Abstract
Adenosine triphosphate, acting through purinergic P2X receptors, has been shown to stimulate ventilation and increase carotid body chemoreceptor activity in adult rats. However, its role during postnatal development of the ventilatory response to hypoxia is yet unknown. Using whole body plethysmography, we measured ventilation in normoxia and in moderate hypoxia (12% fraction of inspired O2, 20 min) before and after intraperitoneal injection of suramin (P2X2 and P2X3 receptor antagonist, 40 mg/kg) in 4-, 7-, 12-, and 21-day-old rats. Suramin reduced baseline breathing (∼20%) and the response to hypoxia (∼30%) in all rats, with a relatively constant effect across ages. We then tested the effect of the specific P2X3 antagonist, A-317491 (150 mg/kg), in rats aged 4, 7, and 21 days. As with suramin, A-317491 reduced baseline ventilation (∼55%) and the hypoxic response (∼40%) at all ages studied. Single-unit carotid body chemoreceptor activity was recorded in vitro in 4-, 7-, and 21-day-old rats. Suramin (100 μM) and A-317491 (10 μM) significantly depressed the sinus nerve chemosensory discharge rate (∼80%) in normoxia (Po2 ∼150 Torr) and hypoxia (Po2 ∼60 Torr), and this decrease was constant across ages. We conclude that, in newborn rats, P2X purinergic receptors are involved in the regulation of breathing under basal and hypoxic condition, and P2X3-containing receptors play a major role in carotid body function. However, these effects are not age dependent within the age range studied.
Keywords: development, hypoxia, P2X receptors, ventilation
carotid body chemoreceptors respond to a decrease in arterial oxygen saturation by increasing action potential (AP) activity in sinus nerve afferent fibers. These fibers terminate in the brain stem, and their stimulation enhances respiratory drive and sympathetic activity. Chemoreceptors undergo significant postnatal maturation. In rats, the chemoreceptor AP response to a given level of hypoxia is weak at birth and matures to adult levels over the first 3 wk of life (25). In addition, the respiratory response to hypoxia is biphasic in newborn mammals, with an initial increase in ventilation, mediated by the carotid bodies, followed by a decrease mediated by central inhibitory mechanisms and a reduction of metabolic rate (7, 17). The two phases involve different neurotransmitters, which may show an age dependence (2, 7).
Although the mechanism by which hypoxia is transduced by the carotid body to increased AP firing frequency is incompletely understood, recent data have implicated a major role of ATP acting through purinergic receptors in the adult cat, rat, and mouse (20, 33, 46). For instance, intracarotid injection of ATP to anesthetized cats increases ventilation and stimulates carotid sinus nerve discharge rate (36). In addition, similar effects are observed with ATP analogs and blocked by the ATP receptor antagonist suramin (37). Pharmacological experiments based on a coculture of rat carotid body chemoreceptor cells and sensory petrosal ganglion neurons provided evidence that the effects of ATP are mainly related to activation of postsynaptic P2X2- and P2X3-containing purinergic receptors (47, 48). Furthermore, RT-PCR and immunofluorescence studies confirmed the presence of P2X receptor subunits in chemoreceptor afferent neurons (3, 21, 34, 48). Finally, the ventilatory and carotid sinus nerve responses to hypoxia were significantly decreased in mice lacking P2X2 receptors and further reduced in P2X2/3 double knockout mice (38).
By contrast, little is known regarding the contribution of the purinergic system to maturation of hypoxic chemosensitivity in the carotid body. This maturational process involves several aspects of carotid body development, including neurogenesis (44), establishment of synapses (9), expression of ion channels (26), and developmental changes of key neurotransmitters, such as dopaminergic and cholinergic systems. These developmental changes typically occur during a period overlapping late fetal development and the first 3 wk following birth in rat pups (2, 11, 31). Our laboratory recently reported that the protein expression level of P2X2 and P2X3 receptors remains stable during postnatal development in the carotid body of cats (3). However, their expression decreases in the petrosal ganglion; expression levels decrease by 50% in 2-mo-old and adult cats compared with 1- and 15-day-old cats (3). These observations led to the hypothesis that functional purinergic signaling in the carotid body changes during development and contributes to postnatal changes in the carotid body response to hypoxia. Accordingly, we designed the present study to test this hypothesis using whole body plethysmography and in vitro recordings of chemosensory activity in developing rats.
MATERIAL AND METHODS
Ethics
We used male and female Sprague-Dawley rats between postnatal (P) days 4 and 21 to conduct our studies in two different laboratories. In vivo recordings of minute ventilation (V̇e) in normoxia and hypoxia were performed at the Centre de Recherche du Centre Hospitalier Universitaire de Québec after approval by the Animal Care Committee at Laval University, in accordance with the Canadian Council on Animal Care guidelines. Recordings of chemoreceptor activity using the in vitro preparation were performed at Yale University School of Medicine and approved by the Institutional Animal Experimentation and Use Committee.
In Vivo Studies: Ventilatory and Metabolic Recordings
Animals.
All animals used were obtained from 15 litters by mating virgin female and male rats purchased from Charles River (St. Constant, Québec). Rats were supplied with food and water ad libitum and maintained under standard laboratory conditions (21°C, 12:12-h dark-light cycle: lights on at 0700 and off at 1900). Litters were culled to 12 pups on P1, with an equal number of each sex when possible.
Measurements.
Respiratory and metabolic parameters were recorded in unrestrained male and female rat pups at P4 (n = 35), P7 (n = 31), P12 (n = 21), and P21 (n = 41) using whole body plethysmography (Emka Technologies, Paris, France), as previously described (23, 31). Using a temperature control system (Physitemp, Clifton, NJ), the temperature inside the plethysmograph was fixed at thermoneutrality for each age studied: 32°C for P4 and P7 and 30°C for P12. Relative humidity was continuously monitored from the outflowing air stream with a high-precision water vapor pressure analyzer (RH-300 Sable Systems International). The inner volume of the plethysmography chamber was 0.22 liter for P4 and P7 pups and 0.75 liter for P12 and P21 pups. Calibration of the respiratory signal was performed by injection of 0.5 or 1 ml of air (according to the size of the chamber), and tidal volume (Vt) was calculated from the pressure deviation by IOX software (Emka Technologies, Paris, France). Oxygen and CO2 analyzers were calibrated following the manufacturer's instructions (AEI Technologies, Naperville, IL). Due to the small size of young rats, body temperature was measured orally in P4 and P7 rats and rectally in P12 and P21 rats using a thermocouple for small rodents (Harvard, Holliston, MA) before and at the end of the recording period. The gas flow through the plethysmograph was set at 0.1 l/min (P4 and P7) or 0.2 l/min (P12 and P21) and measured continuously with a mass flowmeter (4140; TSI, Shoreview, MN). Using a dual-channel analyzer for oxygen and CO2 (AEI Technologies), respiratory gases (oxygen and CO2) were continuously measured during the entire recording time and used later to calculate oxygen consumption (V̇o2) and CO2 production (V̇co2). Calibration was performed per the manufacturer's specification using a one-point or two-point method with certified gases. Respiratory frequency (fR) and Vt were recorded from the plethysmograph signal. Vt was calculated based on barometric pressure, room and body temperature, and humidity (btps) using the Bartlett and Tenney (5) equation. V̇e was calculated as V̇e = fR × Vt. V̇o2 was calculated as V̇o2 = flow × [(O2 in − O2 out) − O2 out × (CO2 out − CO2 in)]/(1 − O2 out) and V̇co2 as V̇co2 = flow × [(CO2 out − CO2 in) − CO2 out × (O2 in − O2 out)]/(1 − CO2 out), corrected to stpd conditions, where O2 in, O2 out, CO2 in, and CO2 out are inflowing and outflowing CO2 and O2, respectively. These values were then used for calculation of the ventilatory equivalent for oxygen (V̇e/V̇o2) and carbon dioxide (V̇e/V̇co2) (1, 17, 30). Vt, V̇o2, and V̇co2 were expressed per 100 g body wt.
