Abstract
Diaphragm caspase-8 activation plays a key role in modulating sepsis-induced respiratory muscle dysfunction. It is also known that double-stranded RNA-dependent protein kinase (PKR) is a regulator of caspase-8 activation in neural tissue. We tested the hypothesis that the PKR pathway modulates sepsis-induced diaphragmatic caspase-8 activation. We first evaluated the time course of diaphragm PKR activation following endotoxin administration in mice. We then determined whether administration of a PKR inhibitor (2-aminopurine) prevents endotoxin-induced diaphragm caspase-8 activation and contractile dysfunction in mice. Finally, we investigated if inhibition of PKR (using either 2-aminopurine or transfection with dominant-negative PKR) blocks caspase-8 activation in cytokine treated C2C12 cells. Endotoxin markedly activated diaphragm PKR (with increases in both active phospho-PKR protein levels, P < 0.03, and directly measured PKR activity, P < 0.01) and increased active caspase-8 levels (P < 0.01). Inhibition of PKR with 2-aminopurine prevented endotoxin-induced diaphragm caspase-8 activation (P < 0.01) and diaphragm weakness (P < 0.001). Inhibition of PKR with either 2-aminopurine or transfection with dominant-negative PKR blocked caspase-8 activation in isolated cytokine-treated C2C12 cells. These data implicate PKR activation as a major factor mediating cytokine-induced skeletal muscle caspase-8 activation and weakness.
Keywords: skeletal muscle, caspase, calpain, proteolysis
two recent studies found that critically ill patients develop severe diaphragm weakness, with diaphragm pressure generation falling to only 20–25% of that observed in normal subjects (12, 28). Such severe weakness could account for the difficulty weaning many critically ill Intensive Care Unit patients from mechanical ventilation (13). The mechanisms responsible for this severe respiratory muscle weakness in Intensive Care Unit patients are poorly understood, but many of these patients are infected, and infections are known to induce severe respiratory muscle weakness (14, 16, 17). In recent animal studies, we showed that endotoxin-induced sepsis activates diaphragmatic caspase-8, and that caspase-8 activation contributes to the development of diaphragmatic weakness (22, 24). Infections can also activate double-stranded RNA-dependent kinase (also known as PKR), and PKR and caspase-8 activation have been linked to one another in several tissues (8, 21). The role of PKR in modulating infection-induced diaphragm caspase-8 activation, however, has never been examined.
The purpose of the present study was to test the hypotheses that endotoxin-induced sepsis leads to PKR activation in the diaphragm and that PKR, in turn, is linked to diaphragm caspase-8 activation. In intact animals (mice), sepsis was induced by endotoxin injection, diaphragm PKR and caspase-8 activation were evaluated using Western blot techniques, and the effects of inhibiting PKR with 2-aminopurine on diaphragm muscle caspase-8 activation and force generation were assessed. In additional experiments, we evaluated the direct effects of addition of cytokines to a mouse muscle cell line (C2C12 cells) and determined if chemical and genetic inhibition of PKR activation (i.e., chemical inhibition of PKR with 2-aminopurine and genetic inhibition via transfection with a dominant-negative PKR construct) prevented caspase-8 activation in these isolated muscle cells.
METHODS
Experimental models and protocols.
Experiments were performed using adult male mice, weighing between 25 and 35 g. Mice had unrestrained access to food and water throughout the period of study. Studies were approved by the university Institutional Animal Care and Use Committee, and animals were monitored throughout experiments by the university animal center staff.
Three sets of studies were performed. In the first study, we assessed the time course of diaphragm PKR activation in animals following endotoxin administration. For this study, animals were killed 2, 4, 8, and 24 h after 12 mg/kg endotoxin (E. coli 055:B5 lipopolysaccharide, Sigma Chemical, St. Louis, MO, injected intraperitoneally in 0.3 ml saline, n = 6/group). Saline-injected animals (0.3 ml given intraperitoneally, n = 6) served as controls. To provide hydration, animals were also injected subcutaneously with 60 ml/kg saline at the time of endotoxin or saline administration. At the time of death, animals were pentobarbital anesthetized (150 mg/kg), diaphragms were removed, and diaphragm PKR activation was determined, as described below.
