Abstract
We examined the antiviral activity of ADAR1 against HIV-1. Our results indicated that ADAR1 in a transfection system inhibited production of viral proteins and infectious HIV-1 in various cell lines including 293T, HeLa, Jurkat T and primary CD4+ T cells, and was active against a number of X4 and R5 HIV-1 of different clades. Further analysis showed that ADAR1 inhibited viral protein synthesis without any effect on viral RNA synthesis. Mutational analysis showed that ADAR1 introduced most of the A-to-G mutations in the rev RNA, in the region of RNA encoding for Rev Response Element (RRE) binding domain and in env RNA. These mutations inhibited the binding of rev to the RRE and inhibited transport of primary transcripts like gag, pol and env from nucleus to cytoplasm resulting in inhibition of viral protein synthesis without any effect on viral RNA synthesis. Furthermore, ADAR1 induced mutations in the env gene inhibited viral infectivity.
Keywords: ADAR1, HIV-1, Antiviral, Cellular Protein
Introduction
ADAR1 (Adenosine Deaminase Acting on RNA 1), an RNA editing enzyme, catalyzes hydrolytic deamination of adenosine to inosine (A-to-I) in completely or partially double-stranded RNA (Hough and Bass, 1994; Kim et al., 1994; O'Connell and Keller, 1994; Patterson and Samuel, 1995). This has a number of effects. The A-to-I substitution affects pre-mRNA splicing by changing the splicing site and decreases the binding of RNA to protein resulting in functional changes of the protein (Bass, 2002; Samuel, 2003). Furthermore, during mRNA translation, Inosine is recognized as Guanosine and therefore it incorporates incorrect amino acid sequence (Bass, 2002; Samuel, 2003). Because of these pluripotent properties of ADAR1, it disrupts replication of a number of RNA viruses. ADAR1 inhibits replication of hepatitis delta virus by editing of adenosine to inosine in pre-mRNA of hepatitis delta virus which eventually affects splicing of untranslated regions (Patterson and Samuel, 1995; Polson, Bass, and Casey, 1996; Sato, Wong, and Lazinski, 2001). ADAR1 was also known to edit double-stranded RNA found in the measles virus, which inhibited virus assembly and release from cells, leading to a persistent infection and development of fatal neuropathic measles infection (Horikami and Moyer, 1995). Taylor, et al. (2005) showed that ADAR1-induced viral RNA editing inhibited Hepatitis C viral replication (Taylor et al., 2005). Recently Suspene et al. (Suspene et al.) have shown ADAR1 induced mutation in seasonal influenza and attenuated measles viruses.
Since HIV-1 genome has several putative double stranded secondary RNA structures throughout its genome, HIV-1 RNA was considered a potential target for ADAR1. Therefore, in this report we investigated the antiviral effect of ADAR1 on HIV-1. We provided evidence that ADAR1 inhibited HIV-1 protein synthesis and viral infectivity in a variety of cells and against HIV-1 of different tropisms and different clades. We further demonstrated that such antiviral activity was at the post transcriptional stage of HIV-1 replication and that ADAR1-induced mutation at the rev and env RNA may be responsible for such posttranscriptional inhibition of viral protein synthesis. In elucidating the mechanism of ADAR1 induced inhibition of HIV-1 protein synthesis we found that ADAR1 induced A-to-G mutations in rev inhibited its transport activity of primary transcripts gag, pol and env from the nucleus to cytoplasm and thereby inhibited viral protein synthesis without any effect on viral RNA synthesis. ADAR1induced mutations in the env gene further attenuate viral infectivity.
Results
ADAR1 inhibits HIV-1 protein synthesis and viral infectivity
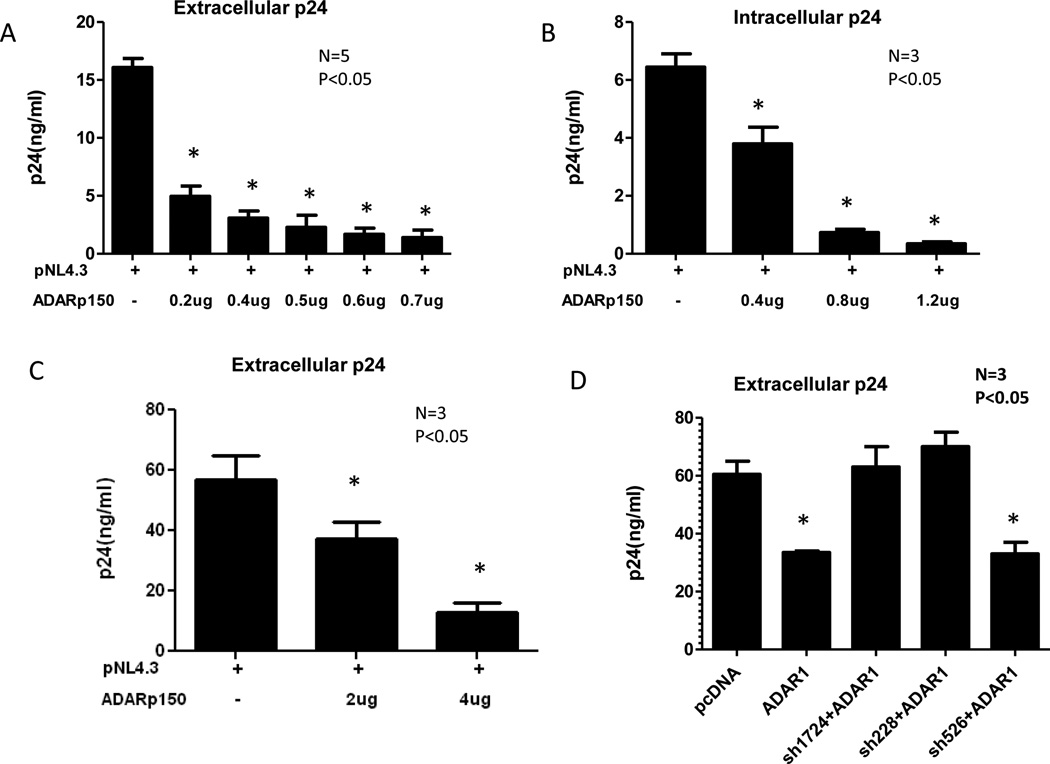
In order to evaluate the effect of ADAR1 on HIV-1 production, 293T cells were co-transfected with 0.1µg pNL4.3 HIV-1 DNA and different amount of ADAR1 DNA. In each transfection assay (this one and subsequent ones), cells were also co-transfected with a luciferase-expressing plasmid DNA to control transfection efficiency. The expression of ADAR1 p150 from transfected ADAR1 DNA was analyzed by Western Blot and normalized against β-actin loading control to show the relative intensity of ADAR1 p150 expression (Supplemental Figure 1A). Following 48 h of transfection, viral protein synthesis was quantified by measuring HIV-1 p24 in culture supernatant and intracellular HIV-1 p24 production in a cell extract by ELISA. ADAR1 inhibited extracellular (Figure 1A) and intracellular (Figure 1B) HIV-1 p24 production in a dose dependent manner. With 0.7µg of ADAR1 containing plasmid there was an 8 fold reduction of extracellular HIV-1 p24 production in culture supernatant as compared to control plasmid pcDNA. The inhibition of viral protein synthesis was not due to cellular toxicity by ADAR1 as evidenced by no change in viable cell numbers in the presence and absence of ADAR1 (MTT assay, data not shown). The antiviral activity of ADAR1 was further evaluated in two other cell lines, Jurkat T and HeLa cells. Inhibition of viral p24 production in culture supernatant (Figure 1C) and intracellular p24 (data not shown) was also observed in Jurkat T cells and in HeLa cells (data not shown).
