Abstract
Objective
Randomized controlled trials in patient education often have difficulty enrolling vulnerable populations – specifically, older, poorer and less educated individuals. We undertook a randomized controlled trial (RCT) of an educational intervention for arthritis management, which included strategies to remove literacy-related barriers to participation. This paper reports on the multi-stage recruitment process and assesses whether refusal to participate was related to education, age, gender, working status or insurance status.
Methods
The recruitment protocol was designed to eliminate literacy-related barriers to participation. Patients were never asked to read or fill out forms. Interactions were oral, using everyday terms and short, clear sentences. Patients who declined during a screening call were considered Stage 1 Refusers. Patients who initially expressed interest but neither completed a baseline questionnaire nor provided consent were considered Stage 2 Refusers. Patients who consented were considered Enrollees. Age, gender, and insurance status were compared between Stage 1 Refusers and Enrollees. A second analysis compared these variables, plus educational attainment and working status, between Stage 2 Refusers and Enrollees.
Results
Of 408 eligible patients, there were 193 (47.3%) Stage 1 Refusers, 81 (19.9%) Stage 2 Refusers and 134 (32.8%) Enrollees. A higher proportion of Stage 1 Refusers than Enrollees were ≥65 years old (58% vs. 37%, p=.0003). Multivariate analysis, adjusting for gender and insurance status, confirmed the effect of older age on refusal (OR=2.3 (1.4, 3.6)). There were no significant differences between Stage 2 Refusers and Enrollees.
Conclusion
We found no evidence of refusal to participate due to educational attainment, working status, insurance status, or gender. Older patients were more likely to refuse participation at the first stage of recruitment.
Practice Implications
Researchers should continue efforts to increase participation among older patients, particularly when studies are designed to be generalized to an elderly population as is the case with arthritis research. Strategies used in this recruitment protocol designed to remove literacy-related barriers to recruitment may be responsible for the observation that subjects with lower education did not have lower refusal rates. Such strategies deserve further study.
Keywords: Recruitment, Patient Education, Age, Randomized Controlled Trial, Arthritis, Selection Bias, Health Literacy
1. Introduction
Randomized controlled trials are critically important for determining the efficacy of educational interventions in patient populations. Recruitment is one of the greatest challenges of randomized controlled trials [1]. Without an adequate sample size and a representative sample, a trial cannot reach definitive conclusions or be generalized to the larger population [2, 3]. An improved understanding of barriers to recruitment would help investigators enhance participation in trials. However, published studies of clinical trials often do not report data about those who refused to participate or do not report on the success of recruitment in specific subgroups of patients [1, 4, 5]. Differing participation rates are important, as they may create selection bias [24] and lead to overgeneralization of findings. Some studies that do examine recruitment data report that older [6–9] and poorer [10–12] individuals are less likely to participate. While some studies report no association between recruitment rates and education [13, 14], other literature suggests that individuals with a lower level of education are less likely to participate in clinical trials [6, 15–21]. As chronic diseases often disproportionately affect the elderly, the poor and those with lower educational attainment, recruiting these populations for clinical trials in chronic diseases is important but may be particularly difficult [22]. Arthritis is an example of a chronic disease that can disproportionately affect populations that may be less likely to participate in research, such as the elderly and those with lower education [23].
The issue of selection bias is especially relevant to trials of patient education interventions, as these trials may seek to enroll individuals with low educational attainment and limited reading skills. Findings from the 1993 National Adult Literacy Survey (NALS) [25] and the more recent findings from the 2005 National Assessment of Adult Literacy (NAAL) [26] indicate that about half of US adults have limited literacy skills. The majority of adults with less than a high school education, many adults with a high school education, and more than 20% of adults with some college education do not have sufficient literacy skills to effectively use print materials required for everyday tasks [27]. Over 800 published analyses in public health and medical journals indicate an ongoing mismatch between the reading grade level of health materials and the average reading skills of US adults [28]. Resources for arthritis patients are no exception [29–31]. With such a large number of patients experiencing limited health literacy skills, it is important that materials used at every step of the research process be accessible to everyone so that study materials themselves do not pose a barrier to participation.
