Abstract
Neurovascular responses to mental stress have been linked to several cardiovascular diseases, including hypertension. Mean arterial pressure (MAP), muscle sympathetic nerve activity (MSNA), and forearm vascular responses to mental stress are well documented in normotensive (NT) subjects, but responses in prehypertensive (PHT) subjects remain unclear. We tested the hypothesis that PHT would elicit a more dramatic increase of MAP during mental stress via augmented MSNA and blunted forearm vascular conductance (FVC). We examined 17 PHT (systolic 120–139 and/or diastolic 80–89 mmHg; 22 ± 1 yr) and 18 NT (systolic < 120 and diastolic < 80 mmHg; 23 ± 2 yr) subjects. Heart rate, MAP, MSNA, FVC, and calf vascular conductance were measured during 5 min of baseline and 5 min of mental stress (mental arithmetic). Mental stress increased MAP and FVC in both groups, but the increases in MAP were augmented (Δ 10 ± 1 vs. Δ14 ± 1 mmHg; P < 0.05), and the increases in FVC were blunted (Δ95 ± 14 vs. Δ37 ± 8%; P < 0.001) in PHT subjects. Mental stress elicited similar increases in MSNA (Δ7 ± 2 vs. Δ6 ± 2 bursts/min), heart rate (Δ21 ± 3 vs. Δ18 ± 3 beats/min), and calf vascular conductance (Δ29 ± 10 vs. Δ19 ± 5%) in NT and PHT subjects, respectively. In conclusion, mental stress elicits an augmented pressor response in PHT subjects. This augmentation appears to be associated with altered forearm vascular, but not MSNA, responses to mental stress.
Keywords: sympathetic nerve activity, mental arithmetic, blood pressure, forearm vasodilation, microneurography
mental stress has been linked to several cardiovascular diseases, including hypertension (12, 13). Hypertensive and prehypertensive individuals, as well as normotensive subjects with a family history of hypertension, have all demonstrated an augmented pressor response to mental stress (14, 17, 22, 28, 33). Although this hypertensive response is well documented, few studies have examined the mechanisms underlying this response.
Mental stress consistently induces forearm vasodilation (2, 3, 8, 26), and evidence suggests that prehypertension blunts this response (28). The mechanism(s) responsible for this attenuated response remain unresolved, but an augmented sympathetic neural response has been suggested. Specifically, Matsukawa et al. (22) reported that muscle sympathetic nerve activity (MSNA) responses to mental stress were augmented in borderline hypertensive compared with normotensive subjects. Unfortunately, concurrent vascular responses were not assessed (22), and MSNA responses to mental stress are highly variable (9).
Therefore, the present study aims to determine both forearm vascular and MSNA responses to mental stress in prehypertensive and normotensive adults. We hypothesize that prehypertension will blunt forearm vasodilation and augment MSNA responses to mental stress. Understanding the mechanisms responsible for the augmented pressor response to mental stress is clinically relevant and may lead to better intervention strategies to help prevent, or at least delay, the development of prehypertension and/or hypertension.
METHODS
Subjects.
Thirty-five healthy men (18 normotensive, age 23 ± 2 yr; 17 prehypertensive, age 22 ± 1 yr) participated in the study. Normotensive subjects were defined as having a resting systolic pressure < 120 mmHg and diastolic pressure < 80 mmHg. Prehypertensive subjects were defined as having a resting systolic pressure of 120–139 mmHg and/or a diastolic pressure of 80–89 mmHg. This is consistent with current blood pressure classifications (10). Subject exclusion criteria included smoking, diabetes, use of blood pressure medication, and autonomic dysfunction. Normotensive (24 ± 1 kg/m2) and prehypertensive (26 ± 1 kg/m2) subjects had similar body mass indexes. Subjects were asked to refrain from exercise, caffeine, and alcohol for 12 h before being tested. This experimental protocol was approved by the Michigan Technological University Institutional Review Board, and all participants signed an informed consent form.
