Abstract
Skeletal muscle exhibits superb plasticity in response to changes in functional demands. Chronic increases of skeletal muscle contractile activity, such as endurance exercise, lead to a variety of physiological and biochemical adaptations in skeletal muscle, including mitochondrial biogenesis, angiogenesis, and fiber type transformation. These adaptive changes are the basis for the improvement of physical performance and other health benefits. This review focuses on recent findings in genetically engineered animal models designed to elucidate the mechanisms and functions of various signal transduction pathways and gene expression programs in exercise-induced skeletal muscle adaptations.
Keywords: endurance exercise, skeletal muscle adaptation, fiber type transformation, angiogenesis, mitochondrial biogenesis
since antiquity, regular exercise has been known to have great benefits, including enhanced performance and healthy longevity. More recently, exercise has been shown to exert significant positive impacts on an increasing number of diseases in humans, including obesity, diabetes, and cardiovascular disease (37, 101, 121, 163, 164), whereas physical inactivity poses major negative influences (22a, 68). Since these chronic diseases are either direct causes or major risks for mortality (139), and there is a strong, negative association between exercise capacity and all-cause mortality (82), regular exercise should become a fundamental strategy in combating many lifestyle disorders. Although exercise inevitably affects all organs in the body, major positive impacts are believed to result directly from skeletal muscle adaptations (84). For example, changes in skeletal muscle phenotype have been associated with the metabolic syndrome in humans (90), a prodrome of type 2 diabetes. An improved understanding of the molecular and signaling mechanisms underlying exercise-induced skeletal muscle adaptation will provide invaluable information for using exercise and designing therapeutic interventions for many of the chronic diseases that affect people worldwide.
In mammals, skeletal muscle is a mosaic of different types of muscle fibers with diverse structural properties and functional capabilities. Muscle fibers have been traditionally classified by myofibrillar actomyosin ATPase histochemistry, immunoblot, and/or immunofluorescence analyses of myosin heavy chain (MHC) isoforms. Based on the expression of the predominant MHC isoforms, rodents have type I, IIa, IId/x, and IIb fibers (34, 167, 171) while humans have three fiber types (I, IIa, and IId/x) (67, 161). Type I slow-twitch, oxidative fibers are slow in force generation (185) and have an oxidative profile (rich in oxidative enzyme expression, mitochondria, and capillary supply) (125, 160). Type IIa fast-twitch, oxidative fibers are fast in force generation (33) but have similar oxidative profiles to the type I fibers. Type IId/x fibers are fast-twitch with a glycolytic metabolic profile (rich in glycolytic enzyme expression and poor in mitochondria and capillary supply) (125). Type IIb fibers have an even more fast-twitch, glycolytic phenotype than type IId/x fibers (134, 135, 167). The relaxation of myofibers also varies dramatically depending on proteins involved in calcium reuptake and sequestration (172, 182).
Adult skeletal muscle fibers retain a robust regulatory network that orchestrates complex processes of phenotypic adaptations in response to changes in functional demands to better respond to future challenges. As a consequence, the adapted muscle has improved performance along with health benefits. It is well known that endurance exercise promotes phenotypic adaptations in skeletal muscle toward a more oxidative phenotype. Specifically, endurance exercise promotes fiber type transformation (type IIb/IId/x to IIa) (36), mitochondrial biogenesis (66, 176), angiogenesis (165), and other phenotypic changes, including improved insulin sensitivity and metabolic flexibility (24, 46, 63, 83, 151, 158, 168). These adaptive processes are analogous to the control of the mechanical, power generation, and fuel supply components of a mechanical engine. A precise control of each individual component in a highly coordinated manner is a prerequisite to an efficient, controllable engine. Skeletal muscle is such a “biological engine.” Several excellent reviews have previously covered this topic (for reviews, see 12, 65, 117, 136, 145, 181). Here, we review the most recent findings in genetically engineered animal models focusing on the molecular signaling network responsible of exercise-induced adaptations.
FIBER TYPE TRANSFORMATION
The experiments of inducible phenotypic changes in skeletal muscle in a cross-innervation study by Buller et al. 50 years ago (14) triggered intense interests in elucidating the underlying mechanisms. The most investigated area is exercise-induced fiber type transformation as this phenotypic property correlates with physical performance (72) and the incidence of chronic diseases (62). Numerous studies have clearly demonstrated that endurance exercise readily promotes transformation from a glycolytic to an oxidative phenotype within the fast-twitch fiber types (type IIb/IId/x to IIa) (5, 49). It is important to note that although MHC I and IIa isoforms coexist in human skeletal muscle fibers following endurance training (81, 150), and professional athletes with extremely intense training for years have increased type I fibers (30, 150), exercise-induced transformation to type I fibers remains to be experimentally confirmed.
The advancement in molecular genetics has empowered recent research in exercise-induced fiber type transformation. An elegant study by Chin et al. (23) marked the beginning of intense research in intracellular signaling-transcription coupling that decodes exercise-induced skeletal muscle adaptation. They showed that rhythmic muscle contractions activated the Ca2+/calmodulin-dependent serine/threonine protein phosphatase, calcineurin (also called protein phosphatase 3). The overall hypothesis is that activation of calcineurin promotes the expression of slow-twitch muscle genes through dephosphorylation and activation of the nuclear factor of activated T-cells (NFAT) (23). In the initial and subsequent studies, inhibition of the calcineurin-NFAT pathway by cyclosporine A (CsA), FK506, calcineurin inhibitory protein (CAIN/CABIN-1), or peptide VIVIT resulted in reduced percentage of slow-twitch fibers and/or blocked slow-twitch muscle promoter activity and gene expression in rodents (103, 108, 154, 170). Later, imaging analysis revealed translocation of NFATc1 to the nucleus on low-frequency electrical stimulation in skeletal muscle (95) through calcineurin-dependent and -independent mechanisms (103, 156). Consistent with an essential function of the calcineurin-NFAT pathway in maintaining slow-twitch muscle phenotype, muscle-specific transgenic mice overexpressing regulator of calcineurin 1 (RCAN1) are devoid of type I fibers in soleus muscle (114). Importantly, deletion of the calcineurin Aα or Aβ gene led to a significant decrease in type I fibers (118), and skeletal muscle-specific deletion of the calcineurin B1 gene impaired fast-to-slow fiber type transformation in a compensatory overloading model in mice (119). More recently, RNA interference-mediated knockdown of NFAT isoforms following somatic gene transfer suggests that all NFAT isoforms are necessary for the expression of slow MHC in regenerating muscle fibers in soleus muscle (16). Collectively, these studies strongly support the notion that contractile activity-induced maintenance and/or transformation of muscle fibers are dependent on the calcineurin-NFAT pathway.
