Abstract
Introduction
Surgical resection remains the only potentially curative treatment for colorectal liver metastases (CLM). However, involvement of both the hepatic lobes or extrahepatic disease (EHD) can be a contra-indication for resection. The aim of the present study was to examine the addition of combined positron emission and computed tomography (PET/CT) to CLM staging to assess the effects upon staging and management.
Methods
All CLM patients referred to a single centre between January 2005 and January 2009 were prospectively included. All underwent routine staging (clinical examination and computed tomography), followed by a whole body 18fluoro-deoxy-glucose (18FDG)-PET/CT scan and Fong clinical risk score calculation.
Results
Sixty-four patients were included [63% male with a median age of 63 years (age range 32–79 years)]. The addition of PET/CT led to disease upstaging in 20 patients (31%) and downstaging in two patients (3%). EHD was found in 24% of low-risk patients (Fong score 0–2) as compared with 44% of high-risk patients (Fong score 3–5) (P = 0.133). There was a trend towards a greater influence upon management in patients with a low score (44% vs. 17%; P = 0.080).
Conclusion
The addition of PET/CT led to management changes in over one-third of patients but there was no correlation between alterations in staging or management and the Fong clinical risk score; suggesting that PET/CT should be utilized, where available, in the pre-operative staging of CLM patients.
Keywords: colorectal metastases, liver resection, liver
Introduction
Over 37 000 diagnoses of colorectal cancer (CRC) are made per annum in the UK, up to 9000 of which will have liver metastases at the time of diagnosis,1 and approximately 50% of which will develop spread to the liver during the course of disease; where they will be responsible for up to two-thirds of all CRC deaths.2 The management of colorectal liver metastases (CLM) continues to evolve but surgical resection remains the only potentially curative treatment and the current approach is that resection is indicated if the metastases are resectable and sufficient residual liver will remain post-operatively to prevent hepatic failure.3–5 Current contra-indications to resection include a non-treatable primary tumour or widespread nodal, locoregional or metastatic (pulmonary, bony and peritoneal) disease; although, certain patients with resectable or ablatable extrahepatic disease (EHD) remain suitable for resection.6
Overall, survival rates after resection for CLM are 26–60% at 5 years,4,7–9 17–26% at 10 years4,10–12 and 26% for those with EHD;13 whereas, post-operative mortality is 1.5–2.8%.4,11 Recurrence within the liver is the commonest cause of treatment failure; however, second or third resections can be performed for recurrent metastases with similar 5-year survival rates to first resection (34% and 32% respectively) and an acceptable post-operative morbidity rate.14
However, even within selected subgroups of patients with resectable disease, there is wide variability in recurrence rates and survival after resection. Independent predictors of poor survival after resection include multiple hepatic metastases, a poorly-differentiated or node positive primary tumour, EHD, tumour diameter ≥5 cm, significantly raised carcinoembryonic antigen levels (CEA), positive resection margins and a short disease-free interval from development of primary to development of metastases (less than 12 months).4,11 Reliable, prognostically accurate and widely-applicable molecular markers for CLM are yet to be identified, and such disease-related clinical variables are often utilized to risk-stratify patients.11,15 In 1999 Fong et al.11 assessed 1001 consecutive CLM patients to become the first group to synthesize a pre-operative clinical risk score. The Fong score incorporated node status, number and size of tumour, disease-free interval and CEA level (see Table 1) in a fashion that was found to be highly predictive of outcome: low scores (two or less out of five) were associated with a favourable outcome, whereas high scores (five out of five) were not associated with a long-term prognosis (see Fig. 1).
Table 1.
Fong score criteria for determination of prognosis in patients with colorectal liver metasatasis (CLM). The patient scores one point for each criteria they meet (adapted from Fong et al.11)
| Lymph node + ve primary tumour |
| Disease-free interval < 12 months |
| >1 Tumour |
| Tumour size > 5 cm |
| CEA level > 200 ng/ml |
CEA, carcinoembryonic antigen.
Figure 1.