In the second set of experiments, we determined whether administration of a PKR inhibitor could prevent endotoxin-induced caspase-8 activation and reductions in diaphragm-specific force generation. Four groups of mice were studied (n = 4–5/group): 1) control animals injected with saline (intraperitoneally, 0.3 ml); 2) animals injected with endotoxin (12 mg/kg, E. coli LPS 055:B5, intraperitoneally in 0.3 ml saline); 3) animals injected intraperitoneally with both 12 mg/kg endotoxin and a PKR inhibitor, 1.5 mmol/kg 2-aminopurine (Sigma Chemical); and 4) animals intraperitoneally injected with 1.5 mmol/kg 2-aminopurine alone. All animals also received 60 ml/kg saline injected subcutaneously. After 24 h, animals were pentobarbital anesthetized and killed by removal of the diaphragm. Excised diaphragms were used for assessment of force generation and caspase-8 levels. Our rationale for assessing caspase-8 levels is based on previous work by our group (22, 24). In this earlier work, we found that endotoxin administration was followed by increases in active caspase-8 protein, active caspase-3 protein, increased caspase-8 function assessed using an activity assay, increased caspase-3 function assessed using an activity assay, and increased formation of caspase-specific spectrin degradation products. Endotoxin did not, however, increase caspase-9, did not significantly increase BAX and did not reduce BCL2 levels. We also found that administration of a caspase-8 inhibitor completely blocked all indexes of caspase-3 activation, implicating formation of caspase-8 protein as the critical diaphragm caspase component activated following endotoxin administration (22, 24). For these reasons, we chose to monitor active caspase-8 protein levels in the present study.
In a third set of studies, we determined whether caspase-8 was activated in C2C12 cells in response to incubation with a mixture of cytokines, and if inhibitors of PKR prevented caspase-8 activation. The reason why cells were exposed to a mixture of cytokines instead of endotoxin alone (as was used in animal studies) is because endotoxin administration to animals results in production of a mixture of cytokines (including TNF-α, IL-1β, IFN-γ) by white cells. As a result, the diaphragm is exposed to a mix of cytokines in vivo following endotoxin injection, and we thought it appropriate to expose cultured myotubes in vitro to a mix of cytokines. Studies were performed on the following groups (n = 4 plates of cells/group): 1) C2C12 myotubes to which 35 μl of sterile saline were added; 2) myotubes treated with a mixture of cytokines [cytomix in a volume of 35 μl, achieving final concentrations of 10 μg/ml LPS, 20 ng/ml TNF-α (Biosource, Carlsbad, CA), 50 U/ml IL-1β (Biosource), and 100 U/ml IFN-γ (Biosource) in media]; 3) myotubes treated with cytomix and a chemical PKR inhibitor, 2-aminopurine, 1.5 mmol/l; and 4) myotubes treated with 2-aminopurine alone. For all cell studies, saline, cytokines, and/or 2-aminopurine were added to Dulbecco's modified Eagle's medium (DMEM), and cells were incubated at 37°C with an atmosphere of 5% carbon dioxide and 95% air. Details about cell growth and differentiation are provided in Cell preparations and transfections section. After 24 h, cells were harvested, and assessment was made of cell caspase-8 concentrations.
In cell studies, we also determined whether genetic inhibition of PKR activation would alter the activation of caspase-8 in C2C12 cells. Studies were conducted on the following groups (n = 4–5 plates of cells/group): 1) control sham transfected cells treated with saline, 35 μl; 2) sham construct transfected cells treated with cytomix, 35 μl; 3) cells transfected with a PKR dominant-negative construct, then treated with saline, 35 μl; and 4) PKR dominant-negative transfected cells treated with cytomix, 35 μl. At 24 h after application of saline or cytomix, cells were harvested, and assessment made of cell caspase-8 concentrations. Details regarding transfection are provided below.
PKR and caspase protein identification by Western blots.
Western blots were used to measure diaphragm and myotube levels of phospho-PKR, total PKR, and caspase-8. We also measured levels of α-tubulin as a loading control. For these determinations, muscle samples were homogenized in buffer (10 mM β-glycerophosphate, 50 mM sodium fluoride, 20 mM HEPES, 2 mM EDTA, 250 mM sodium chloride, 2 μm/ml leupeptin, 2 μm/ml aprotinin, 1 mM PMSF, 0.5 μm/ml benzamidine, and 1 mM DTT; all from Sigma Chemical, St. Louis, MO) in a 1 g/10 ml ratio, centrifuged at 3,000 g for 10 min. Supernatant samples of equal protein content were then diluted 1:1 with loading buffer (126 mM Tris·HCl, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromophenol blue, all from Sigma Chemical, pH 6.8), boiled for 5–7 min, and then loaded onto Tris glycine polyacrylamide gels (Invitrogen, Carlsbad, CA). Sample protein mixtures were separated by electrophoresis (Novex Minicell II, Carlsbad, CA), transferred to polyvinylidene fluoride membranes, and incubated over night at 4°C with primary antibodies to targeted proteins (phospho-PKR, total PKR, from Cell Signaling, Danvers, MA, caspase-8 from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology), and antibody binding was detected using enhanced chemiluminescence. Densitometry of filmed images was performed using a Microtek scanner (Carson, CA) and UN-SCAN-IT software (Silk Scientific, Orem, UT). To verify equal loading across lanes, membranes were stripped and reprobed with antibodies to α-tubulin (Santa Cruz Biotechnology). We chose α-tubulin for this normalization because experiments indicate this protein is not altered in skeletal muscle by sepsis.