Gain-of-function genetic studies further substantiated the importance of this pathway. Transgenic mice overexpressing a constitutively active form of calcineurin in skeletal muscle present increased number of slow-twitch fibers and elevated expression of slow-twitch troponin I, myoglobin, glucose transporter 4 (GLUT4), pyruvate dehydrogenase kinase 4 (PDK4), mitochondrial enzymes and peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) (113, 143). These mice have low carbohydrate oxidation and high lipid oxidation in skeletal muscle (98, 143) along with enhanced performance (76). Somatic gene transfer in adult skeletal muscle with a constitutively active NFATc1 mutant promoted the MHC I (Myhc) promoter activity and protein expression and inhibited the MHC IIb (Myh4) promoter activity (103). Furthermore, gene disruption of calcineurin inhibitory protein calsarcin in a glycolytic muscle resulted in a shift toward slow-twitch, oxidative phenotype along with enhanced NFAT activity and increased expression of RCAN 1–4 (38). Thus enhanced calcineurin-NFAT signaling is sufficient to promote fiber type transformation toward an oxidative phenotype. It should be noted that none of these studies have determined whether exercise-induced IIb/IId/x-to-IIa fiber type transformation is dependent on the calcineurin-NFAT signaling pathway.
Ca2+/calmodulin-dependent protein kinases (CaMK) may also play a role in exercise-induced genetic reprogramming in adult skeletal muscle. Several studies showed synergy between calcineurin and CaMKIV in stimulating the activities of transcriptional factors, myocyte enhancer factor 2 (MEF2) and NFAT (189–191); however, other studies showed that CaMKIV is not expressed in skeletal muscle and therefore not required for exercise-induced IIb/IId/x-to-IIa fiber type transformation (4). CaMKII is known to decode frequency-dependent information (31, 140) and be activated by endurance exercise (162). However, due to the complexity and redundancy of the CaMKII proteins, loss-of-function approach has not been employed to ascertain their function in exercise-induced fiber type transformation.
The mechanism by which CaMK activates MEF2 proteins was elucidated by several elegant studies. Class II histone deacetylases, such as HDAC4, HDAC5, and HDAC9, interact and inhibit MEF2, resulting in repression of the target genes. CaMK prevents the formation of MEF2-HDAC complexes, and induces nuclear export of HDAC4 and HDAC5 through phosphorylation (105) and 14-3-3-mediated nuclear export (106). In fact, low-frequency electrical stimulation-induced nuclear export of HDAC4 is dependent on CaMKII activity (157). More definitive evidence came from a study in which class II HDAC proteins failed to accumulate in oxidative soleus muscle (124), and compound deletion of the Hdac5 and Hdac9 genes led to increased type I and IIa fibers in soleus and plantaris muscles, whereas muscle-specific inducible expression of a nonphosphorylatable HDAC prevented voluntary running-induced fiber type transformation (124). Furthermore, deletion of the Mef2c and Mef2d genes resulted in reduced percentage of type I fibers in soleus muscle, and skeletal muscle-specific overexpression of a constitutively active MEF2C chimera protein led to increased type I fibers and oxidative phenotype (124). These findings support the view that CaMK activates MEF2 through derepression of HDACs in fiber type transformation.
A broad kinase inhibitor, staurosporine, led to increased nuclear accumulation of HDAC4 in the presence of the CaMKII inhibitor KN62 in cultured muscle fibers (157), suggesting that a kinase other than CaMKII is involved in HDAC shuttling. Protein kinase D1 (PKD1), originally called PKCμ, is a member of a family of diacylglycerol (DAG)-stimulated serine/threonine protein kinases (77) that lacks the C2 domain responsible for Ca2+ sensitivity (77, 173) and is activated by PKC-mediated phosphorylation in response to phorbol ester and diacyglycerol (174, 197). Kim et al. (80) showed that PKD1 is predominantly expressed in type I myofibers, and muscle-specific overexpression of a constitutively active form led to increased percentage of type I fibers, enhanced myoglobin, IId/x, and IIa MHC protein expression, and improved fatigue resistance (80). Interestingly, these phenotypic changes are not accompanied by enhanced mitochondrial biogenesis, whereas genetic deletion of PKD1 increases susceptibility to fatigue with no significant impact on fiber type composition (80). The functional role of PKD1 in exercise-induced fiber type transformation remains to be determined.
Metabolic cues may play important functional roles in skeletal muscle adaptation. AMP-activated protein kinase (AMPK), sensitive to metabolic stress and energy deprivation (59), is activated by contractile activity in skeletal muscle and has been linked to metabolic adaptations (39, 54, 58, 183, 184, 186). Although there is ample evidence that AMPK activation promotes mitochondrial biogenesis in skeletal muscle (42, 196), muscle-specific expression of a dominant-negative form of AMPKα2 blocked voluntary running-induced IIb-to-IId/x/IIa fiber type transformation without affecting the induction of PGC-1α expression and mitochondrial enzyme activity (137). Conversely, muscle-specific expression of an active mutant of AMPKγ1 leads to a marked increase in type IIa/x fibers in triceps muscle (137). Therefore, AMPK appears to be functionally important for exercise-induced fiber type transformation and is sufficient, but not necessary, for mitochondrial biogenesis.
PGC-1α plays a pivotal role in endurance exercise-induced muscle adaptation. Skeletal muscle-specific PGC-1α overexpression led to increased percentage of slow-twitch myofibers (91) and improved volitional exercise capacity in mice (17). Global or muscle-specific deletion of the Pgc-1a (Ppargc1a) gene led to a reduced oxidative phenotype in skeletal muscle (55, 89, 92) with only a moderate decrease of type I myofibers. Conversely, muscle-specific deletion of the Pgc-1c gene or the p38γ3mitogen-activated protein kinase (MAPK) (Mapk12) gene, an upstream stress-activated kinase for PGC-1α, led to normal fiber type transformation (type IIb/IId/x to IIa), but attenuated mitochondrial biogenesis and angiogenesis (43, 123). Finally, recent evidence based on the characterization of mice with combined PGC-1α/β deficient skeletal muscle indicates that the PGC-1 coactivators are dispensable for fiber type determination (194). These studies provide evidence for segregated signaling pathways in control of exercise-induced contractile and metabolic adaptations. PGC-1α may influence the maintenance of slow-twitch, type I fibers but is not required for exercise-induced fiber type transformation.
Peroxisome proliferator-activated receptors (PPARs) are ligand-dependent nuclear receptors. Muscle-specific overexpression of an active form of the PPARβ/δ gene (Ppard) led to an increased percentage of type I myofibers with improved volitional exercise capacity in mice (177), while deletion of the PPARβ/δ gene resulted in reduced slow-twitch muscle gene expression (153). Consistent with a function of PPARβ/δ in slow muscle maintenance, mice with gene deletion and overexpression of the RIP40 corepressor, which interacts with and inhibits multiple nuclear receptors, have increased and decreased type I myofibers, respectively (155). It remains to be demonstrated that exercise-induced fiber type transformation is dependent on PPARβ/δ function.
