Abstract
Limb venous compliance decreases with advancing age, even in healthy humans. To test the hypothesis that adrenergic mechanisms contribute to age-associated reductions in limb venous compliance, we measured calf venous compliance before and during acute systemic α- and β-adrenergic blockade in eight young (27 ± 1 yr old, mean ± SE) and eight older healthy men (67 ± 2 yr old). Calf venous compliance was determined in supine subjects by inflating a thigh-collecting cuff to 60 mmHg for 8 min and then decreasing it (1 mmHg/s) to 0 mmHg while calf volume was indexed with a strain gauge. The slope (·10−3) of the pressure-compliance relation (compliance= β1 + 2·β2·cuff pressure), which is the first derivative of the quadratic pressure-volume relation [(Δlimb volume) = β0 + β1·(cuff pressure) + β2·(cuff pressure)2] during reductions in cuff pressure, was used to quantify calf venous compliance. Calf venous compliance was ∼30% lower (P < 0.01) in older compared with young men before adrenergic blockade. In response to adrenergic blockade calf venous compliance did not increase in young (−2.62 ± 0.14 and −2.29 ± 0.18 ml·dl−1·mmHg−1, before and during blockade, respectively) or older men (−1.78 ± 0.27 and −1.68 ± 0.21 ml·dl−1·mmHg−1). Moreover, during adrenergic blockade differences in calf venous compliance between young and older men observed before adrenergic blockade persisted. Collectively, these data strongly suggest that adrenergic mechanisms neither directly restrain calf venous compliance in young or older men nor do they contribute to age-associated reductions in calf venous compliance in healthy men.
Keywords: venous capacitance, aging, autonomic nervous system, orthostasis
limb venous compliance decreases with advancing age in humans (11, 14, 16, 18, 22, 23, 34, 37). Although decreases in limb venous compliance with age are well described, the mechanism(s) underlying these changes remain uncertain. As composition of the venous wall is altered with age, it is likely that structural changes contribute, at least in part, to age-associated reductions in limb venous compliance (2). However, functional factors, such as sympathetic/adrenergic influences, may also play a critical role.
Previously, we reported that acute activation of the sympathetic nervous system, induced via baroreceptor unloading, decreased calf venous compliance in young men (19). Since human aging is associated with tonic increases in sympathetic nervous system outflow (21, 28, 32), as well as increased α-adrenergic tone in the leg (4), it is possible that enhanced adrenergic influences contribute mechanistically to decreased calf venous compliance with age. Consistent with this possibility, increased sympathetic nervous system outflow/adrenergic tone in pathophysiological states, such as hypertension (27), appear to contribute to reduced venous compliance in both animals (9) and humans (33).
Accordingly, the purpose of this study was to determine if adrenergic mechanisms tonically restrain limb venous compliance in an age-dependent manner in healthy men. We hypothesized that acute systemic adrenergic blockade would increase calf venous compliance in both young and older men. Moreover, we hypothesized that the magnitude of increase in calf venous compliance during acute systemic adrenergic blockade would be greater in older compared with young men as a result of enhanced adrenergic influences with advancing age.
METHODS
Subjects
Eight young (23–35 yr old) and eight older (56–77 yr old) healthy Caucasian men were studied. Subjects were healthy based on review of medical history and physical examination. Subjects were not hypertensive [resting arterial blood pressure (BP) < 140/90 mmHg], did not smoke, were nonobese (body mass index < 30 kg/m2), and were not taking any medications known to affect cardiovascular/autonomic function. The Institutional Review Board at the Pennsylvania State University College of Medicine approved the experiments. Signed informed consent was obtained from all subjects before testing.
Protocol
Subjects refrained from caffeine (12 h) and food ingestion (4 h) before testing. At least 30 min before obtaining any measurements subjects were positioned supine and instrumented for the study. Instrumentation included placement of BP cuffs, a thigh-collecting cuff (∼5 cm proximal to the patella) on the left leg, a strain gauge around the maximal circumference of the left calf, and insertion of an intravenous catheter in the left arm for drug infusion. The left leg was elevated and supported above heart level (to promote venous drainage) for the remainder of the study. Baseline measurements (i.e., preblockade) were then obtained. Thirty minutes after completing baseline calf venous compliance measurements adrenergic blockade (see below) was induced before repeating measurements (i.e., during blockade).