Figure 1. Effect of ADAR1 on HIV-1 protein synthesis and infectivity.
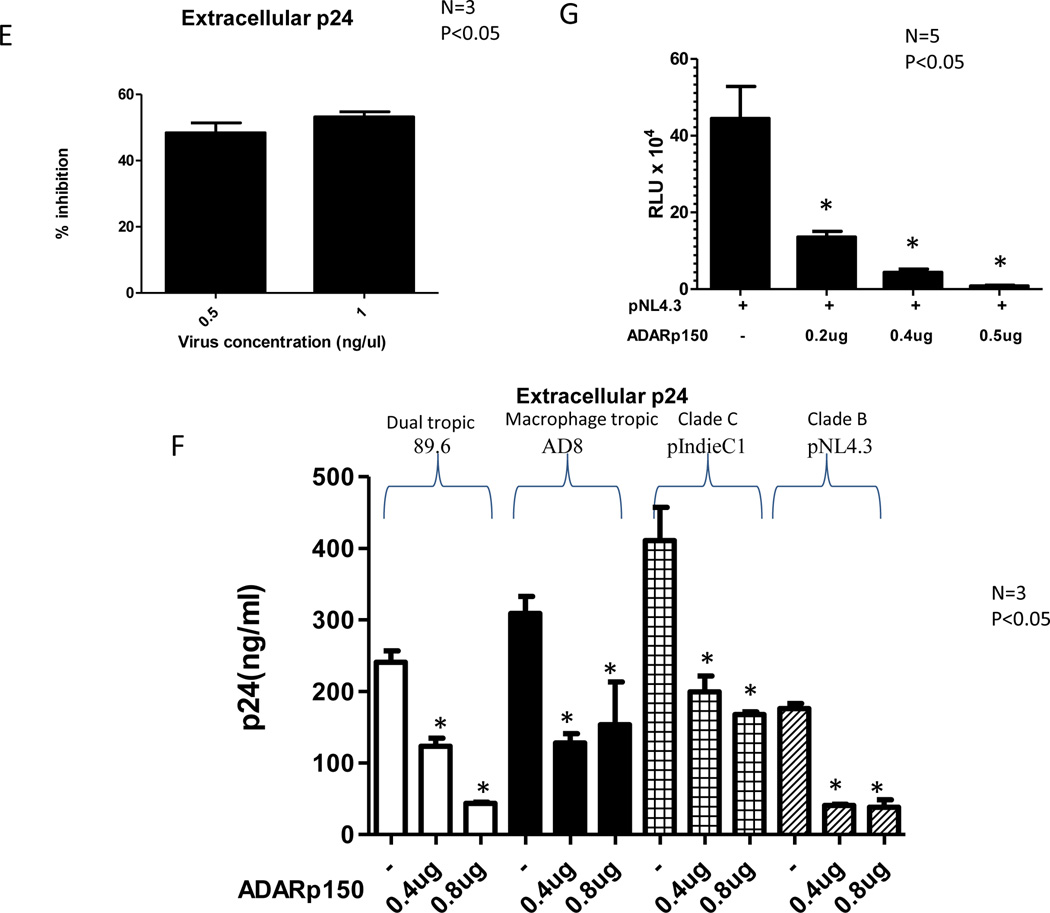
293T cells were co-transfected with 0.1µg pNL4.3 plasmid and different amounts of ADAR1 plasmid and the control Renilla Luciferase plasmid. HIV-1 production and infectivity were evaluated after 48 h. Viral protein synthesis was monitored by measuring HIV p24 in culture supernatant (Panel A) and intracellular HIV p24 production (Panel B). Results are given as means ± standard deviation. Measurement of extracellular HIV-1 p24 production in transfected Jurkat cells in the absence and presence of increasing amounts of ADAR1 in three independent experiments are shown (Panel C). 293T cells were transfected with 0.1µg pNL4.3, 0.5µg ADAR1 in presence and absence of shRNA 228, shRNA 1724 and 526. Viral protein synthesis was monitored by measuring HIV p24 in culture supernatant 48 h post transfection (Panel D). CD8 depleted PBMC were nucleofected with 2µg of ADAR1. After 48 h cells were infected with 0.5ng/µl and 1ng/µl, of pNL4.3 virus for 2 h. HIV-1 production was evaluated after 72 h using p24 ELISA and calculating the percentage of inhibition of p24 production in the presence of ADAR1. 0.0475ng of p24 value was considered as 100% where 0.5ng/ml virus was added and 0.333ng of p24 value was considered as 100% where 1ng/ml virus was added (Panel E). Production of extracellular HIV-1 p24 in 293T cells co-transfected with HIV-1 89.6, AD8, pIndie C or pNL4.3 plasmids and increasing amount of ADAR1 DNA (Panel F). 3ng HIV-1 p24 equivalent viruses obtained from 293T cells transfected with pNL4.3 alone or together with ADAR1 from Panel B were used to infect TZM-bl cell line (Panel G). Productive infection was monitored after 48 h by measurement of luciferase activity. * indicates there is a significant difference (P<0.05) compared to the respective control.
To further demonstrate specificity of ADAR1 mediated inhibition of HIV-1 synthesis, shRNA mediated silencing of ADAR1 was used to block ADAR1 mediated antiviral activity. First we evaluated the effect of shRNA on the expression of ADAR1. 293T cells were co-transfected with ADAR1 in the presence and absence of two shRNA constructs targeting human ADAR1 p150 RNA (sh1724 and sh228). As shown in Supplemental Figure 1B, both of the shRNA constructs (sh1724 and sh228) were able to inhibit expression of ADAR1 p150. As a control a shRNA 526 against an unrelated RNA showed no inhibition of human ADAR1 expression. As shown in Figure 1D, shRNA against ADAR1 (sh1724 and sh228) blocked ADAR1 mediated inhibition of HIV-1 protein synthesis.