Drugs.
All drugs were purchased from Sigma Aldrich Canada (Oakville, ON, Canada). Because selective P2X2 receptor antagonists are not commercially available, we used the nonspecific purinergic P2X receptor antagonist suramin (28) and the selective P2X3 receptor antagonist A-317491 (22), which acts on both homomeric P2X3- and heteromeric P2X2/3-containing receptors. Each drug was dissolved in sterile saline and injected 30 min before the recordings were started. Based on dose-response studies performed in 19- to 21-day-old rats (see below), we administered suramin and A-317491 at doses of 40 and 150 mg/kg, respectively. The choice of a 30-min delay was based on association constant data from the literature (22, 28). For in vitro studies, suramin was added to the perfusion solution at 100 μM, as previously used to assess the chemoreceptor activity in adult rats (21). A-317491 was used at a concentration of 10 μM based on the affinity constant measured for recombinant and native rat P2X3 receptors (22).
Ventilatory dose-response curve for suramin and A-317491 in 19- to 21-day-old rats.
Because there are no data in the literature concerning the ventilatory effects of suramin or A-317491 on young rats, we first determined the ventilatory dose-response curve for each drug in normoxia [inspired O2 fraction (FiO2) = 21%] and in response to hypoxia (FiO2 = 12%) in 19- to 21-day-old rats (data not shown). A total of 55 rats from 6 litters were used for these preliminary studies. Four doses of suramin were tested [20, 40, 80, and 100 mg/kg, injected intraperitoneally (IP), 6 rats/dose], and compared with vehicle (saline injection, n = 10 rats). Compared with vehicle, suramin had no effect on ventilation at 20 mg/kg, but decreased V̇e to similar levels at 40, 80, and 100 mg/kg in normoxia and hypoxia. The magnitude of this decrease was ∼15 and 22% in normoxia and hypoxia, respectively. Accordingly, a dose of 40 mg/kg was chosen for the experimental series. For the selective P2X3-containing receptor antagonist A-317491, four doses were tested: 30 mg/kg (n = 3), 100 mg/kg (n = 3), 150 mg/kg (n = 6), and 300 mg/kg (n = 3) injected IP and compared with vehicle (n = 6). As with suramin, A-317491 reduced V̇e in normoxia and hypoxia at 150 and 300 mg/kg. Each of these doses decreased V̇e ∼42% in normoxia and 45% in hypoxia relative to vehicle. Accordingly, the dose of 150 mg/kg was used for the experimental series. We used both males and females for the dose-response study. Because we found no sex-specific effect, we used male and female rats for recordings throughout the study, but we did not perform further sex-specific analyses.
Experimental protocol.
To avoid pup- and litter-specific effects, rats from each litter were weighed and randomly assigned to one of three groups: vehicle (1 ml/kg, IP saline injection), suramin (40 mg/kg), or A-317491 (150 mg/kg). Treatments were given 30 min before the start of the experiment. Each rat was used for only one experiment to avoid repetitive drug/hypoxic exposures. After each rat's body temperature was measured, it was returned to the plethysmograph. Once it laid quiet, measurements were started under normoxia (FiO2 = 21%) for 10 min. Nitrogen was mixed with air at the gas inlet to produce the desired chamber O2 pressure for hypoxia (FiO2 = 12%), which generally occurred within 1 min and was stable during the 20-min recording period. All recordings were performed under poikilocapnic conditions.
In Vitro Studies: Carotid Body Chemoreceptor Activity Recordings
Animals.
Dams with their 1-day-old pups were purchased and housed in Yale University animal care facilities under standard conditions (light-dark cycle 12:12 h, food and water ad libitum). Male and female rat pups aged P3–P4 (n = 11), P7–P8 (n = 15), and P16–P21 (n = 25) from five different litters were used. For all in vitro experiments, each rat was only used for one recording (i.e., one carotid body per pup per experiment).
Single-unit chemoreceptor preparation.
Each rat was deeply anesthetized with 100% CO2, then decapitated, and the carotid body/carotid sinus nerve/petrosal ganglion complex was harvested en bloc under surgical microscopy, as previously detailed (13, 15). The connective tissue was partially digested with a mixture of collagenase type P (1 mg/ml, Roche Diagnostics, Mannheim, Germany) and protease type IX (0.2 mg/ml, Sigma Aldrich, St. Louis, MO) in Ringer buffer solution (in mM: 120 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 24 NaHCO3, 1 MgSO4, and 5 glucose, pH = 7.4), bubbled with a mixture of 95% O2 and 5% CO2.
After the connective tissue was cleaned, the complex was transferred to the recording chamber (RC-22C, Warner Instruments, Hamden, CT) mounted on the stage of an inverted microscope (AxioVert 10, Carl Zeiss, Germany). The preparation was continuously superfused with 37°C Ringer solution (TC-344B in-line heater, Warner Instruments) at a rate of 3 ml/min (Peristaltic Pump Minipuls 3, Gilson, Middleton, WI). The chamber Po2 near the preparation was continuously monitored (Oxy-Micro with PST-1 probe, World Precision Instrument, FL) during the experiment.