Experimental design.
Subjects reported to the laboratory on 3 consecutive days. Testing occurred at the same time of day to avoid diurnal fluctuations in autonomic measurements. Resting blood pressures were measured in the seated position three times after 5 min of rest on each of the 3 consecutive days and reported as the mean. Height and weight were recorded following the resting blood pressure readings on the 3rd day. After resting measurements were taken on day 3, subjects were instrumented for the mental stress autonomic function test, which included 5 min of supine rest (baseline), 5 min of mental stress (mental arithmetic), and 5 min of supine rest (recovery). Mental arithmetic consisted of subtracting the number 6 or 7 from a 2- to 3-digit number continuously as investigators encouraged the subject to respond quickly. The 2- to 3-digit number was changed every 5–10 s. MSNA, heart rate (HR), beat-to-beat blood pressure, and forearm and calf blood flow were recorded throughout the protocol.
Measurements.
Arterial blood pressure was measured using two techniques. Resting arterial blood pressure was measured three times (separated by ∼1-min intervals) over 3 consecutive days using an automated sphygmomanometer and reported as a mean value (i.e., 9 readings over 3 days). Beat-to-beat arterial blood pressure was recorded continuously via Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) during the mental stress protocol (i.e., baseline, mental stress, and recovery). The Finometer accurately determines relative changes in arterial blood pressure, but should not be used to determine absolute values. Therefore, the Finometer was used to determine precise changes in arterial blood pressure that occurred during mental stress, while the sphygmomanometer allowed us to compare baseline arterial blood pressures (Table 1). Arterial blood pressures are expressed as systolic (SAP), diastolic (DAP), and mean arterial pressures (MAP). HR was recorded with the automated sphygmomanometer during the 3 consecutive days of blood pressure monitoring, and with a three-lead electrocardiogram during the mental stress protocol.
Table 1.
Resting hemodynamics for normotensive and prehypertensive subjects
| Normotensive |
Prehypertensive |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Day 1 | Day 2 | Day 3 | Mean | Day 1 | Day 2 | Day 3 | Mean |
| SAP, mmHg | 113 ± 2 | 112 ± 1 | 111 ± 1 | 112 ± 1 | 127 ± 2* | 126 ± 2* | 127 ± 2* | 127 ± 2* |
| DAP, mmHg | 65 ± 2 | 64 ± 2 | 64 ± 2 | 64 ± 1 | 71 ± 2* | 71 ± 2* | 70 ± 2* | 71 ± 2* |
| MAP, mmHg | 81 ± 1 | 80 ± 1 | 80 ± 1 | 80 ± 1 | 90 ± 2* | 89 ± 2* | 89 ± 2* | 89 ± 2* |
| HR, beats/min | 69 ± 3 | 68 ± 2 | 68 ± 2 | 68 ± 2 | 75 ± 3 | 74 ± 3 | 76 ± 3 | 75 ± 3 |
Values are means ± SE; n = 18 for normotensive, and n = 17 for prehypertensive. Measurements were recorded after 5 min of seated rest at the same time of day over 3 consecutive days with an automated sphygmomanometer. SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial blood pressure; HR, heart rate.
Significantly different (P < 0.05) from corresponding normotensive value.
Forearm and calf blood flow were measured using venous occlusion plethysmography. Changes in forearm and calf blood flow were measured via mercury-in-Silastic strain gauges placed around the subject's forearm and calf at the point of greatest circumference. Cuffs were placed around the subject's left wrist, upper arm, thigh, and ankle. The wrist and ankle cuffs were inflated to 220 mmHg to occlude blood flow to the hand and foot, while the upper arm and thigh cuffs were inflated to 60 mmHg for 8 s and deflated for 7 s (i.e., 15-s blood flow intervals).