Next, we examined the effect of ADAR1 on HIV -1 protein synthesis in primary CD4 enriched T cells in which viral challenge was done by infection rather than by transfection. Since expression of ADAR1 is very low in immune cells like T cells, B cells and macrophages (Yang et al., 2003), we transfected primary CD4 enriched T cells with ADAR1 expressing DNA followed by infection with HIV-1. For this purpose PHA stimulated CD8 depleted PBMC were nucleofected with 2µg of plasmid encoding ADAR1. The cells were infected 48 h post-transfection with 0.5ng/µl, and 1ng/µl of pNL4.3 virus for 2 h. Virus production in culture supernatant was measured using p24 ELISA 72 h post-infection. As shown in Figure 1E, ADAR1 inhibited HIV-1 protein synthesis in CD4 enriched T cells.
Next, the inhibitory effect of ADAR1 was examined in 293T cells against HIV-1 of different tropisms and different clades. As shown in Figure 1F, ADAR1 inhibited production of clade C HIV-1 (pIndie C) in culture supernatant, however the antiviral activity was more pronounced against pNL4.3 (clade B) than pIndie C (clade C). ADAR1 also inhibited production of macrophage tropic HIV-1 AD8 and dual tropic HIV-1 89.6 (Figure 1F).
The infectivity of the virus from the culture supernatant of 293T cells transfected with ADAR1 plasmid or control plasmid (from Figure 1A) was then evaluated for infectivity in TZM-bl cells. A dose-dependent reduction in the viral infectivity in the presence of ADAR1 was observed (Figure 1G), even though equal amount of virus (as measured by p24) were used.
We then examined the effect of ADAR1 expression on infectivity of HIV-1 produced in culture supernatant from 293T cells transfected with HIV-1 having different tropisms and of different clades (used in Figures 1F). Equal amounts of viruses (as measured by p24) obtained from 293T cells transfected with the ADAR1 plasmid or the control plasmid were used to infect TZM-bl cells. As shown in Supplemental Figure 2, viral infectivity was significantly inhibited in the presence of ADAR1, regardless of viral tropisms and clades.
Induction of ADAR1 expression in Jurkat cells following IFNα treatment
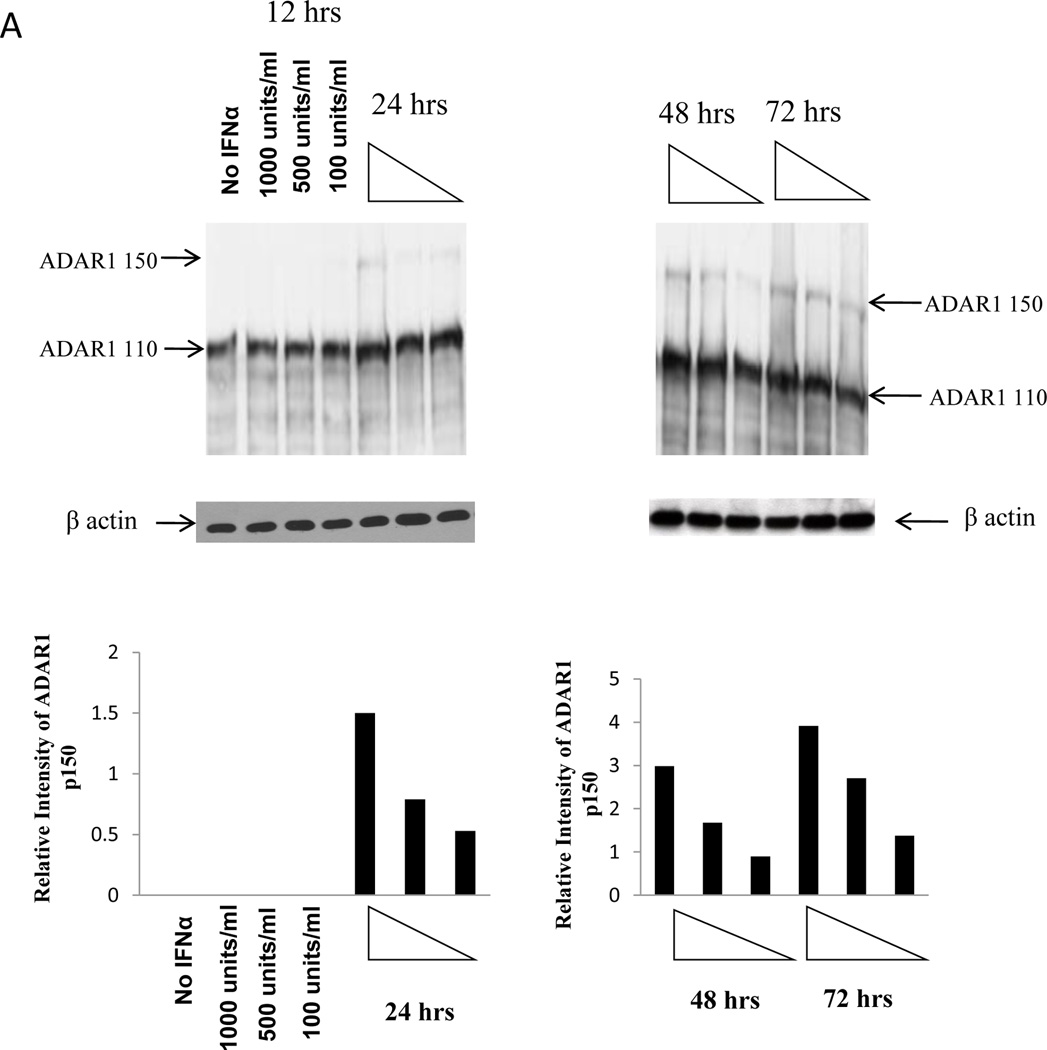
Since ADAR1 is an IFNα-inducible enzyme, the expression of ADAR1 p150 is expected to increase in the presence of IFNα treatment (Patterson et al., 1995). Jurkat cells were stimulated with different concentration of IFNα (100units/ml, 500units/ml and 1000 units/ml) for various lengths of time (12 h, 24 h, 48 h and 72 h). At the indicated time after interferon treatment, cells were lysed and a Western blot was performed using a monoclonal antibody against ADAR1 to evaluate the level of IFNα-induced expression of endogenous ADAR1. Intracellular ADAR1 p150 expression was observed after 24 h of IFNα stimulation with 100, 500 and 1000 units/ml of IFNα and it increased over time (Figure 2A). As ADAR1 p150 is an IFNα-inducing enzyme, the expression of p150 form was increased with increasing amount of IFNα.