Single-unit chemoreceptor afferent nerve fiber activity was recorded from the soma of petrosal neurons projecting to the carotid body by placing a suction electrode in the petrosal ganglion. To facilitate unit identification and measurement of conduction time, a glass pipette filled with 1 M NaCl (0.2-MΩ impedance) was placed in the carotid body, and a cathodal electrical stimulus (100 μA, 0.1-ms duration; BSI-2, BAK Instruments, Rockville, MD) was delivered by a constant current source (BPG-1, BAK instruments) through the pipette to evoke an orthodromic AP. Electrical stimuli were delivered only until evoked single-unit activity was detected and spontaneous APs were displayed on an oscilloscope. The AP discharge activity recorded from the sensory petrosal neuron was continuously acquired, amplified (MD5, BAK instruments), filtered, digitized at a sample rate of 10 kHz (Digidata 1200, Axon Instruments, Foster City, CA), and stored on a computer (Axoscope, Axon Instruments) for further analyses.
Chemoreceptor activity recordings.
Chemoreceptor activity was first recorded under control, normoxic conditions (without drug treatment) for 2–3 min (superfused with Ringer buffer equilibrated with 21% O2 and 5% CO2, balanced with N2, Po2 ∼150 Torr). Then the control hypoxic response was measured for 3–4 min by superfusing the preparation with a Ringer buffer equilibrated with 5% O2 and 5% CO2 balanced with N2, Po2 ∼60 Torr. After returning to normoxic conditions and stable activity, the preparation was superfused for 10 min with normoxic solution containing either suramin or A-317491, and the AP discharge rate was recorded for 2–3 min. The inflowing line was then switched to the hypoxic solution containing the same drug for 3–4 min. The drug was washed out for 10 min, and the hypoxia response retested. Only one drug was tested on a given preparation.
Data Collection and Statistical Analyses
Ventilation.
Ventilatory and metabolic variables during normoxia were measured and calculated each minute during the experiment using the IOX software program (version 1.8.9 EMKA technology, Paris, France). For each rat, the last 5 min of ventilatory (fR, Vt, V̇e) and metabolic (V̇o2, V̇co2, V̇e/V̇o2, and V̇e/V̇co2) data were averaged. A two-way ANOVA (StatView 5.1) using the drug applied (suramin, A-317491, vehicle) and age (4-, 7-, 21-day-old rats) as treatment variables was used to test statistical significance. In these groups, the mean value of V̇e in vehicle-treated rats was arbitrarily made 100. V̇e of each rat in the vehicle, suramin, and A-317491 groups was calculated using the following formula: (x = individual value in normoxia/mean value of vehicle group × 100). This calculation was performed for each of age group studied. Values were expressed as means ± SE.
For the hypoxic response, fR and Vt were obtained on a minute-by-minute basis for the 20 min of recording. We expressed the response to hypoxia as peak and steady state. The peak response is the average of the highest values observed in two successive minutes during the first 10 min of recording, and the steady-state response is the average of the last 5 min of recording. The peak and steady state fR, Vt, and V̇e in response to hypoxia were expressed as percent changes from normoxia.
ANOVAs were used to determine main effects of treatment and age and interactions between treatment and age. Fisher's protected least significant difference tests were used for post hoc testing if ANOVAs yielded a P value < 0.05. Values were expressed as means ± SE.
Chemoreceptor activity.
Chemoreceptor activity was analyzed using an event detection program (CLAMPFIT 9, Molecular Devices, Sunnyvale, CA). The AP occurrence times were converted to frequency (Hz) (Origin 7.5 program, Microcal Software, Northampton, MA) and plotted against oxygen concentration of the recording chamber and time to determine the activity under normoxia and hypoxic conditions. Discharge rates were averaged over the entire 3-min recording under normoxia. In contrast, discharge rates under hypoxia were quantified as the peak value of a 3-s moving average.
Significance was tested using a two-way ANOVA with treatment and age as grouping variables. If a P value < 0.05 was reported, post hoc Fisher's protected least significant difference tests were performed. Values were expressed as means ± SE.
RESULTS
Ventilatory and Metabolic Effects of Purinergic Receptor Antagonists in Developing Rats
Normoxic ventilation.
SURAMIN.
Representative respiratory recordings obtained from P4, P7, and P21 rats at 30 min after suramin injection are shown in Fig. 1, A and B, while ventilatory (fR, Vt, and V̇e) and metabolic variables (V̇o2, V̇co2, V̇e/V̇o2, and V̇e/V̇co2) are presented in Table 1. Compared with vehicle, suramin significantly decreased fR (treatment effect, P = 0.002), significantly decreased V̇e (treatment effect, P = 0.0001), and showed a tendency toward decreased Vt (treatment effect, P = 0.08) over the four ages studied. No significant interaction between age and treatment was detected for fR (age × treatment, P = 0.09) or V̇e (age × treatment, P = 0.4).
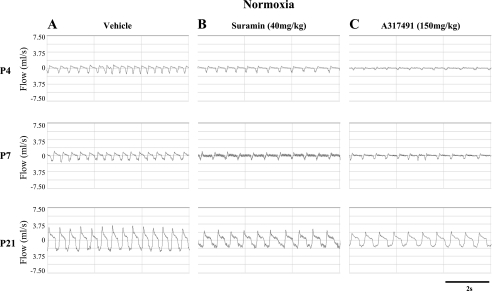
Fig. 1.
Typical respiratory recordings under normoxia [inspired O2 fraction (FiO2) = 21%] from three different pups at postnatal day 4 (P4), P7, and P21 after a single injection of either vehicle (saline; A), suramin (B), or A-317491 (C). These examples were obtained during the last 5 min of recording during normoxia. Note that these are flow traces; tidal volume is calculated from the integrated signal. Inspirations correspond to downward deviations below 0.
Table 1.