Adrenergic blockade was achieved by intravenous infusion of propranolol (priming dose, 0.25 mg/kg) over 15 min immediately followed by phentolamine (priming dose, 0.1428 mg/kg) over 5 min. Propranolol was infused before phentolamine to avoid any unopposed β-mediated effects that may occur during phentolamine infusion. After infusion of the priming doses were complete maintenance doses were begun (propranolol 0.004 mg·kg−1·min−1; phentolamine 0.01428 mg·kg−1·min−1) and maintained until completion of the study. Measurements of calf venous compliance began ∼10 min after the start of the maintenance doses. Doses of propranolol (1, 20) and phentolamine (12, 31) were chosen based on prior studies, which documented effective systemic blockade of both α- and β-adrenergic receptor subtypes. In the present study, effectiveness of α-adrenergic blockade was assessed by measuring the pressor response to bolus intravenous infusion of norepinephrine (3 μg) before (preblockade) and during adrenergic blockade (7). If BP did not increase by ∼15 mmHg (preblockade) during the first bolus (3 μg) a second bolus (4 μg) of norepinephrine was given 10 min later. Only the final dose of norepinephrine used in the preblockade period (3 or 4 μg) was administered during adrenergic blockade.
Measurements
Arterial pressure.
Arterial pressure at rest was measured noninvasively over the brachial artery (Dinamap; GE Medical System, Milwaukee, WI) and on a beat-by-beat basis during the norepinephrine bolus trials using a Finometer (Finapres Medical Systems, Amsterdam, Netherlands). Heart rate was determined via three-lead ECG.
Calf venous compliance.
Calf venous compliance was determined using strain-gauge plethysmography (EC4, Hokanson, Bellevue, WA) (13, 18). After a 3-min baseline period thigh-collecting cuff pressure was increased to 60 mmHg (from 0 mmHg) for 8 min (models AG101 and E20, Hokanson). Thigh-collecting cuff pressure was monitored with a hand-held pressure transducer (Xcaliber Spectramed, Oxnard, CA) placed inline with the thigh-collecting cuff. After this 8-min period thigh-collecting cuff pressure was manually reduced at a rate of 1 mmHg/s to 0 mmHg with the aid of a stopwatch.
Data Collection and Analysis
Data were recorded at 400 Hz (MacLab 8e, ADInstruments). Calf venous compliance was determined from pressure-volume relations developed during the cuff deflation period (Fig. 1), averaged over 2-mmHg pressure increments, using thigh-collecting cuff pressure as a surrogate for intravenous pressure (13, 37). Calf volumes were determined based on strain gauge-derived measures (35). Pressures below 10 mmHg were excluded since resting venous pressure was not measured and thus the assumption that collecting cuff pressure and venous pressure were similar may have been violated (13). Pressure-volume relations were fit using a quadratic regression equation [(Δlimb volume) = β0 + β1·(cuff pressure) + β2·(cuff pressure)2]. Additionally, the first derivative of the pressure-volume curve [compliance = β1 + 2·β2·(cuff pressure)] was calculated to develop a linear pressure-compliance plot. Intragroup (preblockade vs. during blockade) and intergroup (young vs. older) group differences in venous compliance were assessed by comparing the slope of the pressure-compliance relation as well as β1 and β2 (pressure-volume relation). Calf venous capacitance was estimated by visually identifying the point at which the pressure-volume relation appeared to shift from a rapid filling response (capacitance response) to a slower less pronounced increase in volume after venous collecting cuff pressure was applied (16). Venous capacitance is reported as the relative (%) increase in calf volume from pre-cuff inflation levels.
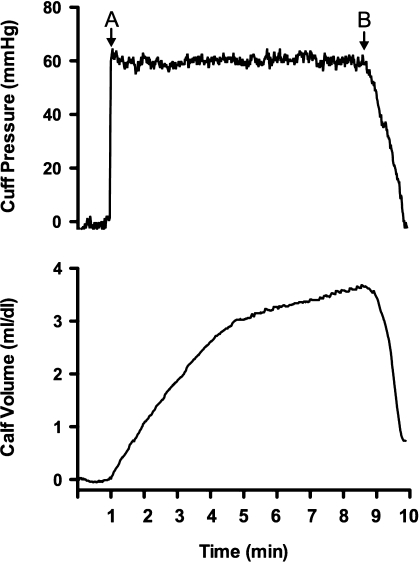
Fig. 1.