Figure 2. Expression of endogenous ADAR1 in Jurkat T cell line and its inhibitory capacity on HIV-1 protein synthesis.
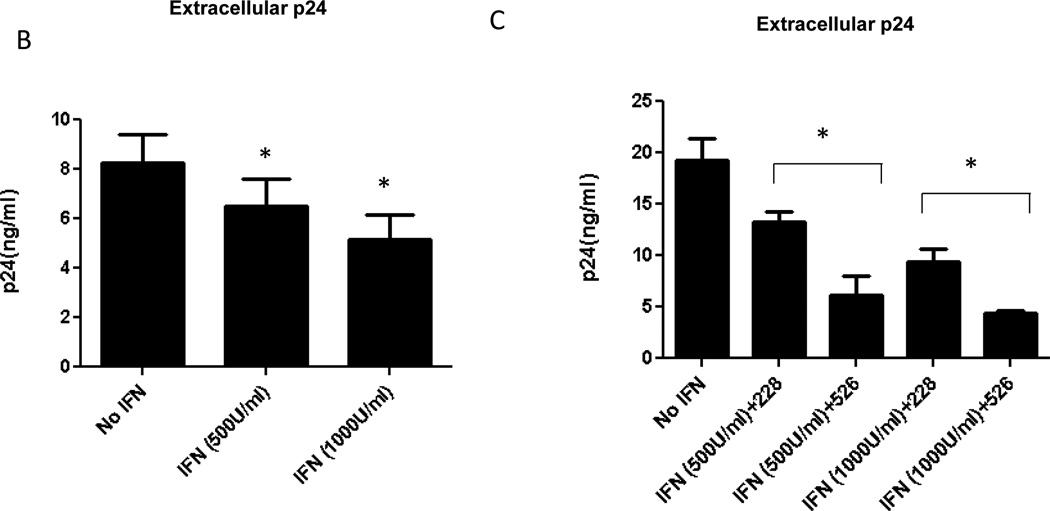
Jurkat T cells were stimulated with 100units/ml, 500units/ml and 1000 units/ml IFNα for different length of time, 12 h, 24 h, 48 h and 72 h. At each time point cells were harvested and expression of ADAR1 was determined by Western blot using ADAR1 antibody and normalized against β-actin loading control. Relative intensity of ADAR1 p150 in the blot was quantitated (Panel A). Jurkat cells were stimulated with IFN α for 72 h and then infected with 500 ng/ml of pNL4.3 virus. After 48 h viral protein synthesis was monitored by measuring the HIV-1 p24 production in the culture supernatant (Panel B). Jurkat cells were stimulated with IFN α for 72 h. After 72 h cells were transfected with 2µg of shRNA 228 against ADAR1 or control scramble shRNA 526 constructs for 48 h. After 48 h cells were infected with 500 ng/ml of virus. 48 h after infection viral protein synthesis was monitored by measuring the p24 production in the culture supernatant (Panel C).
To check whether ADAR1 expression induced by IFNα could inhibit the synthesis of HIV-1 viral protein, cells were induced with 500 units/ml and 1000 units/ml IFNα. After 72 h of interferon treatment cells were infected with 500 ng/ml of pNL4.3 virus. In presence of 500 units/ml and 1000 units/ml of IFNα approximately 25% and 40% reduction in the viral protein synthesis was observed, respectively, as compared to the unstimulated cells (Figure 2B). To verify that the inhibition of HIV-1 synthesis was due to IFNα-induced expression of ADAR1, cells were induced with 500 units/ml and 1000 units/ml IFNα for 72 h. After 72 h, cells were transfected with shRNA against ADAR1 (sh228) and shRNA against an unrelated RNA (sh526). As shown in Figure 2C, IFNα-induced inhibition of viral protein synthesis was at least partially reversed in the presence of shRNA against ADAR1, but not in the presence of shRNA against an unrelated RNA. These results indicate that the inhibition of HIV-1 protein synthesis in presence of IFNα was due to expression of endogenous ADAR1 in Jurkat cells (Figure 2C).
ADAR1 inhibits HIV-1 viral protein synthesis
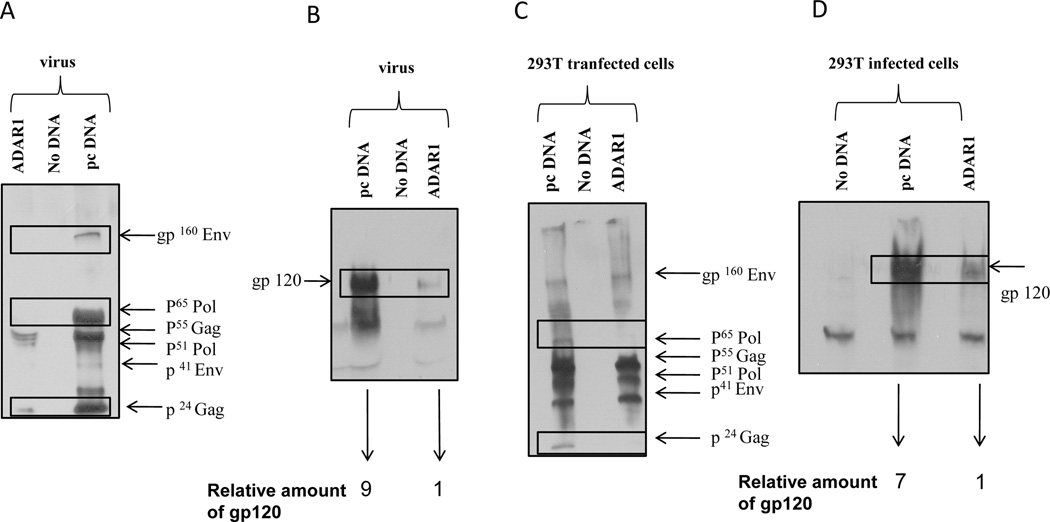
Since expression of ADAR1 inhibited extracellular and intracellular p24 production, we investigated the effect of ADAR1 on production of other HIV-1 protein. 293T cells were co-transfected with pNL4.3 plasmid (0.1µg), and ADAR1 (0.7µg) or control empty vector (pcDNA 3.1, 0.7µg). Cells were also co-transfected with a luciferase-expressing plasmid DNA to control transfection efficiency. Western blots were performed using monoclonal antibody against gp120 and polyclonal serum from a single HIV-infected patient to determine the expression of p24, Pol and Env gp120 proteins in virus from culture supernatant as well as lysates from cells transfected with or without ADAR1 plasmid.