Suramin effect on baseline ventilatory and metabolic parameters in developing rats
| Age, days | Drug | n | Weight, g | Temperature, °C | fR, breaths/min | Vt, ml/100 g | V̇e, ml · min−1 · 100 g−1 | V̇o2, ml · min−1 · 100 g−1 | V̇co2, ml · min−1 · 100 g−1 | V̇e/V̇o2 | V̇e/V̇co2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Vehicle | 10 | 10.2 ± 0.2 | 33.8 ± 0.2 | 166 ± 3 | 1.26 ± 0.1 | 210 ± 15 | 5.9 ± 0.2 | 4.3 ± 0.1 | 44 ± 4 | 52 ± 5 |
| Suramin | 10 | 10.8 ± 0.3 | 33.7 ± 0.4 | 157 ± 3* | 1.11 ± 0.1 | 169 ± 6* | 3.6 ± 0.3* | 2.9 ± 0.3* | 47 ± 4 | 61 ± 9 | |
| 7 | Vehicle | 10 | 17.3 ± 0.5 | 35.2 ± 0.2 | 167 ± 2 | 0.89 ± 0.1 | 157 ± 12 | 4.6 ± 0.7 | 3.8 ± 0.6 | 42 ± 6 | 51 ± 6 |
| Suramin | 12 | 17.7 ± 0.5 | 34.9 ± 0.3 | 155 ± 3* | 0.71 ± 0.1 | 110 ± 5* | 2.2 ± 0.2* | 1.9 ± 0.2* | 44 ± 5 | 50 ± 6 | |
| 12 | Vehicle | 11 | 30.2 ± 0.6 | 35.4 ± 0.2 | 157 ± 2 | 0.93 ± 0.1 | 146 ± 7 | 3.8 ± 0.4 | 2.4 ± 0.1 | 41 ± 5 | 63 ± 4 |
| Suramin | 10 | 30.5 ± 0.6 | 35.5 ± 0.2 | 137 ± 3* | 0.74 ± 0.1 | 101 ± 5* | 2.8 ± 0.4* | 1.7 ± 0.1* | 39 ± 8 | 62 ± 4 | |
| 21 | Vehicle | 14 | 52.1 ± 1.2 | 36.7 ± 0.2 | 147 ± 3 | 0.90 ± 0.1 | 132 ± 8 | 4.7 ± 0.3 | 2.7 ± 0.3 | 27 ± 1 | 43 ± 10 |
| Suramin | 11 | 55.7 ± 3.2 | 36.7 ± 0.1 | 134 ± 2* | 0.87 ± 0.1 | 116 ± 7* | 3.4 ± 0.3* | 2.6 ± 0.2 | 37 ± 5 | 45 ± 3 |
Values are means ± SE; n, no. of rats. Body temperature (°C) was recorded orally (postnatal days 4 and 7) and rectally (postnatal days 12 and 21). Shown are respiratory frequency (fR), tidal volume (Vt), minute ventilation (V̇e), oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and oxygen and carbon dioxide convection ratios (V̇e/V̇o2 and V̇e/V̇co2) after single intraperitoneal injection of vehicle (saline) or suramin (40 mg/kg) in 4-, 7-, 12-, and 21-day-old rats.
P < 0.05 vs. vehicle.
Suramin reduced both V̇o2 (treatment effect, P = 0.03) and V̇co2 (treatment effect, P = 0.01) in P4, P7, P12, and P21 rats (Table 1), with no significant interaction between age and treatment (age × treatment, P = 0.5 and P = 0.8 for V̇o2 and V̇co2, respectively). Neither V̇e/V̇o2 (treatment effect, P = 0.9) nor V̇e/V̇co2 (treatment effect, P = 0.7) or body temperature (treatment effect, P = 0.5) was reduced by suramin compared with vehicle (Table 1), which likely indicated that ventilation was reduced to accommodate the lower metabolic requirements (i.e., suramin did not induce specific hypoventilation in normoxia).
A-317491.
Considering the results obtained with suramin, we studied the effects of the specific P2X3 antagonist A-317491 in P4, P7, and P21 rats. Representative respiratory recordings at each age are shown in Fig. 1C, and mean values for respiratory and metabolic variables are presented in Table 2. At each age studied, A-317491 significantly decreased fR (treatment effect, P = 0.0003), Vt (treatment effect, P = 0.001), and V̇e (treatment effect, P = 0.0001) compared with vehicle (Table 2). A-317491 also decreased V̇o2 (treatment effect, P = 0.004) and V̇co2 (treatment effect, P = 0.009), but V̇e/V̇o2, V̇e/V̇co2, and body temperature remained unchanged (Table 2). There was no significant interaction between treatment and age for fR (age × treatment, P = 0.3), Vt (age × treatment, P = 0.09), or V̇e (age × treatment, P = 0.2).
Table 2.
A-317491 effect on baseline ventilatory and metabolic parameters in developing rats
| Age, days | Drug | n | Weight, g | Temperature, °C | fR, breaths/min | Vt, ml/100 g | V̇e, ml · min−1 · 100 g−1 | V̇o2, ml · min−1 · 100 g−1 | V̇co2, ml · min−1 · 100 g−1 | V̇e/V̇o2 | V̇e/V̇co2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Vehicle | 10 | 10.9 ± 1.1 | 33.7 ± 0.1 | 162 ± 3 | 1.23 ± 0.1 | 202 ± 13 | 5.2 ± 0.4 | 3.6 ± 0.2 | 44 ± 4 | 57 ± 5 |
| A-317491 | 5 | 9.2 ± 0.5 | 33.8 ± 0.4 | 132 ± 3* | 0.70 ± 0.1* | 95 ± 20* | 2.2 ± 0.3* | 1.5 ± 0.2* | 41 ± 5 | 61 ± 6 | |
| 7 | Vehicle | 6 | 17.5 ± 1.3 | 35.7 ± 0.3 | 164 ± 3 | 0.86 ± 0.1 | 149 ± 15 | 4.5 ± 0.3 | 3.2 ± 0.2 | 39 ± 6 | 49 ± 6 |
| A-317491 | 6 | 16.8 ± 1.2 | 34.7 ± 0.2 | 134 ± 7* | 0.46 ± 0.1* | 60 ± 8* | 1.7 ± 0.2* | 1.4 ± 0.1* | 37 ± 5 | 42 ± 5 | |
| 21 | Vehicle | 10 | 50.5 ± 1.7 | 36.7 ± 0.7 | 143 ± 2 | 0.82 ± 0.1 | 129 ± 5 | 3.7 ± 0.2 | 2.3 ± 0.1 | 34 ± 1 | 43 ± 10 |
| A-317491 | 6 | 47.4 ± 2.8 | 36.7 ± 0.1 | 105 ± 3* | 0.66 ± 0.1* | 69 ± 5* | 2.1 ± 0.2* | 1.4 ± 0.2* | 35 ± 5 | 51 ± 7 |
Values are means ± SE; n, no. of rats. Body temperature (°C) was recorded orally (postnatal days 4 and 7) and rectally (postnatal day 21). Shown are fR, Vt, V̇e, V̇o2, V̇co2, V̇e/V̇o2, and V̇e/V̇co2 after single intraperitoneal injection of vehicle (saline) or A-317491 (150 mg/kg) in 4-, 7-, and 21-day-old rats.
P < 0.05 vs. vehicle.
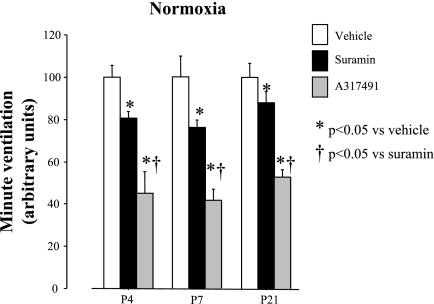
There was a clear treatment effect of the P2X antagonists in normoxia (P = 0.0001), but no interaction between age and treatment (P = 0.9) (Fig. 2). Furthermore, the effects of A-317491 on normoxic V̇e were greater than those of suramin on P4, P7, and P21 rats. This difference might be related to the difference in the dose used (3.75-fold more A-317491 than suramin) or to different binding capacities of these antagonists.
