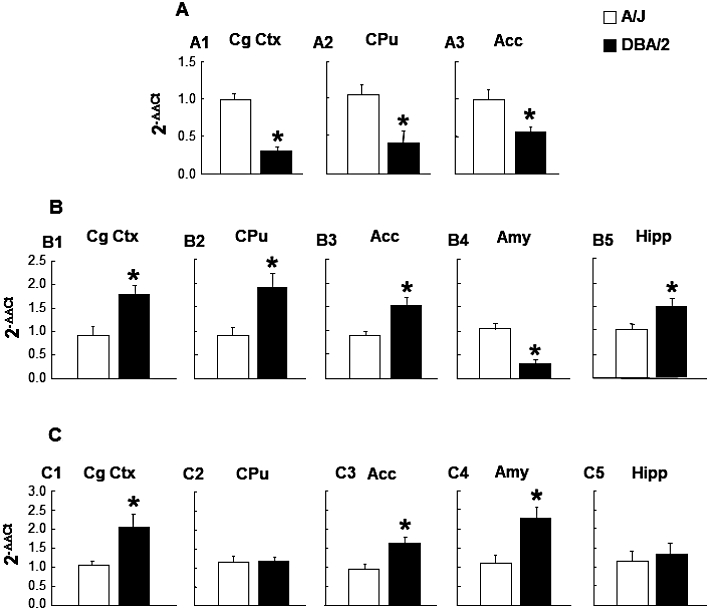
Gene expression analyses by real time-PCR in A/J and DBA/2 mice. Gene expression in DBA/2 mice is expressed relative to A/J. Data are expressed as mean ± SEM of 2−ΔΔCt. (A) D2 receptor gene expression in (A1) CgCtx (A/J n= 5, DBA/2 n= 6) (A2) CPu (A/J n= 9, DBA/2 n= 6) and (A3) Acc (A/J n= 6, DBA/2 n= 6). (B) CB1 receptor gene expression in (B1) CgCtx (A/J n= 5, DBA/2 n= 6) (B2) CPu (A/J n= 8, DBA/2 n= 6) (B3) Acc (A/J n= 7, DBA/2 n= 7) (B4) Amy (A/J n= 8, DBA/2 n= 8) and (B5) Hipp (A/J n= 9, DBA/2 n= 8). (C) CB2 receptor gene expression in (C1) CgCtx (A/J n= 9, DBA/2 n= 7) (C2) CPu (A/J n= 10, DBA/2 n= 10) (C3) Acc (A/J n= 9, DBA/2 n= 9) (C4) Amy (A/J n= 8, DBA/2 n= 9) and (C5) Hipp (A/J n= 10, DBA/2 n= 9). *P < 0.05, significant difference between DBA/2 mice and A/J mice.
CB1 receptor gene expression was significantly higher in the CgCtx (92%) (Student's t-test: t=−3.139, P= 0.012, 9 d.f.), CPu (110%) (Student's t-test: t=−2.958, P= 0.012, 12 d.f.), Acc (66%) (Student's t-test: t=−2.922, P= 0.013, 12 d.f.), and Hipp (47%) (Student's t-test: t=−2.599, P= 0.020, 15 d.f.) of DBA/2 mice, whereas the expression of this gene was markedly lower in the Amy (−68%) (Student's t-test: t= 5.611, P < 0.001, 14 d.f.) compared with A/J mice (Figure 2B).
CB2 receptor gene expression was also investigated in the CgCtx, CPu, Acc, Amy and Hipp. The statistical analyses revealed pronounced increases in the CgCtx (95%) (Student's t-test: t=−2.962, P= 0.010, 14 d.f.), Acc (70%) (Student's t-test: t=−3.244, P= 0.005, 16 d.f.), and Amy (105%) (Student's t-test: t=−3.184, P= 0.006, 15 d.f.) of DBA/2 mice. However, no differences were found in relative gene expression in the CPu (Student's t-test: t=−0.099, P= 0.922, 18 d.f.) or Hipp (Student's t-test: t=−0.455, P= 0.655, 17 d.f.) (Figure 2C).