PKR activity assay.
We measured PKR activity directly by immunoprecipitation of PKR from diaphragm sample homogenates and subsequent incubation of the immunoprecipitate with a PKR substrate [eukaryotic initiation factor (eIF)-2α, Biosource]. Muscle samples were homogenized in lysis buffer (1 g tissue/10 ml buffer) using a polytron. Homogenates were then centrifuged at 3,000 g for 10 min (4°C), the supernatant reserved, and its protein concentration determined. Aliquots of homogenates containing 100 μg (processed from muscles from control and endotoxin-treated animals) were then placed in tubes containing immobilized phospho-PKR MAb (Cell Signaling) to immunoprecipitate PKR. After washing, eIF-2α was added to serve as an exogenous PKR substrate. Following incubation, the reaction products were separated electrophoretically and probed for eIF-2α phosphorylation (i.e., using phospho-eIF-2α antibody, Cell Signaling). Densities of the eIF-2α phosphorylation product were quantitated using UN-SCAN-it software.
Measurement of diaphragm force generation.
To assess force production, diaphragm strips were dissected from the left costal diaphragm and mounted vertically in water-jacketed organ baths (Radnoti Glass, Monrovia, CA) containing curarized Krebs-Henseleit solution at 22°C bubbled with 95% O2-5% CO2 (25). The rib end of strips was attached to the bottom of baths by silk ties, and the tendinous end tied to a Grass FT10 force transducer (West Warwick, RI). Platinum field electrodes were placed around strips and connected to an amplifier (Biomedical Technology of America, Cleveland, OH; this delivers current adjustable to values up to 10 A/channel) attached to a Grass S48 stimulator (West Warwick, RI). After a 15-min equilibrium period, muscle length was adjusted to Lo (the length at which force generation was maximum); stimulation current was adjusted to supramaximal levels (i.e., greater than that sufficient to achieve a maximal force level, generally 0.7 A for the electrode configuration used); and a force-frequency curve was constructed by stimulating strips at 1, 10, 20, 50, 100, and 150 Hz (train duration 800 ms) with a 30-s rest period between adjacent stimulus trains. After force measurements were completed, transducers were calibrated with standard weights, and specific force/cross-sectional area (CSA) was calculated by the approach of Close (3). In brief, this method involves calculating muscle CSA as CSA = muscle strip weight/(Lo × 1.06) and then dividing total muscle force generation by CSA to determine the specific force/CSA.
Cell preparations and transfections.
For these experiments, C2C12 myoblasts were obtained from American Type Culture Collection (Manassas, VA) and were grown to 70% confluency in plastic Petri dishes in DMEM with 10% fetal bovine serum (Sigma Chemical). To produce differentiation, media was switched to DMEM with 2% horse serum (Sigma Chemical) for 5 days.
For transfection experiments, C2C12 myoblasts were first transfected with a genetic construct containing a PKR dominant-negative gene (supplied by Dr. Grover Bagby, Oregon Cancer Institute). This construct was transfected into cells using Ambion Xpress reagent (Invitrogen), and verification of transfection was performed using PCR with primers to the genetic construct. Sham control cells were transfected with an inactive transcript (muscle creatine kinase). After transfection, cells were differentiated for 4 days before exposure to either saline or cytomix.
Statistical analysis.
ANOVA was employed to compare variables (e.g., force) across groups of animals treated with different agents, with post hoc testing (Tukey) to determine differences between groups. A P value of <0.05 was taken as indicating statistical significance. Data are presented as means ± 1 SE.
RESULTS
Effect of endotoxin administration on diaphragm PKR activation.
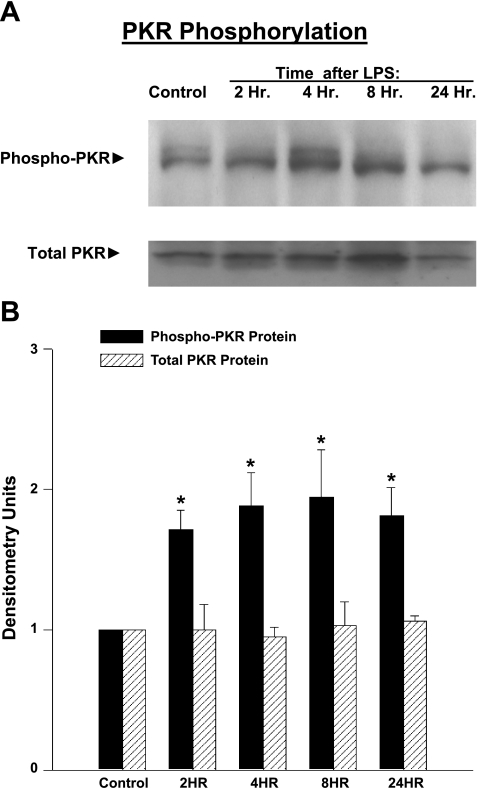
Phospho-PKR levels were increased for diaphragm muscle samples taken from endotoxin-treated animals compared with controls. Examples of individual Western blots for phospho-PKR levels for diaphragms taken from a control animal and animals killed at various points in time after endotoxin administration are shown in Fig. 1A, and mean data for diaphragm phospho-PKR levels are shown in Fig. 1B. On average, phospho-PKR levels increased to 171 ± 14, 188 ± 24, 194 ± 34, and 181 ± 20% of control values 2, 4, 8, and 24 h, respectively, after endotoxin administration (P < 0.02 for each comparison).