We are aware of few studies of whether the literacy level of recruitment materials affects recruitment success in trials. A recent study which randomized methods for presenting the consent form found that participants at or below the 8th grade reading level more effectively recalled a simple version of the consent form than either the original form or a video [45]. Existing health literacy studies [32], suggest that recruitment materials written at above an 8th grade reading level could pose a barrier to trial participation [33, 34]. We have completed enrollment into a randomized controlled trial of two patient education interventions in arthritis management. The interventions were designed to be particularly effective in patients with average and low literacy. Therefore, our recruitment procedures included strategies to remove literacy-related barriers to participation.
The objectives of this report are to describe our experience recruiting arthritis patients into a randomized controlled trial of patient education. The recruitment protocol was a multi-step process that included identifying eligible patients, receiving physician consent to contact, contacting and screening patients, administering a series of baseline questionnaires, and setting up the intervention appointment. We sought to identify subgroups of patients less likely to participate at these different stages in the recruitment process.
We also present our unique strategies to remove any literacy-related barriers to recruitment. We report on the methods and results of the recruitment process separately from the parent study because we wanted to draw attention to literacy as a barrier to recruitment and highlight the predictors and reasons for refusal in a multi-stage recruitment effort.
2. Methods
2.1 Description of parent RCT
Participants were recruited for a randomized controlled trial testing two education strategies designed to improved disease management skills and health outcomes for arthritis patients. The Standard Care Group received usual clinical care from their rheumatologists and pamphlets about their condition developed by the Arthritis Foundation. These materials were written between an 11th and 15th grade reading level as measured by the SMOG index of reading difficulty, a well-regarded measure of text reading level [37]. The Individualized Care Group received materials written in Plain Language and one-on-one education from the study educator. Plain Language is a term that refers to a clear, simple, conversational style for both oral and written interactions, usually at or below an 8th grade reading level [38–40]. Our Plain Language arthritis materials were written at an 8th to 9th grade level as assessed by the SMOG and formatted for ease of reading. Participants in the individualized group also had a second meeting with the study educator, and were encouraged to contact her for information or assistance for six months. Study visits took place immediately after the participants scheduled rheumatology appointments and in the same location as the visit. The hospital’s institutional review board approved this study.
2.2 Recruitment Procedures and Definition of Stage 1 and Stage 2 Refusers
Prospective participants over 18 yrs old with rheumatoid arthritis, psoriatic arthritis or inflammatory polyarthritis (ICD-9 codes 714.0, 696.0 or 714.9, respectively) were identified through the database of a rheumatology clinic at an urban teaching hospital.
A recruitment letter was sent to patients whose participation was approved by their rheumatologist. The letter was signed by the principal investigator and the person’s rheumatologist. An “opt-out” card and a return stamped envelope were included with the letter. Research assistants attempted to reach by phone patients who indicated they wanted to be called or who did not return the “opt–out” card. Up to six attempts were made to reach the patient at various times during the day, evening and throughout the week. During the recruitment/screening call the research assistant explained the study, including a verbal summary of the information in the letter. Those who expressed interest in participating were asked five screening questions to determine eligibility. Patients who decided during this phone call that they did not want to participate in the study were identified as Stage 1 Refusers. At the screening call, reasons given for refusal were recorded verbatim if offered by the patient. If the patient simply said “not interested” or ended the call, no further inquiry was made.
Patients were excluded during the screening call if they did not have an inclusion diagnosis, were not comfortable with English, were felt not to be able to give informed consent, were a medical professional, or had a post-graduate or professional degree (Table 1). Because this was an educational intervention designed to help those patients with lower literacy levels, we initially tried to target patients with educational attainment levels at or below high school. However, halfway through recruitment, to increase enrollment rates, this requirement was relaxed to ≤16 years of education.
Table 1.
Reasons for exclusion from trial
| Reasons For Exclusion
(N=271) |
N | % |
|---|---|---|
| Education level | 188 | 69.4 |
| Medical professional | 31 | 11.4 |
| Diagnosis | 13 | 4.8 |
| Language | 12 | 4.4 |
| In another study | 11 | 4.1 |
| Inability to give informed consent | 6 | 2.2 |
| No reason recorded | 10 | 3.7 |
After the initial screening, interested and eligible participants were administered a 45-minute baseline questionnaire. The questions were read over the phone to the patient by a research assistant either immediately after the initial screening or at a later, more convenient time.