It is extremely important to consider two technical issues in studying exercise-induced fiber type transformation. First, many studies employed myofibrillar actomyosin ATPase histochemistry for determination of fiber type composition (23, 80, 91, 113, 114, 124, 177, 189, 191). This analysis appears to be significantly different from antibody-based analyses. For example, immunoblot and immunofluorescence analyses have shown clear evidence of increased type IIa MHC protein and type IIa fibers in mouse plantaris muscle following voluntary running with no evidence of increased type I MHC protein and type I fibers (4, 43, 123, 137), whereas metachromatic ATPase staining repeatedly showed significant increases of type I fibers in the same muscle following voluntary running (124) and other interventions with increased contractile activities (23, 80, 91, 113, 114, 177, 189, 191). Since myofibrillar actomyosin ATPase histochemistry is vulnerable to subtle changes in pH and requires staining of serial sections, caution should be taken when interpreting the data, particularly with regard to changes in type I fibers. Second, although the molecular and pharmacological approaches to augment or reduce the expression of a gene of interest are powerful for studying fiber type regulation, these interventions may lead to changes in fiber type composition due to their impact on skeletal muscle development and/or fiber type maintenance instead of exercise-induced adaptation. An altered fiber type composition does not warrant a conclusion of gene function in exercise-induced fiber type transformation. A physiological exercise model needs to be employed as was done in some of the aforementioned studies (4, 43, 123, 124, 137).
MITOCHONDRIAL BIOGENESIS
Mitochondria are critical for aerobic ATP synthesis and proper cell function. Mitochondrial DNA (mtDNA) encodes 13 subunits of the electron transport system, 22 tRNAs, and 2 ribosomal RNAs. The vast majority of proteins in oxidative metabolism in mitochondria are nuclear encoded and subsequently transported to the mitochondria (25, 26, 149, 175). Mitochondrial quantity and quality in skeletal muscle are not only important for performance but also relevant to health. Mitochondrial dysfunction in muscle, likely resulting from inactivity (169), is associated with muscle atrophy (138), diabetes (99, 152), and aging (32, 35). Conversely, upregulation of mitochondrial mass and function, also referred to as mitochondrial biogenesis, is instrumental in exercise training-induced improvement of muscle function and whole body metabolic homeostasis (85, 93).
Holloszy (63) in 1967 first showed that endurance exercise training led to mitochondrial biogenesis in skeletal muscle. This original study and several subsequent studies set the stage for investigating molecular mechanisms involved in exercise-induced adaptation (61, 64, 146). Recent studies showed that resistance and interval training regimens could also lead to skeletal muscle mitochondrial biogenesis in humans (10, 15, 44). Great progress has been made since the identification of PGC-1α in brown fat (129). It is now known that PGC-1α interacts with nuclear transcription factors, such as PPARs (112), estrogen-related receptor α (ERRα) (70, 71, 129), thyroid receptor (TR) (129), nuclear respiratory factor 1 (NRF1) (192), NRF2 (109), and MEF2 (57, 107) in stimulating the expression of nuclear-encoded mitochondrial genes. PGC-1α coactivation of NRF1 and NRF2 also induces mitochondrial transcription factor A (TFAM) expression (192), which regulates mtDNA transcription. Therefore, PGC-1α coordinates the expression of both nuclear- and mitochondrial-encoded genes in mitochondrial biogenesis (128, 148, 179).
Pgc-1α mRNA and protein are readily upregulated by endurance exercise (9, 48, 74, 122, 142, 166). However, it has only been determined recently whether PGC-1α is required for exercise-induced mitochondrial biogenesis. Leick et al. (88) observed that mice with global disruption of the Pgc-1α gene (PGC-1α KO) are incapable of upregulating cytochrome c (Cyc) mRNA in soleus muscle after an acute bout of treadmill running. Conversely, these animals present normal upregulation of Cyc, cytochrome oxidase 1 (Cox1), aminolevulinate synthase 1 (Alas 1) mRNA and protein in response to endurance exercise training. Adhihetty et al. (1) reported that PGC-1α KO mice have normal voluntary running activity despite reduced basal mitochondrial respiratory function and that this limitation could be reversed by endurance exercise training. More recently, Leick et al. reported that exercise training-induced attenuation of age-associated decline in citrate synthase (CS) activity in skeletal muscle is absent in PGC-α knockout mice (87), suggesting a critical role for PGC-1α in skeletal muscle in aging. These contrasting findings may be related to compensatory adaptations in different tissues due to the global disruption of the Pgc-1α gene. In fact, PGC-1α knockout mice have lesions in the central nervous system causing hyperactivity and circadian rhythm abnormalities (56, 92, 94). To avoid these complications, muscle-specific Pgc-1α knockout mice (PGC-1α MKO) were employed. These mice have reduced locomotor activity and exercise tolerance, impaired muscle function, and reduced oxidative capacity in skeletal muscles (55, 56); however, voluntary running-induced CYC and COXIV protein expression in plantaris muscle was significantly attenuated in PGC-1α MKO (43). These findings provide strong evidence for a critical role for PGC-1α in exercise-induced mitochondrial biogenesis in skeletal muscle but also suggest some redundancy.
Considering the central role of PGC-1α in exercise-induced mitochondrial biogenesis, it is important to know how PGC-1α is regulated. Current evidence supports a fundamental role of p38γ MAPK. First, different exercises cause activation of p38 MAPK in skeletal muscle in animals and humans (47, 180, 187), phosphorylating and activating PGC-1α (127), MEF2 (195), and activating transcriptional factor 2 (ATF2) (21). In a series of studies, Akimoto et al. demonstrated that p38 MAPK acts through MEF2 and ATF2 in stimulating the Pgc-1α promoter, and muscle specific overexpression of an upstream MAPK kinase (MKK6) promotes the expression of PGC-1α and mitochondrial proteins without a significant change in fiber type composition (3). Pgc-1α transcriptional activation requires functional interaction of ATF2 with the Pgc-1α promoter (2), and forced expression of a kinase dead form of PKD1 or a nonphosphorylatable HDAC5 prevents upregulation of the Pgc-1α gene induced by contractile activity (2). In addition, Wright et al. (188) reported that p38 MAPK and ATF2 phosphorylation occur in parallel with PGC-1α nuclear translocation after an exhaustive bout of swimming exercise and that these events coincide with increases in Cs and Cyc mRNAs before the upregulation in PGC-1α protein. More recently, Pogozelski et al. demonstrated that mice with muscle-specific deletion of the p38γ gene (Mapk12) have attenuated upregulation of PGC-1α and markers of mitochondrial biogenesis (i.e., CYC and COXIV) in response to voluntary running with similar phenotype to the PGC-1α MKO mice (43), but not in p38α or p38β MKO mice (123). These findings underscore that the p38γ MAPK-PGC-1α regulatory axis is required for exercise-induced mitochondrial biogenesis in skeletal muscle. Therefore, PGC-1α activation occurs before the induction of PGC-1α protein expression, which may be mediated by p38 MAPK-dependent phosphorylation. PKD1- and p38γ-mediated activation of ATF2 and MEF2 and their functional interaction with the Pgc-1α promoter are required for the exercise-induced PGC-1α expression in skeletal muscle.