Representative tracings obtained during a venous compliance trial. Thigh-collecting cuff pressure was increased from 0 to 60 mmHg after a 1-min baseline period (point A) and maintained at this level for 8 min (minutes 1–9). After this period collecting cuff pressure was reduced to 0 mmHg over the course of 60 s (starting at point B). Changes in calf volume were measured using strain-gauge plethysmography. Calf venous compliance is determined from pressure and volume data obtained between minutes 8 and 9.
Statistical Analysis
Differences in subject characteristics were determined by t-test, and repeated-measures ANOVA was used to compare responses both within and between groups. Specific contrasts were made using Newman-Keuls post hoc tests. Statistical significance was achieved when P < 0.05. Data are presented as means ± SE.
RESULTS
Subject Characteristics
Subject characteristics are presented in Table 1. In addition to age differences the older subjects BP at rest was significantly higher than levels in the young.
Table 1.
Subject characteristics
| Variable | Young (n = 8) | Older (n = 8) |
|---|---|---|
| Age, yr | 27 ± 1 | 67 ± 2* |
| Height, cm | 180.1 ± 2.8 | 178.3 ± 1.1 |
| Body mass, kg | 80.4 ± 5.2 | 83.0 ± 2.5 |
| BMI, kg/m2 | 24.6 ± 1.0 | 26.1 ± 0.8 |
| Systolic BP, mmHg | 115 ± 3 | 128 ± 3* |
| Diastolic BP, mmHg | 64 ± 2 | 76 ± 2* |
| Mean BP, mmHg | 85 ± 1 | 94 ± 2* |
| Heart rate, beats/min | 57 ± 4 | 63 ± 5 |
Values are means ± SE. BMI, body mass index; BP, blood pressure.
P < 0.05 compared with young.
Effect of Adrenergic Blockade on Heart Rate and BP at Rest
Heart rate and BP responses to α- and β-adrenergic blockade are presented in Table 2. β-Adrenergic blockade decreased (P < 0.05) heart rate at rest in both young and older adults, without influencing BP. Subsequently, when α-adrenergic blockade was performed (i.e., during combined α- and β-adrenergic blockade) heart rate increased (P < 0.05) and BP decreased (P < 0.05) in both young and older adults. The magnitude of this depressor response was greater in older adults (P < 0.05).
Table 2.
Hemodynamic response to adrenergic blockade
| Baseline | β-Blockade | α- and β -Blockade | |
|---|---|---|---|
| Young | |||
| Heart rate, beats/min | 59 ± 4 | 49 ± 2* | 53 ± 2*† |
| ΔHeart rate, beats/min | −10 ± 2* | −6 ± 2*† | |
| Systolic BP, mmHg | 114 ± 3 | 113 ± 2 | 109 ± 3*† |
| ΔSystolic BP, mmHg | −2 ± 2 | −6 ± 2*† | |
| Diastolic BP, mmHg | 63 ± 1 | 61 ± 2 | 58 ± 3*† |
| ΔDiastolic BP, mmHg | −1 ± 1 | −4 ± 2*† | |
| Mean BP, mmHg | 84 ± 1 | 84 ± 1 | 80 ± 2*† |
| ΔMean BP, mmHg | −1 ± 1 | −4 ± 2*† | |
| Older | |||
| Heart rate, beats/min | 63 ± 4 | 55 ± 5*‡ | 59 ± 5*†‡ |
| ΔHeart rate, beats/min | −8 ± 2* | −3 ± 2*† | |
| Systolic BP, mmHg | 129 ± 3‡ | 127 ± 3‡ | 113 ± 2*†‡ |
| ΔSystolic BP, mmHg | −2 ± 2 | −15 ± 2*†‡ | |
| Diastolic BP, mmHg | 76 ± 2‡ | 76 ± 3‡ | 69 ± 2*†‡ |
| ΔDiastolic BP, mmHg | 0 ± 2 | −8 ± 1*†‡ | |
| Mean BP, mmHg | 95 ± 2‡ | 94 ± 2‡ | 86 ± 1*†‡ |
| ΔMean BP, mmHg | −1 ± 1 | −9 ± 1*†‡ |
Data were obtained 1) at baseline (i.e., before beginning adrenergic blockade priming doses), 2) after completion of the β -adrenergic priming dose (β-blockade), and 3) subsequent to completion of the α-blockade priming dose (i.e., during combined α- and β -blockade).