The participant signed the consent form at their next rheumatology visit. The research assistant read the consent form aloud while the participant reviewed it. Some eligible participants who expressed interest during the screening phone call decided at a later date not to take part in the study. They either did not complete the baseline questions, did not meet the research assistant in the clinic, or changed their mind during the consent process. These participants are identified as Stage 2 Refusers. Patients who enrolled in the study are called Enrollees.
Our multi-stage recruitment protocol was specifically designed to reduce any literacy- related barriers to participation. Recruitment materials and oral scripts were written at or below an eighth grade reading level. All recruitment interactions, after the mailing of the initial letter, were done orally and did not require the patient to read any materials. A health literacy expert trained the research assistants and the study educator in appropriate modes of communication, which included speaking in short sentences and maintaining the same amicable tone, voice level and conversational pace. The initial contact letter was written at the sixth grade level and the telephone screening script was written at an eighth grade level. Although it was difficult to incorporate Plain Language into some of the baseline questionnaire’s validated forms (SF 36, HAQ, etc), many of which were written at a higher than desired reading level, simpler words were substituted whenever possible. For example, the word “medicine” was used in place of “medication”. To avoid biasing participation, patients were not told that the study focused on health literacy and the term literacy was never used in any materials or recruitment conversations.
2.3 Analysis Plan for Present Study
Participants were categorized as Stage 1 Refusers, Stage 2 Refusers or Enrollees as described above. We chose to analyze Stage 1 and Stage 2 Refusers as separate groups because characteristics of early and late responders to studies have been shown to differ [41, 42].
Oftentimes, information about patients who refuse to participate is unavailable to researchers. In this case, we were able to obtain information from the arthritis clinic’s database for all patients whose doctor gave us permission to contact them. Age, gender and insurance status were obtained from this database for all three groups examined. Because we did not have any other measures of socioeconomic status, insurance status was used as a proxy for income [47]. Insurance status was coded as either having private insurance, such as having an HMO or Medicare, or not having private insurance, paying out of pocket or having Medicaid. Stage 2 Refusers and Enrollees self-reported the number of years of education they had completed and their employment status in five eligibility questions during the screening call. Stage 1 Refusers opted out of the study during this call, so these variables are not available for this group of patients. Reasons participants were excluded from the study fell into six categories. Reasons for refusal given by Stage 1 Refusers fell into eight categories (Table 2).
Table 2.
Reasons for refusal at Stage 1
| Reasons For Refusal
(N=193) |
N | % |
|---|---|---|
| “Not Interested”/No reason given | 70 | 36.3 |
| Already in a study/didn’t want to be in another | 36 | 18.7 |
| Health issues- self | 25 | 13.0 |
| Too busy/No time | 19 | 9.8 |
| Did not like the study or some aspect of it | 16 | 8.3 |
| Transportation issues/Traffic | 14 | 7.3 |
| Moved/Changed doctors | 8 | 4.1 |
| Health issues- family member | 5 | 2.6 |
A univariate analysis compared age, gender, and insurance status between Stage 1 Refusers and Enrollees. The salient variables from these analyses were introduced into a multivariate logistic regression model. A second analysis compared age, gender, insurance status, educational attainment, and employment status between Stage 2 Refusers and Enrollees. All analyses were performed in SAS version 9.