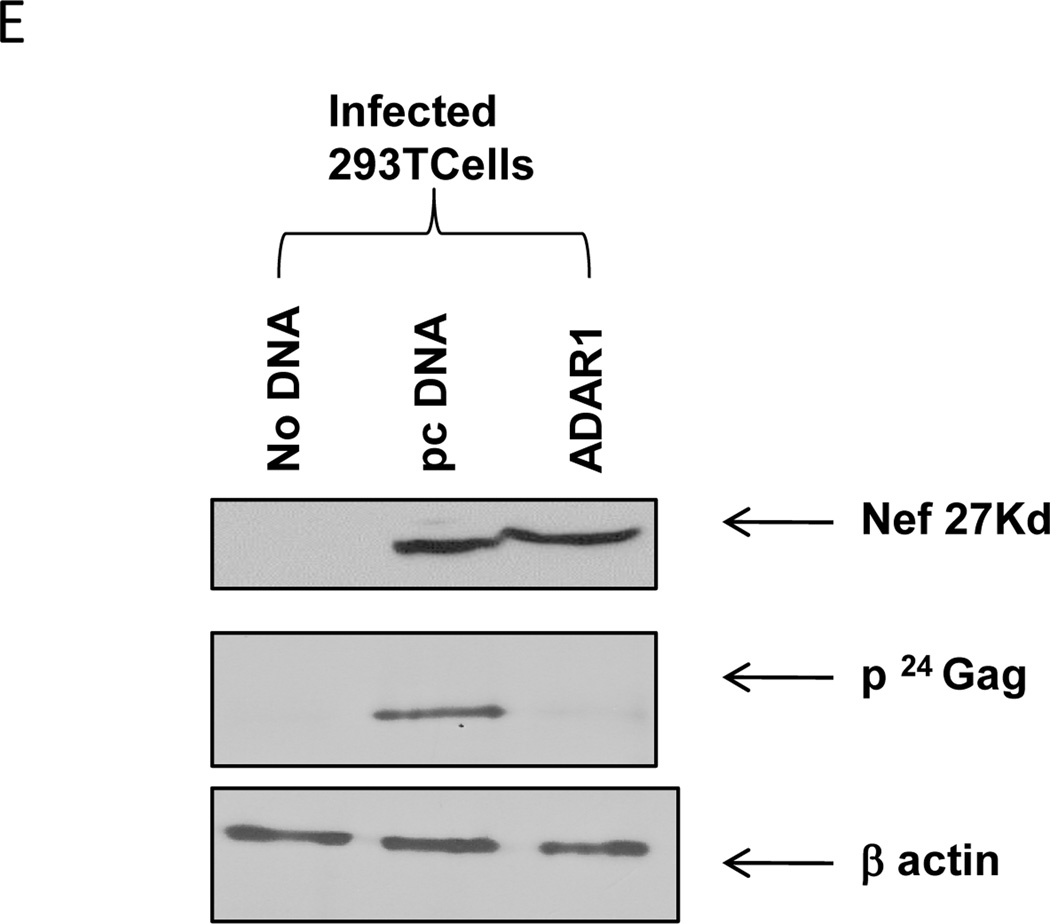
As shown in Figure 3A, the expression of HIV-1 p24, Env gp41, p65 Pol, and Env gp160 proteins in the presence of ADAR1 expression was significantly reduced in culture supernatants as compared to supernatants of cells not expressing ADAR1. The reduction of Env protein in virion in the presence of ADAR1 expression was further confirmed by Western blot analysis using monoclonal antibody against Env gp120 (Figure 3B). In the presence of ADAR1, there was a 9-fold decrease in the expression of virion associated HIV-1 gp120 protein in culture supernatant (Figure 3B). In addition, Western blot analysis of cell lysates using a polyclonal serum from an HIV-1 infected patient showed that the levels of p24 Gag and p65 Pol protein were significantly reduced in the presence of ADAR1 (Figure 3C). Similarly, as shown in Figure 3D, a 7 fold decrease in the expression of intracellular gp120 was observed in the presence of ADAR1 compared to the control DNA. However, ADAR1 showed no effect on the synthesis of an accessory protein Nef (Figure 3E).These results indicated that ADAR1 inhibited expression of intracellular and extracellular HIV-1 structural proteins, Gag, Pol and Env proteins.
Figure 3. HIV-1 viral protein synthesis in the presence of ADAR1.
293T cells were co-transfected with pNL4.3 plasmid (0.1µg), and ADAR1 (0.7µg) or control empty vector (pcDNA 3.1, 0.7µg). After 48 hours and the supernatant was filtered and centrifuged at 220000 rpm for 1 hour and the pellet was lysed with RIPA buffer. The transfected cells were also lysed with RIPA buffer and were subjected to Western blot analysis. Western blot analysis of viral supernatant was done using HIV-1 infected patient’s antisera (Panel A) and gp120 polyclonal antibody (Panel B). Number below panel B shows the relative amount of gp120 in the supernatant by the band density. Western Blot was also performed using the cell lysate from the same experiment using HIV-1 infected patient’s antisera as an antibody (Panel C) and gp120 polyclonal antibody (Panel D). Number below panel B shows the relative amount of gp120 in the infected cells. Expression of Nef protein in presence and absence of ADAR1 in cell lysate was analyzed by Western Blot using anti-nef antibody (Panel E).
ADAR1 does not inhibit HIV-1 RNA synthesis
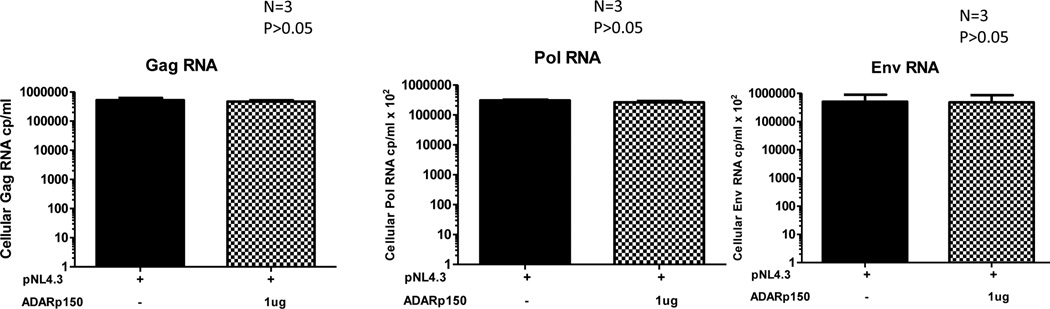
In order to determine whether the inhibition of HIV-1 protein synthesis is due to inhibition of viral RNA synthesis we quantitated the level of gag, pol and env RNA by real-time RT-PCR. Cellular RNA was isolated from cells transfected with ADAR1 or control DNA in the presence of pNL4.3 plasmid and subjected to real-time RT-PCR, as described in Materials and Methods section. As shown in Figure 4 the level of gag, pol and env RNA was not diminished in the presence of ADAR1 expression as compared to cell not expressing ADAR1, whereas the production of the HIV-1 p24 protein (data not shown) was significantly reduced in the presence of ADAR1 expression. These results led us to conclude that the ADAR1-mediated antiviral effect was at a post transcriptional step in the HIV-1 life cycle.