Multifiber recordings of MSNA were made by inserting a tungsten microelectrode into the peroneal nerve of a resting leg. A reference electrode was inserted subcutaneously 2–3 cm from the recording electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000), where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated (time constant, 0.1) to obtain a mean voltage display of nerve activity. Satisfactory recordings of MSNA were defined by spontaneous, pulse synchronous bursts that increased during end-expiratory apnea and did not change during auditory stimulation. A loss or shift of the neurogram during mental stress prevented analysis of MSNA data in 14 subjects; thus we report MSNA burst frequency responses to mental stress in a total of 21 subjects (10 normotensive, 11 prehypertensive). Total MSNA responses to mental stress are presented for 19 subjects (9 normotensive, 10 prehypertensive).
Data analysis.
Data were imported and analyzed in the WinCPRS software program (Absolute Aliens, Turku, Finland). R-waves were detected and marked in the time series. Bursts of MSNA were automatically detected on the basis of amplitude using a signal-to-noise ratio of 3:1, within a 0.5-s search window centered on a 1.3-s expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by one investigator. The average burst area occurring during baseline was normalized to a mean value of 100. MSNA was expressed as bursts per minute, bursts per 100 heartbeats, and total MSNA (i.e., the sum of the normalized burst areas per minute).
Forearm and calf blood flows were analyzed as percent change and used to calculate vascular resistance and vascular conductance. Vascular resistance was calculated as MAP divided by limb blood flow, while vascular conductance was calculated as the reciprocal (i.e., limb blood flow divided by MAP).
Statistical analysis.
All data were analyzed statistically using commercial software (SPSS 15.0, SPSS, Chicago, IL). A two-way repeated-measures ANOVA was utilized to determine if changes in MSNA, SAP, DAP, MAP, HR, and forearm and calf vascular resistance and conductance occurred during baseline and mental stress and across trial groups (normotensive and prehypertensive). Post hoc analyses were performed using least significant difference pairwise comparisons. Resting variables were compared using independent t-tests. Means were considered significantly different when P < 0.05.
All results are expressed as means ± SE. Hemodynamic variables are presented as 5-min averages in results text and 1-min averages in Fig. 1 to provide more detail on differences between groups. Although there were group differences in forearm vascular responses, data are only presented as 5-min averages, as this was more conducive to the sampling technique (venous occlusion plethysmography). Finally, MSNA and calf vascular responses were not different between groups, regardless of analysis (i.e., minute by minute vs. 5-min averages), so data are presented as 5-min averages.
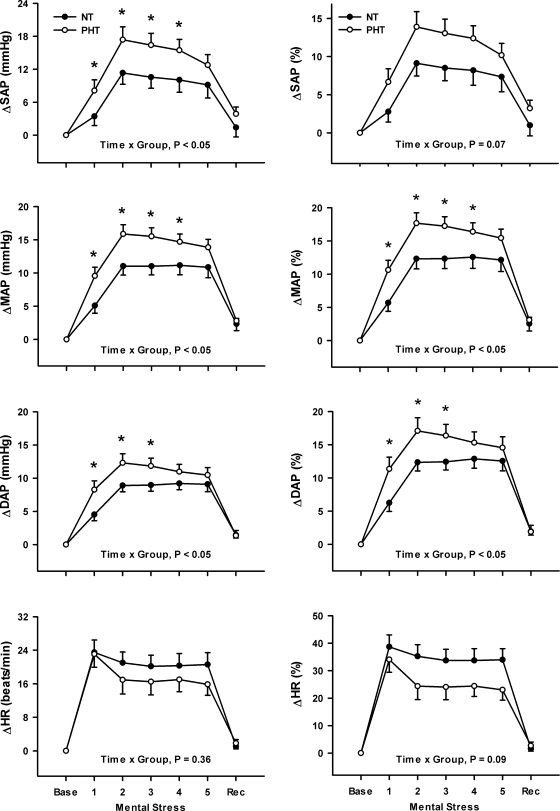
Fig. 1.