Effects of JWH133 and AM630 on impulsive-like behaviours in DBA/2 mice
Locomotor activity
In the open field, the administration of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg·kg−1) to DBA/2 mice did not modify travelled distance (Figure 3A): two-way repeated-measures anova (factor 1: treatment – seven levels: vehicle, JWH133 0.5, JWH133 1, JWH133 3, AM630 1, AM630 2 and AM630 3 mg·kg−1 and factor 2: period – three levels: 0–10, 10–20 and 20–30 min) (treatment: F6,167= 0.683, P= 0.664; period: F2,167= 15.355, P < 0.001; treatment × period interaction: F12,167= 0.407, P= 0.958); nor mean speed (Figure 3B): two-way repeated-measures anova (treatment: F6,167= 1.281, P= 0.283; period: F2,167= 3.442, P= 0.036; treatment × period interaction: F12,167= 0.749, P= 0.701).
Figure 3.
Analysis of motor activity in DBA/2 mice after acute administration of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg·kg−1) in the open-field test (n= 8 per group). Data are expressed as mean ± SEM of (A) travelled distance (cm) and (B) mean speed (cm s−1). No differences were found between treatment groups.
Exploratory level and novelty seeking behaviour
The exploratory level on the hole board (number of head dips) did not differ between treatment groups of DBA/2 mice (Figure 4A) (one-way anova, factor treatment –seven levels: vehicle, JWH133 0.5, JWH133 1, JWH133 3, AM630 1, AM630 2 and AM630 3 mg·kg−1, F6,55= 0.910, P= 0.495). The treatment with single doses of JWH133 (0.5, 1 or 3 mg·kg−1) did not change the percentage of object preference in DBA/2 mice (Figure 4B), whereas the administration of AM630 (1, 2 and 3 mg·kg−1) dose-dependently blocked significantly the novelty seeking behaviour (one-way anova followed by the Holm–Sidak test, factor treatment, F6,55= 4.335, P < 0.001).
Figure 4.
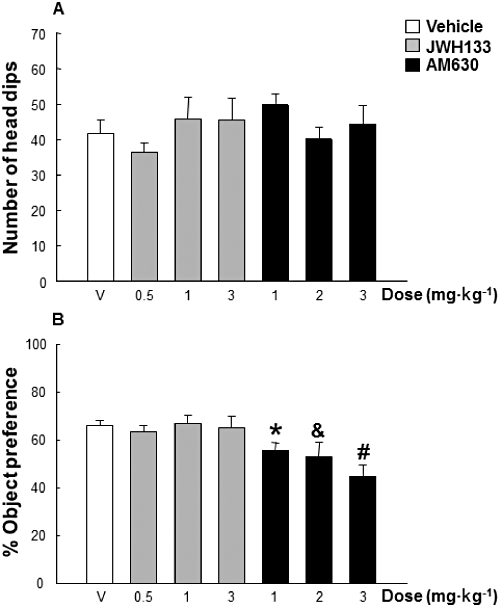
Effects of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg·kg−1) on exploratory level and novelty seeking behaviour of DBA/2 mice (n= 8 per group) on the hole board task. Data are expressed as mean ± SEM of (A) number of head dips and (B) percentage of object preference. *, &, #, P < 0.05, significantly different from the vehicle group, for AM630 (1, 2 or 3 mg·kg−1) treated mice respectively.
Attention deficit
The effects of the acute administration of JWH133 (0.5, 1 and 3 mg·kg−1) and AM630 (1, 2 and 3 mg·kg−1) on the pre-pulse inhibition of DBA/2 mice were measured according to the same protocol described previously (Figure 5). JWH133 did not significantly modify the underlying attention deficit displayed by DBA/2 mice at any pre-pulse sound. On the other hand, AM630 produced a significant improvement of the pre-pulse inhibition of DBA/2 mice at all the pre-pulses (P < 0.05) at the intermediate and highest administered doses (one-way anova, factor treatment – seven levels: vehicle, JWH133 0.5, JWH133 1, JWH133 3, AM630 1, AM630 2 and AM630 3 mg·kg−1, followed by the Holm–Sidak test, 74 dB: F6,55= 2.995, P= 0.014; 82 dB: F6,54= 2.768 P= 0.022; 90 dB: F6,54= 4.896, P < 0.001).
Figure 5.