Fig. 2.
Comparative effects of suramin and A-317491 on minute ventilation (V̇e) in developing rats under normoxia. The mean V̇e in vehicle-treated rats was arbitrarily assigned a value of 100. V̇e of each rat in vehicle (open squares), suramin (dark shaded squares), or A-317491 (light shaded squares) groups was calculated using the following formula: (x = individual normoxia/mean value of vehicle group × 100). This calculation was done for each age group studied. The dose of suramin was 40 mg/kg [intraperitoneal (IP)], and the dose of A-317491 was 150 mg/kg (IP). Values are expressed as means ± SE; n values for vehicle, suramin, and A-317491, respectively, are as follows: 10, 10, and 5 (P4); 6, 12, and 6 (P7); and 10, 11, and 6 (P21). *P < 0.05 vs. vehicle (saline). †P < 0.05 vs. suramin.
Hypoxic response.
SURAMIN.
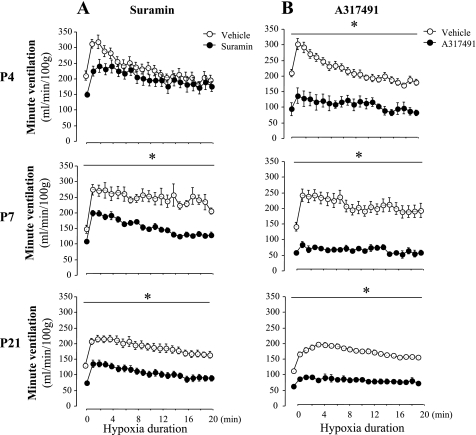
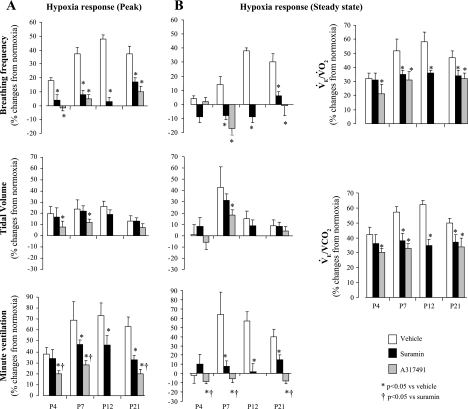
Minute-by-minute ventilation data during hypoxic exposure is presented in Fig. 3A, which shows the clear effects of suramin on the hypoxic response for V̇e in P7 and P21 rats. Percent changes from normoxia are presented in Fig. 4, A and B, which show that suramin significantly reduced the peak (treatment effect, P = 0.01) and steady-state responses (treatment effect, P = 0.001) for fR but not Vt (treatment effect, P = 0.8 and P = 0.6 for peak and steady state, respectively) in P4, P12, P7, and P21 rats. Although the peak V̇e was slightly reduced in P4 rats, a significant effect was only obtained in P7–P21 rats (Fig. 4A). A similar result was observed during the steady-state response to hypoxia (Fig. 4B). There was no significant interaction between age and treatment for the effects of suramin on both the peak and steady-state values for fR (age × treatment, P = 0.3 and P = 0.09 for peak and steady state, respectively) or for V̇e (age × treatment, P = 0.9 and P = 0.5, respectively). Suramin induced a significant decrease in V̇e/V̇o2 and V̇e/V̇co2 in P7–P21 rats compared with vehicle (Fig. 4B), which indicated profound hypoventilation relative to metabolic needs in hypoxia, although suramin induced no significant interaction between age and treatment for V̇e/V̇o2 and V̇e/V̇co2 (P = 0.08).
Fig. 3.
Effects of suramin (A) or A-317491 (B) on minute-by-minute changes of ventilatory response to hypoxia in P4, P7, and P21 rats. The suramin dose was 40 mg/kg (IP), and the A-317491 dose was 150 mg/kg (IP). Figure 1 presents representative tracings from this experimental group. A: n values are as follows for vehicle and suramin, respectively: 10 and 10 (P4), 10 and 12 (P7), and 14 and 11 (P21). B: n values are as follows for vehicle and A-317491, respectively: 10 and 5 (P4), 6 and 6 (P7), and 10 and 6 (P21). The bar with the asterisk indicates significant difference between vehicle (○) and suramin (●; A) or A-317491 (●; B) at each point.
Fig. 4.
Percent changes from normoxia of respiratory frequency, tidal volume, and V̇e responses to hypoxia measured at peak (A) and at steady state (B) after a single injection of vehicle (open bars) or suramin (40 mg/kg; dark shaded bars) in P4, P7, P12, and P21 rats, or after administration of A-317491 (150 mg/kg; light shaded bars) in P4, P7, and P21 rats. B: percent changes from normoxia of ventilatory equivalent for oxygen and carbon dioxide responses to hypoxia were only determined at steady state. V̇o2, oxygen consumption; V̇co2, CO2 production. Values are means ± SE. n values for vehicle, suramin, and A-317491, respectively, are as follows: 10, 10, and 6 (P4); 6, 12, and 6 (P7); 11 (vehicle) and 10 (suramin) (P12); 10, 11, and 6 (P21). *P < 0.05 vs. vehicle (saline).
A-317491.
V̇e during hypoxic exposure was decreased by A-317491 at all ages studied (Fig. 3B) and decreased both the peak and steady-state responses relative to vehicle (Fig. 4, A and B, respectively) (treatment effect, P = 0.02 and P = 0.003, respectively). Like suramin, there was no significant interaction between age and treatment for the effects on the peak or the steady-state responses of V̇e (age × treatment, P = 0.7 and P = 0.3, respectively). As reported for normoxia, A-317491 had a stronger inhibitory effect than suramin at P4, P7, and P21 (Fig. 4). The inhibitory effects of A-317491 in hypoxia resulted in a hypoventilation, as shown by the reduced V̇e/V̇o2 and V̇e/V̇co2 in P4, P7, and P21 rats (Fig. 4B).
Carotid Body Response to Purinergic Receptor Antagonists in Developing Rats
Chemoreceptor activity in response to suramin.