Figure 4. Effect of ADAR1 on HIV-1 RNA synthesis.
Viral gag, pol and env RNA was quantitated in cells transfected with pNL4.3 in the presence and absence of ADAR1 DNA by Real-time RT-PCR.
ADAR1 induces mutation in HIV-1 rev and env RNA
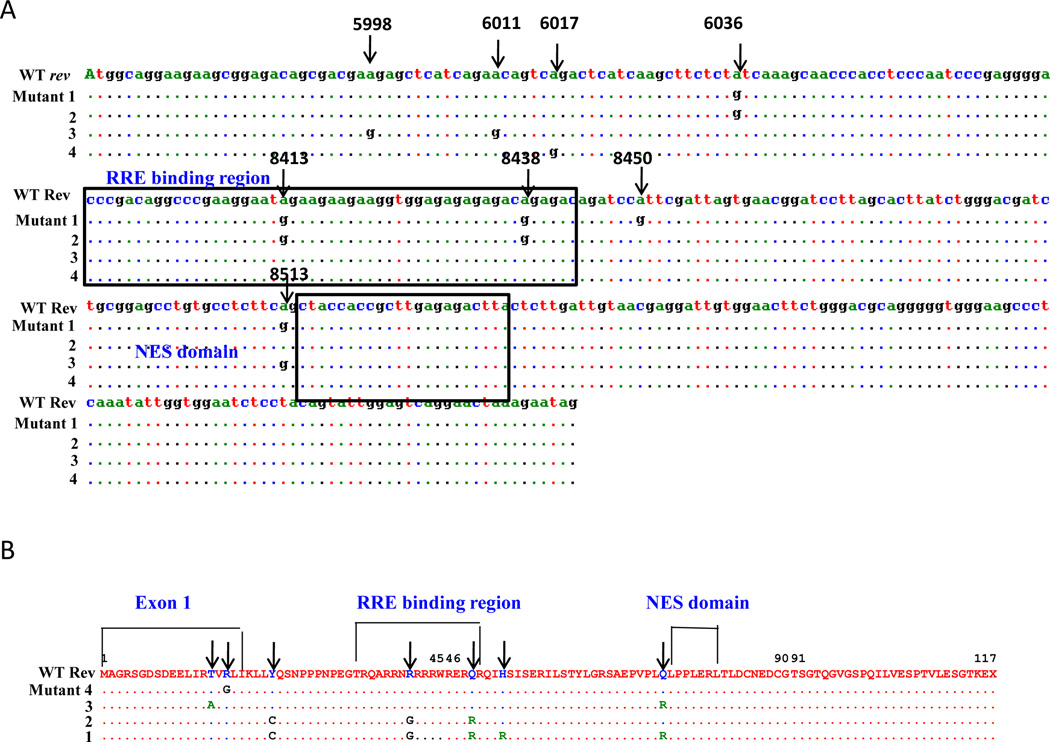
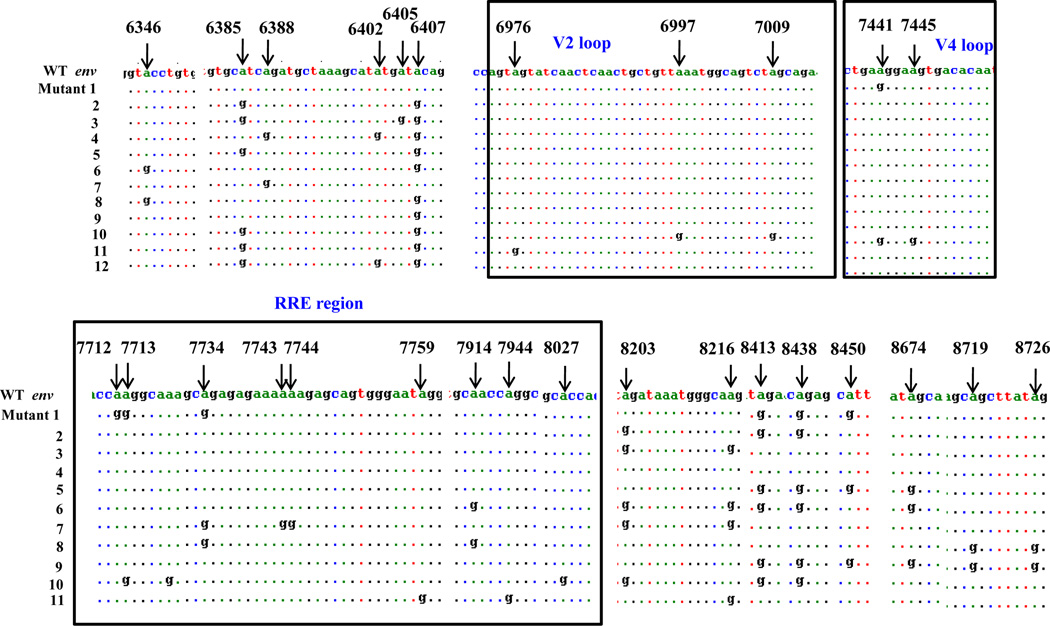
HIV-1 rev RNA is involved in the post-transcriptional modification of the HIV proteins (Felber et al., 1989; Hadzopoulou-Cladaras et al., 1989; Malim and Cullen, 1993; Malim et al., 1989b; Malim et al., 1990; Rosen et al., 1988). Furthermore, Env and Vif expression are required for infectivity of the virus (Buchbinder, 1990; Desrosiers et al., 1998; Simon et al., 1998; Simon et al., 1999). Therefore, we analyzed rev, env and vif RNA for A-to-G mutations, a hallmark of the editing function of ADAR1. For this purpose, 293T cells were transfected with pNL4.3 construct in the presence and absence of ADAR1 expression. Following 48 h post transfection cellular RNA was isolated and was amplified by RT-PCR using gene specific primer pairs. PCR products were cloned in pcDNA3.1 vector and sequenced. As shown in Figure 5A specific A-to-G mutations were noted in the rev sequences. These mutations were specifically observed in the first exon of rev at positions 5998, 6011, 6017, 6036, and N terminus of Rev Response Element (RRE) binding site (positions 8413 and 8438) of rev. These mutations caused significant amino acid changes, like arginine to glycine, and glutamine to arginine in the RRE binding site (Figure 5B). No A-to-G mutations were observed in the LTR, vif, and tat regions of HIV-1 genome (data not shown).