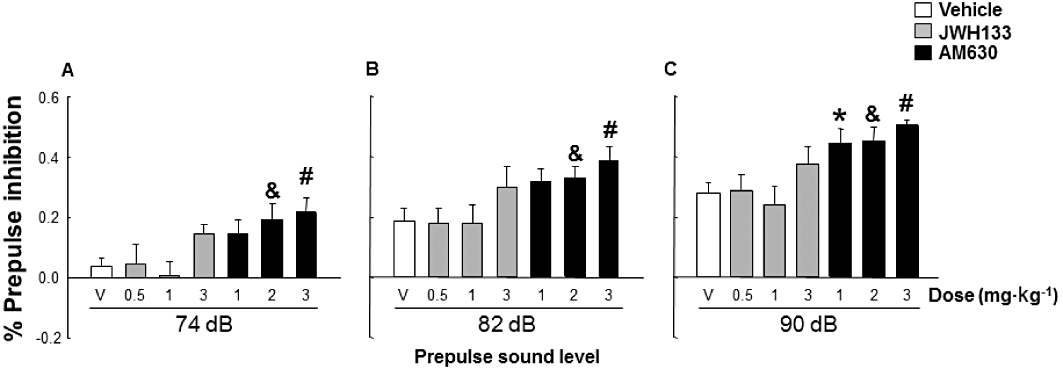
Evaluation of effects of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg·kg−1) on pre-pulse inhibition of DBA/2 mice (n= 8 per group) in the pre-pulse inhibition paradigm. Data are expressed as mean ± SEM of percentage pre-pulse inhibition at (A) 74 dB (B) 82 dB and (C) 90 dB pre-pulse sound levels. *, &, #, P < 0.05, significantly different from the vehicle group, for AM630 (1, 2 or 3 mg·kg−1) treated mice respectively.
Cognitive and motor impulsivity levels
Learning criteria reflecting preference for the large reinforcement which was immediately available as the small reinforcement in the first training phase were achieved after 10 training days. Drug testing began from first delay session with the administration of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg·kg−1). The administration of JWH133 increased, in a dose-dependent way, the percent preference with respect to the control group for the large reinforcement over delays, which reached statistical significance at 24 s (0.5 mg·kg−1), from 12 to 30 s (1 mg·kg−1) and from 12 to 66 s (3 mg·kg−1) time delays (Figure 6A) (two-way repeated-measures anova, factor 1 treatment – seven levels: vehicle, JWH133 0.5, JWH133 1, JWH133 3, AM630 1, AM630 2 and AM630 3 mg·kg−1 and factor 2 delay – 10 levels: 6, 12, 18, 24, 30, 42, 54, 66, 78 and 90 s, followed by the Holm–Sidak test, treatment: F3,351= 2.617, P= 0.071; delay: F10,351= 159.611, P < 0.001; treatment × delay interaction: F30,351= 0.972, P= 0.513). The administration of the antagonist AM630 (1, 2 and 3 mg·kg−1) did not change the percent preference compared with the vehicle group (Figure 6C) (two-way repeated-measures anova followed by the Holm–Sidak test, treatment: F3,351= 0.406, P= 0.750; delay: F10,351= 99.920, P < 0.001; treatment × delay interaction: F30,351= 0.434, P= 0.996).
Figure 6.
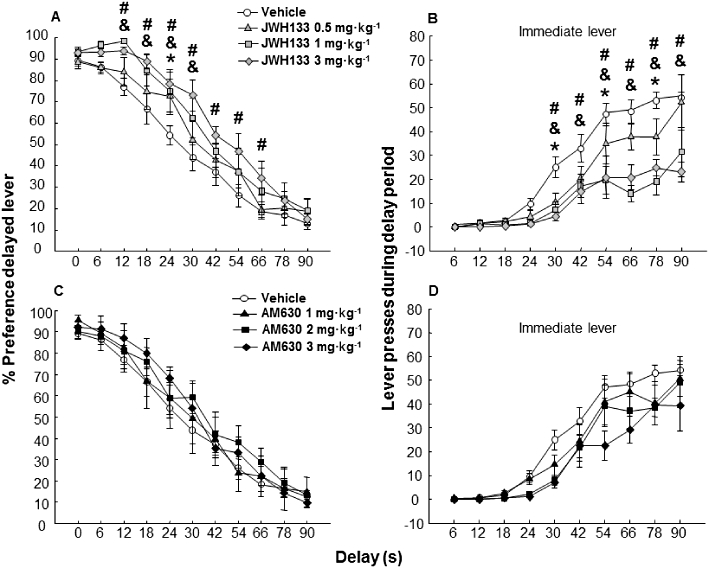
Effects of JWH133 (0.5, 1 and 3 mg·kg−1) or AM630 (1, 2 and 3 mg kg−1) on high cognitive and motor impulsivity level of DBA/2 mice (n= 8 per group) in the delayed reinforcement task in which progressive time delays associated with the large reinforcer were imposed on subsequent days. Data are expressed as mean ± SEM of (A, C) percentage preference for the delayed lever and (B, D) number of inadequate immediate lever presses during time delay. *, &, #, P < 0.05, significantly different from the vehicle group, for JWH133 (0.5, 1 and 3 mg·kg−1) treated mice respectively.
