Abstract
Background
The inclusion of hepatitis B core antibody-positive (HBcAb+) liver donors is a strategy utilized to increase organ availability. This study examined HBcAb+ transplantation practices to identify specific factors influencing outcomes.
Methods
Twenty-five HBcAb+ liver transplants were identified retrospectively among 868 adult transplants performed between 1 January 1997 and 31 December 2009. Twelve (48%) recipients had hepatitis C and five (20%) had hepatitis B. Patient and donor demographics, preoperative morbidity, transplant data and outcomes were examined. Statistical analysis was completed using Student's t-test or the Kaplan–Meier method. A P-value of <0.05 was considered significant.
Results
There was no difference in age, body mass index or comorbidities between HBcAb+ liver recipients and control subjects. Model for End-stage Liver Disease (MELD) scores of >30 were significantly more frequent in HBcAb+ liver recipients (32% vs. 15%; P = 0.04). All patients received immunoglobulin and longterm antiviral therapy as prophylaxis against graft hepatitis B resurgence. No patients who received HBcAb+ livers developed hepatitis B infection on follow-up. Overall survival at 30 days, 1 year and 5 years in HBcAb+ liver recipients was 92%, 74% and 74%, respectively, compared with 96%, 89% and 76%, respectively, in the control group (P = not significant, log-rank test). All except one of the deaths in the HBcAb+ liver recipient group occurred within 90 days postoperatively and in patients with MELD scores >30.
Conclusions
The practice of transplanting HBcAb+ grafts incurs low risk for infection using current methods of prophylaxis. The highest mortality risk was in the early postoperative period, specifically in patients with very high MELD scores. This probably reflects the practice of using positive serology grafts in emergent situations.
Keywords: liver transplantation, hepatitis B, donor
Introduction
In response to an increasing shortage of donor organs, the parameters used to select livers for donation have been widened. The inclusion of donor livers with hepatitis B virus (HBV) core antibody (HBcAb), which were previously considered unsuitable, can reduce the gap between demand and organ availability. These organs are positive for the core antibody against HBV, but negative for HBV core antigen (HBcAg) and surface antigen (HBsAg). This serological pattern indicates prior exposure to HBV infection without evidence of active infection; however, these donors frequently harbour occult disease which can emerge under conditions of host immunosuppression. The prevalence of HBcAb among potential donors is as high as 15% in some urban US centres and a number of strategies to utilize these organs have been developed.1,2 These include matching with specific recipients who are also HBcAb-positive (HBcAb+), as well as the administration of prophylaxis against hepatitis B infection to recipients.2–4
Rates of hepatitis B transfection from infected grafts performed prior to the routine use of prophylaxis therapy were 50–75%.5,6 A number of approaches were attempted to reduce these rates of infection, including transplantation into recipients with evidence of past HBV infection (15% infection rate), and pre-transplant immunization (10% infection rate).2–4,7 The administration of immunoglobulin against HBV, as well as antiviral therapy, has promoted the use of HBcAb+ grafts in non-HBV recipients.8 Since the introduction of immunoglobulin and antiviral treatment strategies, the reported incidence of HBV arising from graft transfection has reduced dramatically.1,9
There have also been conflicting reports on graft and survival outcomes after the use of HBcAb+ livers.5,10 Earlier reports suggested significantly worse graft and patient survival rates in HBcAb+ liver transplants,5 but more recent studies offer opposing results.10,11
We report the results of a retrospective review of the practices and outcomes of HBcAb+ donor liver transplants performed at a single transplant centre. Graft and patient survival in recipients of HBcAb+ organs were determined and compared with overall survival rates for all liver transplants performed at this institution during the study period. We examined the HBV prophylaxis given to recipients and the incidence of HBV infection post-transplant. In addition, we investigated specific recipient and donor factors that may have influenced outcomes.