P < 0.05 vs. baseline (same age group).
P < 0.05 vs. β-blockade (same age group).
P < 0.05 vs. young (same time point).
Calf Venous Compliance
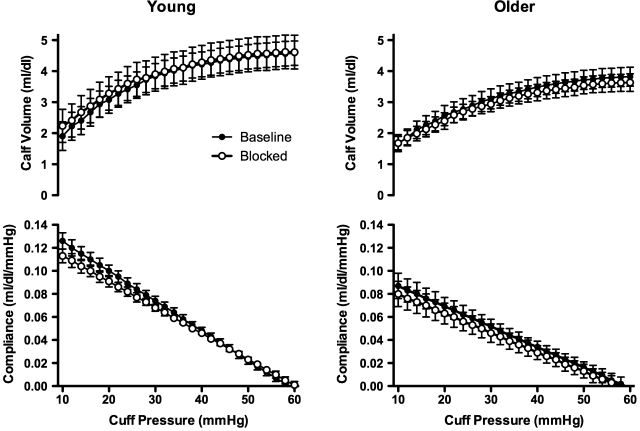
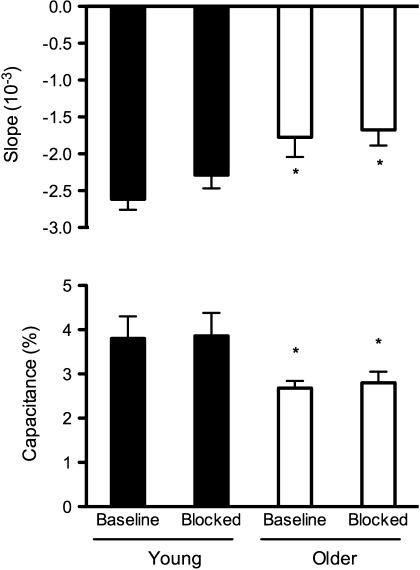
Before adrenergic blockade the slope of the pressure-volume (β1 and β2; Table 3) and pressure-compliance relations (Figs. 2 and 3) were less steep in older compared with young men (P < 0.01), indicating lower venous compliance in the older men. In response to adrenergic blockade calf venous compliance was not altered in young or older men (Table 3 and Figs. 2 and 3). Finally, the age-associated decrease in calf venous compliance observed before adrenergic blockade persisted during adrenergic blockade (Table 3 and Figs. 2 and 3). Calf venous capacitance was lower in older compared with young men before adrenergic blockade (Fig. 3). In response to adrenergic blockade calf venous capacitance was unchanged in both young and older men (i.e., the age-associated differences persisted) (Fig. 3). The rate of reduction in thigh-collecting cuff pressure was similar in young and older men before (−0.97 ± 0.02 vs. −0.98 ± 0.02 mmHg/s for young and older men, respectively; P > 0.05) and during (−0.97 ± 0.02 vs. −0.97 ± 0.01 mmHg/s; P > 0.05) adrenergic blockade.
Table 3.
Pressure-volume regression parameters
| (ΔLimb Volume) = β0 + β1 · (Cuff Pressure) + β2 · (Cuff Pressure)2 | |
|---|---|
| Young | |
| Baseline | ΔLimb volume = (0.799 ± 0.530) + (0.141 ± 0.007) · (cuff pressure) − (0.00125 ± 0.00007) · (cuff pressure)2 |
| Blocked | ΔLimb volume = (0.813 ± 0.324) + (0.125 ± 0.008) · (cuff pressure) − (0.00111 ± 0.00009) · (cuff pressure)2 |
| Older | |
| Baseline | ΔLimb volume = (1.233 ± 0.554) + (0.103 ± 0.013)* · (cuff pressure) − (0.00084 ± 0.00013)* · (cuff pressure)2 |
| Blocked | ΔLimb volume = (0.844 ± 0.301) + (0.094 ± 0.013)* · (cuff pressure) − (0.00080 ± 0.00011)* · (cuff pressure)2 |
Data were obtained before (baseline) and during systemic adrenergic blockade (blocked) in young and older men.
P < 0.05 vs. Young (same time point; baseline or blocked).
Fig. 2.