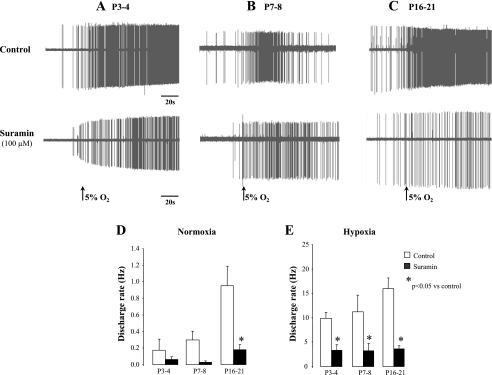
For these experiments, we used 24 carotid body-carotid sinus nerve-petrosal ganglion preparations from P3–P4 (n = 6), P7–P8 (n = 5), and P16–P21 (n = 13) rats. Representative raw activities without (control) and with suramin (100 μM) under normoxic and hypoxic conditions are shown in Fig. 5, A–C. Compared with control, suramin decreased the chemoreceptor activity for each age group measured under conditions of normoxic isocapnic or hypoxic isocapnic superfusion. Under normoxia, the decreased chemoreceptor activity was significant only in P16–P21 (treatment effect, P = 0.03, Fig. 5D) rats, but in hypoxia the chemoreceptor response was significantly decreased at all ages (treatment effect, P = 0.0001, Fig. 5E). In line with the ventilatory data, the effects of suramin on chemoreceptor activity was not age dependent (ANOVA, age × treatment, P = 0.5 and P = 0.6 for normoxia and hypoxia, respectively, see Fig. 8, A and B, respectively). These inhibitory effects of suramin were in line with a previous study using a similar dose that showed a decrease in the frequency of chemoreceptor activity in P19–P21 rats without a significant effect on nerve conduction velocity or AP amplitude (12).
Fig. 5.
Effects of suramin on chemoreceptor activities in developing rats. A, B, and C: typical recordings of single-unit chemosensory activities of petrosal ganglion neurons in response to normoxic-isocapnic and hypoxic-isocapnic superfusion without (control) and with suramin (100 μM) in developing rats at P3–P4 (A), P7–P8 (B), and P16–P21 (C). Suramin depressed the chemosensory discharge rate in normoxic (D) and hypoxic (E) superfusion solutions. Please note the difference in the scale between D and E. Values are means ± SE; n = 6 (P3–P4), 5 (P7–P8), and 13 (P16–P21). *P < 0.05 vs. control.
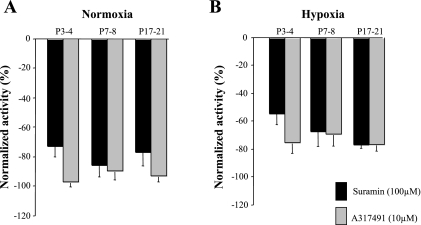
Fig. 8.
Normalized chemosensory discharge activity in developing rats. For each age group studied, discharge rates during superfusion with suramin (dark shaded bars) or A-317491 (light shaded bars) were normalized to control (superfusion without drugs) for normoxic (A) and hypoxic (B) conditions.
Chemoreceptor activity in response to A-317491.
For these experiments, we used 19 carotid body-carotid sinus nerve-petrosal ganglion preparations from P3–P4 (n = 5), P7–P8 (n = 5), and P16–P21 (n = 9) rats.
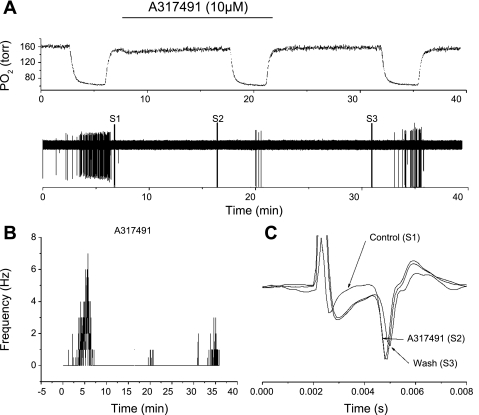
Because no data are currently available in the literature describing the use of A-317491 on peripheral chemoreceptors, we initially tested its specificity on chemoreceptor activity. Figure 6 shows an example of the effect of A-317491 on tissue prepared from P3–P4 rats. The orthodromically evoked APs were recorded after an electrical stimulus at S1, S2, and S3 (Fig. 6A) to evaluate the nerve conduction time and AP amplitude without and with A-317491 (Fig. 6C). Neither the nerve conduction time (time lapsed from the stimulus artifact to the arrival of an afferent AP at the soma of the petrosal neuron) nor the AP amplitude (the amplitude difference between baseline and the AP peak) was affected by A-317491 (Fig. 6C), suggesting that the drug had no anesthetic effect.
Fig. 6.
A: polygraphic recording of experimental run. Top trace: oxygen tension in the recording chamber during the run. Horizontal bar indicates superfusion period with A-317491. Bottom trace: raw recording of action potential activity from the petrosal chemoreceptor neuron with time on abscissa. Stimulations, S1, S2, and S3, indicate the periods in which the electrical stimulation of the carotid body was induced to initiate orthodromic action potentials. B: action potential frequency plotted against time during an experimental run, with A-317491 perfusion time indicated by horizontal bar. C: overlaid traces during orthodromic stimulation for S1, S2, and S3. A-317491 had no effect on action potential amplitude and did not change the conduction time, suggesting no direct anesthetic action on afferent nerve function.
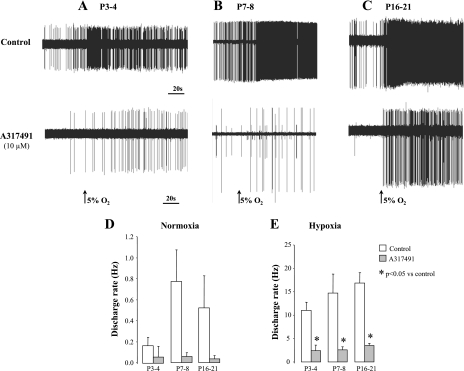
Raw AP activities without (control) and with A-317491 (10 μM) under normoxic and hypoxic conditions are shown in Fig. 7, A–C. As reported for suramin, the discharge rate was inhibited by A-317491 under conditions of normoxic isocapnic or hypoxic isocapnic superfusion (Fig. 7, A–C). However, the decrease in discharge rate under normoxic superfusion was not significant due to the high variability in chemoreceptor activity relative to the low discharge frequency. During hypoxia, the discharge rate was significantly lower than control at each age studied (treatment effect, P = 0.001, Fig. 7E). In addition, there was no interaction between age and treatment (ANOVA age × treatment, P = 0.5 and 0.6, for normoxia and hypoxia, respectively; Fig. 8, A and B, respectively).
Fig. 7.
Effects of A-317491 on chemoreceptors activity in developing rats. A, B, and C: typical recordings of single-unit chemosensory activities of petrosal ganglion neurons in response to normoxic-isocapnic and hypoxic-isocapnic superfusion without (control) and with A-317491 (10 μM) in developing rats at P3–P4 (A), P7–P8 (B), and P16–P21 (C). A-317491 depressed the chemosensory discharge rate in normoxic (D) and hypoxic (E) superfusion solutions. Please note the difference in the scale between D and E. Values are means ± SE; n = 5 (P3–P4), 5 (P7–P8), and 9 (P16–P21). *P < 0.05 vs. control.