Changes (Δ) in systolic (SAP), diastolic (DAP), mean arterial pressures (MAP), and heart rate (HR) (expressed as both absolute and percent change) during 5 min of mental stress in normotensive (NT; n = 18) and prehypertensive (PHT; n = 16) subjects. Mental stress elicited a significant pressor response in both groups, and this response was augmented in PHT subjects. Mental stress increased HR in both NT and PHT subjects, and there was no significant difference between groups. Values are means ± SE. *P < 0.05 from corresponding NT value.
RESULTS
Table 1 reports seated resting blood pressure and HR values taken over 3 consecutive days. Resting SAP, DAP, and MAP were greater in prehypertensive than normotensive subjects, while resting HR was not different across groups. Table 2 reports supine baseline MSNA, along with limb blood flow, vascular resistance, and vascular conductance to the forearm and calf. Forearm blood flow was significantly greater in prehypertensive compared with normotensive subjects, whereas all other baseline variables were not different between groups (Table 2).
Table 2.
Baseline values for normotensive and prehypertensive subjects
| Variable | Normotensive | Prehypertensive | P Value |
|---|---|---|---|
| MSNA, bursts/min | 10 ± 2 | 11 ± 2 | 0.78 |
| MSNA, bursts/100 heartbeats | 17 ± 3 | 16 ± 3 | 0.88 |
| FBF, units | 2.5 ± 0.3 | 3.4 ± 0.3* | 0.03 |
| FVR, mmHg/units | 35 ± 2 | 30 ± 4 | 0.28 |
| FVC, units/mmHg | 0.03 ± 0.003 | 0.04 ± 0.004 | 0.13 |
| CBF, units | 2.1 ± 0.2 | 2.4 ± 0.2 | 0.25 |
| CVR, mmHg/units | 42 ± 3 | 42 ± 4 | 0.99 |
| CVC, units/mmHg | 0.03 ± 0.002 | 0.03 ± 0.002 | 0.80 |
Values are means ± SE. MSNA, muscle sympathetic nerve activity (n = 15 for normotensive and n = 16 for prehypertensive); FBF, forearm blood flow (n = 17 for normotensive and n = 16 for prehypertensive); FVR, forearm vascular resistance; FVC, forearm vascular conductance; CBF, calf blood flow (n = 17 for normotensive and n = 15 for prehypertensive); CVR, calf vascular resistance; CVC, calf vascular conductance; units, ml · 100 ml tissue−1 · min−1.
Significantly different between groups.
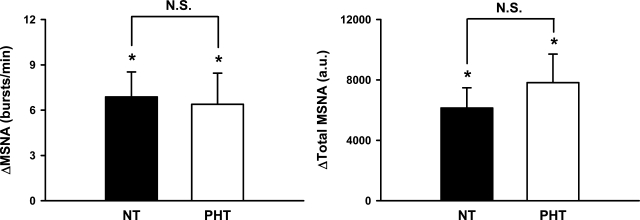
Figure 1 demonstrates that increases (Δ) in SAP (Δ9 ± 2 vs. Δ14 ± 2 mmHg; P < 0.05), DAP (Δ8 ± 1 vs. Δ11 ± 1 mmHg; P < 0.05), and MAP (Δ10 ± 1 vs. Δ14 ± 1 mmHg; P < 0.05) during mental stress were significantly greater in prehypertensive compared with normotensive subjects. Figure 2 demonstrates that mental stress significantly increased MSNA from baseline when expressed as bursts per minute (normotensive: 11 ± 2 to 18 ± 3 bursts/min, P < 0.05; prehypertensive: 11 ± 3 to 18 ± 3 bursts/min, P < 0.05) or total MSNA (normotensive: 3,918 ± 718 to 10,064 ± 1,295 arbitrary units, P < 0.05; prehypertensive: 5,591 ± 1,360 to 13,417 ± 2,684 arbitrary units, P < 0.05). In contrast to blood pressure, MSNA responses to mental stress were not different between groups (Fig. 2).
Fig. 2.