Fig. 1.
A: time course of diaphragm double-stranded RNA-dependent protein kinase (PKR) activation following endotoxin treatment. Representative Western blots display protein levels of phospho-PKR and total PKR as a function of time after endotoxin administration. Phospho-PKR protein levels increased above control levels between 4 and 24 h after endotoxin administration. Total PKR was similar for the five time points. B: mean phospho-PKR protein levels over time. Diaphragm phospho-PKR levels increased significantly between 4 and 24 h after endotoxin administration compared with control levels (P < 0.02 for each time point compared with control). Total PKR levels did not change over time. Values are means ± SE. *Significant statistical difference compared with control levels.
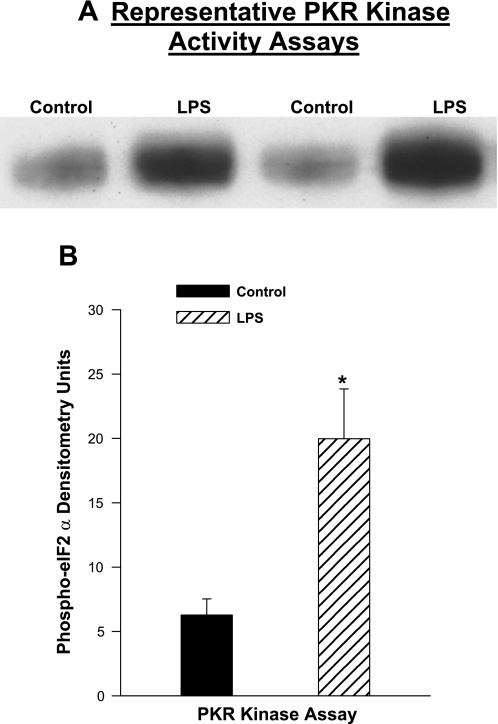
To further assess PKR activation, we directly measured the PKR activity by incubating a known PKR substrate, eIF-2α, with PKR immunoprecipitated from diaphragm samples and then assessed levels of phospho-eIF-2α. As shown in Fig. 2, diaphragm samples taken from endotoxin-treated animals (at 8 h after endotoxin administration) had substantially greater PKR activity, generating phospho-eIF-2α at 318% of the level observed for control samples (P < 0.01).
Fig. 2.
PKR activity assays for diaphragms from endotoxin-treated animals. A: blot presents PKR assay data for diaphragm samples from controls and at 8 h after endotoxin administration. B: mean densitometric analysis of assay product levels. Endotoxin administration was associated with a large increase in PKR activity (P < 0.01). Values are means ± SE. *Significant statistical difference compared with control levels.
Effects of endotoxin and 2-aminopurine administration on diaphragm force generation.
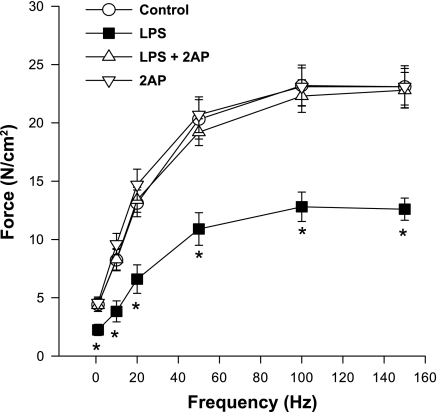
Administration of endotoxin evoked a marked reduction in diaphragm-specific force generation, as shown in Fig. 3. Concomitant administration of 2-aminopurine, a PKR inhibitor, completely prevented endotoxin-induced reductions in diaphragm force generation. For example, force generated in response to 20-Hz stimulation averaged 13.1 ± 1.1, 6.6 ± 1.2, 13.4 ± 1.1, and 14.6 ± 1.3 N/cm2, respectively, for control, endotoxin, endotoxin/2-aminopurine, and 2-aminopurine inhibitor alone treated groups of animals (P < 0.001). A similar pattern was observed for forces generated in response to 150-Hz stimulation, which averaged 23.1 ± 1.8, 12.6 ± 1.0, 22.8 ± 1.5, and 23.1 ± 1.6 N/cm2, respectively, for control, endotoxin, endotoxin/2-aminopurine, and 2-aminopurine alone treated groups of animals (P < 0.001).