Materials and methods
Study population
Data were collected retrospectively in this cohort study for all adult patients who underwent liver transplantation between 1 January 1997 and 31 December 2009. We used the liver transplant database at our institution to identify recipients of HBcAb+ liver grafts and confirmed these with data held on the United Network for Organ Sharing (UNOS) database.
Information collected in the database included: patient demographics; preoperative medical and surgical histories; operative details; postoperative condition and recovery status; standard pre- and postoperative laboratory values; Model for End-stage Liver Disease (MELD) scores; data on immunosuppression therapy; data on postoperative complications; data on any retransplantation, and information on whether the patient survived during the study period. The causes of recipient death were also reviewed. The treatments used to prevent recipient HBV infection were reviewed. Donor characteristics including age, cause of death and hepatitis B serology were also obtained.
Inclusion criteria
All adult patients who underwent liver transplantation at our institute from 1 January 1997 to 31 December 2009 were included in the study.
Exclusion criteria
Adult patients who underwent liver transplantation before 1 January 1997 were excluded because donor hepatitis serologies were not uniformly maintained prior to that date. Paediatric patients were excluded because most of them received living donor organs.
Statistical methods and analysis
Cox regression analysis was performed to determine the respective influence of these data points on outcome. Fisher's exact test and Student's t-test were used for comparative analysis as applicable. Analyses of variance (anovas) were performed for multiple group comparisons. Survival curves were generated using Kaplan–Meier methods and compared using log-rank tests. A Cox proportional hazard model was used for survival analysis.
Results
Recipient characteristics
Twenty-five (2.9%) of the 868 recipients of liver transplants performed at this institution between 1 January 1997 and 31 December 2009 received an HBcAb+ liver. The utilization of HBcAb+ donors increased significantly over the period under study. During 1997–2004, only five of 376 transplants (1.3%) used HBcAb+ livers, whereas 20 of the 492 liver transplants (4.1%) performed during 2005–2009 used HBcAb+ organs (P = 0.01).
The HBcAb+ organ recipient group were similar in age (55.1 ± 7.0 years vs. 52.0 ± 10.3 years) and body mass index (BMI) (29.2 ± 5.2 vs. 27.9 ± 5.4) to the control group. However, the mean MELD score at the time of operation was higher in the HBcAb+ organ recipient group than in the control group (25 ± 12 vs. 21 ± 9; P = 0.03) (Table 1). There was no restriction policy on the use of HBcAb+ livers. The most common indication for liver transplantation in both groups was hepatitis C (48% in the HBcAb+ organ group, 35% in the control group). Hepatitis B virus was more frequently the reason for transplantation in recipients of HBcAb+ livers (20% vs. 4%). Eleven HBcAb+ liver recipients (44%) had hepatocellular carcinoma (HCC), compared with 190 (22%) control group recipients (P = 0.03). The waiting time from listing to transplantation was longer in the HBcAb+ liver recipient group (385 ± 749 vs. 230 ± 367 days; P = 0.04). The median wait time was 89 days in the control group and 139 days in the HBcAb+ liver recipient group.
Table 1.
Recipient characteristics
| Characteristic | Control group (n = 843) | HBcAb+ organ recipients (n = 25) | P-value |
|---|---|---|---|
| Age, years, median (range) | 53 (18–75) | 54 (38–68) | 0.14a |
| Age, years, mean ± SD | 52.0 ± 10.3 | 55.1 ± 7.0 | |
| BMI, median (range) | 27.3 (14.4–47.3) | 29 (19.7–38.6) | 0.24a |
| BMI, mean ± SD | 27.9 ± 5.4 | 29.2 ± 5.2 | |
| Indication for transplantation, n (%) | |||
| Hepatitis C virus | 295 (35%) | 12 (48%) | |
| Hepatitis B virus | 33 (4%) | 5 (20%) | |
| Alcohol-related | 140 (17%) | 1 (4%) | |
| Primary biliary cirrhosis | 37 (4%) | 2 (8%) | |
| Primary sclerosing cholangitis | 42 (5%) | 0 | |
| Non-alcoholic steatohepatitis | 30 (4%) | 0 | |
| Cryptogenic | 71 (8%) | 3 (12%) | |
| Other | 205 (24%) | 4 (16%) | |
| MELD score, median (range) | 20 (5–50) | 22 (8–48) | 0.03a |
| MELD score, mean ± SD | 21 ± 9 | 25 ± 12 | |
| MELD score ≤30 | 737 (86%) | 17 (68%) | 0.01b |
| MELD score >30 | 106 (13%) | 8 (32%) | |
| Retransplantation, n (%) | 53 (6%) | 3 (12%) | 0.21b |
| Wait time, days, median (range) | 89 (1–2934) | 139 (18–359) | 0.04a |
| Wait time, days, mean ± SD | 230 ± 367 | 385 ± 749 | |
Unpaired t-test, two-tailed P-value.