Exercise elicits other intracellular signals contributing to PGC-1α regulation and mitochondrial biogenesis. Transgenic overexpression of a constitutively active calcineurin in skeletal muscle increases myoglobin, GLUT4, pyruvate dehydrogenase kinase 4 (PDK4), mitochondrial enzymes, and PGC-1α expression (113, 143) with enhanced lipid oxidation in glycolytic muscles (98, 143) and improved volitional exercise capacity (76) similar to muscle-specific PGC-1α transgenic mice (17, 91, 179). In contrast, animals treated with the calcineurin inhibitor, CsA, have normal upregulation of PGC-1α and mitochondrial enzymes in response to endurance exercise (41). A direct role of the calcineurin-NFAT pathway remains to be ascertained in the upregulation of PGC-1α and metabolic adaptations in skeletal muscle in the context of exercise.
CaMKs have been shown to act synergistically with calcineurin in activating slow muscle gene expression in myocytes (190), and forced expression of a constitutively active form of CaMKIV in skeletal muscle in transgenic mice increases expression of the Pgc-1α gene and enhances mitochondrial biogenesis along with reduced fatigability of EDL muscle (189). However, genetic disruption of the Camk4 gene did not prevent exercise-induced upregulation of PGC-1α (4), and CaMKIV is not detectable in skeletal muscle (141). CaMKII is the main CaMK isoform in skeletal muscle (140, 141) and is activated by endurance exercise (130, 140, 141, 162), and pharmacological inhibition of CaMKII blocks exercise- and intracellular calcium-induced Glut4 gene transcription (110, 162). Due to the presence of multiple isoforms, verification of functional importance of this protein kinase has not been obtained in exercise-induced mitochondrial biogenesis models.
AMPK is activated by contractile activity (39, 54, 58, 183, 184, 186), and multiple mechanisms have been postulated for AMPK-mediated PGC-1α regulation. Exercise-induced AMPK activation is associated with HDAC5 phosphorylation and nuclear export in human skeletal muscle (104). PGC-1α phosphorylation at threonine-177 and serine-538 (75) and subsequent deacetylation by histone deacetylase SIRT1 (19) are required for AMPK action, including upregulation of PGC-1α and mitochondrial genes (19, 74). Endurance exercise in humans can also stimulate SIRT1 (28, 40), and a single bout of exercise induces PGC-1α deacetylation in glycolytic muscles along with upregulation of PGC-1α target genes, such as Pdk4, Glut4, and carnitine palmitoyl transferase 1B (Cpt1b) (18), where SIRT1 activity, rather than expression, appears to play a prominent role (50). On the contrary, muscle-specific expression of a dominant-negative form of AMPKα2 fails to block the induction of PGC-1α expression and mitochondrial enzyme activity (136), and genetic deletion of functional AMPK isoforms fails to block exercise-induced PGC-1α gene expression in skeletal muscle (78). More recently, gain-of-function mutation of regulatory γ3 subunit of AMPK in mouse glycolytic fibers presents an increased expression of PGC-1α and mitochondrial biogenesis (42), and exercise-induced PGC-1α activation (deacetylation) is blunted in AMPKγ3 knockout mice (20). Therefore, the issue regarding the role of AMPK in exercise-induced mitochondrial biogenesis in skeletal muscle remains controversial.
Skeletal muscle contraction increases the production of reactive oxygen species (ROS) (27, 79, 102, 120, 132, 159). Production of hydrogen peroxide (H2O2) in contracting skeletal muscle has been shown to be required for PGC-1α upregulation (186), and increased H2O2 production has been proposed as a mechanism by which lactate, a by-product of glycolysis, upregulates PGC-1α (60). ROS may be functionally important for endurance exercise-induced PGC-1α expression and metabolic adaptation in skeletal muscle (45, 79, 133). Pharmacological inhibition of xanthine oxidase (XO) with allopurinol suppresses the exercise-induced upregulation in PGC-1α in parallel to reduced activation (i.e., phosphorylation) of p38 MAPK. These findings suggest that ROS, most likely H2O2 as a by-product of XO, acts upstream of p38 MAPK in the induction of PGC-1α in response to contraction in vivo (79). Interestingly, a recent study showed that antioxidant supplementation in humans does not alter endurance training adaptation (193), clearly indicating the complexity in the role of redox signaling in muscle adaptation.
Finally, micro RNA-dependent regulation of PGC-1α in response to exercise has also been postulated. An acute bout of endurance exercise downregulates the expression of miR-23, a putative regulator (inhibitor) of PGC-1α translation, which is negatively correlated with PGC-1α mRNA after exercise (144). This is clearly a fertile area of research, which will provide new insights into exercise-induced mitochondrial biogenesis in the near future.
The precise coordination of all the aforementioned regulatory pathways relevant to exercise training and their importance to PGC-1α expression and mitochondrial biogenesis remains to be fully elucidated. There is no doubt that the mechanisms responsible for mitochondrial biogenesis in response to exercise are extremely elegant and complex and will likely provide a venue for investigation for years to come.
ANGIOGENESIS
Exercise requires an increase in blood flow to the skeletal muscle to provide additional supply of oxygen and nutrients, which cannot solely be achieved by an increase in the cardiac output (7). The major vascular adaptations in skeletal muscle in response to endurance exercise include an increase in flow capacity due to an increase in the radius of large-caliber vessels and an increase of muscle capillarity through angiogenesis (for review, see 126). Here we focus on the adaptive process of angiogenesis, an expansion of the capillary network from preexisting capillaries that improves gas and nutrient exchange in peripheral tissues, which has been speculated to contribute significantly to improved physical performance (147). The expansion of the capillary network occurs primarily as intussusception, which involves the longitudinal division of the capillary within the lumen, and sprouting, which refers to the branching out of endothelial cells from an existing capillary (126). Endurance exercise-induced angiogenesis is thought to be mediated by a combination of growth factors, hypoxia and shear and mechanical stresses (126).
Early studies showed that exercise training induces an increase in capillarity in skeletal muscle (22, 29, 100), which could be recapitulated by chronic motor nerve stimulation (69, 111, 131). The fact that capillary-to-fiber ratio differs among different types of muscle fibers (73, 178) and exercise training results in fiber type-dependent angiogenic responses (52, 53, 178) strongly suggests that the signals for angiogenesis originate from within the contracting muscle fibers. In fact, a single bout of endurance exercise is sufficient to induce mRNA expression of multiple angiogenic growth factors and related receptors in skeletal muscle, including vascular endothelial growth factor (Vegfa) mRNA (13, 51), in a fiber type-specific manner (96). Existing evidence strongly supports that muscle fiber expression and secretion of VEGF promote angiogenesis through its paracrine effects. Of particular interest are the findings that treadmill running in rats induces significant increases in Vegfa mRNA in type IIb myofibers (11), and voluntary running in mice induces angiogenesis in type IIb/IId/x fibers before switching to type IIa fibers (178). The findings raise the questions as to whether induced expression of VEGF plays an essential role in exercise-induced angiogenesis in skeletal muscle and whether exercise-induced angiogenesis dictates fiber type transformation.