JWH133 treatment significantly reduced the number of immediate lever presses of DBA/2 mice (Figure 6B) (two-way repeated-measures anova followed by the Holm–Sidak test, treatment: F3,319= 9.575, P < 0.001; delay: F9,319= 57.289, P < 0.001; treatment × delay interaction: F27,319= 2.851, P < 0.001), but AM630 did not have any effect on motor impulsivity (Figure 6D) (two-way repeated-measures anova followed by the Holm–Sidak test, treatment: F3,319= 2.995, P= 0.0.048; delay: F9,319= 65.977, P < 0.001; treatment × delay interaction: F27,319= 0.782, P= 0.773).
Expression of the genes for CB2 receptors after sub-chronic JWH133 treatment
The sub-chronic administration of JWH133 (0.5, 1 and 3 mg·kg−1) during the delay phase of the delayed reinforcement task reduced the CB2 receptor gene expression in the brain regions of high-impulsive DBA/2 mice in which there was a higher basal level in comparison with the low-impulsive A/J mice (Figure 7) (one-way anova, factor treatment – four levels: vehicle, JWH133 0.5, JWH133 1 and JWH133 3 mg·kg−1, followed by the Holm–Sidak test, CgCtx: F3,23= 5.934, P= 0.005; Acc: F3,27= 3.311, P= 0.037; Amy: F3,25= 4.478, P= 0.013). Post hoc comparisons revealed that this reduction achieved statistical significance only with the highest dose of JWH133 (3 mg kg−1).
Figure 7.
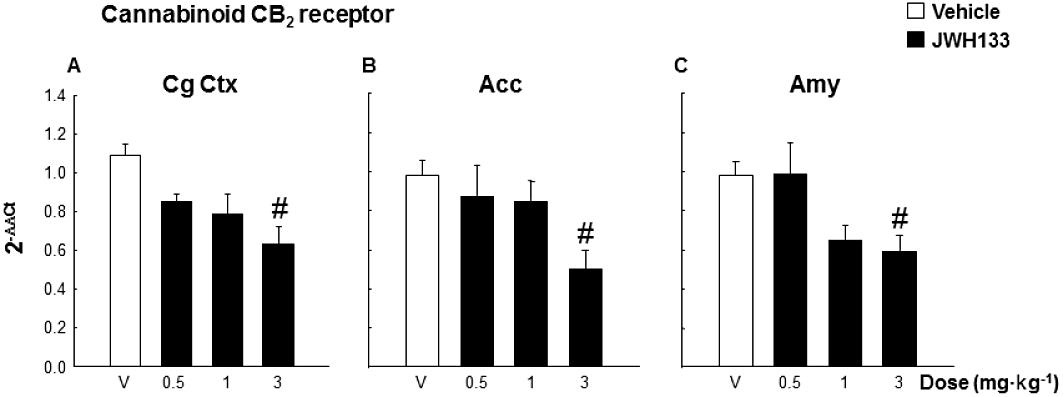
Effects of JWH133 (0.5, 1 and 3 mg kg−1) on CB2 receptor gene expression of DBA/2 mice (n= 8 per group) after sub-chronic treatment during delay phase of the delayed reinforcement task in (A) CgCtx (B) Acc and (C) Amy. Data are expressed as mean ± SEM of 2−ΔΔCt. #P < 0.05, significantly different from the vehicle group.