Fisher's exact test, two-tailed P-value.
HBcAb+, hepatitis B core antibody-positive; SD, standard deviation; BMI, body mass index; MELD score, Model for End-stage Liver Disease score.
Donor characteristics
Donors positive for HBcAb were older (49.6 ± 14.8 years vs. 41.5 ± 17.6 years; P = 0.002) and were more likely to be male and African American (Table 2). Mean cold ischaemic time was lower among HBcAb+ donor organs (5.2 ± 2.3 h vs. 6.4 ± 2.5 h; P = 0.02).
Table 2.
Donor characteristics
| Characteristic | Control group (n = 843) | HBcAb+ organ recipients (n = 25) | P-value |
|---|---|---|---|
| Age, years, median (range) | 43 (7–80) | 50 (21–76) | 0.002a |
| Age, years, mean ± SD | 41.5 ± 17.6 | 49.6 ± 14.8 | |
| Gender, n (%) | 0.10b | ||
| Male | 462 (55%) | 18 (72%) | |
| Female | 381 (45%) | 7 (28%) | |
| Ethnicity, n (%) | |||
| White | 708 (84%) | 11 (44%) | |
| African American | 118 (14%) | 11 (44%) | |
| Other | 17 (2%) | 3 (12%) | |
| CIT, h, median (range) | 6.2 (0.3–11.9) | 4.9 (0.4–11.3) | 0.01a |
| CIT, h, mean ± SD | 6.4 ± 2.5 | 5.2 ± 2.3 | |
Unpaired t-test, two-tailed P-value.
Fisher's exact test, two-tailed P-value.
HBcAb+, hepatitis B core antibody-positive; SD, standard deviation; CIT, cold ischaemic time.
Operative and hospital course after HBcAb+ liver transplantation
Operative time and transfusion requirements were similar in both the HBcAb+ organ recipient and control groups. There was no difference in warm ischaemic time between the groups. Postoperatively, both groups had similar lengths of intensive care unit and hospital stay (Table 3).
Table 3.
Hospital course
| Control group (n = 843) | HBcAb+ organ recipients (n = 25) | P-value | |
|---|---|---|---|
| Operative time, h, median (range) | 6.6 (3.3–14.4) | 6.1 (3.7–10.8) | 0.15a |
| Operative time, h, mean ± SD | 6.8 ± 1.7 | 6.3 ± 1.8 | |
| PRBC in OR, units, median (range) | 3 (0–42) | 4 (0–37) | 0.52a |
| PRBC in OR, units, mean ± SD | 5.2 ± 6.2 | 6.0 ± 7.2 | |
| WIT, min, median (range) | 35 (15–80) | 33 (18–48) | 0.08a |
| WIT, min, mean ± SD | 36 ± 8.4 | 33 ± 6.9 | |
| ICU stay, days, median (range) | 2 (1–121) | 2 (1–12) | 0.93a |
| ICU stay, days, mean ± SD | 5.9 ± 12 | 5.7 ± 12 | |
| Hospital stay, days, median (range) | 7 (6–145) | 7 (4–56) | 0.67a |
| Hospital stay, days, mean ± SD | 11.5 ± 12.9 | 12.6 ± 12.5 | |
Unpaired t-test, two-tailed P-value.