In addressing the functional importance of VEGF, Lloyd et al. (97) showed that treadmill running-induced angiogenesis in skeletal muscle is partially blocked by the employment of a VEGF receptor inhibitor, ZD4190, in rat in vivo. It is not clear if the incomplete inhibition of exercise-induced angiogenesis is due to the partial dependency of this process on VEGF function or the partial potency of the inhibitor in vivo. More recently, it was reported that skeletal muscle-specific deletion of the Vegfa gene led to significantly reduced capillarity in skeletal muscle with compensatory increases in oxidative enzymes, but reduced volitional exercise capacity on the treadmill (115). More importantly, muscle deficiency in VEGF attenuates exercise training-induced angiogenesis and improvement of physical performance (116). These findings strongly support the notion that exercise-induced VEGF expression from contracting muscle fibers plays a pivotal role in directing angiogenesis around them through paracrine-like actions.
A remaining question is what signaling cascade within the muscle fibers decodes muscle contractile activity signals in regulating VEGF expression. PGC-1α has recently emerged as one of the key regulators of angiogenesis in skeletal muscle under the condition of hypoxia in a hypoxia-inducible factor (HIF)-independent manner (8). In this process, PGC-1α coactivates the orphan nuclear receptor estrogen-related receptor-α (ERRα) (8). Whole body knockout of the Pgc-1α gene led to reduced VEGF protein expression and blunted response to acute and chronic exercise training (86). More convincing data of the functional importance of PGC-1α in exercise-induced angiogenesis in skeletal muscle were obtained in mice with muscle-specific deletion of the Pgc-1α gene, showing significant attenuation of contractile activity-induced VEGF expression and exercise-induced angiogenesis (24, 43). Mechanistically, the functional role of PGC-1α in exercise-induced VEGF expression and angiogenesis is dependent on the upstream p38γ MAPK (123) and the downstream ERRα (24). Interestingly, transgenic mice with muscle-specific expression of an inactive form of AMPK have lower capillarity compared with the wild-type littermates, but have normal induced angiogenesis in response to voluntary running exercise (198).
SUMMARY
In summary, a sophisticated signaling-transcription network within individual muscle fibers mediates exercise-induced skeletal muscle adaptation (Fig. 1). Current experimental evidence from various genetically engineered animal models supports the view that multiple regulatory factors sense Ca2+ (calcineurin and CaMK) and metabolic stress (AMPK and PKD1) converging on transcriptional factors (NFAT and MEF2) and repressors (HDACs) in mediating endurance exercise-induced slow-twitch muscle gene expression and type IIb/IId/x to IIa fiber type transformation. On the other hand, oxidative and metabolic stresses induced by contractile activity stimulate PGC-1α activity and expression, which in turn promote mitochondrial biogenesis through interactions with transcription factors (NRF1, NRF2, and Tfam) on nuclear-encoded and mitochondria-encoded genes. PGC-1α also promotes angiogenesis through an interaction with ERRα in activating the gene that encodes VEGF. Continued research efforts using more precisely controllable animal models, such as tissue-specific inducible transgenic and knockout mice, will elucidate the highly coordinated remodeling processes in skeletal muscle and will unveil the mysteries of this beautiful “biological engine” in the body.
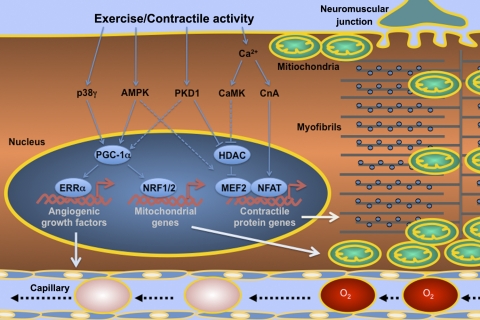
Fig. 1.
Schematic presentation of the current understanding of the signaling and molecular mechanisms underlying endurance exercise-induced adaptation in skeletal muscle. The figure depicts an adult skeletal muscle fiber with neuromuscular junction, capillary and intracellular metabolic (subsarcolemmal and intermyofibrillar mitochondria) and contractile (myofibrils) apparatuses. Muscle contractile activity, or endurance exercise, activates various protein phosphatase and kinases, which in turn regulate transcriptional factors, coactivators, and repressors in the control of contractile protein genes in fiber type transformation, mitochondrial genes in mitochondrial biogenesis, and angiogenic growth factor genes in angiogenesis. Solid lines between the regulatory factors depict the findings that have been confirmed by targeted gene deletion studies in animal models, while dashed lines depict findings by transgenic approaches in animal models, but not yet by targeted gene deletion studies. The relationships that have been confirmed by gene deletion studies not present in animal models in response to endurance exercise training are not depicted in this figure, such as the regulation of fiber type transformation by peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and mitochondrial biogenesis by CaMKIV. ERRα, estrogen-related receptor α; NFAT, nuclear factor of activated T-cells; NRF1/2, nuclear respiratory factor 1/2; MEF2, myocyte enhancer factor 2; CnA, calcineurin A.