Pressure-volume (top panels) and pressure-compliance relations (bottom panels) in young (left panels) and older men (right panel) before (filled symbols) and during systemic adrenergic blockade (blocked; open symbols). Before adrenergic blockade calf venous compliance was reduced in older compared with young men. In response to adrenergic blockade calf venous compliance did not increase in young or older men, suggesting that adrenergic tone neither contributes to calf venous compliance in young or older men nor does it contribute to age-associated decreases in calf venous compliance in healthy men.
Fig. 3.
Venous compliance derived from the slope of the pressure-compliance relation in young and older men at baseline and during adrenergic blockade (top). These data indicate that calf venous compliance is reduced in older men compared with young men (before blockade). Moreover, these data suggest that age-associated differences in calf venous compliance are not explained by increased adrenergic influences as age-associated differences persist after adrenergic blockade. Calf venous capacitance (bottom; percent change in calf volume) was determined at the transition between the steep filling and slower plateau phase of changes in calf volume during application of thigh-collecting cuff pressure during the venous compliance trials. Calf venous capacitance is reduced with age in men but was not altered by adrenergic blockade in either young or older men. *P < 0.05 vs. young at the same time point.
Adequacy of Adrenergic Blockade
Bolus infusion of norepinephrine elicited a pronounced and similar increase in systolic (Δ20 ± 2 vs. Δ25 ± 3 mmHg for young and older subjects, respectively: change P > 0.05 young vs. older) and diastolic BP (Δ12 ± 1 vs. Δ15 ± 2 mmHg for young and older subjects, respectively: change P > 0.05 young vs. older) before adrenergic blockade in young and older men. During adrenergic blockade the pressor response to norepinephrine was largely and similarly attenuated in young (Δ7 ± 2 and Δ6 ± 1 mmHg for systolic and diastolic BP, respectively; P < 0.05 compared with preblockade response in each group) and older subjects (Δ10 ± 2 mmHg and Δ6 ± 1 mmHg; P < 0.05 compared with preblockade response in each group).
DISCUSSION
The primary new finding from this study is that acute adrenergic blockade does not increase calf venous compliance in young or older healthy men. Thus increased sympathetic/adrenergic influences with advancing age do not appear to directly contribute to, or explain, age-associated decreases in calf venous compliance in healthy men.
Important interactions exist between the venous and sympathetic nervous systems. For instance, veins are richly innervated with sympathetic nerve endings and respond robustly to sympathetic neurotransmitters (24, 25). Consistent with these findings, increases in sympathetic nervous system outflow contribute mechanistically to increased venous tone and decreased venous compliance in pathological states, such as hypertension (9, 15, 17, 33). Thus the possibility exists that the sympathetic nervous system activation that has consistently been shown to accompany healthy human aging (28) may contribute to and/or explain decreases in limb venous compliance with age (11, 14, 16, 18, 22, 23, 34, 37).
The present study indicates that adrenergic blockade does not acutely increase calf venous compliance in young men. In contrast, we previously reported that acute sympathoexcitation, elicited by baroreflex unloading, decreased calf venous compliance in young men (19). A possible explanation for these apparently divergent findings could be that although marked increases in sympathetic outflow may influence venous function, withdrawal of low tonic (basal) levels of sympathetic outflow, present in young healthy men at rest (21), may be insufficient to influence venous function.
With advancing age, pronounced sympathetic activation occurs, even in healthy adults (28). Specifically, directly measured muscle sympathetic nerve activity in the leg at rest is markedly elevated with age (21, 32). Moreover, increases in femoral artery blood flow, which is reduced at rest with age (3), are greater after whole leg α-adrenergic blockade in older compared with young men (4). These findings indicate enhanced sympathetic/adrenergic influences in the leg with advancing age in men. However, despite increased sympathetic/adrenergic influences, it appears that these factors do not contribute to age-associated decreases in calf venous compliance. Thus other mechanism(s), such as structural changes in the venous wall with age (i.e., decreased elastin-to-collagen ratio, increased collagen crosslinking, etc.) (2), or other yet to be studied functional factors, appear to be the primary mechanism(s) contributing to age-associated decreases in limb venous compliance in healthy men.
