Abstract
Lung inflammation and alterations in endothelial cell (EC) permeability are key events to development of acute lung injury (ALI). Protective effects of atrial natriuretic peptide (ANP) have been shown against inflammatory signaling and endothelial barrier dysfunction induced by gram-negative bacterial wall liposaccharide. We hypothesized that ANP may possess more general protective effects and attenuate lung inflammation and EC barrier dysfunction by suppressing inflammatory cascades and barrier-disruptive mechanisms shared by gram-negative and gram-positive pathogens. C57BL/6J wild-type or ANP knockout mice (Nppa−/−) were treated with gram-positive bacterial cell wall compounds, Staphylococcus aureus-derived peptidoglycan (PepG) and/or lipoteichoic acid (LTA) (intratracheal, 2.5 mg/kg each), with or without ANP (intravenous, 2 μg/kg). In vitro, human pulmonary EC barrier properties were assessed by morphological analysis of gap formation and measurements of transendothelial electrical resistance. LTA and PepG markedly increased pulmonary EC permeability and activated p38 and ERK1/2 MAP kinases, NF-κB, and Rho/Rho kinase signaling. EC barrier dysfunction was further elevated upon combined LTA and PepG treatment, but abolished by ANP pretreatment. In vivo, LTA and PepG-induced accumulation of protein and cells in the bronchoalveolar lavage fluid, tissue neutrophil infiltration, and increased Evans blue extravasation in the lungs was significantly attenuated by intravenous injection of ANP. Accumulation of bronchoalveolar lavage markers of LTA/PepG-induced lung inflammation and barrier dysfunction was further augmented in ANP−/− mice and attenuated by exogenous ANP injection. These results strongly suggest a protective role of ANP in the in vitro and in vivo models of ALI associated with gram-positive infection. Thus ANP may have important implications in therapeutic strategies aimed at the treatment of sepsis and ALI-induced gram-positive bacterial pathogens.
Keywords: cytoskeleton, pulmonary endothelium, inflammation, vascular leak
sepsis is the 10th leading cause of death in the United States (43). Analysis of national age-adjusted sepsis mortality in the US shows ∼200,000 incidents per year (67). Sepsis is a major contributor to development of acute respiratory distress syndrome, which yields unacceptably high mortality rates, reaching 35–40% (42). About 50% of all cases of sepsis have been reported to be caused by gram-positive bacteria (53). The presence of Staphylococcus aureus has also been associated with the acquisition of late-onset ventilator-associated pneumonia (VAP) in critically ill patients and represented 21% of recovered organisms in the bronchoalveolar lavage (BAL) (24). In the other report, Staphylococcus aureus (of which 65.8% are methicillin resistant) was defined as a major risk factor for VAP, representing 27.1% of the principal microorganisms causing VAP (25).
Although the role of lipopolysaccharide (LPS) in development of gram-negative septic shock is well established, pathological mechanisms triggered by gram-positive organisms leading to lung dysfunction or septic shock are less well understood. Peptidoglycan (PepG) and lipoteichoic acid (LTA) are two major cell wall components in gram-positive bacteria. Both PepG and LTA stimulate inflammatory responses in vivo and in vitro via activation of toll-like receptors (TLRs) (32, 68). In the lungs, both LTA and PepG induce acute pulmonary inflammation in a dose-dependent way, as characterized by neutrophilic influx and IL-6 production in the BAL fluid (36). LTA and PepG may also synergize to cause shock and multiple systems failure (17).
Of the 10 TLRs known, only TLR-2 is clearly shown to be involved in the host defense against gram-positive bacteria, but it also recognizes lipoproteins from other bacterial species (48, 58). TLR activation in the endothelial cells (ECs) induces phosphorylation/activation of downstream targets, including mitogen-activated protein kinases (MAPK) p42/p44, JNK1/2 and p38, and nuclear factor-κB (NF-κB) (2). NF-κB normally localizes to the cytoplasm, where it is bound by the inhibitory IκB proteins (IκBα, IκBβ, IκBϵ). Activation of inflammatory signaling leads to IκB phosphorylation by IκB kinase and subsequent degradation by the proteasome. IκB degradation causes NF-κB release and translocation to the nucleus, where it triggers the transcription of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 (16). Consistent with its key role in mediating inflammatory signaling from gram-positive bacteria, short interfering RNA-induced knockdown of TLR-2 decreases Raf phosphorylation and suppresses TLR-2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB cascade (13).
Small Rho GTPases appear to be activated by TLR signaling (54), as stimulation of TLR-2 receptor caused rapid activation of Rho GTPase (61). Because Rho pathway plays a major role in the endothelial cytoskeletal remodeling and actomyosin contraction via increased phosphorylation of myosin light chains (MLC), which leads to lung EC barrier failure (5, 9, 52), this study examined the effects of LTA and PepG on Rho-dependent phosphorylation of cytoskeletal Rho target, myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT-1), and the levels of phosphorylated MLC.
Natriuretic peptides (atrial, brain, and C-type) regulate a variety of physiological functions, including vascular tone, plasma volume, and renal function. In addition to well-established diuretic, natriuretic, and vasodilatory effects in the cardiovascular system (see Ref. 3 for review), atrial natriuretic peptide (ANP) exhibits other important biological activities. ANP may protect endothelial barrier function in vivo and in vitro apart from its vasodilatory and natriuretic effects (22, 27, 28, 45). These modalities suggest a potential role for ANP in the regulation of the lung function in the settings of acute lung injury (ALI) associated with sepsis, inflammation, and prolonged mechanical ventilation (19, 45).
We hypothesized that ANP may exhibit more general protective effects on lung inflammation and EC barrier dysfunction caused by gram-negative and gram-positive bacterial compounds by suppressing inflammatory cascades and barrier disruptive mechanisms, which may be shared by gram-negative and gram-positive pathogens. We tested signaling pathways activated by LTA and PepG in the pulmonary ECs and in lung tissue, linked them with changes in vascular permeability, and examined effects of ANP in the modulation of lung vascular dysfunction induced by components of gram-positive bacteria.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery ECs (HPAECs) were obtained from Lonza (Allendale, NJ). Cells were maintained in a complete culture medium, according to the manufacturer's recommendations and used for experiments at passages 5–9. ANP was purchased from Ana Spec (San Jose, CA). Phospho-HSP, phospho-p38, phospho-ERK1/2, phospho-MLC, phospho-IκBα, and IκBα antibodies were obtained from Cell Signaling (Beverly, MA); phospho-MYPT antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). SB-203580 was obtained from Calbiochem (La Jolla, CA). Unless specified, biochemical reagents, including LTA and PepG, were obtained from Sigma (St. Louis, MO).