Fig. 3.
Effect of a PKR inhibitor [2-aminopurine (2AP)] on the diaphragm force-frequency curve. Force generation was significantly lower for diaphragms from LPS-treated animals (■) than for control animals (○) (P < 0.05 for all frequencies tested). Diaphragms from animals given both 2AP, a PKR inhibitor (▵), and LPS generated forces higher than diaphragms from animals given LPS alone (P < 0.05 for all frequencies). Force generation for muscles taken from animals given the PKR inhibitor alone (▿) were similar to control levels. Values are means ± SE. *Significant statistical difference between LPS and all other groups.
Endotoxin-induced diaphragm caspase-8 activation.
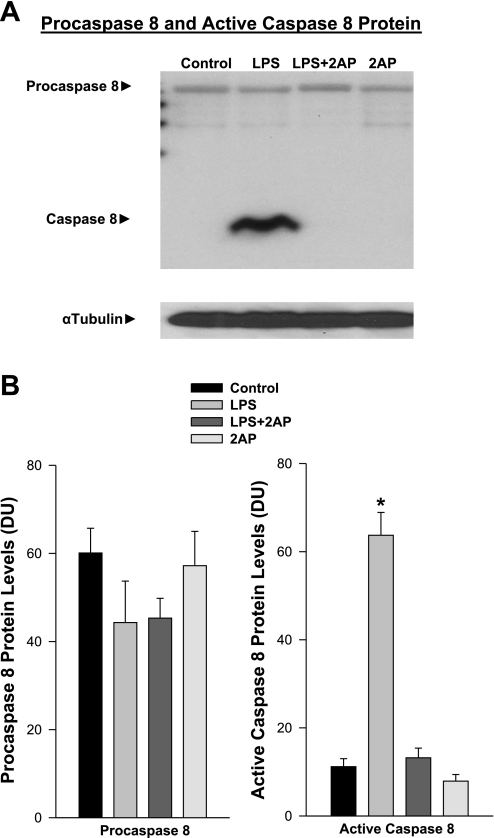
We have previously shown that endotoxin administration evokes significant caspase-3 and caspase-8 activation in the diaphragm, with caspase-3 activation downstream and dependent on caspase-8 activation (22, 24). Moreover, caspase activation in muscle is known to cleave contractile proteins, inducing force loss, initiating proteolysis, and facilitating muscle wasting. To determine whether PKR contributes to caspase-8 activation following endotoxin administration, we assessed the effect of administration of 2-aminopurine on diaphragm levels of active caspase-8 protein. As shown in Fig. 4, endotoxin induced a large increase in active caspase-8, and concomitant 2-aminopurine administration attenuated endotoxin-induced caspase-8 activation (P < 0.01 for comparison of control to endotoxin, and P < 0.01 for comparison of endotoxin to endotoxin plus 2-aminopurine groups).
Fig. 4.
A: effect of PKR inhibition on caspase-8 protein levels. Top: procaspase-8 and active caspase-8 protein for representative diaphragm samples. Bottom: α-tubulin protein levels (loading control). LPS administration elicited a large increase in active cleaved caspase-8 protein compared with the control, and administration of a PKR chemical inhibitor, 2AP, prevented this increase. B: mean data examining the effect of 2AP on diaphragm caspase activation. Left: procaspase-8 levels were similar for the four experimental groups. Right: in contrast, diaphragm active caspase-8 protein levels increased dramatically for endotoxin (LPS)-treated animals (P < 0.01), and administration of 2AP to endotoxin-treated animals prevented this increase (P < 0.01 for comparison between endotoxin and endotoxin plus 2AP groups). Values are means ± SE. *Significant statistical difference between endotoxin and all other groups. DU, densitometry units.
Cytomix-induced caspase activation in C2C12 cells.
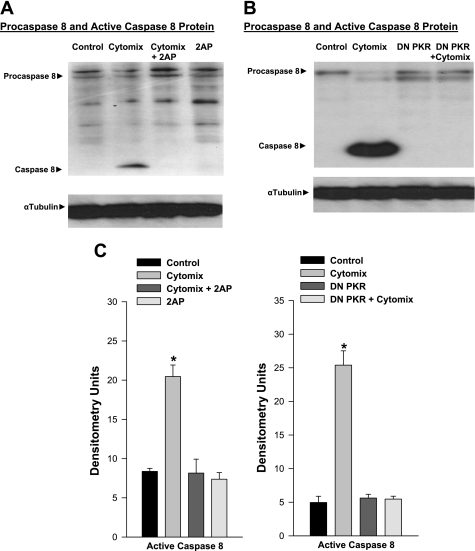
To further assess the effects of 2-aminopurine, a PKR inhibitor, on muscle caspase-8 activation, we examined the effect of this agent on caspase-8 activation in a muscle cell line, i.e., C2C12 myotubes, exposed to a mixture of cytokines (cytomix, which includes LPS, TNF-α, IL-1β, and IFN-γ). We found that incubation of C2C12 cells with cytomix induced marked caspase-8 activation, as shown by representative and mean Western blot data in Fig. 5, A and C (P < 0.01). Chemical inhibition of PKR with 2-aminopurine, however, resulted in virtually no cytomix-induced caspase-8 activation in C2C12 cells. We also examined the effect of cytomix incubation on caspase-8 levels in C2C12 cells transfected with either an inactive construct or a PKR dominant-negative construct (Fig. 5, B and C). Cytomix again induced a large increase in active caspase-8 levels for cells transfected with the control genetic construct (P < 0.01 for comparison of caspase-8 levels in saline-exposed and cytomix-exposed control transfected cells), but cytomix did not induce a increase in active caspase-8 for cells transfected with the dominant-negative PKR construct (nonsignificant).