Discussion
The results of the present study show that differences in the expression of the genes for D2 receptors, CB1 receptors and CB2 receptors could underlie the differences in impulsivity levels between A/J and DBA/2 strains and suggest for the first time that the CB2 receptor is involved in the regulation of impulsive-like behaviours, as treatment with a highly selective CB2 receptor agonist and antagonist was able to modulate certain impulsive markers. These conclusions were based on several findings. First, DBA/2 mice presented motor hyperactivity, increased exploration, novelty seeking behaviour, attention deficit and greater delay discounting and behavioural disinhibition than A/J mice. Significant changes in D2 receptor, CB1 and CB2 receptor gene expressions were found in specific brain regions closely related with impulsive behaviour between A/J and DBA/2 mice. Treatment with the CB2 receptor antagonist AM630 reduced object preference on the hole board task and improved attention in DBA/2 mice. Finally, sub-chronic treatment with the CB2 receptor agonist JWH133 decreased impulsive choice and the number of inadequate responses during the delay phase in DBA/2 mice, an effect that was accompanied by a normalization of the CB2 receptor gene expression (down-regulation).
In the present study, a greater cognitive and motor impulsivity level with increased motor/exploratory activity and impairment of attentional processes was found in the DBA/2 strain. Travelled distance and average speed in the open field test as well as the number of head dips on the hole board were significantly higher in DBA/2 compared to A/J mice. In addition, DBA/2 mice displayed significant impairments in attentional processes in the pre-pulse inhibition paradigm at all three pre-pulses tested. One of the circuits that probably plays a major role in the regulation of motor activity is the nigrostriatal dopaminergic system, which has cell bodies in the substantia nigra that project their axons to the CPu. This region contains a high density of D2 receptors regulating mainly motor activity. The results of this study showed that the most active strain of mice (DBA/2) displayed 61% less D2 receptor gene expression in the CPu compared with A/J mice. The involvement of D2 receptors in impulsive behaviour is based on several findings. Several D2 receptor gene variants were strongly correlated with impulsiveness in ADHD and addictive compulsive behaviours (Blum et al., 1995; Eisenberg et al., 2007). In addition, D2 receptor availability was significantly reduced in the Acc (ventral striatum) of high-impulsive rats using micro-PET (Dalley et al., 2007). In methamphetamine-dependent subjects displaying high impulsivity scores, there was a negative relationship between impulsiveness and striatal D2/D3 receptor availability in the CPu and Acc, measured through PET scanning with [(18)F]fallypride (Lee et al., 2009). Our results, showing decreased (43%) D2 receptor gene expression in the Acc of DBA/2 mice, are in agreement with this previous study. In addition, trait impulsivity in healthy human volunteers was inversely correlated with D2/D3 receptor availability in the substantia nigra/ventral tegmental area (Buckholtz et al., 2010). Similarly, in the CgCtx, a brain region closely involved in higher-order cognitive functions such as reward-related learning, D2 receptor gene expression was significantly lower (69%) in DBA/2 compared with A/J mice. Furthermore, it has been reported that pharmacological modulation by D2 receptor antagonists induced impulsive choice, suggesting that these receptors normally promote choice of the delayed reinforcement (Wade et al., 2000), which is consistent with the results obtained in the delayed reinforcement task and the opposing D2 receptor gene expression patterns observed in A/J and DBA/2 mice.
Recently, it has been established that the endocannabinoid system plays an important role in the control of motor activity (Zimmer et al., 1999), anxiety and cognition (Wotjak, 2005; Lafenetre et al., 2007), emotional behaviour (Valverde, 2005) and memory (Varvel et al., 2002). Cannabinoid neurotransmission has also been implicated in impulsivity (Pattij and Vanderschuren, 2008), since different forms of impulsive behaviours may be pharmacologically dissociated in rats by using selective agonist or antagonist of the CB1 receptor (Pattij et al., 2007). The results of our study revealed a significant increase of CB1 receptor gene expression in CgCtx, Acc, CPu and Hipp, and decreased expression of this gene in the Amy of DBA/2 mice. In contrast, Adriani and collaborators pointed out a reduction in CB1 receptor density in the prefrontal cortex of the SHR (a model of ADHD) (Adriani et al., 2003; Adriani and Laviola, 2004). There are several possible explanations of this discrepancy, as summarised here. First, although rats and mice present similar CB1 receptor brain distribution (Gatley et al., 1996), there are no studies evaluating differences in CB1 receptor gene and protein expression between these species that could be related with behavioural and brain functional differences. Adriani et al. used a gross general prefrontal cortex dissection, whereas in our present study, the CgCtx sub-region of DBA/2 mice was microdissected from 500 µm-thick coronal brain slices using a Ø 22 µm needle. In SHR rats, membrane CB1 receptor protein expression was determined by Western blotting, whereas we measured the CB1 receptor mRNA in DBA/2 mice, by real time-PCR. The different sensitivities of these techniques and intracellular metabolic alterations, such as protein synthesis regulation at a post-transcriptional level or translocation of mRNA transcripts to another neuronal compartment, which can affect mRNA translation rate, could account for differences between gene transcription and final receptor expression on cell membranes.