GRANTS
This review article was made possible by National Institutes of Health Grant AR-050429 to Z. Yan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1α on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol 297: C217–C225, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Akimoto T, Li P, Yan Z. Functional interaction of regulatory factors with the Pgc-1α promoter in response to exercise by in vivo imaging. Am J Physiol Cell Physiol 295: C288–C292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akimoto T, Pohnert S, Li P, Zhang M, Gumbs C, Rosenberg P, Williams R, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Akimoto T, Ribar T, Williams R, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol 287: C1311–C1319, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Andersen P, Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand 99: 123–125, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arany Z, Foo S, Ma Y, Ruas J, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala S, Baek K, Rosenzweig A, Spiegelman B. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Baar K, Wende A, Jones T, Marison M, Nolte L, Chen M, Kelly D, Holloszy J. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Balakrishnan VS, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F, Castaneda-Sceppa C. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 996–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birot O, Koulmann N, Peinnequin A, Bigard X. Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J Physiol 552: 213–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Booth FW, Baldwin KM. Muscle plasticity: energy demand and supply processes. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc, 1996, sect. 12, chapt. 24, p. 1075–1123 [Google Scholar]
- 13. Breen E, Johnson E, Wagner H, Tseng H, Sung L, Wagner P. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurone and muscles in respect of the characteristic speeds of their responses. J Physiol 150: 417–439, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA 106: 13335–13340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol 104: 1304–1312, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantó C, Gerhart-Hines Z, Feige J, Lagouge M, Noriega L, Milne J, Elliott P, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057–3067, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrow RE, Brown RE, Van Huss WD. Fiber sizes and capillary to fiber ratios in skeletal muscle of exercised rats. Anat Rec 159: 33–39, 1967 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of physical inactivity during leisure time among overweight persons—1994. JAMA 275: 905, 1996 [PubMed] [Google Scholar]
- 23. Chin E, Olson E, Richardson J, Yang Q, Humphries C, Shelton J, Wu H, Zhu W, Bassel-Duby R, Williams R. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12: 2499–2509, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA 106: 21401–21406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clayton DA. Mitochondrial DNA replication: what we know. IUBMB Life 55: 213–217, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Clayton DA. Vertebrate mitochondrial DNA—a circle of surprises. Exp Cell Res 255: 4–9, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Close GL, Ashton T, Cable T, Doran D, Noyes C, McArdle F, MacLaren DP. Effects of dietary carbohydrate on delayed onset muscle soreness and reactive oxygen species after contraction induced muscle damage. Br J Sports Med 39: 948–953, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church T, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is Induced by Exercise in Humans. Am J Physiol Endocrinol Metab 298: E117–E126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cotter M, Hudlicka O, Vrbova G. Growth of capillaries during long-term activity in skeletal muscle. Bibl Anat 11: 395–398, 1973 [PubMed] [Google Scholar]
- 30. Coyle EF. Improved muscular efficiency displayed as Tour de France champion matures. J Appl Physiol 98: 2191–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 31. De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279: 227–230, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Dufour E, Larsson NG. Understanding aging: revealing order out of chaos. Biochim Biophys Acta 1658: 122–132, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Eddinger TJ, Moss RL. Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol Cell Physiol 253: C210–C218, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Ennion S, Sant'ana Pereira J, Sargeant AJ, Young A, Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil 16: 35–43, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Figueiredo PA, Mota MP, Appell HJ, Duarte JA. The role of mitochondria in aging of skeletal muscle. Biogerontology 9: 67–84, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Fitzsimons D, Diffee G, Herrick R, Baldwin K. Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J Appl Physiol 68: 1950–1955, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Franklin BA, Wrisley D, Johnson S, Mitchell M, Rubenfire M. Chronic adaptations to physical conditioning in cardiac patients. Implications regarding exercise trainability. Clin Sports Med 3: 471–512, 1984 [PubMed] [Google Scholar]
- 38. Frey N, Frank D, Lippl S, Kuhn C, Kögler H, Barrientos T, Rohr C, Will R, Müller OJ, Weiler H, Bassel-Duby R, Katus HA, Olson EN. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J Clin Invest 118: 3598–3608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fujii N, Hayashi T, Hirshman M, Smith J, Habinowski S, Kaijser L, Mu J, Ljungqvist O, Birnbaum M, Witters L, Thorell A, Goodyear L. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun 273: 1150–1155, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14: 661–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia-Roves P, Huss J, Holloszy J. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab 290: E1172–E1179, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Roves PM, Osler ME, Holmstrom MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem 283: 35724–35734, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol 298: C572–C579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gibala M. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab 34: 428–432, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol Endocrinol Metab 271: E403–E408, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274: 350–354, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Green HJ, Thomson JA, Daub WD, Houston ME, Ranney DA. Fiber composition, fiber size and enzyme activities in vastus lateralis of elite athletes involved in high intensity exercise. Eur J Appl Physiol Occup Physiol 41: 109–117, 1979 [DOI] [PubMed] [Google Scholar]
- 50. Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol 587: 1817–1828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H679–H685, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Gute D, Fraga C, Laughlin M, Amann J. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol 81: 619–626, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Gute D, Laughlin M, Amann J. Regional changes in capillary supply in skeletal muscle of interval-sprint and low-intensity, endurance-trained rats. Microcirculation 1: 183–193, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Hancock CR, Janssen E, Terjung RL. Contraction-mediated phosphorylation of AMPK is lower in skeletal muscle of adenylate kinase-deficient mice. J Appl Physiol 100: 406–413, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur N, Yan Z, Spiegelman B. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Handschin C, Rhee J, Lin J, Tarr P, Spiegelman B. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 100: 7111–7116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21: 2602–2612, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Henriksson J. Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol 270: 661–675, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hickey M, Carey J, Azevedo J, Houmard J, Pories W, Israel R, Dohm G. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol Endocrinol Metab 268: E453–E457, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 64. Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38: 273–291, 1976 [DOI] [PubMed] [Google Scholar]
- 65. Hood DA. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90: 1137–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Hoppeler H, Luthi P, Claassen H, Weibel E, Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflügers Arch 344: 217–232, 1973 [DOI] [PubMed] [Google Scholar]
- 67. Horton MJ, Brandon CA, Morris TJ, Braun TW, Yaw KM, Sciote JJ. Abundant expression of myosin heavy-chain IIB RNA in a subset of human masseter muscle fibres. Arch Oral Biol 46: 1039–1050, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 351: 2694–2703, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Hudlicka O, Myrhage R, Cooper J. Growth of capillaries in adult skeletal muscle after chronic stimulation. Bibl Anat 508–509, 1977 [PubMed] [Google Scholar]