HBcAb+, hepatitis B core antibody-positive; SD, standard deviation; PRBC, packed red blood cells; OR, operating room; WIT, warm ischaemic time; ICU, intensive care unit.
Patient and graft survival
The mean length of follow-up in HBcAb+ organ recipients was significantly shorter than in control recipients (2.3 ± 2.0 years vs. 4.3 ± 3.6 years; P = 0.006), which probably reflects our increased utilization of HBcAb+ grafts in the later years of the study period. Six deaths (24%) occurred in the HBcAb+ organ recipient group and 232 deaths (28%) occurred in the control group. All but one of the HBcAb+ organ recipient deaths occurred in patients with MELD scores of >30 at transplantation. The cause of death in four of the six patients was sepsis and five of the six patients died within 90 days of surgery (Table 4). Sepsis with multi-organ failure accounted for 66 of the 232 deaths (28%) in the control group. Most deaths in the control group occurred later. The mean time to death was 2.8 ± 3.2 years in the control group and 0.17 ± 0.22 years in the HBcAb+ liver recipient group (P = 0.04).
Table 4.
Recipient deaths
| Control group (n = 843) | HBcAb+ organ recipients (n = 25) | P-value | |
|---|---|---|---|
| Deaths, n (%) | 232 (28%) | 6 (24%) | 0.82b |
| Transplant to death, years, median (range) | 1.5 (1–12.8) | 0.09 (0.01–0.60) | 0.04a |
| Transplant to death, years, mean ± SD | 2.8 ± 3.2 | 0.17 ± 0.22 | |
| Cause of death, n (%) | |||
| Sepsis/multi-organ failure | 66 (28%) | 4 (67%) | |
| Non-liver cancers | 18 (8%) | ||
| Recurrent liver cancer | 8 (3%) | ||
| Graft failure | 40 (17%) | ||
| Cardiac or stroke | 27 (12%) | 1 (17%) | |
| Pneumonia | 7 (3%) | ||
| Intraoperative death | 9 (4%) | ||
| Unnatural death | 6 (3%) | ||
| Other | 51 (22%) | 1 (17%) | |
Unpaired t-test, two-tailed P-value.
Fisher's exact test, two-tailed P-value.
HBcAb+, hepatitis B core antibody-positive; SD, standard deviation.
There was no significant difference in patient survival between the two recipient groups (P = 0.16, log-rank test). Overall survival rates at 1 month, 1 year and 5 years in HBcAb+ organ recipients were 92%, 74% and 74%, respectively, compared with 96%, 89% and 76%, respectively, in the control group (Fig. 1). One patient in the study group was retransplanted for graft failure caused by ischaemic cholangiopathy. Graft survival did not differ statistically between the groups (P = 0.15, log-rank test). Graft survival at 1 month, 1 year and 5 years was 92%, 74% and 65%, respectively, in the HBcAb+ organ group and 94%, 86% and 73%, respectively, in the control group (Fig. 2).
Figure 1.
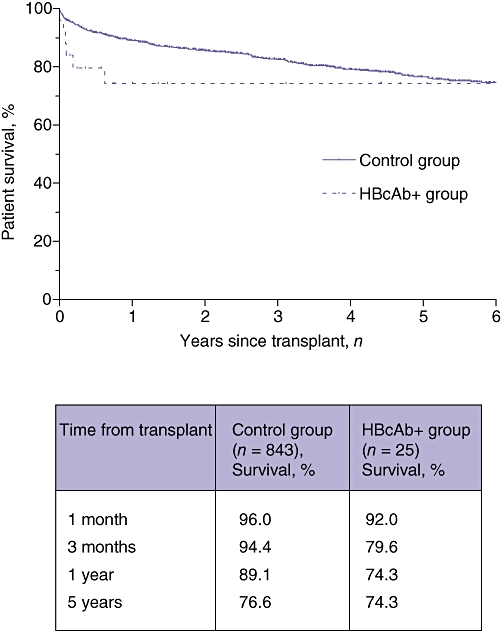
Kaplan–Meier curves for overall survival in recipients of hepatitis B core antibody-positive (HBcAb+ group) and HBcAb− (control group) organs. Statistical analysis using the log-rank test did not indicate a significant difference between survival curves (P = 0.16). The table shows survival percentages in the two groups at 1 and 3 months, and 1 and 5 years
Figure 2.