Finally, unlike the ventilatory responses (Figs. 2 and 4), there was no significant difference between suramin and A-317491 with respect to their effects on chemosensory activity in normoxia (treatment effect, P = 0.06, Fig. 8A) and hypoxia (treatment effect, P = 0.1, Fig. 8B), although the dose of suramin used was 10-fold higher than that of A-317491.
DISCUSSION
The primary result from the present study is that antagonism of P2X- (suramin) and P2X3-containing receptors (A-317491) exerts a significant effect on normoxia and hypoxia-stimulated ventilation across the range of ages studied. These actions are mediated, in part, by peripheral chemoreceptors, which are inhibited by both suramin and A-317491. This result is broadly consistent with previous studies that demonstrated a critical role of P2X receptors in sensory transduction at the carotid body in mature animals (38). Furthermore, the effects of antagonists were relatively constant across the range of ages. Thus, despite significant changes in respiratory control in early life, the contribution of P2X receptors to the overall ventilatory level remains relatively constant.
Technical Limitations
There are some technical considerations inherent in the present experiments. First, accurate measurement of Vt using whole body plethysmography on very small animals is problematic (30). However, as previously discussed (23, 31), the use of a vehicle group for each age category studied was appropriate because all experiments were performed under similar conditions. Furthermore, rat pups were asleep for 70–80% of the time (4, 8), so we cannot deny the possibility that our recordings were made while the rats were asleep. Interestingly, the results of our in vitro experiments were in line with the plethysmography ventilation recordings. Second, the drugs tested here may have crossed the blood-brain barrier, so the ventilatory effects of the drugs may involve both peripheral and central blockade. Findings on whether suramin may cross (35) or not cross (39) the blood-brain barrier have been inconsistent. Currently available data indicate that A-317491 does not cross the blood-brain barrier (45). In addition, the efficiency of the blood-brain barrier changes developmentally (27), so central effects of the drugs may have been present at some ages and not others. This issue could be addressed using chemodenervated rat pups, but this surgery is exceptionally challenging in rats of young ages and is associated with a high mortality rate (40). Interestingly, P4 and P7 rats that received suramin or A-317491 showed respiratory patterns under normoxic condition that were similar to chemodenervated pups (40). The results of treatment with A-317491 or suramin being consistent between the in vivo and in vitro experiments suggest that the ventilatory results were mostly related to peripheral rather than central activation of purinergic receptors.
Finally, with respect to single-unit chemoreceptor recording, the number of sampled chemoreceptor neurons was relatively small and may not reflect the level of carotid chemoreceptor activity for the entire population. However, this limitation is likely to be minor because there was good agreement in the present experiments between the chemoreceptor recordings and the observed ventilatory changes produced by hypoxia or drug treatment.
Implication of ATP and Purinergic Receptors on Respiratory Control
A role for ATP acting through purinergic receptors is well established within the mature carotid body. The chemoreceptor afferent fibers within the carotid body show immunoreactivity to P2X2 and P2X3 receptors (34). In addition, the messenger RNA for P2X receptors is present in high concentrations in the carotid body and petrosal ganglia, which contain the soma for chemoreceptor afferent neurons (3). ATP is rapidly released from the carotid body during stimulation with hypoxia (10, 16), and antagonism of P2X receptors with suramin or pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulfonate reduces chemoafferent activity (21, 34, 43) and postsynaptic activity in petrosal neurons co-cultured with carotid body glomus cells (48). Similarly, knockout mice lacking the P2X2 receptors showed a clear decrease in the carotid sinus nerve chemoreceptor activity and in the ventilatory response to acute hypoxia. These responses are further reduced when both P2X2 and P2X3 subunits are deleted in the P2X2/3 double-knockout mice (18, 38). In petrosal neurons, αβ-methylene-ATP (a specific agonist of P2X3 receptors) produces fast and long-lasting inward depolarizing currents, demonstrating the functional presence of P2X3 receptors in these neurons (48).
All of these observations suggest an important role of activation of P2X3-containing receptors in specific neurons to affect the ventilatory and chemoreceptor activity of adult animals. In addition to mediating an excitatory role at the carotid body, ATP is rapidly released in the brain stem and plays an important role in mediating central processing of respiratory input (18, 19, 41). Purinergic receptors for ATP, present as homomeric and heteromeric forms on cellular membranes (32), have been identified in the nucleus tractus solitarius (19, 24), which receives the central projections of the carotid sinus nerve.
Purinergic Receptors Mediate Potent Tonic Drive Under Normoxia in Rat Pups
In rat pups aged P4–P21, suramin and A-317491 inhibited ventilation during normoxia, as previously reported in adult rats (19, 42). The V̇e/V̇o2 and V̇e/V̇co2 were not altered by P2X blockade, indicating that ventilation is reduced in proportion to the reduced V̇o2 and V̇co2. Thus blood gases were likely not affected by the drugs. It seems likely that the reduced carotid body activity (as observed in vitro) contributed to this reduction of ventilation, but this assertion requires confirmation. The mechanism by which suramin reduces metabolic rate is not known, but suramin has been shown to decrease the activity of glycolytic enzymes in Trypanosoma Brucei (the parasite that causes African sleeping sickness) (14) and reduces respiration in isolated liver mitochondria (6). Whether these effects are also mediated by A-317491 is currently unknown.
Across all of the ages studied, the reduction in normoxic chemoreceptor activity was ∼80% (Fig. 8A), a level consistent with that observed in mice lacking the heteromeric forms of P2X2/3 receptors. In mice lacking homomeric P2X2 receptors, the decrease in resting activity was reported to be ∼40% (18). Hence, data obtained here, together with those in the literature, are in line with the tonic role of ATP and the implication that P2X3-containing receptors regulate ventilation at rest in both newborn and adult rats. The magnitude of the inhibition in V̇e and the reduction of chemoreceptor activity by purinergic antagonists were relatively constant over the age range studied (Figs. 2 and 8A), suggesting that the contribution of ATP receptors to normoxic chemoreceptor activity is constant over development.
Purinergic Receptors Are Involved in Hypoxic Ventilatory Responses in Rat Pups
Both suramin and A-317491 produced a significant reduction in the ventilatory response to acute hypoxia, and this effect was most pronounced between P7 and P21. A-317491 produced a significant reduction in ventilation at P4, while suramin produced no statistically significant effect on ventilation in P4 rats. The larger effects in older rats were likely due to the enhanced peripheral chemoreceptor activity following maturation, which largely takes place over the first week of life and continues through the second and third weeks (25). The ventilatory changes were consistent with the large decrease in chemoreceptor activity during hypoxia following treatment with suramin and A-317491 (Figs. 5E and 7E). As in normoxia, the relative decrease in chemoreceptor activity following treatment with P2X blockers was relatively constant across ages, suggesting that the role of P2X stimulation is constant across early development. However, although the relative role is constant, the absolute role played by P2X receptors might actually increase with development, as shown by the change in absolute AP discharge rates following suramin or A-317491, which were greater at P16–P21 than P3–P4 (Figs. 5E and 7E). This finding suggests that ATP receptors contribute to the higher spiking activity of the mature organ.