- 70. Huss J, Torra I, Staels B, Giguere V, Kelly D. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24: 9079–9091, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem 277: 40265–40274, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Inbar O, Kaiser P, Tesch P. Relationships between leg muscle fiber type distribution and leg exercise performance. Int J Sports Med 2: 154–159, 1981 [DOI] [PubMed] [Google Scholar]
- 73. Ingjer F. Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance-trained men. A histochemical and ultrastructural study. Eur J Appl Physiol Occup Physiol 40: 197–209, 1979 [DOI] [PubMed] [Google Scholar]
- 74. Irrcher I, Adhihetty P, Sheehan T, Joseph A, Hood D. PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 284: C1669–C1677, 2003 [DOI] [PubMed] [Google Scholar]
- 75. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jiang LQ, Garcia-Roves P, de Castro Barbosa T, Zierath JR. Constitutively active calcineurin in skeletal muscle increases endurance performance and mitochondrial respiratory capacity. Am J Physiol Endocrinol Metab 298: E8–E16, 2010 [DOI] [PubMed] [Google Scholar]
- 77. Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem 269: 6140–6148, 1994 [PubMed] [Google Scholar]
- 78. Jorgensen S, Wojtaszewski J, Viollet B, Andreelli F, Birk J, Hellsten Y, Schjerling P, Vaulont S, Neufer P, Richter E, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J 19: 1146–1148, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med 47: 1394–1400, 2009 [DOI] [PubMed] [Google Scholar]
- 80. Kim MS, Fielitz J, McAnally J, Shelton JM, Lemon DD, McKinsey TA, Richardson JA, Bassel-Duby R, Olson EN. Protein kinase D1 stimulates MEF2 activity in skeletal muscle and enhances muscle performance. Mol Cell Biol 28: 3600–3609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, Clausen T, Saltin B. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflügers Arch 416: 470–472, 1990 [DOI] [PubMed] [Google Scholar]
- 82. Kokkinos P, Myers J, Doumas M, Faselis C, Manolis A, Pittaras A, Kokkinos JP, Singh S, Fletcher RD. Exercise capacity and all-cause mortality in prehypertensive men. Am J Hypertens 22: 735–741, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Koves T, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm G, Yan Z, Newgard C, Muoio D. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CW, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 33: 1774–1784, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JFP, Hidalgo J, Pilegaard H. PGC-1α mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab 297: E92–E103, 2009 [DOI] [PubMed] [Google Scholar]
- 87. Leick L, Lyngby SS, Wojtasewski JF, Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol 45: 336–342, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Leick L, Wojtaszewski JFP, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294: E463–E474, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Leone T, Lehman J, Finck B, Schaeffer P, Wende A, Boudina S, Courtois M, Wozniak D, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy J, Medeiros D, Schmidt R, Saffitz J, Abel E, Semenkovich C, Kelly D. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lillioja S, Young A, Culter C, Ivy J, Abbott W, Zawadzki J, Yki-Jarvinen H, Christin L, Secomb T, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin J, Wu H, Tarr P, Zhang C, Wu Z, Boss O, Michael L, Puigserver P, Isotani E, Olson E, Lowell B, Bassel-Duby R, Spiegelman B. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 92. Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299: E145–E161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447: 477–481, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Liu Y, Cseresnyes Z, Randall W, Schneider M. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol 155: 27–39, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lloyd P, Prior B, Yang H, Terjung R. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol 284: H1668–H1678, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol 288: H759–H768, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Long YC, Glund S, Garcia-Roves PM, Zierath JR. Calcineurin regulates skeletal muscle metabolism via coordinated changes in gene expression. J Biol Chem 282: 1607–1614, 2007 [DOI] [PubMed] [Google Scholar]
- 99. Lowell B, Shulman G. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 100. Mai J, Edgerton V, Barnard R. Capillarity of red, white and intermediate muscle fibers in trained and untrained guinea pigs. Experientia 26: 1222–1223, 1970 [DOI] [PubMed] [Google Scholar]
- 101. Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 268: 63–67, 1992 [PubMed] [Google Scholar]
- 102. McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280: C621–C627, 2001 [DOI] [PubMed] [Google Scholar]
- 103. McCullagh K, Calabria E, Pallafacchina G, Ciciliot S, Serrano A, Argentini C, Kalhovde J, Lomo T, Schiaffino S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 101: 10590–10595, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McGee S, Hargreaves M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 53: 1208–1214, 2004 [DOI] [PubMed] [Google Scholar]
- 105. McKinsey T, Zhang C, Lu J, Olson E. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408: 106–111, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McKinsey T, Zhang C, Olson E. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc Natl Acad Sci USA 97: 14400–14405, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Michael L, Wu Z, Cheatham R, Puigserver P, Adelmant G, Lehman J, Kelly D, Spiegelman B. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98: 3820–3825, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Miyazaki M, Hitomi Y, Kizaki T, Ohno H, Haga S, Takemasa T. Contribution of the calcineurin signaling pathway to overload-induced skeletal muscle fiber-type transition. J Physiol Pharmacol 55: 751–764, 2004 [PubMed] [Google Scholar]
- 109. Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101: 6570–6575, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mukwevho E, Kohn TA, Lang D, Nyatia E, Smith J, Ojuka EO. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am J Physiol Endocrinol Metab 294: E582–E588, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Myrhage R, Hudlicka O. Capillary growth in chronically stimulated adult skeletal muscle as studied by intravital microscopy and histological methods in rabbits and rats. Microvasc Res 16: 73–90, 1978 [DOI] [PubMed] [Google Scholar]
- 112. Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell 134: 405–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Naya F, Mercer B, Shelton J, Richardson J, Williams R, Olson E. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275: 4545–4548, 2000 [DOI] [PubMed] [Google Scholar]
- 114. Oh M, Rybkin II, Copeland V, Czubryt M, Shelton J, van Rooij E, Richardson J, Hill J, De Windt L, Bassel-Duby R, Olson E, Rothermel B. Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol Cell Biol 25: 6629–6638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059–R1067, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Olson E, Williams R. Calcineurin signaling and muscle remodeling. Cell 101: 689–692, 2000 [DOI] [PubMed] [Google Scholar]
- 118. Parsons S, Wilkins B, Bueno O, Molkentin J. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol Cell Biol 23: 4331–4343, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem 279: 26192–26200, 2004 [DOI] [PubMed] [Google Scholar]
- 120. Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37: 1064–1072, 2004 [DOI] [PubMed] [Google Scholar]
- 121. Perseghin G, Price T, Petersen K, Roden M, Cline G, Gerow K, Rothman D, Shulman G. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996 [DOI] [PubMed] [Google Scholar]
- 122. Pilegaard H, Saltin B, Neufer P. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE 4: e7934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Potthoff M, Wu H, Arnold M, Shelton J, Backs J, McAnally J, Richardson J, Bassel-Duby R, Olson E. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Prince FP, Hikida RS, Hagerman FC, Staron RS, Allen WH. A morphometric analysis of human muscle fibers with relation to fiber types and adaptations to exercise. J Neurol Sci 49: 165–179, 1981 [DOI] [PubMed] [Google Scholar]
- 126. Prior B, Yang H, Terjung R. What makes vessels grow with exercise training? J Appl Physiol 97: 1119–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 127. Puigserver P, Rhee J, Lin J, Wu Z, Yoon J, Zhang C, Krauss S, Mootha V, Lowell B, Spiegelman B. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8: 971–982, 2001 [DOI] [PubMed] [Google Scholar]
- 128. Puigserver P, Spiegelman B. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003 [DOI] [PubMed] [Google Scholar]
- 129. Puigserver P, Wu Z, Park C, Graves R, Wright M, Spiegelman B. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 130. Raney MA, Turcotte LP. Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J Appl Physiol 104: 1366–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 131. Reichmann H, Hoppeler H, Mathieu-Costello O, von Bergen F, Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflügers Arch 404: 1–9, 1985 [DOI] [PubMed] [Google Scholar]
- 132. Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73: 1797–1804, 1992 [DOI] [PubMed] [Google Scholar]
- 133. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rivero JL, Talmadge RJ, Edgerton VR. Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J Muscle Res Cell Motil 19: 733–742, 1998 [DOI] [PubMed] [Google Scholar]