Changes in muscle sympathetic nerve activity (MSNA) bursts per minute and total MSNA during 5 min of mental stress in NT and PHT subjects. Mental stress increased MSNA burst frequency and total MSNA in both groups. These responses were not different between groups (burst frequency: time × group, P = 0.43; total MSNA: time × group, P = 0.29). Values are means ± SE. *P < 0.05 from baseline. N.S., no significance; a.u., arbitrary units per minute.
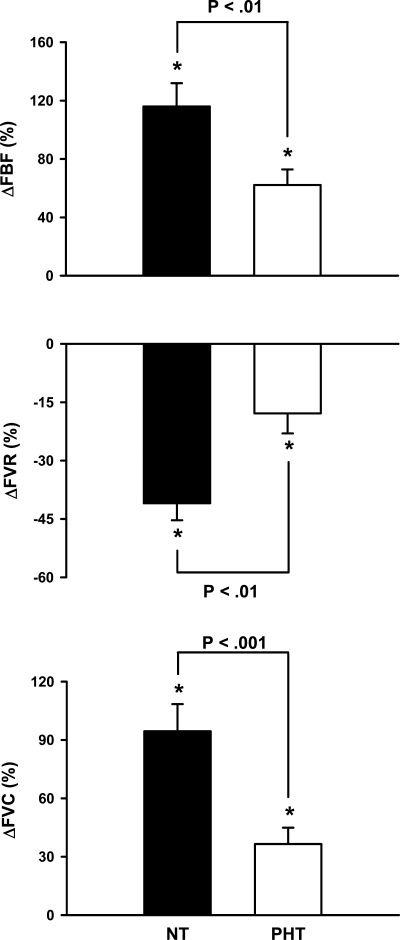
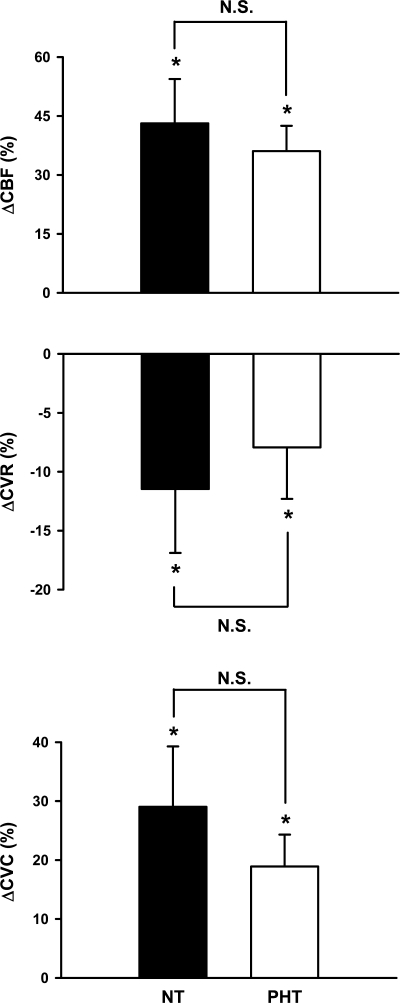
Figure 3 represents the forearm blood flow responses during mental stress in normotensive and prehypertensive subjects. Although mental stress increased forearm blood flow in both groups, increases were significantly blunted in prehypertensive subjects (Δ116 ± 16 vs. Δ62 ± 11%; P < 0.01). Likewise, decreases in forearm vascular resistance (Δ −41 ± 4 vs. Δ −18 ± 5%; P < 0.01) and increases in conductance (Δ95 ± 14 vs. Δ37 ± 8%; P < 0.001) during mental stress were blunted in prehypertensive subjects. In contrast, calf blood flow (Δ43 ± 11 vs. Δ36 ± 6%), resistance (Δ −12 ± 5 vs. Δ −8 ± 4%), and conductance (Δ29 ± 10 vs. Δ19 ± 5%) responses during mental stress were similar between groups (Fig. 4).