Fig. 5.
A: effect of PKR inhibition with 2AP on caspase-8 protein levels in C2C12 cells. Top: procaspase-8 and cleaved, active caspase-8 protein for representative C2C12 myotube samples. Bottom: α-tubulin protein levels. Cytomix administration elicited a large increase in active cleaved caspase-8 protein, and administration of a PKR chemical inhibitor, 2AP, prevented this increase. B: effect of dominant-negative (DN) PKR transfection on caspase-8 protein levels in C2C12 cells. Top: procaspase-8 and cleaved, active caspase-8 protein for representative C2C12 myotube samples. Bottom: α-tubulin protein levels. Cytomix administration elicited a large increase in active cleaved caspase-8 protein, while cytomix administration to cells transfected with DN PKR did not manifest an increase in active caspase-8 protein. C: mean data examining the effect of PKR inhibition on C2C12 cell caspase activation. Data from experiments in which PKR was inhibited using 2AP are presented on the left, while data for studies in which cells were transfected with DN PKR constructs to inhibit PKR are shown on the right. Procaspase-8 levels were similar for all four groups in both sets of experiments, and these data are not shown. Active caspase-8 levels rose in both sets of experiments in response to administration of cytomix (P < 0.05). Administration of 2AP (left) or transfection with a DN PKR construct (right) prevented caspase-8 activation in response to cytomix administration (P < 0.01 for comparison between cytomix and cytomix plus 2AP groups, left; P < 0.05 for comparison of cytomix to cytomix plus DN PKR transfection, right). Values are means ± SE. *Significant statistical difference compared with the other groups.
DISCUSSION
Recent work by our group implicates caspase-8 activation as a key factor involved in sepsis-induced diaphragm dysfunction, as we found that endotoxin administration rapidly increases diaphragm caspase-8 activation and that caspase-8 inhibitors prevent endotoxin-induced diaphragm dysfunction (22, 24). The present study extends this previous work, examining one of the upstream signaling pathways, PKR, that is thought to be linked to death receptor activation and death receptor-mediated caspase-8 activation. The main observations of the present study are that endotoxin-induced sepsis elicits activation of PKR in the diaphragm, and administration of a PKR inhibitor prevents both endotoxin-induced diaphragm caspase-8 activation and endotoxin-induced reductions in diaphragm-specific force generation. Our data also indicate that PKR is required for cytokine-induced activation of caspase-8 in isolated skeletal muscle cells, as both a chemical inhibitor (2-aminopurine) and genetic inhibitor (transfection using a PKR dominant-negative construct) of PKR blocked caspase-8 activation in C2C12 cells during cytokine exposure.
Infection-induced respiratory muscle weakness.
Recent studies have shown that infections rapidly induce severe reductions in respiratory skeletal muscle strength in both human patients and animal models of infection (e.g., pseudomonas induced pneumonia, cecal ligation perforation induced sepsis, E. coli injection, endotoxin administration) (2, 4, 7, 14, 16, 17). The mechanisms by which infection produces these reductions in respiratory muscle strength are incompletely understood. Studies performed to date using animal models of infection indicate that several processes interact to modulate the development of respiratory muscle dysfunction, including activation of TNF-α receptors, generation of high levels of reactive oxygen species, and caspase-3 activation (22, 23, 24).
With regard to this last factor, we have performed several recent studies examining the role of caspase-3 activation in mediating diaphragm dysfunction in animal models of infection. As part of this work, we found that the extrinsic pathway of caspase-3 activation, involving death receptor-linked activation of caspase-8, was the major pathway of diaphragm caspase-3 activation in endotoxin-induced sepsis. This conclusion was reached because we found that endotoxin potently and rapidly increases diaphragm caspase-8 activity, administration of selective caspase-8 inhibitors prevents caspase-3 activation following endotoxin administration, and caspase-8 inhibitor administration also reduces endotoxin-induced muscle weakness (22, 24). In contrast, we observed no inhibition of endotoxin-induced diaphragm caspase-3 activation in response to caspase-9 inhibitor administration and no improvement in diaphragm force generation with caspase-9 inhibition. Our previous studies also found that TNF receptor 1 (TNFR1) receptors were required for endotoxin administration to elicit caspase activation, since TNFR1-deficient mice did not manifest diaphragm caspase activation when given endotoxin (22, 24). Importantly, our work is consistent with the notion that caspase-3 activation in skeletal muscle induces disassembly and dysfunction of the contractile machinery in that we find that inhibition of caspase-3 activation almost completely prevents sepsis-induced reduction in diaphragm force-generating capacity (5, 22, 24).