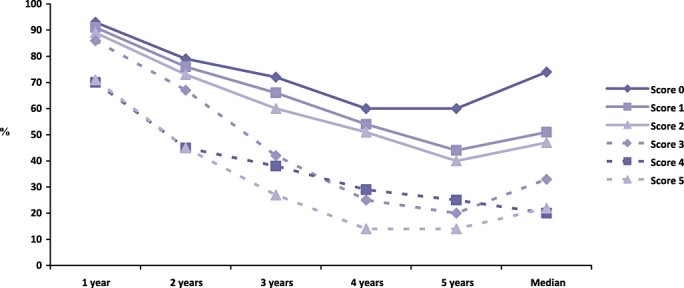
The Fong clinical risk score predicts survival up to 5 years in patients with colorectal liver metastases, as calculated from 1001 consecutive patients by Fong et al. (1999) (adapted from Fong et al.11)
The accuracy of pre-operative staging is pivotal for the correct implementation of such scoring systems and a number of modalities are routinely utilized to image the liver, including ultrasound (US), contrast-enhanced multi-detector computed tomography (CEMDCT) and magnetic resonance imaging (MRI).16 However, many of these techniques remain limited as the tumour burden is often underestimated, particularly in extra-hepatic disease.17 Combined positron emission and computerized tomography (PET/CT), utilizing 18fluro-deoxy glucose (18FDG) produces a fusion image combining conventional cross-sectional, anatomical imaging of CT with the biological, functional imaging of PET.18 It can be utilized to identify and stage primary colorectal tumours19 as well as metastases18 and has also been used to great effect in the staging of anal squamous cell,20 pancreatic21 and lung22 cancers.
The present study examined the pre-treatment staging of CLM by PET/CT to investigate whether it could alter the staging of local or distant disease and management. Further, we aimed to compare the Fong clinical risk score with PET/CT yield in an attempt to stratify the use of PET/CT in patients with CLM.
Methods
Patients and staging
All patients referred to a single centre for potential resection of CLM, between January 2005 and January 2009, were prospectively included in the present study. All had previously undergone R0 (complete histological clearance) resection of a primary colorectal tumour and all patients underwent routine staging, consisting of clinical examination and a CEMDCT scan of the thorax, abdomen and pelvis. All patients also underwent a whole body 18FDG-PET/CT scan performed within 2 weeks of CEMDCT at the same institution, as part of their standard pathway of care (therefore, no ethical approval was sought for the study).
A clinical risk score was calculated for each patient (range 0–5), as per the Fong criteria (see Table 1 and Fig. 1):11 a clearly-defined and widely-applicable score of clinical criteria for the selection of patients likely to benefit from resection of CLM. The patients were divided into low risk (Fong score 0–2; five-year survival 40% or higher) and high risk for recurrence (Fong score 3–5; five-year survival 20% or less), as per the original scoring system.
All patients were reviewed in a specialist hepatobiliary, multi-disciplinary team (MDT) meeting, consisting of hepatobiliary surgeons, oncologists, pathologists and radiologists with an interest in radiofrequency ablation (RFA). The MDT was initially blinded to the results of the PET/CT scans, which were reported by a dual-trained nuclear medicine and radiology physician with over 5 years experience of reviewing PET/CTs, and who in turn was blinded to the results of the standard imaging. A PET/CT lesion was identified as being ‘positive’ according to the considered opinion of the reporting physician. An initial management decision was made based upon standard imaging and the results of the PET/CT scan were subsequently revealed and a new management plan created. The results of the PET/CT scan results were then correlated with the Fong clinical risk score.