Fig. 3.
Changes in forearm blood flow (FBF), vascular resistance (FVR), and vascular conductance (FVC) during 5 min of mental stress. Mental stress elicited forearm vasodilation in both groups, but these responses were blunted in PHT (n = 15) compared with NT (n = 17) subjects (time × group = P < 0.01, all). Values are means ± SE. *P < 0.05 from baseline.
Fig. 4.
Changes in calf blood flow (CBF), vascular resistance (CVR), and vascular conductance (CVC) during 5 min of mental stress. Mental stress elicited calf vasodilation in NT (n = 17) and PHT (n = 14) groups, and these responses were not different between groups (time × group, P > 0.20, all). Values are means ± SE. *P < 0.05 from baseline.
DISCUSSION
The present study compared neurovascular responses to mental stress in prehypertensive and normotensive subjects, and our data reveal three major findings. First, mental stress elicited an augmented pressor response in prehypertensive subjects. Second, this augmented pressor response was associated with an attenuated forearm vasodilation. These findings are consistent with previous work (17, 22, 28). Third, prehypertension did not alter MSNA responses to mental stress. This finding is novel and provides new mechanistic insight into the well-documented, yet poorly understood, link between mental stress and hypertension. Collectively, our data indicate that blunted forearm vasodilation, not augmented MSNA, contributes to a more dramatic increase of arterial blood pressure during mental stress in prehypertensive subjects.
Several studies have shown augmented blood pressure responses to mental stress in prehypertensive compared with normotensive subjects (17, 22, 28). Similar results have also been seen in subjects with a family history of hypertension (25, 33). Furthermore, an augmented pressor response to stress in normotensive subjects may even help predict the development of hypertension (12, 23). In the present study, we report an augmented pressor response to mental stress in prehypertensive subjects. Thus our findings are consistent with previous studies and strengthen the rationale to determine potential mechanisms that may be responsible for this augmented response.
Matsukawa et al. (22) reported an elevated blood pressure response to mental stress in borderline hypertensive subjects and suggested that the difference was likely due to a decrease in MSNA in normotensive subjects and no change in the borderline hypertensive subjects. However, recent evidence (9) suggests that the findings of Matsukawa et al. (22) may not represent typical MSNA responses to mental stress. Specifically, a recent retrospective analysis of 82 neurograms from normotensive subjects indicated that nearly 90% of subjects demonstrated an increase or no change in MSNA, while ∼10% were classified as “negative” MSNA responders (less than or equal to Δ −3 bursts/min) (9). Therefore, the findings of Matsukawa et al. (22), which reported a significant sympathoinhibition of MSNA during mental stress in normotensive subjects, may not represent a normal distribution with regards to typical MSNA responses to mental stress.
The present study demonstrates similar increases of MSNA during mental stress in normotensive and prehypertensive patients. We attribute differences between our data and the findings of Matsukawa et al. (22) to the inherent variability of MSNA responses to mental stress in humans (9). However, it is important to note that in the present study, 10 of 10 normotensive subjects and 10 of 11 prehypertensive subjects demonstrated an increase or no change in MSNA during mental stress. These ratios are consistent with a recent large-scale study that reported MSNA either increases or does not change during mental stress in ∼90% of adults (9). Therefore, we are confident in our data and conclude that MSNA responses to mental stress are similar in normotensive and prehypertensive populations.
Whereas prehypertension did not alter MSNA responses to mental stress, it did alter forearm vascular responses. Specifically, prehypertension blunted the classic forearm vasodilatory response associated with mental stress. Blunted forearm vascular responses to physiological stressors are associated with increased incidence or risk of hypertension (4, 28, 29). For example, young normotensive individuals with a family history of hypertension display a reduction in peak forearm blood flow (4) and an increased forearm vascular resistance during (29) and after (4) reactive hyperemia. Moreover, borderline hypertensive subjects demonstrate increased forearm vascular resistance and MAP during mental stress (28). Thus the present study, which demonstrates blunted forearm vascular responses to mental stress in prehypertensive subjects, is consistent with previous work (28) and offers new insights by demonstrating that the blunted forearm vascular response to mental stress is not accompanied by altered MSNA.