Measurement of transendothelial electrical resistance.
Measurements of transendothelial electrical resistance (TER) across confluent HPAEC monolayers were performed using electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY), as previously described (7, 10). In brief, cells were cultured on small gold electrodes (10−2 mm2), and culture media were used as electrolyte. The total electrical resistance was measured dynamically across the monolayer and was determined by the combined resistance between the basal surface of the cell and the electrode, reflective of focal adhesion, and the resistance between cells. The small gold electrodes and the larger counter electrodes (100 mm2) were connected to a phase-sensitive lock-in amplifier (5301A; EG&G Instruments, Princeton, NJ) with a built-in differential preamplifier (5316A; EG&G Instruments). A 1-V, 4,000-Hz alternating current signal was supplied through a 1-MΩ resistor to approximate a constant-current source. Voltage and phase data were stored and processed with a personal computer that controlled the output of the amplifier and relay switches to different electrodes. Experiments were conducted only on wells that achieved >1,000 Ω (10 microelectrodes/well) of steady-state resistance. Resistance was expressed by the in-phase voltage (proportional to the resistance), which was normalized to the initial voltage and expressed as a fraction of the normalized resistance value.
Immunoblotting.
After stimulation, cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies, as described elsewhere (4, 10).
Immunofluorescence.
Endothelial monolayers plated on glass coverslips were treated with agonist of interest, then fixed and subjected to immunofluorescence staining for F-actin, as previously described (6, 7).
In vivo model of ALI.
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6J mice, 8–10 wk old, with average weight of 20–25 g (Jackson Laboratories, Bar Harbor, ME), were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Bacterial LPS (0.63 mg/kg body wt; Escherichia coli O55:B5), LTA (2.5 mg/kg), PepG (2.5 mg/kg), both from Sigma (St. Louis, MO), a mixture of LTA and PepG, or sterile saline solution were injected intratracheally in a small volume (20–30 μl) using a 20-gauge catheter (Penn-Century, Philadelphia, PA). Mice were randomized to concurrently receive sterile saline solution or ANP (2 μg/kg) by intravenous injection in the external jugular vein to yield the experimental groups: control, (LTA + PepG), ANP, and ANP + (LTA + PepG). At 24 h, animals were killed by exsanguination under anesthesia. Tracheotomy was performed, and the trachea was cannulated with a 20-gauge intravenous catheter, which was tied into place. Measurements of cell count and protein concentration in BAL fluid were performed as previously described (8, 20). Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 h before termination of ventilation to assess vascular leak, as described previously (47, 50). Histological assessment of lung injury was performed, as descried elsewhere (20, 50).
ANP knockout mice.
Mice with targeted disruption of ANP gene (strain B6.129P2-Nppatm1Unc/J) were purchased from the Jackson Laboratories (Bar Harbor, ME). Genotyping of Nppa−/− mice was performed by PCR analysis of genomic DNA extracted from tail snips with assistance of animal core facility. Similar to experiments described above, male 8- to 10-wk-old homozygous Nppa−/− and wild-type animals were anesthetized, and a mixture of LTA (2.5 mg/kg) and PepG (2.5 mg/kg) was administered intratracheally, with or without intravenous ANP (2 μg/kg) injection. At 24 h, animals were killed by exsanguination under anesthesia, and parameters of lung injury were evaluated by measurements of BAL cell count and protein concentration.
Statistical analysis.
Results are presented as means ± SD of three to six independent experiments. Stimulated samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way ANOVA, followed by the post hoc Tukey test, were used. P < 0.05 was considered statistically significant.
RESULTS
LTA induces barrier dysfunction in pulmonary endothelium.
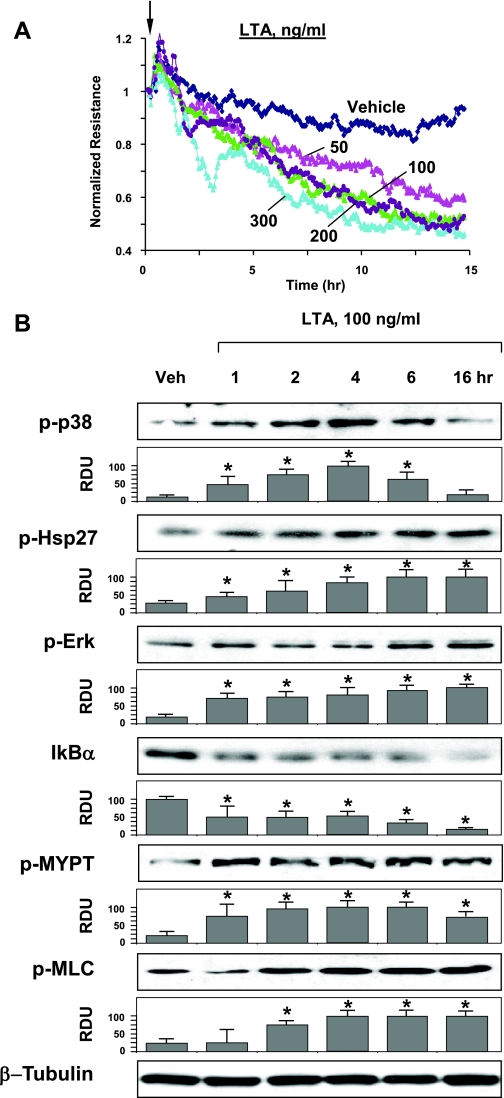
Dose-dependent (50–300 ng/ml) effects of LTA derived from Staphylococcus aureus on pulmonary EC permeability were assessed by measurements of TER, as described in detail in materials and methods. All LTA concentrations significantly decreased TER after 4–5 h, and maximal response was observed after 10 h of treatment (Fig. 1A). All following in vitro experiments were performed using 100 ng/ml LTA. Activation of intracellular signaling by LTA was evaluated by increases in phosphorylation of signaling proteins involved in stress and inflammatory cascades.
Fig. 1.