Caspase and PKR.
While caspase-8 activation requires attachment of specific ligands to death receptors (e.g., attachment of Fas ligand to the Fas receptor), the generation of active caspase-8 in response to ligand-receptor attachment is modulated by the activity of accessory proteins that associate with the death receptor (10). In addition, there appear to be critical death receptor-death receptor interactions, such that activation of one death receptor (e.g., the TNFR1) can trigger activation of a second type of death receptor (e.g., the Fas receptor) with subsequent cleavage of procaspase-8 attached to the second receptor to generate active cleaved caspase-8 (27). These two processes, accessory protein attachment and death receptor-death receptor interactions, are thought to be modulated by the activity of several kinases (29). As a result, caspase-8 generation from attachment of a ligand to a death receptor is not an all or none phenomenon, but is a regulated process, dependent on the status of several signaling kinase pathways.
The present study establishes a previously unrecognized role for one such signaling kinase, PKR, as a regulator of caspase-8 generation in skeletal muscle (the diaphragm) following endotoxin administration. While no previous study has demonstrated a role for PKR in mediating caspase-8 activation in skeletal muscle, work studying several other cell types has shown that PKR clearly regulates procaspase-8 cleavage to active caspase-8 in other tissues (1, 9). For example, Gil and Esteban (9) found that activation of PKR induces Fas-associated death domain-mediated caspase-8 activation in kidney cells, inducing apoptosis. In other work, Balachandran et al. (1) found that inducible overexpression of functional PKR in murine fibroblasts sensitized these cells to apoptosis induced by influenza virus. In contrast, fibroblasts transfected with a dominant-negative variant of PKR were completely resistant to viral-induced apoptosis in this study. Moreover, these authors found that this virus-induced, PKR-dependent apoptosis was secondary to PKR-mediated activation of caspase-8.
While the current report is the first to demonstrate that PKR modulates skeletal muscle caspase-8 activation, PKR has been shown to have a number of other important actions in skeletal muscle. One target of activated PKR is eIF-2α, a key enzyme required for protein synthesis (18). Under normal conditions, eIF-2α functions in the early stages of protein translation by combining with GTP, tRNA, the 40S ribosomal unit, and mRNA to form the 43S preinitiation complex (24). Hydrolysis of GTP bound to eIF-2α results in release of eIF-2-GDP. Subsequently, a reaction catalyzed by eIF-2β regenerates eIF-2α, which is then available to initiate another round of protein translation. Phosphorylation of eIF-2α by PKR prevents this recycling reaction, blocking formation of 43S preinitiation complexes, and thereby shutting down protein synthesis. In addition, Russell and Tisdale (20) have recently shown that PKR activates p38 in skeletal muscle. In turn, skeletal muscle p38 activation has been shown to activate key components of the proteasome pathway (15). Specifically, p38 phosphorylates and initiates degradation of the NF-κB inhibitory protein κB (I-κB); loss of I-κB then results in translocation of NF-κB to the nucleus, initiating upregulation of E proteins required for initiation of proteasomally mediated degradation of proteins (15). Taken together, these results suggest that PKR simultaneously regulates several pathways involved in protein turnover in muscle, with activation of PKR reducing protein synthesis due to eIF-2α phosphorylation, increasing disassembly of the contractile apparatus via caspase activation, and increasing protein degradation secondary to p38-mediated proteasomal activation. Arguably, PKR may, therefore, be a central mediator of sepsis-induced reductions in skeletal muscle protein content and contractile function.