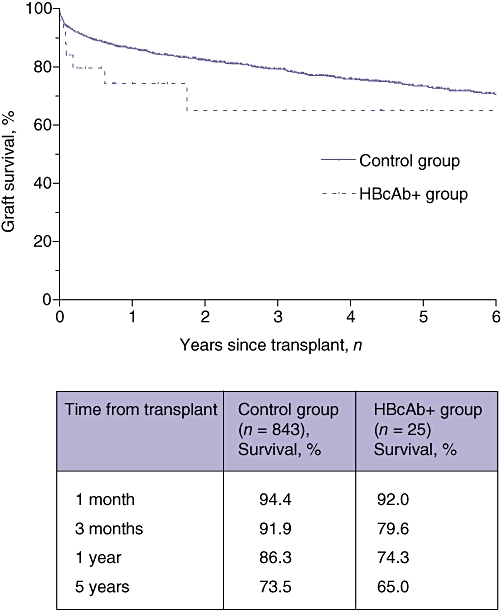
Kaplan–Meier curves for graft survival in recipients of hepatitis B core antibody-positive (HBcAb+ group) and HBcAb− (control group) organs. Statistical analysis using the log-rank test did not indicate a significant difference between the curves (P = 0.15). The table shows survival percentages in the two groups at 1 and 3 months, and 1 and 5 years
Transmission of HBV infection and prophylaxis
This institution operated a general policy of HBV vaccination prior to transplantation; however, seroconversion was not mandatory for listing or consideration for transplantation using HBcAb+ livers. All patients, with the exception of two who died early in the post-transplant period, received postoperative hepatitis B immunoglobulin (HBIG) prophylaxis irrespective of their vaccination outcome. The duration of treatment varied over the period studied. The mean length of treatment was 5.9 ± 7.3 months. All patients received an initial dose of immunoglobulin intraoperatively. Twenty-two (88%) patients received antiviral prophylaxis. Initially, the preferred antiviral therapy for HBcAb+ organ recipients was lamivudine. However, more recent recipients of HBcAb+ livers have been treated with adefovir or entecavir. The mean length of treatment was 16.6 ± 19.5 months. The current protocol stipulates that recipients are treated with HBIG intraoperatively and are then given intermittent infusions to maintain HBsAb levels at >150 IU/ml (and >300 IU/ml if the recipient is positive for HBV). The study patients were monitored for HBV infection using serology and, more recently, viral DNA by polymerase chain reaction. None of the HBV-naïve patients developed HBV infection from HBcAb+ donors.
Transplantation of HBcAb+ livers into recipients with high MELD scores
The proportion of patients with MELD scores >30 was significantly higher in the HBcAb+ liver recipient group (n = 8, 32%) than in the control group (n = 106, 13%) (P = 0.01). Five of the six deaths in recipients of HBcAb+ livers occurred in patients with MELD scores >30 (Table 4). Survival comparisons using univariate analysis show that overall outcome was significantly worse in HBcAb+ liver recipients with high MELD scores compared with the other HBcAb+ liver recipients (P = 0.003) and also with control recipients with MELD scores >30 (P = 0.002) (Fig. 3).
Figure 3.