Perspectives
This study clearly demonstrates that P2X receptors are involved in the hypoxic ventilatory response in developing rats and highlights the important role of P2X3-containing receptors. The lack of difference between the effect of suramin and A-317491 on chemosensory neuronal activities, together with the lack of age-dependent effects of either drug, are in line with previous data showing no changes in levels of P2X2 or P2X3 receptors in the carotid body and petrosal ganglion during the first 2 wk of life in cats (3). Because ventilation and the chemosensory response to hypoxia were not completely abolished by suramin or A-317491, other excitatory pathways, such as metabotropic P2Y receptors (29) or other transmitters (2), are necessarily involved. In particular, we have recently demonstrated that cholinergic receptors might be critically involved in postnatal maturation of the hypoxic ventilatory response (31). Furthermore, interactions between different receptors might also play a role in the process of carotid body maturation, because receptor-receptor interaction has been recently suggested to play a role at rest and in response to hypoxia (46). Finally, how ATP and its receptors interact with the other modulatory factors, such as a persistent Na+ current in chemoafferent fibers (15), needs to be better understood.
GRANTS
This study was supported in part by Téléthon des Étoiles to A. Bairam and National Heart, Lung, and Blood Institute Grant HL084520 to D. F. Donnelly. L. M. Niane holds a PhD fellowship from the Respiratory Health Network of Fonds de la Recherché en Santé Québec.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Merlin M. Njoya for advice on statistical analyses (MSc in biostatistics, Centre de recherche du Centre Hospitalier Universitaire de Québec), Van Diep Doan for technical assistance, and Mélanie Pelletier and Sylvie Viger for animal care.
REFERENCES
- 1. [Anon] Glossary on respiration and gas exchange. J Appl Physiol 34: 549–558, 1973 [DOI] [PubMed] [Google Scholar]
- 2. Bairam A, Carroll JL. Neurotransmitters in carotid body development. Respir Physiol Neurobiol 149: 217–232, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bairam A, Joseph V, Lajeunesse Y, Kinkead R. Developmental profile of cholinergic and purinergic traits and receptors in peripheral chemoreflex pathway in cats. Neuroscience 146: 1841–1853, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Balbir A, Lande B, Fitzgerald RS, Polotsky V, Mitzner W, Shirahata M. Behavioral and respiratory characteristics during sleep in neonatal DBA/2J and A/J mice. Brain Res 1241: 84–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol 10: 384–395, 1970 [DOI] [PubMed] [Google Scholar]
- 6. Bernardes CF, Fagian MM, Meyer-Fernandes JR, Castilho RF, Vercesi AE. Suramin inhibits respiration and induces membrane permeability transition in isolated rat liver mitochondria. Toxicology 169: 17–23, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278: R1391–R1400, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A 102: 14860–14864, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci 19: 2131–2142, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun 322: 82–87, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Donnelly DF. Development of carotid body/petrosal ganglion response to hypoxia. Respir Physiol Neurobiol 149: 191–199, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Donnelly DF. Nicotinic acetylcholine receptors do not mediate excitatory transmission in young rat carotid body. J Appl Physiol 107: 1806–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J Appl Physiol 88: 1489–1495, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Fairlamb AH, Bowman IB. Uptake of the trypanocidal drug suramin by bloodstream forms of Trypanosoma brucei and its effect on respiration and growth rate in vivo. Mol Biochem Parasitol 1: 315–333, 1980 [DOI] [PubMed] [Google Scholar]
- 15. Faustino EV, Donnelly DF. An important functional role of persistent Na+ current in carotid body hypoxia transduction. J Appl Physiol 101: 1076–1084, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald RS, Shirahata M, Chang I, Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res 1270: 39–44, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol 568: 715–724, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signaling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J Physiol 552: 197–211, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci 32: 241–248, 2009 [DOI] [PubMed] [Google Scholar]
- 21. He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol 100: 157–162, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A 99: 17179–17184, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am J Physiol Regul Integr Comp Physiol 294: R1356–R1366, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407: 11–32, 1999 [PubMed] [Google Scholar]
- 25. Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol 453: 461–473, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim I, Boyle KM, Carroll JL. Postnatal development of E-4031-sensitive potassium current in rat carotid chemoreceptor cells. J Appl Physiol 98: 1469–1477, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Lajtha A. The development of the bloodbrain barrier. J Neurochem 1: 216–227, 1957 [DOI] [PubMed] [Google Scholar]
- 28. Leff P, Wood BE, O'Connor SE. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br J Pharmacol 101: 645–649, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Botzinger complex inspiratory rhythm generating network in vitro. J Neurosci 27: 993–1005, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Niane L, Joseph V, Bairam A. Role of cholinergic-nicotinic receptors on hypoxic chemoreflex during postnatal development in rats. Respir Physiol Neurobiol 169: 323–332, 2009 [DOI] [PubMed] [Google Scholar]
- 32. North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol 537: 667–677, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rees S, Constantopoulos G, Brady RO. The suramin-treated rat as a model of mucopolysaccharidosis. Variation in the reversibility of biochemical and morphological changes among different organs. Virchows Arch B Cell Pathol Incl Mol Pathol 52: 259–272, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Reyes EP, Fernandez R, Larrain C, Zapata P. Carotid body chemosensory activity and ventilatory chemoreflexes in cats persist after combined cholinergic-purinergic block. Respir Physiol Neurobiol 156: 23–32, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Reyes EP, Fernandez R, Larrain C, Zapata P. Effects of combined cholinergic-purinergic block upon cat carotid body chemoreceptors in vitro. Respir Physiol Neurobiol 156: 17–22, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23: 11315–11321, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanderson L, Khan A, Thomas S. Distribution of suramin, an antitrypanosomal drug, across the blood-brain and blood-cerebrospinal fluid interfaces in wild-type and P-glycoprotein transporter-deficient mice. Antimicrob Agents Chemother 51: 3136–3146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol 91: 1298–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol 89: 53–59, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J Physiol 523: 441–447, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varas R, Alcayaga J, Iturriaga R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res 988: 154–163, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Wang ZY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol 149: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, Ilyin VI. A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eur J Pharmacol 504: 45–53, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Zapata P. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir Physiol Neurobiol 157: 106–115, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Zhang M, Nurse CA. CO2/pH chemosensory signaling in co-cultures of rat carotid body receptors and petrosal neurons: role of ATP and ACh. J Neurophysiol 92: 3433–3445, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525: 143–158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]