3. Results
3.1 Enrollment
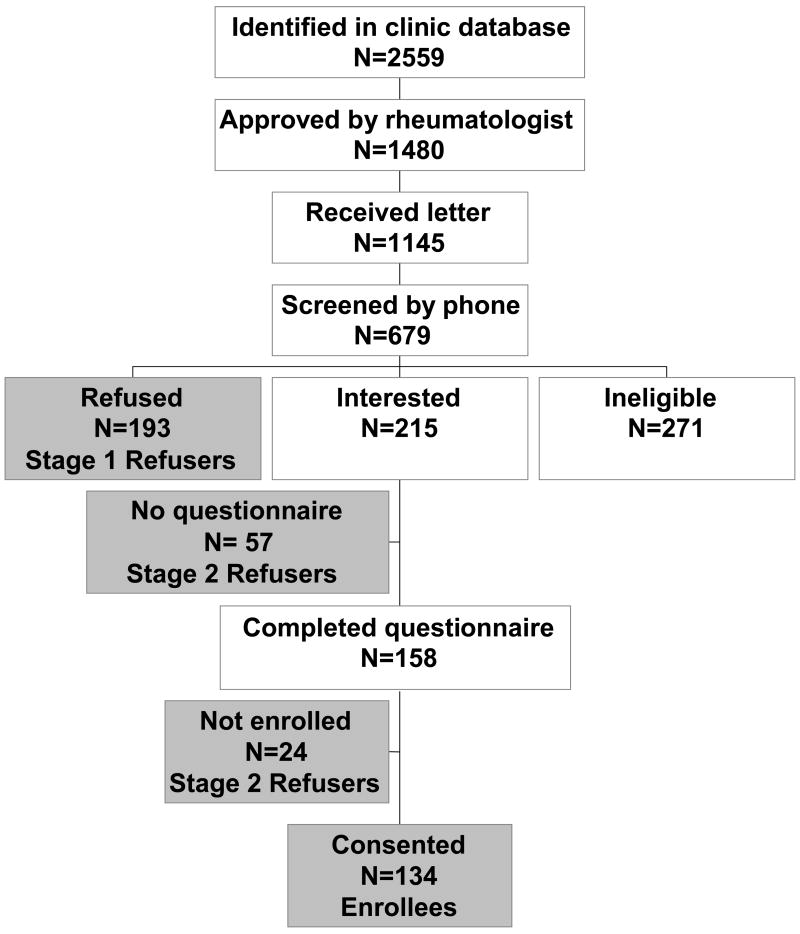
The recruitment period lasted from March 2003 to April 2005. We obtained permission from the rheumatologists to contact 1480 (57.8%) of the 2559 patients identified from the rheumatology clinic’s database (see Figure 1.) Patients with no scheduled appointment during the recruitment period were not sent a recruitment letter (N=335, 22.6%). Recruitment letters were sent to 1145 patients before their next rheumatology appointment. Of these, 679 (59.3%) were reached by phone for screening. One hundred ninety-three (28.4% of those reached by phone) said they were not interested (Stage 1 Refusers), and 271 (39.9%) were ineligible. Two hundred and fifteen patients (31.7%) were interested and eligible after answering the five screening questions. Of these 215, 158 patients (73.5%) went on to complete the baseline questionnaire. Of those who completed the baseline questionnaire, 134 patients (84.8%) were eventually enrolled in the study. The 57 (26.5%) patients who did not complete the baseline questionnaire and the 24 (15.2%) who did not enroll in the study after completing the questionnaire are the Stage 2 Refusers. Our final enrollment of 134 participants represents 11.7% of those who received letters, 19.7% of those who received a screening phone call and 32.8% of those screened as eligible for the trial.
Figure 1.
Overview of patient recruitment
3.1.1 Reasons for exclusion
At the screening call, 271 patients were found to be ineligible (see Table 1). Among the reasons for exclusion were they had attained more than the maximum number of years of formal education (69.4%), did not have one of the requisite diagnoses (4.8%), or were a medical professional (11.4%). Reasons for exclusion were unavailable for ten participants.
3.1.2 Reasons for refusal
Of those who refused at Stage 1 of the recruitment process, 70 (36.3%) said “not interested” or did not give a reason (see Table 2). Reasons given for refusal also included: participation in another study (18.7%), lack of interest in the study or some aspect of it (education, taped visits, time involved after visit) (8.3%), being too busy (9.8%), having health issues themselves or dealing with the health issues of family (15.5%) or having difficulty with transportation or traffic (7.3%).
3.2 Predictors of Refusal
3.2.1 Univariate predictors of refusal at Stage 1
A higher proportion of Stage 1 Refusers than Enrollees were ≥65 years old (58% vs. 37%, p=.0003, OR=2.3 (1.5, 3.6)) (Table 3). No other significant differences were seen between Stage 1 Refusers and Enrollees. A multivariate analysis, adjusting for gender and insurance, confirmed the effect of older age on refusal (OR=2.3 (1.4, 3.6)).
Table 3.