Human studies support the notion of CB1 receptor gene disruption and potential alterations in impulsive behaviours. Indeed, increased impulsiveness was significantly associated with the six-repeat allele of the triplet repeat polymorphism (AATn/A6), as well as four single nucleotide polymorphisms in or near the CB1 receptor gene in Southwest Californian Indians (Ehlers et al., 2007). Recently, it has been proposed that several CB1 receptor polymorphisms are associated with central effects of the endocannabinoid system, including impulsivity and substance addiction (Dinu et al., 2009). Furthermore, an association between CB1 and childhood ADHD in Spanish male alcoholic patients was also reported, suggesting an important role of this gene in novelty seeking (Ponce et al., 2003), a behaviour that appears to be strongly related to impulsivity (Van Laere et al., 2009) and drug abuse (Kelly et al., 2006). DBA/2 mice showed a clear object preference as evaluated through the head dipping task on the hole board, whereas A/J mice explored holes with or without a novel object equally.
The results of this study revealed that DBA/2 mice have a higher CB2 receptor gene expression in the CgCtx, Acc and Amy compared with A/J mice, but no changes were found in the CPu and Hipp between both strains. Brain regions presenting high CB2 receptor gene expression have been closely related with impulsive behaviour. Furthermore, human studies found evidence for the relevance of the Amy in decision-making processes, considered as a part of an ‘impulsive system’ that triggers emotional responses to immediate outcomes (Bechara, 2005). On the other hand, lesions and functional brain imaging studies have demonstrated a critical involvement of the Acc pattern of neuronal activity in impulsivity (Basar et al., 2010). A few reports suggest enhancement of CB2 receptor gene expression in the brain after chronic mild stress (Onaivi et al., 2008) or reduction of the expression after consumption of ethanol or other drugs of abuse (Ishiguro et al., 2007). Therefore, it appears that functional alterations of the CB2 receptor gene in the brain may be associated with increased vulnerability for anxiety, depressive-like behaviours and consumption of drugs of abuse, pathological states closely related with impulsive behaviour.
As several earlier studies have revealed the involvement of dopamine D2 receptors and CB1 receptors in impulsivity, this study was focused on the potential role of the CB2 receptor in this personality trait. Consequently, the effects of the CB2 receptor agonist JWH133 (0.5, 1 and 3 mg·kg−1) and antagonist AM630 (1, 2 and 3 mg·kg−1) were explored in DBA/2 mice, a strain displaying a high impulsive-like behaviour, employing behavioural paradigms that measure distinct varieties of impulsivity (hyperactivity, high exploration, novelty seeking, attention deficit, high delay discounting and low behavioural inhibition).
Neither travelled distance and mean speed in the open field nor the number of head dips on the hole board parameters were significantly modified by JWH133 or AM630 treatment, excluding any effect on motor or exploratory activity that could interfere with the interpretation of the rest of measured behaviours. Object preference on the hole board task was not affected by the administration of the agonist JWH133, whereas the antagonist AM630 blocked this impulsive-related behaviour in a significant and dose-dependent way. There are no previous studies about the effects of these drugs on novelty seeking behaviour, but the role of the endocannabinoid system in this impulsivity aspect has been recently evaluated through the CB1 receptor. Low baseline CB1 receptor availability in human brains was related to a high novelty seeking personality (Van Laere et al., 2009), emphasizing that this negative correlation is most pronounced in the amygdala in accordance with the results obtained with DBA/2 mice.
The administration of JWH133 did not significantly change the percentage of pre-pulse inhibition with respect to the vehicle group, whereas AM630 significantly attenuated the attention deficit in DBA/2 mice. Several studies have shown that the activation of the endocannabinoid system through synthetic or natural ligands that mainly activate CB1 receptors, impairs sensorimotor gating (Schneider and Koch, 2002; Wegener et al., 2008; Long et al., 2009). On the other hand, the administration of CB1 receptor antagonists produces an opposite effect (Ballmaier et al., 2007). The results of the present study point out that the notable role of the endocannabinoid system in the modulation of sensorimotor gating is not only mediated by CB1 receptors but also by CB2 receptors, as blockade of the latter receptors improved the pre-pulse inhibition of DBA/2 mice.