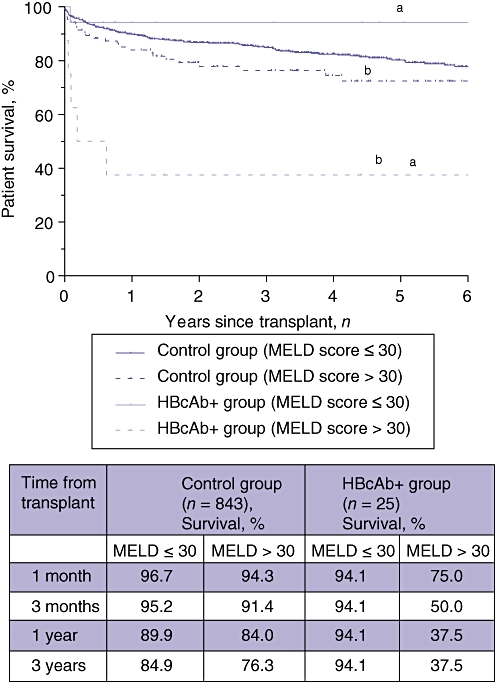
Kaplan–Meier overall survival curves for recipients of hepatitis B core antibody-positive (HBcAb+ group) and HBcAb− (control group) organs stratified according to actual Model for End-stage Liver Disease (MELD) scores at surgery. Statistical analysis using the log-rank test indicated a significant difference between survival curves. Recipients of HBcAb+ grafts with high MELD scores had worse outcomes. aP = 0.003; bP = 0.002
Discussion
Organ shortages represent one of the greatest challenges facing transplantation and much effort is focused on increasing the number of eligible donors. The past decade has seen increased use of marginal donors, including older donors, steatotic donors and donors with positive serologies for viral hepatitis. The identification of HBcAb on donor serologies was once considered a contraindication to organ use in patients without prior exposure to HBV or HBV as an indication for transplant.3,6 However, adherence to this policy led to the discarding of otherwise suitable organs at many centres. Recent data on HBV infection found that 4.7% of the US population are positive for HBcAb; this incidence increases in older people (≥50 years) (7.7%) and African Americans (12.2%).12 In large urban communities, an estimated 12–15% of the population will have previous exposure to HBV and positive serology for HBcAb.1,2,12 In the past 10 years, efforts to transplant HBcAb+ livers have increased. In keeping with the national trend, we noted a significant increase in HBcAb+ grafts transplanted at our institution over the decade (rising from 1.3% to 4.0% of all liver transplants). However, this donor resource remains underused and mechanisms that safely increase its utilization would expand donor numbers.8,13
The greatest barriers to HBcAb+ graft utilization in HBV-naïve recipients have been concern that survival outcomes are lower after HBcAb+ organ transplantation1 and the risk for recipient infection with HBV.2,3,11,14 Many of the initial studies into patient outcomes demonstrated survival outcomes that were lower than expected in HBcAb+ organ recipients.3,5,6 However, when these earlier studies were performed, HBcAb+ organs were commonly used in critically ill or HCC patients.1–3,6 Dickson et al.5 compared recipients of HBcAb+ and HBcAb− organs and found decreased 4-year survival in HBcAb+ organ recipients (55.9% vs. 75.8%). However, the significant discrepancy in outcome between the groups was probably confounded by increased disease acuity in HBcAb+ organ recipients.5 Furthermore, this study was conducted prior to routine prophylaxis treatment with lamivudine or HBIG and its sample featured a significantly greater rate of de novo infection (78%) with progression to liver dysfunction. Prieto et al. (2003) compared patient survival in HBcAb+ organ and control recipients and, by contrast, failed to demonstrate a significant difference in 4-year survival rates (68% vs. 76%).10 More recently, Yu et al.11 examined UNOS data for all liver transplants performed between 1994 and 2006 using HBcAb+ donors and compared these with data for negative controls.11 It is unclear how many of the HBcAb+ organ recipients received antiviral prophylaxis, but the use of HBcAb+ donors was not independently associated with worse post-transplant survival.11 In the current study we found no significant difference in overall or graft survival between recipients of HBcAb+ and HBcAb− organs, despite, interestingly, higher MELD scores at surgery in the HBcAb+ organ recipient group.