Association between patient characteristics and refusal at Stage 1
| Stage 1 Refusers
(N=193) |
Enrollees
(N=134) |
P- Value | |
|---|---|---|---|
| Age | .0003* | ||
| <65 | 82 (42%) | 84 (63%) | |
| ≥65 | 111 (58%) | 50 (37%) | |
| Gender | .35 | ||
| Male | 49 (25%) | 28 (21%) | |
| Female | 144 (75%) | 106 (79%) | |
| Insurance Status | .86 | ||
| Medicaid or self pay | 19 (10%) | 14 (10%) | |
| Insurance | 174 (90%) | 120 (90%) | |
| Education | NA | ||
| ≤12 | NA | 68 (51%) | |
| >12 | NA | 66 (49%) | |
| Employment Status | NA | ||
| Working | NA | 56 (42%) | |
| Not Working | NA | 78 (58%) |
Statistically significant, α =.005
3.2.2 Univariate predictors of refusal at Stage 2
No significant differences were seen between Stage 2 Refusers and participants on any variable (Table 4).
Table 4.
Association between patient characteristics and refusal at Stage 2
| Stage 2 Refusers
(N=81) |
Enrollees
(N=134) |
P- Value | |
|---|---|---|---|
| Age | .97 | ||
| <65 | 51 (63%) | 84 (63%) | |
| ≥65 | 30 (37%) | 50 (37%) | |
| Gender | .38 | ||
| Male | 13 (16%) | 28 (21%) | |
| Female | 68 (84%) | 106 (79%) | |
| Insurance Status | .06 | ||
| Medicaid or self pay | 16 (20%) | 14 (10%) | |
| Insurance | 65 (80%) | 120 (90%) | |
| Education | .65 | ||
| ≤12 | 38 (47%) | 68 (51%) | |
| >12 | 42 (53%) | 66 (49%) | |
| Employment Status | .18 | ||
| Working | 26 (32%) | 56 (42%) | |
| Not Working | 54 (68%) | 78 (58%) |
4. Discussion and Conclusion
4.1 Discussion
We analyzed data on recruitment of patients into a trial of an educational intervention for patients with arthritis. Our question was whether patients from vulnerable populations (elderly, poor, less educated) were more likely to refuse to participate at various stages of the recruitment process. We found no association between refusal to participate at either stage of recruitment and insurance status or gender. Education level and employment status were not predictive of refusal at the later stage of recruitment. Patients aged 65 or greater were more likely to refuse participation at the initial stage of recruitment, but not later on in recruitment.
Our overall recruitment of 32.8% of eligible participants was typical of other educational RCT recruitment efforts. For example, an educational intervention trial in lupus patients at the same arthritis clinic recruited 37% of eligible patients [43]. It is important to understand the characteristics of the roughly sixty to seventy percent of eligible participants that fail to participate in a trial. However, little is published about the reasons for or predictors of refusal. As Gross and colleagues point out, recruitment processes are often not mentioned in writing about methods and results of a trial, making it difficult for the reader to determine if enrolled participants are representative of a specific subgroup of patients [5]. In addition to the effect of recruitment on the validity and generalizability of study results, issues related to recruitment can have important implications for clinical practice and policy that should not be overlooked [44].
According to Kurt Lewin’s theory of behavioral change, a critical first step in bringing about any change is the removal of barriers [35, 36]. In order to increase participation in research, the priority should be placed on reducing barriers to participation before increasing internal and external facilitating factors, such as offering greater incentives or using more aggressive recruiting strategies.
In recruitment for this RCT, we attempted to reduce one possible barrier to recruitment: the burden of high-literacy recruitment materials. Our strategies included reading aloud all recruitment materials, training recruiters to speak slowly and carefully, and using Plain Language whenever possible in both written and oral communications. One limitation of this report is that the present analysis was not a randomized trial of recruitment strategies; therefore, no causal conclusions are possible. However, the findings suggest that the recruitment approach used did not result in selective under recruitment of poorer or less educated patients. These results are encouraging and suggest that a randomized controlled trial of recruitment protocols is warranted. In the meantime, we suggest researchers consider implementing these strategies to remove potential literacy-related barriers to recruitment.
In many cases these strategies involve almost no additional resources. Using simple language when developing recruiting materials and scripts does not require a lot of additional effort and may increase enrollment of individuals with limited literacy skills. Staff time needed for oral administration of all data collection instruments may prove more financially burdensome in many research settings. However, researchers might consider offering at least the option of oral administration to all potential participants. This may be especially cost-effective for studies seeking to enroll individuals with lower education or limited literacy skills.