Effects of lipoteichoic acid (LTA) on pulmonary endothelial cell (EC) barrier function and inflammatory signaling. A: EC grown on microelectrodes to confluence were treated with LTA (50, 100, 200, or 300 ng/ml, indicated by arrow) and used for measurements of transendothelial electrical resistance (TER). B: human pulmonary artery EC (HPAEC) were challenged with LTA (100 ng/ml) for indicated periods of time. Phosphorylation (p) of p38, Hsp27, ERK1/2, myosin-associated phosphatase type (MYPT), and myosin light chain (MLC) was determined by Western blot with corresponding phospho-specific antibodies. Degradation of IκBα was detected using pan IκBα antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. Results are representative of three to six independent experiments. Result of densitometry are shown as means ± SD. *P < 0.05, compared with vehicle (Veh) control. RDU, relative densitometric units.
LTA caused time-dependent phosphorylation of stress-activated p38 MAP kinase and its downstream target Hsp27, and increased phosphorylation of ERK1/2 MAP kinase at later time points (Fig. 1B, top panels). LTA caused degradation of IκBα, an inhibitory subunit of NF-κB complex (Fig. 1B, middle panels), which leads to activation of NF-κB-dependent transcription.
Recent reports, including our works, show the role of Rho signaling in the increased lung vascular endothelial permeability induced by LPS (60), protease-activated receptor 1 activators (10, 29), transforming growth factor-β (5, 15), or ventilation at high tidal volume (50). Treatment of EC with LTA caused time-dependent phosphorylation of MYPT-1 at the Rho kinase-specific site, leading to increased MLC phosphorylation (Fig. 1B, bottom panels). These parameters reflect EC contraction associated with activation of Rho pathway (10, 64).
PepG increases endothelial permeability.
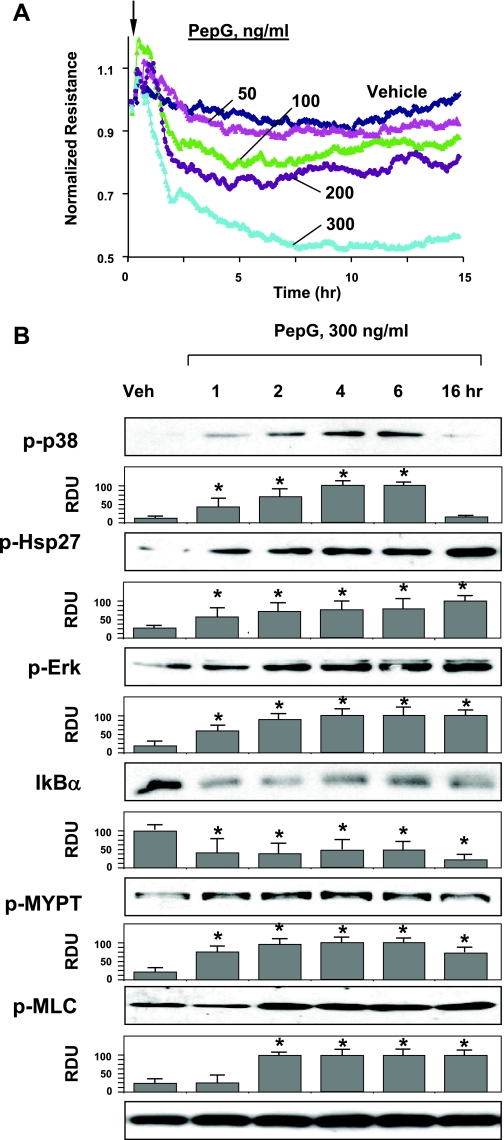
The following experiments tested effects of the other proinflammatory compound derived from Staphylococcus aureus, PepG, on the lung EC permeability. Cells were incubated in the range of PepG concentrations (50–300 ng/ml) and used for measurements of TER. No significant effects were observed at lower PepG concentration (50 ng/ml), while higher concentrations induced pronounced permeability response with maximal levels observed at 300 ng/ml PepG (Fig. 2A). Maximal TER decline was observed after 5–6 h of PepG challenge. PepG at 300 ng/ml was used for the further experiments. Activation of inflammatory and barrier disruptive signaling by PepG was further assessed by Western blot analysis of stress kinase, Rho, and NF-κB cascade activation. Similar to LTA, PepG caused time-dependent activation of ERK1/2 and p38 MAP kinase and increased phosphorylation of p38 MAPK downstream effector Hsp27 (Fig. 2B, top panels). PepG also induced degradation of IκBα (Fig. 2B, middle panels) and promoted sustained phosphorylation of MYPT-1 and MLC after 2 h of treatment (Fig. 2B, bottom panels). Collectively, these data show significant barrier disruptive effect of PepG on pulmonary endothelium.
Fig. 2.
Effects of peptidoglycan (PepG) on EC barrier function and inflammatory signaling. A: EC monolayers grown on microelectrodes were treated with PepG (50, 100, 200, or 300 ng/ml, indicated by arrow) and used for permeability measurements. B: HPAEC were stimulated with PepG (300 ng/ml) for indicated periods of time. Phosphorylation of p38, Hsp27, ERK1/2, MYPT, and MLC was determined by Western blot with corresponding phospho-specific antibodies. Degradation of IκBα was detected using pan IκBα antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. Results are representative of three to six independent experiments. Result of densitometry are shown as means ± SD. *P < 0.05, compared with vehicle control.
ANP attenuates hyperpermeability induced by LTA and PepG.
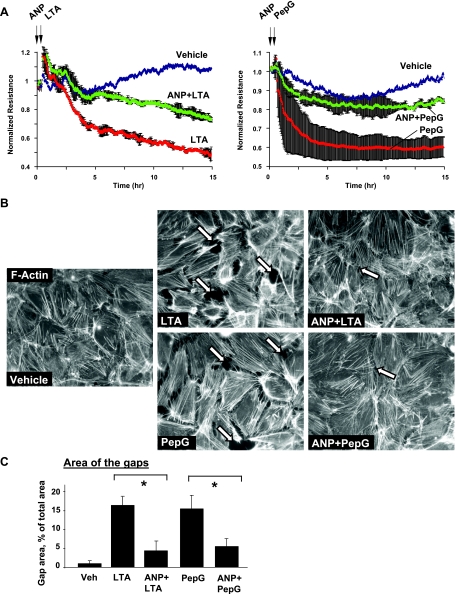
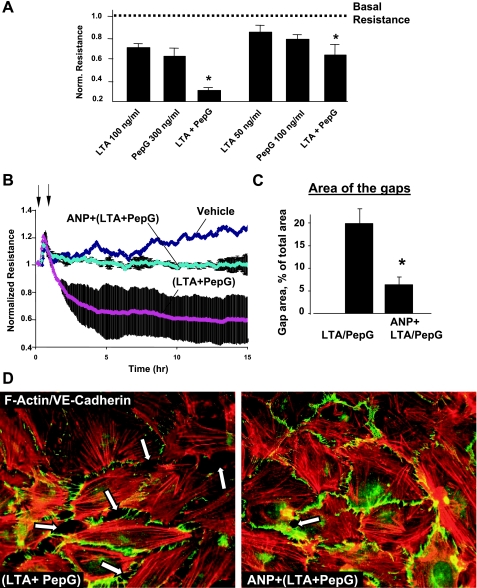
Effects of ANP on EC dysfunction induced by LTA or PepG were further examined using the measurements of TER. HPAEC were pretreated with ANP (20 min), followed by incubation with LTA or PepG for up to 15 h. ANP dramatically attenuated disruptive effects of both LTA and PepG (Fig. 3A). Effects of ANP on the EC cytoskeletal remodeling induced by LTA or PepG were next examined by immunofluorescence staining and visualization of actin cytoskeleton in pulmonary EC after 6 h of LTA or PepG treatment, with the time point corresponding to pronounced increase in EC monolayer permeability. Consistent with permeability data, treatment of lung EC with LTA or PepG induced formation of actin stress fibers and cell contraction, leading to paracellular gap formation, which reflects EC monolayer barrier compromise (Fig. 3, B and C). These results depict potent protective effects of ANP against EC barrier dysfunction caused by components of gram-positive bacteria.