Numerous strategies are used to prevent HBV transmission, including passive immunization with HBIG and antiviral therapies.2,4,9,14–17 Previous studies have demonstrated that, in the absence of prophylactic therapy, HBV-naïve recipients (HBsAb– HBcAb–) had an approximately 76% chance of developing HBV infection and no instances of HBsAb+ HBcAb+ patients developing HBV after transplantation have been reported.2,5 Consequently, many transplant centres have restricted the use of HBcAb+ grafts to HBV+ recipients.8,18 The administration of HBIG has been shown to help prevent HBV transmission, but it has several limitations including the provision of low protection in HBV-naïve recipients, low levels of compliance and high costs.14 Lamivudine has been demonstrated to decrease the rate of recipient infection in HBV-naïve recipients to 2.6%.2 Much debate remains regarding the use of combined antiviral and HBIG therapy.2,3,8,14,15,17 Although the addition of HBIG significantly increases the cost of treatment, its administration appears to be common practice at most transplant centres.7,17 There are no data to confirm lower rates of transmission with combined treatment.2,14 More recently, the use of tenofovir and entecavir as prophylactic antivirals of choice has become more widespread.16,19 We currently treat all of our patients with HBIG for 3 months and subsequently provide lifetime antiviral therapy. Similarly to other recent studies,17,19 we report no recipient HBV infections.
There are several limitations to the current report. This study examined outcomes at a single transplant centre and therefore the number of patients included was relatively small. In addition, the mean duration of follow-up was only 2.3 years in the study patients and was significantly shorter than in the control group. Demographic data for the 25 recipients of HBcAb+ livers were similar to those for the control recipients, although indications for transplantation and severity of disease at transplantation differed between the groups. The average MELD score was higher in the HBcAb+ organ recipient group and an increased percentage of HBcAb+ organ recipients had MELD scores >30 at the time of surgery. The average wait time for a liver was significantly lower in the group with high MELD scores (91 ± 223 days, median = 12.5 days). This suggests a disproportionate utilization of HBcAb+ grafts in critically ill patients with high MELD scores.
Within the scope of this single-centre retrospective study, it is difficult to demonstrate either statistically or clinically significant differences between subgroups of HBcAb+ organ recipients. In our cohort of HBcAb+ organ recipients, we noted an increased number of deaths and worse survival in recipients with MELD scores >30. This difference was apparent when this group was compared with HBcAb+ organ recipients with lower MELD scores and also when it was compared with control recipients with MELD scores >30. Potentially confounding variables relate to the severity of patient disease and the non-randomized design of this study.
In summary, we demonstrate in this study that liver transplantation using HBcAb+ grafts can be performed with similar outcomes to transplants that use HBcAb− grafts. Treatment with combined antiviral and HBIG prophylaxis leads to very low risk for recipient infection. Although it remains to be determined whether specific recipient subgroups should avoid HBcAb+ grafts, it is clear that this underused resource can be safely utilized to expand the pool of donor organs.
Conflicts of interest
None declared.