However, although cost-effective, these literacy-related recruitment strategies did not seem to have an affect on the recruitment of older participants into the study. The reasons for refusal shed some light on barriers to participation in elderly patients. Transportation issues were cited as a reason for nonparticipation in the trial. These issues could be particularly relevant for older patients who often rely on family members or community rideshare programs to bring them for doctors’ appointments and therefore cannot extend their visit to participate in research. Stage 1 Refusers also cited their poor health or the poor health of a family member in their care as a reason for not wanted to participate in this research. Elderly patients may feel that participation in research adds an extra burden. Further research should identify additional factors that lead to nonparticipation of older patients, especially during the initial stages of refusal, so that these barriers can be reduced.
Patients also cited participation in another research study as a primary reason for refusal. Although research in volunteerism has suggested that participating in one study is a likely predictor of participation in another study, this may not always be the case. In fact, in a teaching hospital such as this one, where multiple trials are going on at once, patients may feel overwhelmed when contacted multiple times and may not want to participate in more than one research program. This is a problem common at academic medical centers, which encourage and fund research programs. At the time of recruitment to this particular study, there were at least three other studies recruiting out of this patient pool. One of which was a large scale effort attempting to recruit all rheumatoid arthritis patients with an appointment during our study’s time frame. Participants in this study were asked to become part of a longitudinal rheumatoid arthritis registry by answering questions and providing a blood sample. Although neither study asked participants to come to the clinic solely for study purposes, both studies asked participants to spend some time after their scheduled appointment to participate. Therefore, it is natural that some patients chose not to do both. Research institutions, such as the hospital where this research was conducted, should continue to study how contacting patients for multiple studies impacts recruitment. Perhaps patients typically say no when contacted a second time or perhaps certain participants, when recruited for more than one type of research, say no to particular types of studies. In this case, it could be that participants valued biologically based research more than an educational intervention. Again, this is an area for future research.
One of the strengths of this study is that we recorded and classified reasons for refusal. This allowed us to examine and discuss the potential barriers to participation that could be alleviated in future research. However, a limitation is that many Stage 1 Refusers, 36%, still failed to offer a reason for refusal. Attempts have been made through focus groups to gain insight into why certain patients are not interested in research 46], but more research is needed to understand these patients, who by their very refusal to participate, make them difficult to study.
4.2 Conclusions
Studying and reporting on recruitment efforts, whether in separate reports such as this one, or as part of any write-up of research, is important to understand internal and external validity. Strategies to reduce barriers to participation should also be implemented whenever possible. Our study found that older individuals were less likely to participate at the earlier stage of recruitment. Thus strategies for increasing recruitment of older subjects are especially needed.
4.3 Practice Implications
These findings emphasize the importance of identifying and lowering barriers to recruitment of subjects into randomized trials, especially the elderly who refused disproportionately to participate in this trial.
Acknowledgments
The authors wish to thank the research assistants that helped in participant recruitment.
Role of funding: The research was supported by grant NIH/NIAMS P60 AR 47782, K24 AR 02123 to the Section of Clinical Sciences, Division of Rheumatology, Immunology and Allergy and Department of Orthopaedic Surgery, Brigham and Women’s Hospital. The funding source was not involved in data collection, analysis or manuscript preparation.
Footnotes
Conflict of Interest: There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Danielle Blanch, Northeastern University, Boston, MA.
Rima Rudd, Harvard School of Public Health, Boston, MA.
Elizabeth Wright, Brigham and Women’s Hospital, Boston, MA.
Victoria Gall, Brigham and Women’s Hospital, Boston, MA; New England Baptist Hospitals, Boston, MA.