Prior studies suggest that limb venous compliance is not altered by acute sympathetic activation in older adults. Specifically, in response to sympathetic activation, induced via handgrip exercise and cold stress, limb venous compliance was not acutely decreased in older adults (37). These data, although important, do not address the issue of whether sympathetic outflow tonically restrains venous compliance in older adults. The only way(s) to address whether adrenergic influences tonically restrain venous function is to acutely reduce sympathetic outflow, such as would occur during head-down tilt, or to antagonize adrenergic influences pharmacologically, as done in the present study. There are numerous examples where responses to stimulation and blockade of sympathetic influences provide divergent results. For example, with aging acute α-adrenergically mediated vasoconstriction is blunted in the leg (30), whereas vasodilator responses to α-adrenergic blockade are enhanced (4). Thus the present study is the first to address the question of whether increased sympathetic/adrenergic influences with age contribute to age-associated decreases in limb venous compliance.
Additionally, a prior study indicates that acute reductions in vascular smooth muscle tone, achieved via systemic administration of nitroglycerin, do not increase limb venous compliance in healthy older adults (37). These data may suggest that increased sympathetic/adrenergic influences do not contribute to age-related decreases in limb venous compliance, as responses to both perturbations could be thought to exert their influences through effects on venous smooth muscle tone. However, such an extrapolation may not be justified, as sympathetic outflow would be expected to reflexively increase during nitroglycerin administration. The net effect of such changes on venous smooth muscle tone in the limb of adults is not easy to determine. Thus the data derived from the present study provide direct evidence of the effect of altered adrenergic influences per se rather than general effects of smooth muscle tone on limb venous compliance in humans.
The significance of identifying mechanism(s) underlying decreased venous compliance with age may lie in the fact that venous compliance is a primary determinant of mean circulatory filling pressure, and thus venous tone (25, 36). Decreased venous compliance should compromise the ability to centralize blood volume during reductions in venous pressure (23, 25), thus reducing effective blood volume and possibly compromising BP regulation (26). Such changes could contribute to an impaired ability of older adults to maintain BP when blood volume is reduced (i.e., during dehydration or in response to hemorrhage) (10, 23, 29).
Previous studies reported that the limb vascular response (12), as well as the systemic pressor response (31), to cold stress was completely abolished by the same dose of phentolamine used in the present study. In contrast, in the present study the systemic pressor response to bolus intravenous infusion of norepinephrine was reduced by approximately two-thirds in both young and older adults by combined β- and α-adrenergic blockade. These data may indicate more complete α-adrenergic blockade in the former studies (12, 31) than in the present study. However, it is also possible that differences may relate to the fact that β-adrenergic receptors, which could result in β-mediated vasodilation during cold stress, were not blocked in these prior studies (12, 31). Although complete α-adrenergic blockade does not appear to have occurred in the present study, we did achieve a comparably large percentage of blockade in both young and older adults. Despite this there was no tendency for venous compliance to be increased in either group making it unlikely that adrenergic mechanisms play a major role in age-related decreases in calf venous compliance.
A limitation of this study is that only men were studied. Our choice to study men was based in part on the results of our prior studies in which we documented decreased calf venous compliance with age (18) and acute modulation of venous compliance by sympathetic nervous system activation (19) in healthy men. It is possible that the results obtained may differ had other venous segments been studied. Additionally, it is possible that tonic sympathetic/adrenergic restraint of limb venous compliance may occur in individuals with exceedingly high levels of sympathetic outflow (i.e., beyond levels excepted to be present as the result of healthy aging). We cannot exclude this possibility. However, it is important to emphasize that the magnitude of sympathetic activation with age is quite pronounced (8, 21, 28, 32) and of similar magnitude to that observed in hypertension (5, 6, 8, 27), in which sympathetic restraint of venous function has been reported (9, 15, 17).
In conclusion, our findings indicate that acute adrenergic blockade neither increases calf venous compliance in young men nor does it diminish age-associated reductions in calf venous compliance in healthy men. Although the results of the present study do not identify a specific mechanism underlying age-associated decreases in calf venous compliance in healthy men, it does demonstrate that increases in sympathetic outflow and/or enhanced adrenergic influences with age in the leg are unlikely to directly contribute to this well-described effect of aging. Collectively, these findings suggest that the primary mechanism(s) underlying decreased calf venous compliance with age involves structural and/or other functional factors independent of direct sympathetic/adrenergic influences.
GRANTS
Grants from the National Institutes of Health (HL-92309, AG-24420, and M01-RR-10732) supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the study participants for volunteering to participate in the study. Additionally, we wish to thank Robert Feehan and the nurses of the General Clinical Research Center for providing technical assistance and nursing support.