Fig. 3.
Effects of atrial natriuretic peptide (ANP) on agonist-induced EC permeability and adherens junction remodeling. A: HPAEC were treated with ANP (100 ng/ml, 20 min, marked by first arrow). At the time point indicated by second arrow, cells were stimulated with LTA (100 ng/ml; left) or PepG (300 ng/ml; right), and TER was monitored over time. Results are representative of three to five independent experiments. B: endothelial monolayers were pretreated with vehicle or ANP (100 nM, 20 min) and stimulated with LTA or PepG for 6 h. Cytoskeletal remodeling was assessed by immunofluorescence staining for F-actin with Texas red phalloidin. Paracellular gaps are marked by arrows. C: quantitative analysis of gap formation in control and treated HPAEC. Data are expressed as means ± SD of three independent experiments. *P < 0.05.
ANP suppresses inflammatory signaling activated by LTA and PepG.
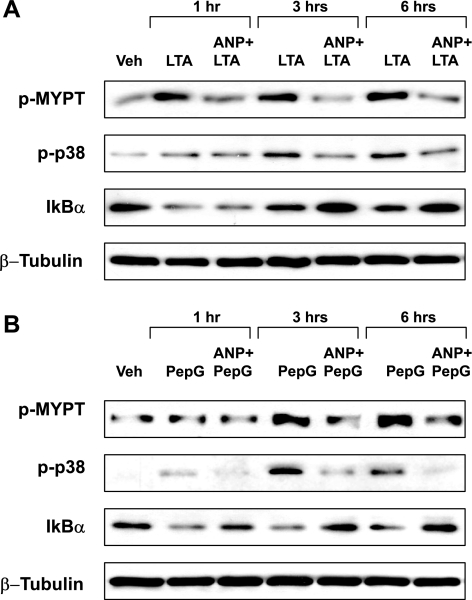
Protective effects of ANP on pulmonary EC barrier were further linked to ANP-induced attenuation of signaling pathways activated by LTA and PepG. HPAEC were pretreated with ANP and stimulated with LTA or PepG for 1, 3, or 6 h. Effects of ANP on activation of p38 MAPK, Rho, or NF-κB pathways were evaluated by Western blot. ANP did not affect the ERK1/2 MAPK phosphorylation induced by LTA challenge (data not shown), but significantly attenuated LTA-induced p38 MAPK and MYPT phosphorylation and IκBα subunit degradation (Fig. 4A). Similar inhibitory effects of ANP on inflammatory and Rho-mediated signaling were observed in the PepG-treated cells (Fig. 4B).
Fig. 4.
Effects of ANP on inflammatory cascade activation induced by LTA or PepG. EC were pretreated with ANP (100 nM, 20 min), followed by treatment with LTA (100 ng/ml; A) or PepG (300 ng/ml; B) for 1, 3, or 6 h. Phosphorylation of p38, MYPT, and IκBα was determined by Western blot with corresponding antibodies. Degradation of IκBα was detected using pan IκBα antibodies. Equal protein loading was confirmed by probing of membranes with β-tubulin antibodies. Results are representative of five to seven independent experiments.
Combined LTA and PepG treatment augments EC barrier dysfunction.
Because both LTA and PepG are present during gram-positive infection, the next experiments examined potential synergy between LTA and PepG barrier disruptive effects and evaluated protective effects of ANP in this model. Permeability changes in pulmonary EC cultures treated with various doses of LTA, PepG, or their combination were monitored by the measurements of TER. Combination of LTA (100 ng/ml) and PepG (300 ng/ml) caused more pronounced decline in TER compared with the EC treatment with either LTA or PepG alone (Fig. 5A, left). Immunofluorescence analysis of (LTA + PepG) EC monolayers showed significant cell collapse (data not shown), and lower LTA and PepG doses were further tested. Combined LTA and PepG cotreatment at submaximal doses (50 and 100 ng/ml, respectively) again caused more prominent permeability increase, compared with LTA or PepG alone (Fig. 5A, right). ANP pretreatment significantly decreased hyperpermeability induced by combined stimulation with LTA and PepG (Fig. 5B). In agreement with these data, ANP pretreatment also markedly inhibited paracellular gap formation and disruption of cell-cell junctions in EC monolayers treated with combination of LTA and PepG (Fig. 5, C and D).
Fig. 5.
Effects of ANP on LTA- and PepG-induced EC permeability. A: ECs grown on microelectrodes to confluence were treated with LTA alone, PepG, or combination of LPA and PepG, followed by measurements of TER. Bar graphs represent polled TER data after 6 h of treatment. *P < 0.05, compared with LTA or PepG alone. B: lung EC monolayers were treated with ANP (100 ng/ml, 20 min, marked by first arrow). At the time point indicated by second arrow, cells were stimulated with a mixture of LTA (50 ng/ml) and PepG (100 ng/ml), and TER was monitored over the time. C: quantitative analysis of gap formation in control and treated HPAEC. Data are expressed as means ± SD of three independent experiments. *P < 0.05, compared with LTA/PepG. D: HPAEC were pretreated with vehicle or ANP (100 nM, 20 min), followed by stimulation with LTA and PepG for 6 h. Cytoskeletal remodeling was assessed by immunofluorescence staining for F-actin with Texas red phalloidin and VE-cadherin (merged images). Paracellular gaps are marked by arrows.