- 135. Rivero JL, Talmadge RJ, Edgerton VR. Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem Cell Biol 111: 277–287, 1999 [DOI] [PubMed] [Google Scholar]
- 136. Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60: 145–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Röckl KSC, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56: 2062–2069, 2007 [DOI] [PubMed] [Google Scholar]
- 138. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69–e171, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Rose A, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol 553: 303–309, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574: 889–903, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Russell A, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier C, Bell D, Kralli A, Giacobino J, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003 [DOI] [PubMed] [Google Scholar]
- 143. Ryder J, Bassel-Duby R, Olson E, Zierath J. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem 278: 44298–44304, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS ONE 4: e5610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve 4: 94–105, 1981 [DOI] [PubMed] [Google Scholar]
- 146. Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand 96: 289–305, 1976 [DOI] [PubMed] [Google Scholar]
- 147. Saltin B, Rowell LB. Functional adaptations to physical activity and inactivity. Fed Proc 39: 1506–1513, 1980 [PubMed] [Google Scholar]
- 148. Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann NY Acad Sci 1147: 321–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008 [DOI] [PubMed] [Google Scholar]
- 150. Schantz PG, Dhoot GK. Coexistence of slow and fast isoforms of contractile and regulatory proteins in human skeletal muscle fibres induced by endurance training. Acta Physiol Scand 131: 147–154, 1987 [DOI] [PubMed] [Google Scholar]
- 151. Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology 22: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 152. Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50: 113–120, 2007 [DOI] [PubMed] [Google Scholar]
- 153. Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4: 407–414, 2006 [DOI] [PubMed] [Google Scholar]
- 154. Serrano A, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci USA 98: 13108–13113, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, Poutanen M, White R, Parker M. The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab 6: 236–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shen T, Liu Y, Cseresnyés Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell 17: 1570–1582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shen T, Liu Y, Randall WR, Schneider MF. Parallel mechanisms for resting nucleo-cytoplasmic shuttling and activity dependent translocation provide dual control of transcriptional regulators HDAC and NFAT in skeletal muscle fiber type plasticity. J Muscle Res Cell Motil 27: 405–411, 2006 [DOI] [PubMed] [Google Scholar]
- 158. Short K, Vittone J, Bigelow M, Proctor D, Rizza R, Coenen-Schimke J, Nair K. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003 [DOI] [PubMed] [Google Scholar]
- 159. Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763: 969–976, 2006 [DOI] [PubMed] [Google Scholar]
- 160. Sjogaard G, Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol Regul Integr Comp Physiol 243: R271–R280, 1982 [DOI] [PubMed] [Google Scholar]
- 161. Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol Cell Physiol 267: C1723–C1728, 1994 [DOI] [PubMed] [Google Scholar]
- 162. Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab 295: E698–E704, 2008 [DOI] [PubMed] [Google Scholar]
- 163. Stamler R, Stamler J, Gosch FC, McDonald AM. Primary prevention of hypertension—a randomized controlled trial. Ann Clin Res 16, Suppl 43: 136–142, 1984 [PubMed] [Google Scholar]
- 164. Streja D, Mymin D. Moderate exercise and high-density lipoprotein-cholesterol. Observations during a cardiac rehabilitation program. JAMA 242: 2190–2192, 1979 [PubMed] [Google Scholar]
- 165. Svedenhag J, Henriksson J, Juhlin-Dannfelt A. β-Adrenergic blockade and training in human subjects: effects on muscle metabolic capacity. Am J Physiol Endocrinol Metab 247: E305–E311, 1984 [DOI] [PubMed] [Google Scholar]
- 166. Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 296: 350–354, 2002 [DOI] [PubMed] [Google Scholar]
- 167. Termin A, Staron RS, Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92: 453–457, 1989 [DOI] [PubMed] [Google Scholar]
- 168. Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol 292: C729–C739, 2007 [DOI] [PubMed] [Google Scholar]
- 169. Timmons JA, Norrbom J, Scheele C, Thonberg H, Wahlestedt C, Tesch P. Expression profiling following local muscle inactivity in humans provides new perspective on diabetes-related genes. Genomics 87: 165–172, 2006 [DOI] [PubMed] [Google Scholar]
- 170. Torgan CE, Daniels MP. Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol Biol Cell 12: 1499–1508, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tsika RW, Herrick RE, Baldwin KM. Subunit composition of rodent isomyosins and their distribution in hindlimb skeletal muscles. J Appl Physiol 63: 2101–2110, 1987 [DOI] [PubMed] [Google Scholar]
- 172. Tupling AR, Asahi M, MacLennan DH. Sarcolipin overexpression in rat slow twitch muscle inhibits sarcoplasmic reticulum Ca2+ uptake and impairs contractile function. J Biol Chem 277: 44740–44746, 2002 [DOI] [PubMed] [Google Scholar]
- 173. Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA 91: 8572–8576, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Van Lint JV, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J Biol Chem 270: 1455–1461, 1995 [DOI] [PubMed] [Google Scholar]
- 175. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wallberg-Henriksson H, Gunnarsson R, Henriksson J, DeFronzo R, Felig P, Ostman J, Wahren J. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes 31: 1044–1050, 1982 [DOI] [PubMed] [Google Scholar]
- 177. Wang Y, Zhang C, Yu R, Cho H, Nelson M, Bayuga-Ocampo C, Ham J, Kang H, Evans R. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Waters R, Rotevatn S, Li P, Annex B, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol 287: C1342–C1348, 2004 [DOI] [PubMed] [Google Scholar]
- 179. Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem 282: 36642–36651, 2007 [DOI] [PubMed] [Google Scholar]
- 180. Widegren U, Jiang X, Krook A, Chibalin A, Bjornholm M, Tally M, Roth R, Henriksson J, Wallberg-Henriksson H, Zierath J. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J 12: 1379–1389, 1998 [DOI] [PubMed] [Google Scholar]
- 181. Williams RS, Neufer PD. Regulation of gene expression in skeletal muscle by contractile activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 25, p. 1124–1150 [Google Scholar]
- 182. Wilwert JL, Madhoun NM, Coughlin DJ. Parvalbumin correlates with relaxation rate in the swimming muscle of sheepshead and kingfish. J Exp Biol 209: 227–237, 2006 [DOI] [PubMed] [Google Scholar]
- 183. Winder W, Hardie D. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab 270: E299–E304, 1996 [DOI] [PubMed] [Google Scholar]
- 184. Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000 [DOI] [PubMed] [Google Scholar]
- 185. Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. J Appl Physiol 53: 335–345, 1982 [DOI] [PubMed] [Google Scholar]
- 186. Wojtaszewski J, Nielsen P, Hansen B, Richter E, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol 528: 221–226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wright D, Geiger P, Han D, Jones T, Holloszy J. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282: 18793–18799, 2007 [DOI] [PubMed] [Google Scholar]
- 188. Wright D, Han D, Garcia-Roves P, Geiger P, Jones T, Holloszy J. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282: 194–199, 2007 [DOI] [PubMed] [Google Scholar]
- 189. Wu H, Kanatous S, Thurmond F, Gallardo T, Isotani E, Bassel-Duby R, Williams R. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296: 349–352, 2002 [DOI] [PubMed] [Google Scholar]
- 190. Wu H, Naya F, McKinsey T, Mercer B, Shelton J, Chin E, Simard A, Michel R, Bassel-Duby R, Olson E, Williams R. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J 19: 1963–1973, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya F, Shelton J, Hutcheson K, DiMaio J, Olson E, Bassel-Duby R, Williams R. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20: 6414–6423, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla R, Spiegelman B. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 193. Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 42: 1388–1395, 2010 [DOI] [PubMed] [Google Scholar]
- 194. Zechner C, Lai L, Fong J, Geng T, Yan Z, Rumsey J, Collia D, Chen Z, Wozniak D, Leone T, Kelly D. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol 19: 21–30, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zong H, Ren J, Young L, Pypaert M, Mu J, Birnbaum M, Shulman G. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 99: 15983–15987, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J 15: 6220–6230, 1996 [PMC free article] [PubMed] [Google Scholar]
- 198. Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol 586: 6021–6035, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]