PET/CT protocol
One hour before the examination, 370MBq of 18FDG was injected intravenously. Utilizing a dedicated combined GE Discovery LS PET/CT unit (GE Advance PET scanner and the GE Light-speed CT; General Electric Company, Fairfield, CT, USA), whole-body examinations were performed with the patient supine and arms held above the head. CT was performed using four 3.75-mm detectors, a pitch of 1.5 and a 5-mm collimation. The CT exposure factors for all examinations were 140 kVp and 80 mA in 0.8 s. Maintaining the patient position, a whole body PET emission scan was performed, covering an area identical to that of a CT (5–6 bed positions). All acquisitions were carried out in a 2-dimensonal (2D) mode and consisted of emission scans of 5 min per bed position. PET images were reconstructed using CT for attenuation correction by employing CT maps. Transaxial emission images of 4.3 × 4.3 × 4.25 mm3 (in plane matrix size 128 × 128) were reconstructed using ordered subsets expectation maximization (OSEM) with two iterations and 28 subsets. The axial field of view was 148.75 mm, resulting in 35 slices per bed position.
Statistical analysis
Statistical analysis was performed using an SPSS (Statistical Package for the Social Sciences Inc., Chicago, IL, USA) software package. Two-tailed P-values less than 0.05 were considered statistically significant.
Results
Demographics
Sixty-four consecutive patients with colorectal liver metastases, all of which had undergone PET/CT as part of their standard pathway of care, were included in the present study. Forty (63%) were male and the median age was 63 years (age range 32–79 years). None of the patients underwent neoadjuvant chemotherapy.
Effect of PET/CT inclusion in the staging protocol
Inclusion of PET/CT in the staging protocol resulted in additional information in 28 patients (43%) and a change of management in 22 patients (34%).
Management changes occurred secondary to disease upstaging in 20 patients (31%) and downstaging in two patients (3%).
Disease upstaging occurred as a result of the discovery of peritoneal disease in six patients, multiple lung metastases in six patients, retroperitoneal lymph nodes in four patients, mediastinal lymph nodes in two patients, porta hepatis nodes in one patient and a previously undiagnosed thyroid cancer with bone metastases in a further patient. Thirteen out of 20 lesions identified were new, whereas seven had been labelled as indeterminate or suspicious on CT alone (two patients with peritoneal disease, two with retroperitoneal lymphadenopathy, two with lung lesions and one with porta hepatis nodes). The patient with thyroid cancer underwent surgical resection to remove the thyroid lesion; however, primary treatment for CLM in upstaged patients was changed from surgical resection to chemotherapy.
The two patients downstaged were reported to have lung metastases on CEMDCT; however, the lung lesions were not FDG avid and were therefore thought to represent chronic airways disease after PET/CT. Both patients were subsequently deemed fit for surgery after pre-operative optimization.
Comparison with Fong clinical risk scores
Of the 64 patients, 46 (72%) had Fong scores of 0–2 (low-risk of recurrence) and 18 (28%) had scores of 3–5 (high-risk of recurrence).
Eleven out of 46 low-risk patients were found to have EHD on PET/CT, as compared with eight out of 18 high-risk patients (Fisher's exact test: P = 0.133). However, although not significant, there was a trend towards a greater influence upon management in those patients with a low clinical risk score (0–2): 20 out of 46 low-risk patients underwent management alterations after PET/CT, as compared with only three out of 18 high-risk patients with a clinical risk score of 3–5 (Fisher's exact test: P = 0.080).
Discussion
The results of the present study demonstrate that the use of PET/CT in CLM patients is associated with alterations in patient management in approximately one-third of patients (34%) (particularly owing to disease upstaging) but that there was no correlation between alterations in staging or management and the Fong clinical risk score.
Biological and multi-modality imaging, in the form of PET and PET/CT, are now being increasingly utilized within the UK. PET/CT works via the preferential accumulation and metabolic trapping of a radiolabelled glucose analogue, 2-[18 F] fluoro-2-deoxy-D-glucose (FDG), within malignant cells.18,23 The functional nature of the image rendered, which relies upon a metabolic evaluation of glucose uptake, rather than size or morphological criteria alone, may allow the evaluation of smaller tumours at an earlier point in their natural history, and a true integrated PET/CT system also allows good anatomical definition by circumventing the lack of an anatomical reference frame found with isolated PET.