ANP attenuates lung injury induced by LTA and PepG in vivo.
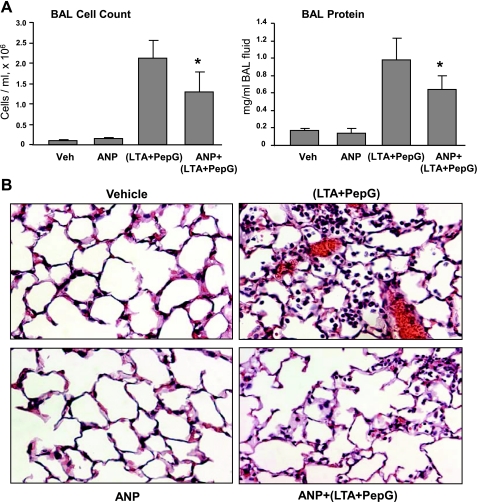
To further verify protective ANP effects against EC barrier failure in septic models, we used an animal model of ALI induced by intratracheal instillation of LTA and PepG. Mice were treated with LTA or PepG for 24 h, and lung injury was assessed by measurements of cell counts and protein content in BAL fluid. A separate group treated with LPS was used as a reference point to compare the magnitudes of LTA- and PepG-induced injurious response. LTA alone caused modest increase in BAL cell count (0.56 ± 0.09 vs. 0.11 ± 0.03 × 106 cells/ml in control, P < 0.05) and protein concentration (0.28 ± 0.07 vs. 0.17 ± 0.03 mg/ml in control, P < 0.05). PepG caused more pronounced increase in both BAL cell count and protein concentration, but it still was lower compared with mice challenged with bacterial wall LPS (0.98 ± 0.24 vs. 1.51 ± 0.60 × 106 cells/ml for LPS, P < 0.005; 0.47 ± 0.20 vs. 0.69 ± 0.05 mg/ml for LPS, P < 0.005, respectively). These results indicate that maximal injurious response to gram-positive bacterial compounds may be achieved by combined LTA and PepG treatment. Indeed, concurrent administration of LTA and PepG caused dramatic acute inflammatory response in the lungs. BAL analysis revealed 20-fold increase in BAL cell counts (2.13 ± 0.43 vs. 0.11 ± 0.03 × 106 cells/ml in controls, P < 0.001) and 6-fold increase in BAL protein concentration (0.98 ± 0.24 vs. 0.17 ± 0.03 mg/ml in controls, P < 0.001) (Fig. 6A). A single ANP injection significantly attenuated BAL cell counts (1.30 ± 0.58 vs. 2.13 ± 0.43 × 106 cells/ml for LTA + PepG, P < 0.05) and protein content (0.69 ± 0.17 vs. 0.98 ± 0.24 mg/ml for LTA + PepG, P < 0.05) in the animals treated with combination of LTA and PepG.
Fig. 6.
Role of ANP on the development of lung inflammation induced by LTA and PepG. A: C57BL/6J mice were treated with a mixture of LTA (2.5 mg/kg) and PepG (2.5 mg/kg), with or without concurrent treatment with ANP (2 μg/kg) or sterile saline solution for 24 h. Control animals were treated with sterile saline solution or ANP alone. Cell count and protein concentration were measured in bronchoalveolar lavage (BAL) fluid taken from control and experimental animals. Values are means ± SD; n = 6 per condition. *P < 0.05, compared with LTA/PepG treatment. B: lung specimens were obtained from control, LTA/PepG-treated, ANP-treated, and ANP + LTA/PepG-treated C57BL/6J mice. Animal lungs were fixed in formaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosion. Original magnification: ×20. Images are representative of five to eight lung specimens for each condition.
Histological analysis of lung tissue sections stained with hematoxylin and eosin revealed that, in contrast to control or ANP-treated animals, combined treatment with LTA and PepG induced neutrophil infiltration in the lung parenchyma and caused appearance of areas of alveolar hemorrhage indicative of vascular disruption. Consistent with BAL data, ANP administration abolished these effects (Fig. 6B).
ANP protects against lung vascular leak induced by LTA and PepG.
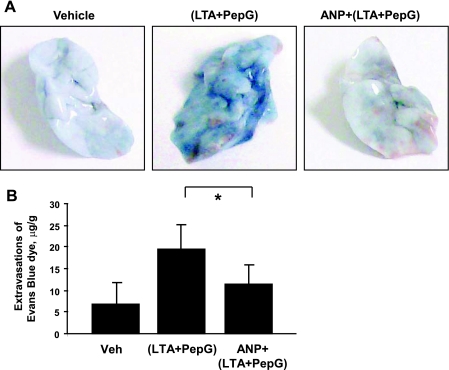
Effects of ANP on the lung vascular leak induced by LTA and PepG were evaluated by measurements of Evans blue extravasation into the lung tissue. Evans blue dye was intravenously injected 2 h before termination of the experiment. Combined administration of LTA and PepG induced prominent Evans blue leakage from the vascular space into the lung parenchyma, which was dramatically suppressed by a single injection of ANP (Fig. 7A). Quantitative analysis of Evans blue-labeled albumin extravasation in the lung tissue confirmed these results and showed significant increase of Evans blue dye accumulation in the samples from the animals treated with LTA-PepG combination (19.68 ± 5.49 vs. 6.96 ± 4.80 μg/g wet weight lung in nontreated controls, P < 0.05). These effects were diminished in ANP-treated animals (11.37 ± 4.12 vs. 19.68 ± 5.49 μg/g wet weight lung in LTA/PepG, P < 0.05), (Fig. 7B).
Fig. 7.
Effects of ANP on LTA- and PepG-induced lung vascular leak. C57BL/6J mice were treated with vehicle or a mixture of LTA (2.5 mg/kg) and PepG (2.5 mg/kg), with or without concurrent administration of ANP (2 μg/kg) for 24 h. Evans blue dye (30 ml/kg iv) was injected 2 h before termination of the experiment. A: lung vascular permeability was assessed by Evans blue accumulation in the lung tissue. B: the quantitative analysis of Evans blue-labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples. Values are means ± SD; n = 4 per condition. *P < 0.05.
ANP-deficient mice develop more severe injury in response to LTA and PepG.