In the present study, treatment with JWH133 during the delay phase decreased the degree of impulsive choice in DBA/2 mice at the beginning of the task with the intermediate dose (1 mg·kg−1) and also during 42, 54 and 66 s delay times with the highest dose (3 mg·kg−1). On the other hand, AM630 failed to alter DBA/2 cognitive impulsivity level at all tested doses. In addition, JWH133 dose-dependently decreased the number of inadequate responses at the final time delays. Unexpectedly, AM630 treatment showed a similar tendency without reaching statistical significance. It is possible that the increased CB2 receptor gene expression in CgCtx, Acc and Amy found in DBA/2 mice was related to the level of impulsive behaviour. On this basis, acute blockade of CB2 receptors would tend to reduce impulsivity level (as observed in the object preference and the pre-pulse inhibition tasks), whereas the acute activation of the receptor would enhance this behaviour. However, in the delayed reinforcement task, administration of JWH133 improved impulsivity level, whereas AM630 was without effects. These apparently opposite effects of the CB2 receptor agonist JWH133 may be related to the neuroadaptative receptor regulation occurring after repeated treatment (10 days' treatment during the test phase) that would produce a down-regulation. As expected, treatment with the CB2 receptor agonist JWH133 was able to down-regulate CB2 receptor gene expression in CgCtx, Acc and Amy, reaching statistical significance only with the highest dose (3 mg·kg−1). These results seem to indicate that the modulation of cognitive and motor impulsivity achieved with JWH133 treatment would be related to the regulation of CB2 receptors. However, it is possible that, despite using highly selective CB2 receptor ligands, these effects might not be exclusively mediated by CB2 receptors, and further studies combining both ligands (agonist and antagonist) are necessary to confirm the specific participation of the CB2 receptor. In addition, the precise molecular mechanisms by which CB2 receptor ligands are regulating impulsive behaviours remains to be further explored.
In conclusion, the present study provides new information about gene expression differences underlying opposing impulsivity patterns and the involvement of the CB2 receptor in the modulation of different varieties of impulsive behaviour, such as novelty seeking, attention deficit or impulsive choice/response, suggesting the possible therapeutic usefulness of this target in impulsivity-related disorders.
Acknowledgments
This study was supported by grant 2007/061 from ‘Plan Nacional Sobre Drogas’ (PNSD, Spanish Ministry of Health) to JM and by ‘Red Temática de Investigación Cooperativa en Salud’ (RETICS, Instituto de Salud Carlos III, MICINN and FEDER, Madrid, Spain, ‘Red de Trastornos Adictivos’, RD06/0001/1004) to JM. FN is a pre-doctoral fellow supported by MICINN (Ministerio de Ciencia e Innovación). JMP-O is a postdoctoral fellow supported by Fundación sociosanitaria de Castilla La Mancha. We thank Patricia Rodríguez and Analía Rico for excellent technical assistance. AR and PR are technicians supported by RETICS and Fundación sociosanitaria de Castilla La Mancha respectively.
Glossary
Abbreviations
- Acc
accumbens
- ADHD
attention-deficit hyperactivity disorder
- Amy
amygdala
- CgCtx
cingulate cortex
- CPu
caudate-putamen
- Hipp
hippocampus
- SHR
spontaneously hypertensive rats
- SMART
spontaneous motor activity recording and tracking
Conflicts of interest
All authors report no biomedical financial interests or potential conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Mean number of completed trials and mean response time analysis between AJ and DBA/2 mouse strains in the training and test phases of the delayed reinforcement task. Data are expressed as mean ± SEM of (A) mean number of completed trials during each 30 min session and (B) mean waiting spontaneous time during ITI before initiating a new trial (A/J n = 12, DBA/2 n = 12). *, values from DBA/2 mice that are significantly different (P < 0.05) from A/J mice.
Figure S2 Evaluation of the effects of JWH133 on the mean number of completed trials and mean response time parameters between JWH133-treated and vehicle groups in the training and test phases of the delayed reinforcement task. Data are expressed as mean ± SEM of (A) the mean number of completed trials during each 30 min session and (B) the mean waiting spontaneous time during ITI before initiating a new trial (vehicle group: n = 8; JWH133 groups (0.5, 1 and 3 mg·kg−1): n = 8).