A meta-analysis performed by Kinkel et al. in 200224 found that FDG-PET was the most sensitive method for the detection of liver metastases from multiple gastrointestinal origins, with a mean weighted sensitivity of 90–92%, a result confirmed by Arulampalam et al.25,26 in two separate studies: one investigating 42 patients with suspected recurrent CRC to discover that FDG-PET was more accurate than CT (sensitivity 100% vs. 45%; specificity 100% for both) and one in 28 patients with confirmed CLM to demonstrate a sensitivity and specificity of FDG-PET of 100% and 91%, respectively, as compared with 47% and 91% for CT. More recent meta-analyses confirm these initial results. A meta-analysis of 25 articles published between 2000 and 2008 demonstrated that sensitivity and specificity, on a per-patient basis, for ultrasound, CT, MRI and FDG-PET in the detection of CLM were 63.0% and 97.6%, 74.8% and 95.6%, 81.1% and 97.2, and 93.8% and 98.7%, respectively; whereas, on a per lesion basis sensitivity was 86.3%, 82.6%, 86.3% and 86.0%, respectively.27 Further, Wiering et al.28 found that FDG-PET had a pooled sensitivity and specificity of 88.0% and 96.1%, respectively, for hepatic disease, and 91.5% and 95.4% for EHD, results that were significantly higher than CT (82.7% and 84.1% for hepatic; 60.9% and 91.1% for EHD).
Integrated PET/CT has also been shown to be superior to PET alone in the assessment of primary colorectal tumours,19 as well as recurrent and metastatic disease;29 and results to date suggest that it may also be superior to many imaging modalities in the assessment of liver metastases. Cohade et al.19 performed a retrospective review of 45 CRC patients to show that more definitely normal and definitely abnormal lesions were identified with PET/CT than with PET alone. The accuracy of staging and restaging also improved from 78% to 89%.
Grassetto et al.30 similarly assessed a mixed cohort of patients with solitary liver metastases (18 CRC, 15 non-small cell lung cancer, six breast carcinoma and four ovarian cancer) to demonstrate that PET/CT restaged disease and changed therapy in 28% of patients. Chua et al.31 compared CEMDCT and 18FDG-PET/CT in 131 patients with liver metastases (75 CRC and 56 other tumours) to show that PET/CT yielded a sensitivity and specificity of 94% and 75%, compared with 91% and 25%, respectively, for CEMDCT. Improvements in staging accuracy were associated with alterations in patient management in 25% of patients.
Discovery of such EHD has been shown to be crucial in preventing unnecessary intervention. Wiering et al.32 prospectively compared 100 CLM patients staged via conventional techniques, with 103 patients staged with an additional FDG-PET to demonstrate a reduction in the number of negative laparotomies by more accurate prediction of EHD. Similarly, Ruers et al.33 randomized 150 CLM patients selected for surgical resection by CT imaging alone (n = 75) or CT plus FDG-PET (n = 75) to demonstrate a significantly decreased number of futile laparotomies (45% vs. 28%; P = 0.042).
In those deemed unsuitable for operative intervention, systemic chemotherapy regimens can be used in a subset (12.5%34) of patients to reduce tumour load and potentially allow certain patients to subsequently undergo resection.34–36 The use of FDG-PET in such circumstances may have additive effects in the monitoring of tumour metabolism and response: Vriens et al.37 examined the effects upon CLM functional tumour metabolism assessed by FDG-PET, prior to and following three chemotherapy cycles in 23 CLM patients to find that glucose metabolic rates decreased significantly during treatment (P < 0.001).
Currently, PET/CT is not readily available within the UK: the technology remains relatively novel, as well as being expensive and constantly evolving, and there is a shortage of PET/CT scanners, as well as trained and experienced staff available to operate them.38 Further, new technologies are being constantly discovered and investigated, with some groups even looking at computer-automated discovery of liver lesions.39,40
After the development of the Fong score, five large predictive models have been published with considerable overlap of key independent predictors of long-term outcome. Utilization of such prognostic scores yields stark differences between patients in good- and poor-risk categories, with 5-year survival rates ranging from 60% to <15%, respectively.4,11,15 However, there remains a need for a universally-adopted predictive model,41 which may be used to pre-operatively improve selection for surgery, and post-operatively risk-stratify patients who may benefit from intensive surveillance and adjuvant therapies.