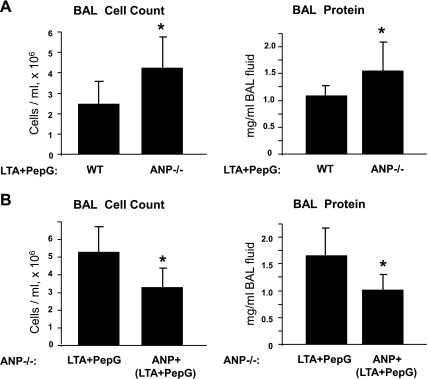
We evaluated parameters of ALI induced by (LTA + PepG) in ANP knockout mice (strain B6.129P2-Nppatm1Unc/J). After 24 h of intratracheal administration of (LTA + PepG), parameters of lung injury in ANP−/− mice and matched controls were analyzed by measurements of BAL cell count and protein content. Compared with wild-type controls, ANP−/− mice developed more severe lung injury in response to LTA and PepG with a 1.7-fold increase in cell counts (4.29 ± 1.60 vs. 2.52 ± 1.11 × 106 cells/ml in wild-type controls, P < 0.02) and nearly 1.5-fold increase in protein concentration in BAL fluid samples (1.54 ± 0.54 vs. 1.10 ± 0.19 mg/ml in wild-type controls, P < 0.04) (Fig. 8A).
Fig. 8.
Assessment of LTA- and PepG-induced lung injury in ANP-deficient mice. Wild-type or ANP knockout mice (strain B6.129P2-Nppatm1Unc/J) were treated with a mixture of LTA (2.5 mg/kg) and PepG (2.5 mg/kg) for 24 h. Control animals were treated with sterile saline solution. A: cell count and protein concentration were measured in BAL fluid taken from control and experimental animals. n = 6 per condition. *P < 0.04, compared with wild type. B: effects of single ANP injection (2 μg/kg iv) on LTA/PepG-induced lung injury in Nppa−/− mice were assessed by measurements of cell count and protein concentration in the BAL fluid. n = 4–6 per group. *P < 0.05, compared with LTA/PepG treatment. Values are means ± SD.
To further evaluate the role of ANP in the development of ALI, we performed rescue experiments. ANP knockout mice were injected with ANP simultaneously with intratracheal administration of LTA/PepG for 24 h, and parameters of lung injury were compared between ANP knockout mice treated with ANP or vehicle. A single ANP injection decreased parameters of LTA/PepG-induced lung injury, as detected by BAL cell counts (3.24 ± 1.11 vs. 5.27 ± 1.47 × 106 cells/ml in LTA/PepG-treated Nppa−/− mice, P < 0.05), and protein content (1.05 ± 0.28 vs. 1.66 ± 0.49 mg/ml in LTA/PepG-treated Nppa−/− mice, P < 0.05) (Fig. 8B).
DISCUSSION
The main finding of this study is protective effects of ANP in the model of lung injury induced by components of gram-positive pathogens, LTA and PepG. TLRs play an essential role in the activation of innate immunity by recognizing specific patterns of microbial components (30). The present results and our laboratory's previous report (70) demonstrate common proinflammatory and barrier-disrupting mechanisms triggered by gram-negative (LPS) and gram-positive (LTA, PepG) bacterial products, which mainly engage TLR-2 and TLR-4 and, by inflammatory cytokine, TNF-α acting via its own receptor. All four agents caused sustained activation of p38 and ERK1/2 MAP kinases, increased phosphorylation and degradation of negative regulator of NF-κB signaling IkBα, and increased Rho-kinase-mediated phosphorylation of MYPT-1, leading to accumulation of phosphorylated MLCs. Consistent with protective effects on EC permeability and monolayer integrity, ANP dramatically attenuated activation of inflammatory and barrier-disruptive signaling. These data suggest that ANP may target a signaling hub shared by LTA, PepG, LPS, and TNF-α signaling pathways, thus leading to inhibition of NF-κB, p38 MAPK, and Rho barrier-disruptive signaling. These events may determine protective effects of ANP in vitro and in vivo. Further studies are required to more precisely elucidate specific targets affected by ANP.
Studies using combined treatment with LPS and LTA/PepG show that blood-gas exchange impairment and pulmonary vascular hyperpermeability are dramatically enhanced in mice given PepG after LPS stimulation, indicating development of more severe ALI. This occurred because LPS significantly upregulated TLR-2 mRNA and protein levels in mouse lungs. That study suggested that the priming effect of LPS on PepG-induced lung injury and death was achieved by NF-κB-mediated upregulation of TLR-2 (41). Because our studies show that ANP efficiently suppresses signaling mediated by both TLR-2 and TLR-4 receptors, it may abolish LPS-induced priming of TLR-2 expression and thus more efficiently suppress severe ALI on combined infection by gram-negative and gram-positive bacteria.
Our results show enhanced LTA/PepG-induced lung injury in the ANP−/− mice and thus further suggest protective effects of endogenous ANP against septic agents. In turn, stress, sepsis, and inflammation also stimulate natriuretic peptide expression in vivo by transcriptional mechanisms (57, 65). These findings suggest that, although plasma levels of ANP and BNP become elevated 10- to 30-fold in patients with septic shock (62), the precise causal relationship between ANP elevation and severity of ALI remains to be investigated. For example, introduction of ANP reduced mortality rate, showed beneficial effect in patients with ALI (46), and improved endothelial barrier function (11, 23, 35).
It is important to note that control of vascular permeability by ANP depends on specific pathophysiological conditions. One basic physiological function of ANP is control of the body hypovolemic reactions and basal control of circulating fluid. Inhibition of ANP signaling in mice with endothelial-specific knockout of ANP receptor-A (NPR-A) leads to significant arterial hypertension and cardiac hypertrophy due to increased total plasma volume, despite full preservation of the direct vasodilating effects of ANP (55). In turn, intravenous ANP administration acutely enhanced the rate of albumin clearance from the circulatory system in control but not in EC-specific NPR-A knockout mice. ANP barrier-protective effects were observed in ALI and endothelial dysfunction caused by acid aspiration (59), LPS or oleic acid administration (28, 44), or oxidant generation (38), but other studies describe increased vascular endothelial barrier dysfunction, tissue injury, and mortality. In isolated mouse lungs, exogenous ANP added to the perfusate caused sevenfold increase in the filtration coefficient, only if lungs were subjected to ischemia-reperfusion (18). During experimental coronary occlusion in mice, ANP increased P-selectin, neutrophil infiltration, infarct size, expression of inflammatory surface molecule P-selectin, and mortality (26). Taken together, these contrasting data emphasize the importance of the understanding of specific signaling mechanisms targeted by ANP and suggest that beneficial effects of ANP may depend on particular pathological condition and ANP concentration.