References
- 1.Manzarbeitia C, Reich DJ, Ortiz JA, Rothstein KD, Araya VR, Munoz SJ. Safe use of livers from donors with positive hepatitis B core antibody. Liver Transpl. 2002;8:556–561. doi: 10.1053/jlts.2002.33451. [DOI] [PubMed] [Google Scholar]
- 2.Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272–279. doi: 10.1016/j.jhep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Muñoz SJ. Use of hepatitis B core antibody-positive donors for liver transplantation. Liver Transpl. 2002;8(10) Suppl. 1:82–87. doi: 10.1053/jlts.2002.35783. [DOI] [PubMed] [Google Scholar]
- 4.Marugán RB, García-Hoz F, Romero MV, Nash R, Mateos M, Alonso RG, et al. Prevention of de novo hepatitis B infection in liver allograft recipients with previous hepatitis B infection or hepatitis B vaccination. Am J Gastroenterol. 2002;97:2398–2401. doi: 10.1111/j.1572-0241.2002.05994.x. [DOI] [PubMed] [Google Scholar]
- 5.Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, et al. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology. 1997;113:1668–1674. doi: 10.1053/gast.1997.v113.pm9352871. [DOI] [PubMed] [Google Scholar]
- 6.Dodson SF, Issa S, Araya V, Gayowski T, Pinna A, Eghtesad B, et al. Infectivity of hepatic allografts with antibodies to hepatitis B virus. Transplantation. 1997;64:1582–1584. doi: 10.1097/00007890-199712150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Roque-Afonso AM, Feray C, Samuel D, Simoneau D, Roche B, Emile JF, et al. Antibodies to hepatitis B surface antigen prevent viral reactivation in recipients of liver grafts from anti-HBc positive donors. Gut. 2002;50:95–99. doi: 10.1136/gut.50.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programmes. Liver Transpl. 2009;15:223–232. doi: 10.1002/lt.21675. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska I, Pawlowska J, Teisseyre M, Kaliciński P, Kamiński A, Czubkowski P, et al. Prevention of de novo hepatitis B virus infection by vaccination and high hepatitis B surface antibodies level in children receiving hepatitis B virus core antibody-positive living related donor liver: case reports. Transplant Proc. 2007;39:1511–1512. doi: 10.1016/j.transproceed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Prieto M, Gómez MD, Berenguer M, Córdoba J, Rayón JM, Pastor M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001;7:51–58. doi: 10.1053/jlts.2001.20786. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Koepsell T, Manhart L, Ioannou G. Survival after orthotopic liver transplantation: the impact of antibody against hepatitis B core antigen in the donor. Liver Transpl. 2009;15:1343–1350. doi: 10.1002/lt.21788. [DOI] [PubMed] [Google Scholar]
- 12.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 13.United Network for Organ Sharing (UNOS) OPTN/SRTR Annual Report 2008. http://optn.transplant.hrsa.gov [Accessed 1 March 2011.]
- 14.Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl. 2010;16:300–307. doi: 10.1002/lt.21998. [DOI] [PubMed] [Google Scholar]
- 15.Nery JR, Nery-Avila C, Reddy KR, Cirocco R, Weppler D, Levi DM, et al. Use of liver grafts from donors positive for antihepatitis B-core antibody (anti-HBc) in the era of prophylaxis with hepatitis-B immunoglobulin and lamivudine. Transplantation. 2003;75:1179–1186. doi: 10.1097/01.TP.0000065283.98275.FE. [DOI] [PubMed] [Google Scholar]
- 16.Bárcena R, Campo SD, Moraleda G, Casanovas T, Prieto M, Buti M, et al. Study on the efficacy and safety of adefovir dipivoxil treatment in post-liver transplant patients with hepatitis B virus infection and lamivudine-resistant hepatitis B virus. Transplant Proc. 2005;37:3960–3962. doi: 10.1016/j.transproceed.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 17.Prakoso E, Strasser SI, Koorey DJ, Verran D, McCaughan GW. Longterm lamivudine monotherapy prevents development of hepatitis B virus infection in hepatitis B surface-antigen negative liver transplant recipients from hepatitis B core-antibody-positive donors. Clin Transplant. 2006;20:369–373. doi: 10.1111/j.1399-0012.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 18.Burton JR, Shaw-Stiffel TA. Use of hepatitis B core antibody-positive donors in recipients without evidence of hepatitis B infection: a survey of current practice in the United States. Liver Transpl. 2003;9:837–842. doi: 10.1053/jlts.2003.50157. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Perez M, Saez-Gomez AB, Mongil Poce L, Lozano-Rey JM, de la Cruz-Lombardo J, Rodrigo-Lopez JM. Efficacy and safety of entecavir and/or tenofovir for prophylaxis and treatment of hepatitis B recurrence post-liver transplant. Transplant Proc. 2010;42:3167–3168. doi: 10.1016/j.transproceed.2010.05.127. [DOI] [PubMed] [Google Scholar]