Figure 5. ADAR1 induced mutations in the HIV-1 rev RNA.
293T cells were transfected with pNL4.3 and the ADAR1 plasmid DNAs. The RNA isolated from transfected cells were subjected to RT PCR amplification. Amplicons were cloned in pcDNA3.1 and 12–25 clones were picked (in the presence and absence of ADAR1) and sequenced. Mutational analysis of the nucleotide sequence of rev region of HIV-1 genome (Panel A). Four sequences are the representative of all clones with identical mutations. Comparison of amino acid sequences of HIV-1 rev region in the presence and absence of ADAR1 (Panel B).
We then investigated whether there was an association between the level of ADAR1-mediated inhibition of protein synthesis and the frequency of ADAR1-induced mutations in rev RNA. Cells transfected with a lower amount of ADAR1 plasmid (0.2µg/µl) resulted in a lower level of inhibition of viral protein synthesis (36%) and a lower frequency (30%) of clones with A-to-G mutations in rev gene. In contrast, cells transfected with a higher amount of ADAR1 plasmid (1µg/µl) led to a higher level of inhibition (80%) of viral protein synthesis and to a higher frequency (56%) of clones with A-to-G mutations in the rev gene.
Since in the presence of ADAR1 virus infectivity was diminished and there was inhibition of viral Env synthesis, we also examined the editing activity of ADAR1 on env RNA, in 293T cells. Since the HIV-1 env gene is of 2564bp in length we analyzed the env sequences in 4 segments: Segment 1(6221 – 6873), Segment 2 (6872–7521), Segment 3 (7522–8187), and Segment 4 (8188–8803). Specific A-to-G mutations were located in 29 different places in env gene. No A-to-G mutations were observed in the absence of ADAR1. It is noteworthy that a number of these mutations were observed in the RRE (Rev Response Element), a region needed for binding of Rev for its function, and in variable region 2 (V2) and V4 region (Kowalski et al., 1987)(Figure 6).
Figure 6. ADAR induced mutations in HIV-1 env gene.
Mutational analysis of the env region of HIV-1 genome in the presence and absence of ADAR1. 293T cells were transfected with pc DNA or ADAR1 constructs and RNA was isolated from infected cells. RT-PCR was done and c DNA of env was cloned in pc DNA vector. Each segment of the env gene was cloned individually (Segment 1 (6221 – 6873), Segment 2 (6872–7521), Segment 3 (7522–8187), and Segment 4 (8188–8803). 24 clones were randomly picked from each segment in presence and absence of ADAR1 and sequenced. In case of env 24 sequences were computed into 11 groups.
Rev mutants rescue replication of HIV-1 Δrev
To determine whether the Rev mutants were functional, we examined the capacity of each mutants to rescue Rev function in rev deleted HIV-1 construct (pMrev(−)).The rev mutant constructs pMrev(−) was derived from an infectious proviral clone of HIV-1IIIB in which Rev in not synthesized, but functionally active Tat is expressed. For this purpose 293T cells were co-transfected with 0.2µg pMrev(−) construct and 0.3µg wild type rev or mutant rev-expressing plasmid. RLuc was used as a transfection control. Virus-containing supernatants were collected approximately 48 h after transfection, and virus production was quantified by p24 production in cell culture supernatant by ELISA (Supplemental Figure 4).
As expected in the absence of Rev, low level of viral protein synthesis was observed which increased by 8-fold in the presence of wild type Rev. Mutant constructs 3 and 4 also rescued viral protein synthesis to wild type level. However, mutant constructs 1 and 2 did not rescue the viral protein synthesis compared to wild type Rev. Mutant 1 and 2 had mutations at position 6036 and in the RRE binding region of Rev, respectively. Thus, these mutations in rev that were induced by ADAR1 expression decreased Rev function.
A-to-G mutation in RRE region in env affects the binding of rev
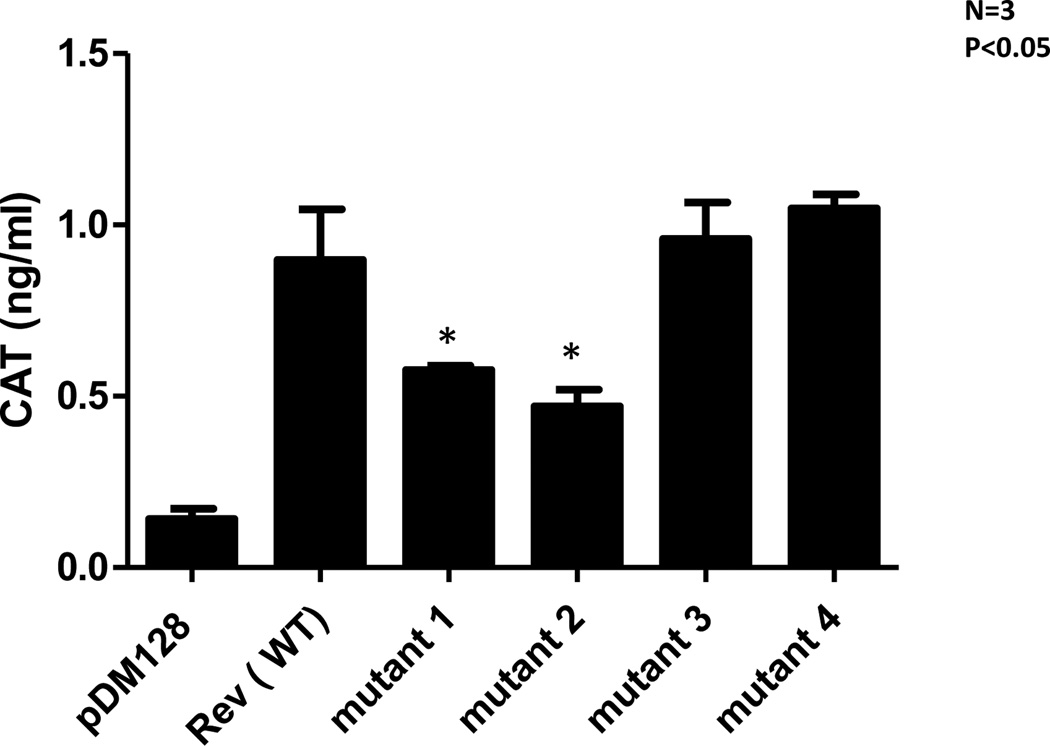
It is known that HIV-1 rev binds to the RRE region in env gene. Because ADAR1 induced specific A-to-G mutations were observed in RRE binding region in rev we examined whether mutations in RRE binding region have any effect in binding of rev to this RRE region to env gene. For this purpose we used pDM128, a reporter plasmid derived from the env region of HIV-1 SF2. The transcripts produced by pDM128 harbor a single intron containing the CAT coding sequence, which is excised when the RNA is spliced. Cells transfected with pDM128 alone express only the spliced viral transcripts in the cytoplasm and, thus produce only trace levels of CAT enzyme activity. Co-transfection with a plasmid encoding wild type Rev, however, permits the unspliced transcripts to enter the cytoplasm, thereby increasing CAT activity. 293T cells were co-transfected with 0.6µg pDM128 construct and 8µg wild type Rev or Rev mutant plasmids. After 48 h post-transfection, cells were lysed and CAT assay was performed on cell lysate (Figure 7). There was a 45% inhibition in the CAT activity in the case of mutants 1 and 2 compare to wild type. Expression of mutants 3 and 4 led to similar levels of CAT activity as compared to wild type Rev expression. The binding activity to Rev to the env RRE region were consistent with rescue activity of the mutants, suggesting that A-to-G mutations in rev RRE present in mutants 1 and 2 decreased the binding of Rev to RRE, resulting in a defect in functional (binding) activity of Rev.