Jeffrey N. Katz, Brigham and Women’s Hospital, Boston, MA
References
- 1.Hunninghake DB, Darby CA, Probstfield JL. Recruitment experience in clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1987;8:6S–30S. doi: 10.1016/0197-2456(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 2.Lovato LC, et al. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–52. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 3.Simon SD. Is the randomized clinical trial the gold standard of research? J Androl. 2001;22:938–43. doi: 10.1002/j.1939-4640.2001.tb03433.x. [DOI] [PubMed] [Google Scholar]
- 4.Britton A, et al. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4:112–21. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 5.Gross CP, et al. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med. 2002;137:10–6. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ahsan H, et al. Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 7.Corbie-Smith G, et al. Influence of race, clinical, and other socio-demographic features on trial participation. J Clin Epidemiol. 2003;56:304–9. doi: 10.1016/s0895-4356(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Williams F, et al. Why are patients in clinical trials of heart failure not like those we see in everyday practice? J Clin Epidemiol. 2003;56:1157–62. doi: 10.1016/s0895-4356(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 9.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 10.Gross CP, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–91. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz CE, Fox BH. Who says yes? Identifying selection biases in a psychosocial intervention study of multiple sclerosis. Soc Sci Med. 1995;40:359–70. doi: 10.1016/0277-9536(94)e0092-7. [DOI] [PubMed] [Google Scholar]
- 12.Unson CG, et al. Barriers to eligibility and enrollment among older women in a clinical trial on osteoporosis: effects of ethnicity and SES. J Aging Health. 2004;16:426–43. doi: 10.1177/0898264304264211. [DOI] [PubMed] [Google Scholar]
- 13.Gitanjali B, et al. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference? J Postgrad Med. 2003;49:109–13. [PubMed] [Google Scholar]
- 14.Creel AH, et al. An assessment of willingness to participate in a randomized trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp Clin Trials. 2005;26:169–78. doi: 10.1016/j.cct.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Advani AS, et al. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 16.Ahluwalia JS, et al. African American smokers interested and eligible for a smoking cessation clinical trial: predictors of not returning for randomization. Ann Epidemiol. 2002;12:206–12. doi: 10.1016/s1047-2797(01)00305-2. [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M, et al. Random versus volunteer selection for a community-based study. J Gerontol A Biol Sci Med Sci. 1998;53:M39–46. doi: 10.1093/gerona/53a.1.m39. [DOI] [PubMed] [Google Scholar]
- 18.Lerman C, et al. Recruiting high risk women into a breast cancer health promotion trial. Cancer Epidemiol Biomarkers Prev. 1994;3:271–6. [PubMed] [Google Scholar]
- 19.Lewis CE, et al. Recruitment strategies in the women’s health trial: feasibility study in minority populations. WHT:FSMP Investigators Group. Women’s Health Trial:Feasibility Study in Minority Populations. Control Clin Trials. 1998;19:461–76. doi: 10.1016/s0197-2456(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 20.Onitilo AA, et al. Race, education, and knowledge of bone marrow registry: indicators of willingness to donate bone marrow among African Americans and Caucasians. Transplant Proc. 2004;36:3212–9. doi: 10.1016/j.transproceed.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Rimer BK, et al. Participation in a women’s breast cancer risk counseling trial. Who participates? Who declines? High Risk Breast Cancer Consortium Cancer. 1996;77:2348–55. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2348::AID-CNCR25>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T. Limitations of randomized clinical trials to recognize possible advantages of combination therapies in rheumatic diseases. Semin Arthritis Rheum. 1993;23:2–10. doi: 10.1016/s0049-0172(10)80002-2. [DOI] [PubMed] [Google Scholar]
- 23.de Jong Z, et al. Differences between participants and nonparticipants in an exercise trial for adults with rheumatoid arthritis. Arthritis Rheum. 2004;51:593–600. doi: 10.1002/art.20531. [DOI] [PubMed] [Google Scholar]
- 24.Rupp I, et al. Selection bias due to non-response in a health survey among patients with rheumatoid arthritis. Eur J Public Health. 2002;12:131–5. doi: 10.1093/eurpub/12.2.131. [DOI] [PubMed] [Google Scholar]