Conclusion
This article presents 64 prospectively-studied CLM patients to demonstrate that the addition of PET/CT to staging provided additional information, in particular the discovery of EHD that led to management changes in 34% of patients. These data suggest that PET/CT should be used where available in the pre-operative staging of these patients.
The influence on management provided by the addition of PET/CT was undoubtedly greater in the lower risk group (44%) than the high risk group (14%) but this failed to reach statistical significance. Based on this series, the Fong clinical risk score should not be used to rationalize the use of PET/CT in those patients being investigated for potential resection of CLM.
Acknowledgments
This article and its contents have not been submitted simultaneously to another journal, have not been accepted for publication elsewhere and have not already been published in their present format.
J.R.A.S., who is supported as the ‘Jason Boas HPB Fellow’ by the No Surrender Charitable Trust, and A.H.E. drafted the manuscript. All authors have significantly contributed to, read and approved the final manuscript.
Conflicts of interest
None declared.
References
- 1.CRUK. Liver surgery leads to 'remarkable' boost for bowel cancer survival: National Cancer Intelligence Network Press Release. 2010. http://info.cancerresearchuk.org/news/archive/pressrelease/2010-06-02-liver-surgery-boost-bowel-survival [Accessed 04 November 2011]
- 2.Faivre J, Manfredi S, Bouvier AM. [Epidemiology of colorectal cancer liver metastases] Bull Acad Natl Med. 2003;187:815–822. discussion 822–823. [PubMed] [Google Scholar]
- 3.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 6.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto Tand Okinaga K. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79–83. doi: 10.1007/s00534-002-0778-7. [DOI] [PubMed] [Google Scholar]
- 9.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 10.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpizo DR, D'Angelica M. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138–2146. doi: 10.1245/s10434-009-0521-6. [DOI] [PubMed] [Google Scholar]
- 14.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. doi: 10.1097/01.sla.0000098112.04758.4e. discussion 883–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minagawa M, Yamamoto J, Kosuge T, Matsuyama Y, Miyagawa S, Makuuchi M. Simplified staging system for predicting the prognosis of patients with resectable liver metastasis: development and validation. Arch Surg. 2007;142:269–276. doi: 10.1001/archsurg.142.3.269. discussion 277. [DOI] [PubMed] [Google Scholar]
- 16.Robinson PJ. Imaging liver metastases: current limitations and future prospects. Br J Radiol. 2000;73:234–241. doi: 10.1259/bjr.73.867.10817037. [DOI] [PubMed] [Google Scholar]
- 17.Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis–meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 18.Papathanassiou D, Bruna-Muraille C, Liehn JC, Nguyen TD, Curé H. Positron Emission Tomography in oncology: present and future of PET and PET/CT. Crit Rev Oncol Hematol. 2009;72:239–254. doi: 10.1016/j.critrevonc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18) F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 20.Engledow AH, Skipworth JR, Blackman G, Groves A, Bomanji J, Warren SJ, et al. The role of 18fluoro-deoxy glucose combined position emission and computed tomography in the clinical management of anal squamous cell carcinoma. Colorectal Dis. 2011;13:532–537. doi: 10.1111/j.1463-1318.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- 21.Tann M, Sandrasegaran K, Jennings SG, Skandarajah A, McHenry L, Schmidt CM. Positron-emission tomography and computed tomography of cystic pancreatic masses. Clin Radiol. 2007;62:745–751. doi: 10.1016/j.crad.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Goerres GW, Kamel E, Seifert B, Burger C, Buck A, Hany TF, et al. Accuracy of image coregistration of pulmonary lesions in patients with non-small cell lung cancer using an integrated PET/CT system. J Nucl Med. 2002;43:1469–1475. [PubMed] [Google Scholar]