A recent study reports similar MSNA responses to mental stress in the arm and leg (8), making it reasonable to assume that other mechanisms beyond MSNA may be responsible for the blunted forearm vasodilation to mental stress in our prehypertensive subjects. However, it should be noted that other studies have reported a divergence in arm and leg MSNA (1) and decreases of arm MSNA (15) during mental stress. We recognize the absence of arm MSNA data as a limitation to the present study, but do not believe this lessens the impact of the data. Our primary focus remains on mechanisms responsible for the augmented pressor response to mental stress in prehypertensive subjects, not the mechanisms underlying forearm vasodilation. Both leg MSNA and forearm vascular conductance have been independently proposed as potential mechanisms contributing to the augmented pressor response, and the present study advanced our knowledge by demonstrating that blunted forearm vasodilation, not augmented MSNA, is a primary contributor. This is important as it had previously been assumed that augmented MSNA was a likely contributor (22). However, we recognize that other potential mechanisms might include vasoconstriction to nonmuscular beds, including renal, splanchnic, and skin (5, 19, 30, 31); future work will have to address these vascular beds.
As previously noted, passive MSNA withdrawal has been suggested as a potential mechanism for the forearm vasodilation associated with mental stress (15), but this remains debatable (8) and other mechanisms have been proposed (7, 11, 21). Specifically, both nitric oxide (6, 7, 11) and circulating epinephrine (21) have been shown to contribute to forearm vasodilation during mental stress. Of interest to the present study, Cardillo et al. (6) reported that nitric oxide-mediated vasodilation during mental stress was attenuated in hypertensive subjects. We did not assess nitric oxide levels in the present study, but it seems reasonable to speculate that the blunted forearm vasodilation during mental stress in prehypertensive subjects may be related to altered nitric oxide responses.
Although mental stress does not typically modulate calf vasculature (8, 27), calf vasodilation has been observed during mental stress (19). Therefore, the modest, yet significant, calf vasodilation reported in the present study is reasonable and consistent with prior work. Importantly, calf vasodilatory responses to mental stress were similar in normotensive and prehypertensive subjects. Thus prehypertension appears to elicit divergent limb vascular responses to mental stress. The clinical consequence of this divergent vascular response remains unclear, but it is consistent with other studies reporting divergent limb vascular responses during physiological “stressors” in both animals (34) and humans (20, 24).
In conclusion, the present study demonstrates that prehypertension elicits a more dramatic pressor response to mental stress compared with normotensive subjects. This augmented pressor response appears to be related to blunted forearm vasodilation, but not augmented MSNA. These findings provide new insight into the complex relationship between mental stress and hypertension.
Perspectives.
Despite attempts to recruit young, prehypertensive women, we were only successful in the recruitment of 17 prehypertensive men. Accordingly, we caution the extrapolation of the present data to women. A growing body of evidence suggests that the relations between MSNA and arterial blood pressure regulation differ in men and women (18), and we cannot speculate how these differences might translate to the present findings. Although young, otherwise healthy women have a lower incidence of prehypertension than their young, male counterparts (16), they have a much higher incidence of anxiety and panic disorder (32). The long-term impact of these more “chronic” stressors on arterial blood pressure remains unclear, but evidence suggests that such chronic stressors are linked to essential hypertension via autonomic mechanisms (12). Whereas the present study focused on neurovascular responses to acute mental stress in prehypertensive men, future investigations might focus on not only prehypertensive women, but also chronic stress.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grants HL-088689 and HL-098676.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Jenna Klein, Ashley Yenior, Kristen Reed, Huan Yang, and Sarah Stream for assistance in data collection and analysis for this project. We also thank all of the subjects for participation and cooperation.