Figure S3 Evaluation of the effects of AM630 on the mean number of completed trials and mean response time parameters between AM630-treated and vehicle groups in the training and test phases of the delayed reinforcement task. Data are expressed as mean ± SEM of (A) mean number of completed trials during each 30 min session and (B) mean waiting spontaneous time during ITI before initiate a new trial (vehicle group: n = 8; AM630 groups (1, 2 and 3 mg·kg−1): n = 8). **, values from DBA/2 AM630 2 mg·kg−1 treated mice significantly different (P < 0.05) from the vehicle group.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Bortolato M, Rizzetti C, Zoli M, Gessa G, Heinz A, et al. Cannabinoid receptor antagonists counteract sensorimotor gating deficits in the phencyclidine model of psychosis. Neuropsychopharmacology. 2007;32:2098–2107. doi: 10.1038/sj.npp.1301344. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, Mcnamara R, Theobald DE, Castel A, Beckett VL, et al. Dissociable Control of Impulsivity in Rats by Dopamine D2/3 Receptors in the Core and Shell Subregions of the Nucleus Accumbens. Neuropsychopharmacology. 2009;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behav Genet. 2000;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann NY Acad Sci. 2008;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinu IR, Popa S, Bicu M, Mota E, Mota M. The implication of CNR1 gene's polymorphisms in the modulation of endocannabinoid system effects. Rom J Intern Med. 2009;47:9–18. [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the cannabinoid receptor gene (CNR1) and impulsivity in southwest California Indians. Twin Res Hum Genet. 2007;10:805–811. doi: 10.1375/twin.10.6.805. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Campbell B, Mackillop J, Lum JK, Wilson DS. Season of birth and dopamine receptor gene associations with impulsivity, sensation seeking and reproductive behaviors. PLoS ONE. 2007;2:e1216. doi: 10.1371/journal.pone.0001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A, Rizzo C, McGeary JE, Knopik VS. Associations of the DRD2 TaqIA polymorphism with impulsivity and substance use: preliminary results from a clinical sample of adolescents. Pharmacol Biochem Behav. 2009;93:306–312. doi: 10.1016/j.pbb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–212. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T, et al. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67:974–982. doi: 10.1016/j.biopsych.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal Dopamine D2/D3 Receptor Availability Is Reduced in Methamphetamine Dependence and Is Linked to Impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, Mcgregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol. 2009;13:861–876. doi: 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- Mcdonald J, Schleifer L, Richards JB, De Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/ hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otobe T, Makino J. Impulsive choice in inbred strains of mice. Behav Processes. 2004;67:19–26. doi: 10.1016/j.beproc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Punch sampling biopsy technique. Methods Enzymol. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Schepers I, Gonzalez-Cuevas G, De Vries TJ, Schoffelmeer AN. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 2007;193:85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press. Harcourt Science and Technology Company; 2001. [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Ponce G, Hoenicka J, Rubio G, Ampuero I, Jimenez-Arriero MA, Rodriguez-Jimenez R, et al. Association between cannabinoid receptor gene (CNR1) and childhood attention deficit/hyperactivity disorder in Spanish male alcoholic patients. Mol Psychiatry. 2003;8:466–467. doi: 10.1038/sj.mp.4001278. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The cannabinoid agonist WIN 55,212-2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol. 2002;13:29–37. doi: 10.1097/00008877-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG. Impulsivity: a link between bipolar disorder and substance abuse. Bipolar Disord. 2004;6:204–212. doi: 10.1111/j.1399-5618.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Valverde O. Participation of the cannabinoid system in the regulation of emotional-like behaviour. Curr Pharm Des. 2005;11:3421–3429. doi: 10.2174/138161205774370780. [DOI] [PubMed] [Google Scholar]
- Van Gaalen MM, Van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Bormans G, Casteels C, Mortelmans L, De Hoon J, et al. Relationship of type 1 cannabinoid receptor availability in the human brain to novelty-seeking temperament. Arch Gen Psychiatry. 2009;66:196–204. doi: 10.1001/archgenpsychiatry.2008.530. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wade TR, De Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wegener N, Kuhnert S, Thuns A, Roese R, Koch M. Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl) 2008;198:375–385. doi: 10.1007/s00213-008-1148-1. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem. 2005;5:659–670. doi: 10.2174/1389557054368763. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biol Psychiatry. 2007;62:616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