Activation of stress-induced MAP kinases p38 and JNK increases endothelial permeability via weakening of cell-cell junctions and remodeling of actin cytoskeleton, and these changes can be reversed by corresponding pharmacological inhibitors (31, 51, 63). Inhibition of p38 MAPK by specific inhibitor SB-203580 attenuated the pulmonary inflammatory responses, neutrophil recruitment, total protein content in BAL fluid, activation of NF-κB, and inflammatory cytokine release, and reduced the mortality rate of LPS-induced ALI (34, 37). The results of this study show that components of gram-positive bacteria LTA and PepG also induced phosphorylation of IκB, p38, and ERK1/2 MAP kinases, which may be efficiently attenuated by injection of exogenous ANP.
Protective effects of ANP may be associated in part with its anti-inflammatory activities. Indeed, pretreatment with ANP attenuated effects of LTA and PepG on p38 MAPK and IkB, but not ERK1/2 MAPK phosphorylation. These data are in agreement with previous findings of ANP-mediated inhibition of vascular endothelial permeability, TNF-α secretion, and inflammatory signaling induced by LPS, hypoxia, or TNF-α challenge (22, 28, 33). Although inhibitory effects of ANP on inflammatory signaling induced by LPS or LTA/PepG shown in this study are evident, precise molecular mechanisms underlying anti-inflammatory ANP effects remain to be investigated.
This study also demonstrates the involvement of Rho-dependent signaling in the inflammatory responses induced by Staphylococcus aureus-derived components. How may Rho become activated by TLR-2 ligation? Recent study showed that both Src and Rho were required for full NF-κB activation caused by TLR-2 ligands. Rho activation was reported downstream of TLR-2-MyD88 (40). The authors proposed a possible mechanism of TLR-2-dependent Rho activation via Rho-specific guanine nucleotide exchange factor AKAP13 recruited by activated TLR-2 (40). This mechanism requires further verification. A role of Rho signaling in promotion of endothelial permeability is well known. Rho and its downstream target, Rho-associated kinase (Rho-kinase), may catalyze direct MLC phosphorylation, or act indirectly via inactivation of MLC phosphatase by phosphorylating its amino acid residues Thr695, Ser894, and Thr850 (21, 64, 66), the events leading to actomyosin-driven cell contraction and EC barrier compromise. In addition, Rho-driven cell contraction is associated with disassembly of endothelial adherens junctions (12, 69). In addition to direct effects on EC permeability, Rho activation by endotoxin may stimulate transcription of proinflammatory genes, while inhibition of Rho signaling reduced expression of TNF-α, CXC chemokines, leukocyte infiltration, and endotoxin-induced lung edema (56, 60).
What are potential mechanisms driving ANP-dependent attenuation of LTA/PepG-induced Rho activity? Recent reports have evaluated protective effects of ANP against human pulmonary macrovascular EC permeability induced by thrombin and described Rac-dependent, barrier-protective mechanism via ANP-mediated inhibition of Rho. In that case, ANP-induced Rac activation was mediated by PKA- and Epac-Rap1/Tiam1/Vav2-dependent signaling cascades (11, 35). A recent report demonstrates that ANP suppresses Rho signaling and lung dysfunction caused by LPS (70). Our data show that LTA/PepG-induced activation of Rho pathway in human pulmonary EC is also attenuated by ANP. This attenuation markedly reduced LTA/PepG-triggered EC permeability in vitro and lung dysfunction in vivo. Collectively, these data strongly suggest that suppression of barrier-disruptive Rho signaling caused by edemagenic agonists, endotoxin, or compounds of gram-positive bacteria may be a common barrier-protective mechanism elicited by ANP. Of note, live bacteria may trigger additional inflammatory cascades and engage other cell types and cellular processes, such as bacteria killing and phagocytosis. Thus further studies are required for comprehensive evaluation of beneficial effects of ANP in the models of lung dysfunction caused by Staphilococcus aureus.
Published studies suggest that different vascular compartments in the lung may exhibit differential permeability responses to pathological interventions or specific pharmacological compounds (39). For example, the direct activation of store-operated channel Ca2+ entry increased extra-alveolar, but not alveolar, EC permeability (14). Thus certain agonists differentially affect micro- and macrovascular permeability (reviewed in Ref. 1), reflecting phenotypic differences of endothelium from these beds, while other inflammatory stimuli induce global endothelial dysfunction in the lung vasculature. We observed fluid accumulation in both the alveolar space and the areas surrounding larger vessels. These results suggest that LTA/PepG-induced ALI was associated with macro- and microvascular endothelial barrier dysfunction. It is also possible that transition from increased extra-alveolar to capillary endothelial permeability may also depend on the duration of insult. For example, perivascular cuffing occurred first and was followed by alveolar flooding in isolated dog lungs under conditions of increased hydrostatic pressure (49). Thus global permeability increase in the LTA/PepG model of ALI observed after 16–24 h in this study does not exclude the possibility of differential changes in lung macro- and microvascular permeability at earlier times.