- 25.Kirsh IS, et al. Adult literacy in America. US Department of Education; Washington, DC: 1993. [Google Scholar]
- 26.Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Washington, DC: US Department of Education, Institute of Education Sciences, National Center for Education Statistics; National Assessment of Adult Literacy (NAAL) [Google Scholar]
- 27.Sum A, Kirsch I, Taggart R. A Policy Information Center Report. Princeton, NJ: Educational Testing Service; The Twin Challenges of Mediocrity and Inequality: Literacy in the United States from an International Perspective. [Google Scholar]
- 28.Rudd R, Moeykens B, Colton T. In: Health and Literacy: A Review of Medical and Public Health Literature, in Annual Review of Adult Learning and Literacy. Comings J, Garners B, Smith C, editors. New York: Jossey-Bass; 1999. [Google Scholar]
- 29.Gordon MM, et al. Illiteracy in rheumatoid arthritis patients as determined by the Rapid Estimate of Adult Literacy in Medicine (REALM) score. Rheumatology (Oxford) 2002;41:750–4. doi: 10.1093/rheumatology/41.7.750. [DOI] [PubMed] [Google Scholar]
- 30.Larson I, Schumacher HR. Comparison of literacy level of patients in a VA arthritis center with the reading level required by educational materials. Arthritis Care Res. 1992;5:13–6. doi: 10.1002/art.1790050105. [DOI] [PubMed] [Google Scholar]
- 31.Ansani NT, et al. Quality of arthritis information on the Internet. Am J Health Syst Pharm. 2005;62:1184–9. doi: 10.1093/ajhp/62.11.1184. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen-Bohlman L, Panzer A, Kindig D. Health Literacy: A Prescription to End Confusion. Washington, DC: Institute of Medicine of the National Academies; 2004. [PubMed] [Google Scholar]
- 33.Dibartolo MC, McCrone S. Recruitment of rural community-dwelling older adults: barriers, challenges, and strategies. Aging Ment Health. 2003;7:75–82. doi: 10.1080/1360786031000072295. [DOI] [PubMed] [Google Scholar]
- 34.Gorkin L, et al. Clinical trial enrollers vs. nonenrollers: the Cardiac Arrhythmia Suppression Trial (CAST) Recruitment and Enrollment Assessment in Clinical Trials (REACT) project. Control Clin Trials. 1996;17:46–59. doi: 10.1016/0197-2456(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 35.Lewin K. Field Theory and Experiment in Social Psychology: Concepts and Methods. Amer J Sociology. 1939;44:868–896. [Google Scholar]
- 36.Lewin K. In: Field Theory and Learning, in Field Theory in Social Science. Cartwright D, editor. New York: Harper & Row; 1942. pp. 60–86. [Google Scholar]
- 37.McLaughlin G. SMOG grading: A new readability formula. Journal of Reading. 1969;12:639–646. [Google Scholar]
- 38.Rudd R, de Jong W. Plain Language: The need for effective communication in medicine. Boston: Harvard School of Public Health, National Center for the Study of Adult Learning and Literacy; [Google Scholar]
- 39.Kimble J. Plain English: A Charter for Clear Writing. Thomas M Cooley Law Review. 1992;1:11–14. [Google Scholar]
- 40.Kimble J. The Elements of Plain Language. Michigan Bar Journal. 2002 October;:44–45. [Google Scholar]
- 41.Rodes A, et al. Recruitment methods and differences in early, late and non-respondents in the first MONICA-Catalonia population survey. Rev Epidemiol Sante Publique. 1990;38:447–53. [PubMed] [Google Scholar]
- 42.Voigt LF, Koepsell TD, Daling JR. Characteristics of telephone survey respondents according to willingness to participate. Am J Epidemiol. 2003;157:66–73. doi: 10.1093/aje/kwf185. [DOI] [PubMed] [Google Scholar]
- 43.Karlson EW, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:1832–41. doi: 10.1002/art.20279. [DOI] [PubMed] [Google Scholar]
- 44.Froelicher ES, Lorig K. Who cares about recruitment anyway? Patient Educ Couns. 2002;48:97. doi: 10.1016/s0738-3991(02)00168-4. [DOI] [PubMed] [Google Scholar]
- 45.Campbell FA, et al. The effect of format modifications and reading comprehension on recall of informed consent information by low-income parents: a comparison of print, video, and computer-based presentations. Patient Educ Couns. 2004;53:205–16. doi: 10.1016/S0738-3991(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 46.Costenbader KH, et al. Factors determining participation in prevention trials among systemic lupus erythematosus patients: a qualitative study. Arthritis Rheum. 2007;57:49–55. doi: 10.1002/art.22480. [DOI] [PubMed] [Google Scholar]
- 47.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Ann Rev Pub Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]