REFERENCES
- 1. Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab 86: 4440–4444, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bouissou H, Julian M, Pieraggi M, Maurel E, Thiers JC, Lounge L. Structure of healthy and varicose veins. In: Return Circulation and Norepinephrine: An Update, edited by Vanhoutte PM. Paris: John Libbey Eurotext, 1991, p. 139–150 [Google Scholar]
- 3. Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esler M, Jennings G, Korner P, Blombery P, Burke F, Willett I, Leonard P. Total, and organ-specific, noradrenaline plasma kinetics in essential hypertension. Clin Exp Hypertens A 6: 507–521, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev 70: 963–985, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Esler MD, Julius S, Randall OS, Ellis CN, Kashima T. Relation of renin status to neurogenic vascular resistance in borderline hypertension. Am J Cardiol 36: 708–715, 1975 [DOI] [PubMed] [Google Scholar]
- 8. Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol Regul Integr Comp Physiol 268: R278–R285, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension 35: 464–469, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev 73: 725–764, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Gascho JA, Fanelli C, Zelis R. Aging reduces venous distensibility and the venodilatory response to nitroglycerin in normal subjects. Am J Cardiol 63: 1267–1270, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol 89: 1830–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Halliwill JR, Minson CT, Joyner MJ. Measurement of limb venous compliance in humans: technical considerations and physiological findings. J Appl Physiol 87: 1555–1563, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez JP, Franke WD. Age- and fitness-related differences in limb venous compliance do not affect tolerance to maximal lower body negative pressure in men and women. J Appl Physiol 97: 925–929, 2004 [DOI] [PubMed] [Google Scholar]
- 15. King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension 48: 927–933, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Lanne T, Olsen H. Decreased capacitance response with age in lower limbs of humans: a potential error in the study of cardiovascular reflexes in aging. Acta Physiol Scand 161: 503–507, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Martin DS, Rodrigo MC, Appelt CW. Venous tone in the developmental stages of spontaneous hypertension. Hypertension 31: 139–144, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Monahan KD, Dinenno FA, Seals DR, Halliwill JR. Smaller age-associated reductions in leg venous compliance in endurance exercise-trained men. Am J Physiol Heart Circ Physiol 281: H1267–H1273, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Monahan KD, Ray CA. Gender affects calf venous compliance at rest and during baroreceptor unloading in humans. Am J Physiol Heart Circ Physiol 286: H895–H901, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Monroe MB, Seals DR, Shapiro LF, Bell C, Johnson D, Parker Jones P. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am J Physiol Endocrinol Metab 280: E740–E744, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Olsen H, Lanne T. Reduced venous compliance in lower limbs of aging humans and its importance for capacitance function. Am J Physiol Heart Circ Physiol 275: H878–H886, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Olsen H, Vernersson E, Lanne T. Cardiovascular response to acute hypovolemia in relation to age. Implications for orthostasis and hemorrhage. Am J Physiol Heart Circ Physiol 278: H222–H232, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther 90: 179–230, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev 63: 1281–1342, 1983 [DOI] [PubMed] [Google Scholar]
- 26. Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993 [Google Scholar]
- 27. Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 43: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shannon RP, Wei JY, Rosa RM, Epstein FH, Rowe JW. The effect of age and sodium depletion on cardiovascular response to orthostasis. Hypertension 8: 438–443, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol 293: H1466–H1472, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Sundlof G, Wallin BG. Human muscle sympathetic nerve activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takeshita A, Mark AL. Decreased venous distensibility in borderline hypertension. Hypertension 1: 202–206, 1979 [DOI] [PubMed] [Google Scholar]
- 34. Tsutsui Y, Sagawa S, Yamauchi K, Endo Y, Yamazaki F, Shiraki K. Cardiovascular responses to lower body negative pressure in the elderly: role of reduced leg compliance. Gerontology 48: 133–139, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Whitney RJ. The measurement of volume changes in human limbs. J Physiol 121: 1–27, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto J, Trippodo NC, Ishise S, Frohlich ED. Total vascular pressure-volume relationship in the conscious rat. Am J Physiol Heart Circ Physiol 238: H823–H828, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Young CN, Stillabower ME, DiSabatino A, Farquhar WB. Venous smooth muscle tone and responsiveness in older adults. J Appl Physiol 101: 1362–1367, 2006 [DOI] [PubMed] [Google Scholar]