In summary, these data show potent in vitro and in vivo protective effects of ANP against lung injury and pulmonary endothelial leak induced by components of gram-positive bacteria and suggest a mechanism of such protection via ANP-mediated inhibition of p38 stress MAP kinase, decreased IkB degradation, and reduction of Rho pathway activated by LTA and PepG. ANP markedly attenuates these pathological mechanisms and thus may have important implications in therapeutic strategies aimed at the treatment of sepsis and ALI-induced gram-negative and gram-positive bacterial pathogens, or their combination. However, care should be taken in consideration of ANP therapy in other conditions, such as ventilator-induced lung injury, where excessive cGMP elevation caused by ANP may have detrimental effects.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute grant HL89257 and the American Heart Association Midwest Affiliate Grant-in-Aid.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aird WC. Endothelial cell heterogeneity. Crit Care Med 31: S221–S230, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Baxter GF. The natriuretic peptides. Basic Res Cardiol 99: 71–75, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453–464, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 40: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell 10: 9–22, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu WT, Lin YL, Chou CW, Chen RM. Propofol inhibits lipoteichoic acid-induced iNOS gene expression in macrophages possibly through downregulation of toll-like receptor 2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chem Biol Interact 181: 430–439, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal 11: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest 86: 9–22, 2006 [DOI] [PubMed] [Google Scholar]
- 17. De Kimpe SJ, Kengatharan M, Thiemermann C, Vane JR. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci U S A 92: 10359–10363, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dodd-o JM, Hristopoulos ML, Kibler K, Gutkowska J, Mukaddam-Daher S, Gonzalez A, Welsh-Servinsky LE, Pearse DB. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am J Physiol Lung Cell Mol Physiol 294: L714–L723, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Eison HB, Rosen MJ, Phillips RA, Krakoff LR. Determinants of atrial natriuretic factor in the adult respiratory distress syndrome. Chest 94: 1040–1045, 1988 [DOI] [PubMed] [Google Scholar]
- 20. Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22: 32–39, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res 96: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Furst R, Bubik MF, Bihari P, Mayer BA, Khandoga AG, Hoffmann F, Rehberg M, Krombach F, Zahler S, Vollmar AM. Atrial natriuretic peptide protects against histamine-induced endothelial barrier dysfunction in vivo. Mol Pharmacol 74: 1–8, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Gacouin A, Barbarot N, Camus C, Salomon S, Isslame S, Marque S, Lavoue S, Donnio PY, Thomas R, Le Tulzo Y. Late-onset ventilator-associated pneumonia in nontrauma intensive care unit patients. Anesth Analg 109: 1584–1590, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Hortal J, Giannella M, Perez MJ, Barrio JM, Desco M, Bouza E, Munoz P. Incidence and risk factors for ventilator-associated pneumonia after major heart surgery. Intensive Care Med 35: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Houng AK, McNamee RA, Kerner A, Sharma P, Mohamad A, Tronolone J, Reed GL. Atrial natriuretic peptide increases inflammation, infarct size, and mortality after experimental coronary occlusion. Am J Physiol Heart Circ Physiol 296: H655–H661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imamura T, Ohnuma N, Iwasa F, Furuya M, Hayashi Y, Inomata N, Ishihara T, Noguchi T. Protective effect of alpha-human atrial natriuretic polypeptide (alpha-hANP) on chemical-induced pulmonary edema. Life Sci 42: 403–414, 1988 [DOI] [PubMed] [Google Scholar]
- 28. Irwin DC, Tissot van Patot MC, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol 288: L849–L859, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116: 1606–1614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawai T, Akira S. TLR signaling. Cell Death Diff 13: 816–825, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Kevil CG, Oshima T, Alexander JS. The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium 8: 107–116, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun 73: 7428–7435, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kiemer AK, Weber NC, Furst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res 90: 874–881, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology 225: 36–47, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Klinger JR, Warburton R, Carino GP, Murray J, Murphy C, Napier M, Harrington EO. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp Cell Res 312: 401–410, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med 165: 1445–1450, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Liu S, Feng G, Wang GL, Liu GJ. p38 MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol 584: 159–165, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Lofton CE, Baron DA, Heffner JE, Currie MG, Newman WH. Atrial natriuretic peptide inhibits oxidant-induced increases in endothelial permeability. J Mol Cell Cardiol 23: 919–927, 1991 [DOI] [PubMed] [Google Scholar]
- 39. Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res 143: 70–77, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol 182: 3522–3529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsuda N, Yamazaki H, Takano K, Matsui K, Takano Y, Kemmotsu O, Hattori Y. Priming by lipopolysaccharide exaggerates acute lung injury and mortality in responses to peptidoglycan through up-regulation of toll-like receptor-2 expression in mice. Biochem Pharmacol 75: 1065–1075, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care 13: R28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mitaka C, Hirata Y, Habuka K, Narumi Y, Yokoyama K, Makita K, Imai T. Atrial natriuretic peptide improves pulmonary gas exchange by reducing extravascular lung water in canine model with oleic acid-induced pulmonary edema. Crit Care Med 30: 1570–1575, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Mitaka C, Hirata Y, Nagura T, Sakanishi N, Tsunoda Y, Amaha K. Plasma alpha-human atrial natriuretic peptide concentration in patients with acute lung injury. Am Rev Respir Dis 146: 43–46, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Mitaka C, Hirata Y, Nagura T, Tsunoda Y, Amaha K. Beneficial effect of atrial natriuretic peptide on pulmonary gas exchange in patients with acute lung injury. Chest 114: 223–228, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res 150: 253–265, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol 75: 749–755, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Noble WH, Kay JC, Obdrzalek J. Lung mechanics in hypervolemic pulmonary edema. J Appl Physiol 38: 681–687, 1975 [DOI] [PubMed] [Google Scholar]
- 50. Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nwariaku FE, Chang J, Zhu X, Liu Z, Duffy SL, Halaihel NH, Terada L, Turnage RH. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock 18: 82–85, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun 306: 244–249, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Pinner RW, Teutsch SM, Simonsen L, Klug LA, Graber JM, Clarke MJ, Berkelman RL. Trends in infectious diseases mortality in the United States. JAMA 275: 189–193, 1996 [PubMed] [Google Scholar]
- 54. Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol Res 34: 33–48, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest 115: 1666–1674, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Slotta JE, Braun OO, Menger MD, Thorlacius H. Fasudil, a Rho-kinase inhibitor, inhibits leukocyte adhesion in inflamed large blood vessels in vivo. Inflamm Res 55: 364–367, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Sun HC, Qian XM, Nie SN, Wu XH. Serial analysis of gene expression in mice with lipopolysaccharide-induced acute lung injury. Chin J Traumatol 8: 67–73, 2005 [PubMed] [Google Scholar]
- 58. Takeda K, Akira S. TLR signaling pathways. Semin Immunol 16: 3–9, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Tanabe M, Ueda M, Endo M, Kitajima M. The effect of atrial natriuretic peptide on pulmonary acid injury in a pig model. Am J Respir Crit Care Med 154: 1351–1356, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol 32: 504–510, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Teusch N, Lombardo E, Eddleston J, Knaus UG. The low molecular weight GTPase RhoA and atypical protein kinase Czeta are required for TLR2-mediated gene transcription. J Immunol 173: 507–514, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Ueda S, Nishio K, Akai Y, Fukushima H, Ueyama T, Kawai Y, Masui K, Yoshioka A, Okuchi K. Prognostic value of increased plasma levels of brain natriuretic peptide in patients with septic shock. Shock 26: 134–139, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem 279: 11789–11797, 2004 [DOI] [PubMed] [Google Scholar]
- 64. van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Vesely DL, de Bold AJ. Cardiac natriuretic peptides gene expression and secretion in inflammation. J Investig Med 57: 29–32, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 9: 2639–2653, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G. National variation in United States sepsis mortality: a descriptive study. Int J Health Geogr 9: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 20: 402–414, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res 79: 26–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]