REFERENCES
- 1. Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension 9: III114–III119, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Barcroft H, Brod J, Hejl BZ, Hirsjarvi EA, Kitchin AH. The mechanism of the vasodilatation in the forearm muscle during stress (mental arithmetic). Clin Sci 19: 577–586, 1960 [PubMed] [Google Scholar]
- 3. Blair DA, Glover WE, Greenfield AD, Roddie IC. Excitation of cholinergic vasodilator nerves to human skeletal muscles during emotional stress. J Physiol 148: 633–647, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boutcher YN, Park YJ, Boutcher SH. Vascular and baroreceptor abnormalities in young males with a family history of hypertension. Eur J Appl Physiol 107: 653–658, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Brod J. Haemodynamic basis of acute pressor reactions and hypertension. Br Heart J 25: 227–245, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Impairment of the nitric oxide-mediated vasodilator response to mental stress in hypertensive but not in hypercholesterolemic patients. J Am Coll Cardiol 32: 1207–1213, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol 80: 1070–1074, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol 564: 321–327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol 35: 498–502, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand 177: 275–284, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Falkner B, Onesti G, Angelakos ET, Fernandes M, Langman C. Cardiovascular response to mental stress in normal adolescents with hypertensive parents. Hemodynamics and mental stress in adolescents. Hypertension 1: 23–30, 1979 [DOI] [PubMed] [Google Scholar]
- 15. Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol 504: 211–220, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izzo JL., Jr Prehypertension: demographics, pathophysiology, and treatment. Curr Hypertens Rep 9: 264–268, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Jern S, Bergbrant A, Hedner T, Hansson L. Enhanced pressor responses to experimental and daily-life stress in borderline hypertension. J Hypertens 13: 69–79, 1995 [PubMed] [Google Scholar]
- 18. Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol 104: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawrence JE, Klein JC, Carter JR. Menstrual cycle elicits divergent forearm vascular responses to vestibular activation in humans. Auton Neurosci 154: 89–93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindqvist M, Davidsson S, Hjemdahl P, Melcher A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiol Scand 158: 7–14, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand 141: 157–165, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Monahan KD, Ray CA. Limb neurovascular control during altered otolithic input in humans. J Physiol 538: 303–308, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Luscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation 93: 866–869, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Roddie IC. Human responses to emotional stress. Ir J Med Sci 146: 395–417, 1977 [DOI] [PubMed] [Google Scholar]
- 27. Rusch NJ, Shepherd JT, Webb RC, Vanhoutte PM. Different behavior of the resistance vessels of the human calf and forearm during contralateral isometric exercise, mental stress, and abnormal respiratory movements. Circ Res 48: I118–I130, 1981 [PubMed] [Google Scholar]
- 28. Santangelo K, Falkner B, Kushner H. Forearm hemodynamics at rest and stress in borderline hypertensive adolescents. Am J Hypertens 2: 52–56, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Takeshita A, Imaizumi T, Ashihara T, Yamamoto K, Hoka S, Nakamura M. Limited maximal vasodilator capacity of forearm resistance vessels in normotensive young men with a familial predisposition to hypertension. Circ Res 50: 671–677, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Tidgren B, Hjemdahl P. Renal responses to mental stress and epinephrine in humans. Am J Physiol Renal Fluid Electrolyte Physiol 257: F682–F689, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Wallin BG, Delius W, Hagbarth KE. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res 33: 9–21, 1973 [DOI] [PubMed] [Google Scholar]
- 32. Westenberg HG, Liebowitz MR. Overview of panic and social anxiety disorders. J Clin Psychiatry 65, Suppl 14: 22–26, 2004 [PubMed] [Google Scholar]
- 33. Widgren BR, Wikstrand J, Berglund G, Andersson OK. Increased response to physical and mental stress in men with hypertensive parents. Hypertension 20: 606–611, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Vestibular inputs elicit patterned changes in limb blood flow in conscious cats. J Physiol 575: 671–684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]