- 23.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 24.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 25.Arulampalam T, Costa D, Visvikis D, Boulos P, Taylor I, Ell P. The impact of FDG-PET on the management algorithm for recurrent colorectal cancer. Eur J Nucl Med. 2001;28:1758–1765. doi: 10.1007/s002590100646. [DOI] [PubMed] [Google Scholar]
- 26.Arulampalam TH, Francis DL, Visvikis D, Taylor I, Ell PJ. FDG-PET for the pre-operative evaluation of colorectal liver metastases. Eur J Surg Oncol. 2004;30:286–291. doi: 10.1016/j.ejso.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Floriani II, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- 28.Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658–2670. doi: 10.1002/cncr.21569. [DOI] [PubMed] [Google Scholar]
- 29.Metser U, You J, McSweeney S, Freeman M, Hendler A. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. AJR Am J Roentgenol. 2010;194:766–771. doi: 10.2214/AJR.09.3205. [DOI] [PubMed] [Google Scholar]
- 30.Grassetto G, Fornasiero A, Bonciarelli G, Banti E, Rampin L, Marzola MC, et al. Additional value of FDG-PET/CT in management of ‘solitary’ liver metastases: preliminary results of a prospective multicenter study. Mol Imaging Biol. 2010;12:139–144. doi: 10.1007/s11307-009-0249-5. [DOI] [PubMed] [Google Scholar]
- 31.Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, et al. The impact of 18F-FDG PET/CT in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2007;34:1906–1914. doi: 10.1007/s00259-007-0518-y. [DOI] [PubMed] [Google Scholar]
- 32.Wiering B, Krabbe PF, Dekker HM, Oyen WJ, Ruers TJ. The role of FDG-PET in the selection of patients with colorectal liver metastases. Ann Surg Oncol. 2007;14:771–779. doi: 10.1245/s10434-006-9013-0. [DOI] [PubMed] [Google Scholar]
- 33.Ruers TJ, Wiering B, van der Sijp JR, Roumen RM, de Jong KP, Comans EF, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med. 2009;50:1036–1041. doi: 10.2967/jnumed.109.063040. [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahajpal A, Vollmer CM, Jr, Dixon E, Chan EK, Wei A, Cattral MS, et al. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol. 2007;95:22–27. doi: 10.1002/jso.20632. [DOI] [PubMed] [Google Scholar]
- 36.Scoggins CR, Campbell ML, Landry CS, Slomiany BA, Woodall CE, McMasters KM, et al. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann Surg Oncol. 2009;16:35–41. doi: 10.1245/s10434-008-0190-x. [DOI] [PubMed] [Google Scholar]
- 37.Vriens D, van Laarhoven HW, van Asten JJ, Krabbe PF, Visser EP, Heerschap A. Chemotherapy response monitoring of colorectal liver metastases by dynamic Gd-DTPA-enhanced MRI perfusion parameters and 18F-FDG PET metabolic rate. J Nucl Med. 2009;50:1777–1784. doi: 10.2967/jnumed.109.064790. [DOI] [PubMed] [Google Scholar]
- 38.CancerResearchUK (2010) PET-CT scan [WWW document]. URL http://www.cancerhelp.org.uk/about-cancer/tests/petct-scan#uk [Accessed 4 November 2011]
- 39.Hahn S, Heusner T, Zhou X, Zhan Y, Peng Z, Hamami M, et al. Computer-aided detection (CAD) and assessment of malignant lesions in the liver and lung using a novel PET/CT software tool: initial results. Rofo. 2010;182:243–247. doi: 10.1055/s-0028-1109833. [DOI] [PubMed] [Google Scholar]
- 40.Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B, et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51:692–699. doi: 10.2967/jnumed.109.068510. [DOI] [PubMed] [Google Scholar]
- 41.Welsh FK, Tekkis PP, John TG, Rees M. Predictive models in colorectal liver metastases–can we personalize treatment and outcome? Dig Surg. 2008;25:406–412. doi: 10.1159/000184731. [DOI] [PubMed] [Google Scholar]