In the present study, skeletal muscle PKR was found to be activated in an animal model of sepsis, but work has shown that this enzyme is also active in muscle in other important pathophysiological conditions. Recent work indicates that both phospho-PKR and phospho-eIF-2α levels are significantly increased in skeletal muscle samples taken from patients with esophageal cancer who lose weight (6). Moreover, muscle levels of myosin declined as weight loss increased in these patients, and there were linear relationships between phospho-PKR and phospho-eIF-2α levels (r = 0.76, P = 0.005) and between phospho-eIF-2α and myosin levels (r = 0.77, P = 0.004). These findings suggest that PKR activation may play an important role in mediating skeletal muscle protein loss and wasting in cancer patients. Other work using a muscle cell line exposed to high levels of exogenous glucose in vitro found that this stimulus led to PKR activation and loss of protein, and that inhibition of PKR blocked hyperglycemia-induced muscle cell protein loss (19). As a consequence, PKR activation may also play a role in hyperglycemia-induced muscle dysfunction, a key factor thought to be responsible for weakness in critically ill medical patients. Finally, PKR activity was recently measured on leukocytes from blood samples in patients with the chronic fatigue syndrome (CFS), the chemical sensitivity syndrome (CSS), and normal controls (26). All patients from both syndrome groups complained of muscle symptoms, including easy fatigability. CFS patients had PKR activity levels that were 1.3- to 13.5-fold greater than that of controls, while CSS patients had PKR levels 1.3- to 11.6-fold greater than that of controls. As a result, there is an association between systemic PKR activation and the presence of enhanced muscle fatigue in these two common syndromes (CFS and CSS).
Implications.
The present study indicates that PKR is activated in the diaphragm in an animal model of infection. In addition, we found that PKR activation is linked to caspase-8 activation in skeletal muscle, with inhibition of PKR blocking endotoxin-induced caspase-8 activation in intact animals and inhibiting cytokine-induced caspase-8 activation in an isolated skeletal muscle cell line. Caspase pathway activation in skeletal muscle has been shown to induce cleavage of selected contractile proteins, resulting in a reduction in the force-generating capacity of the contractile lattice (5). As indicated earlier, PKR activation has additional effects to block protein synthesis (via eIF-2α phosphorylation) and promote proteasomally mediated protein degradation (via p38 activation). Taking all of these findings together, it seems reasonable to speculate that administration of PKR inhibitors may represent a rational therapeutic approach to the prevention of sepsis-induced skeletal muscle dysfunction. Moreover, since PKR activation appears to be present in a number of other diseases associated with muscle dysfunction (i.e., cancer, hyperglycemia, the CFS), administration of inhibitors of this pathway may prove to be a useful treatment for a wide spectrum of disease processes associated with abnormal skeletal muscle function.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants 80429, 81525, 63698, 80609, and 69821.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol 74: 1513–1523, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski GS. Free radical induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24: 210–217, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Close RI. The dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 4. Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D, Comtois AS, Petrof BJ. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169: 679–686, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eley HL, Skipworth RJ, Deans DA, Fearon KC, Tisdale MJ. Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2alpha may signal skeletal muscle atrophy in weight-losing cancer patients. Br J Cancer 98: 443–449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujimura N, Sumita S, Aimono M, Masuda Y, Schichinohe Y, Narimatsu E, Namiki A. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 162: 2159–2165, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5: 107–114, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Gil J, Esteban M. The interferon-induced protein kinase (PKR), triggers apoptosis through FADD-mediated activation of caspase-8 in a manner independent of Fas and TNF-alpha receptors. Oncogene 19: 3665–3674, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J 23: 1625–1637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimball SR, Jefferson LS. Role of amino acids in the translational control of protein synthesis in mammals. Semin Cell Dev Biol 16: 21–27, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med 68: 10–48, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Lanone S, Mebazaa A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J. Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med 162: 2308–2315, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mier-Jedrzejowicz M, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis 138: 5–7, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Poponick J, Jacobs I, Supinski G, DiMarco A. Effects of upper respiratory tract infections on respiratory muscle strength in patients with neuromuscular disease. Am J Respir Crit Care Med 156: 659–664, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Raven JF, Koromilas AE. PERK and PKR: old kinases learn new tricks. Cell Cycle 7: 1146–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Russell ST, Rajani S, Dhadda RS, Tisdale MJ. Mechanism of induction of muscle protein loss by hyperglycaemia. Exp Cell Res 315: 16–25, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methyl butyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 21: 125–34, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol 316: 253–292, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 100: 1770–1777, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 102: 2056–2063, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Supinski GS, Ji X, Wang W, Callahan LA. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol 102: 1649–1657, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Supinski G, Nethery D, Stofan D, DiMarco A. Comparison of the effects of endotoxin on limb, respiratory, and cardiac muscles. J Appl Physiol 81: 1370–1378, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Vojdani A, Lapp CW. Interferon-induced proteins are elevated in blood samples of patients with chemically or virally induced chronic fatigue syndrome. Immunopharmacol Immunotoxicol 21: 175–202, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Wallach D, Arumugam TU, Boldin MP, Cantarella G, Ganesh KA, Goltsev Y, Goncharov TM, Kovalenko AV, Rajput A, Varfolomeev EE, Zhang SQ. How are the regulators regulated? The search for mechanisms that impose specificity on induction of cell death and NF-kappaB activation by members of the TNF/NGF receptor family. Arthritis Res 4: S189–S196, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wenon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Yeh JH, Hsu SC, Han SH, Lai MZ. Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein-mediated apoptosis by induced FLICE-inhibitory protein expression. J Exp Med 188: 1795–1802, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]