Figure 7. A-to-G mutation in RRE region in env affects the binding of Rev.
293T cells were transfected with 0.6µg pDM128 construct with 8µg wild type rev or mutants rev constructs 1, 2, 3 and 4. Cells were lysed after 48 hours and CAT ELISA assay was performed. Renilla luciferase plasmid was used to normalize the internal transfection efficiency.
ADAR1-induced A-to-G mutations affect the export of gag, pol and env RNA transcripts from nucleus to cytoplasm
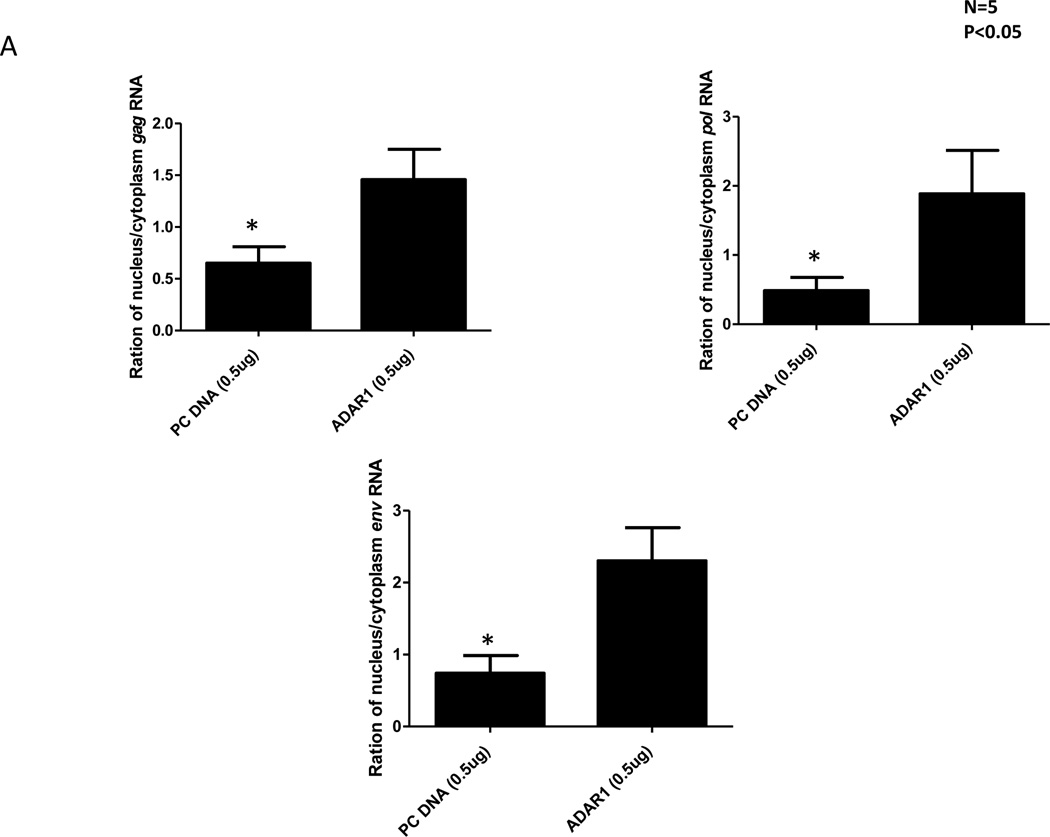
It is known that the Rev protein is involved in controlling synthesis of the structural proteins Gag, Pol and Env protein by facilitating transport of their partially and fully spliced message into the cytoplasm (Felber et al., 1989; Malim et al., 1989b). We examined whether the ADAR1 induced A-to-G mutations in the rev inhibited transport of partially spliced (4Kb) and fully unspliced RNA from nucleus to cytoplasm. 293T cells were co-transfected with 0.1µg of pNL4.3 and 0.5µg control pcDNA or 0.5µg ADAR1 DNA. After 48 h of transfection RNA and protein from cytoplasm and nuclei were isolated. To determine whether there was any cross-contamination between RNA in isolated nuclear and cytoplasmic fractions, we examined the presence of β-actin, a cytoplasmic protein, and histone 3, a nuclear protein. Western Blot analysis (data not shown) showed no contamination between the nuclear and cytoplasmic fraction. Real-time RT-PCR of gag, pol and env RNA was performed using RNA isolated from both the cytoplasmic and nuclear fractions. The ratio of nuclear RNA: cytoplasm RNA was calculated for cells transfected with pcDNA (control) or ADAR1 plasmid. In general in the presence of ADAR1 there were more gag (approximately 3-fold), pol and env (approximately 4-fold) RNA copies present in the nucleus to those measured in the cytoplasm (Figure 8).
Figure 8. A-to-G mutations introduced by ADAR1 affects the export of gag, pol and env RNA transcripts from nucleus to cytoplasm.
293T cells were co-transfected with 0.1µg of pNL4.3 and 0.5µg control pc DNA or 0.5µg ADAR1. After 48 hours PARIS kit (Ambion) was used to isolate RNA and protein from cytoplasm and nucleus of transfected cells. Real-time PCR of gag, pol and env RNA was performed using RNA isolated from cytoplasm and nucleus fraction. The ratio of nuclear RNA: cytoplasmic RNA was plotted for both control pcDNA and ADAR1.
These data together indicate that ADAR1-induced mutations in rev cause inefficient binding of rev to the RRE region of HIV-1 which leads to reduced transport of HIV-1 RNA from nucleus to cytoplasm, resulting in inhibition of viral protein synthesis without any effect on viral RNA synthesis.
A-to-G mutations in the proviral genome at position 6036, 8413 and 8438 in rev gene affect viral protein synthesis
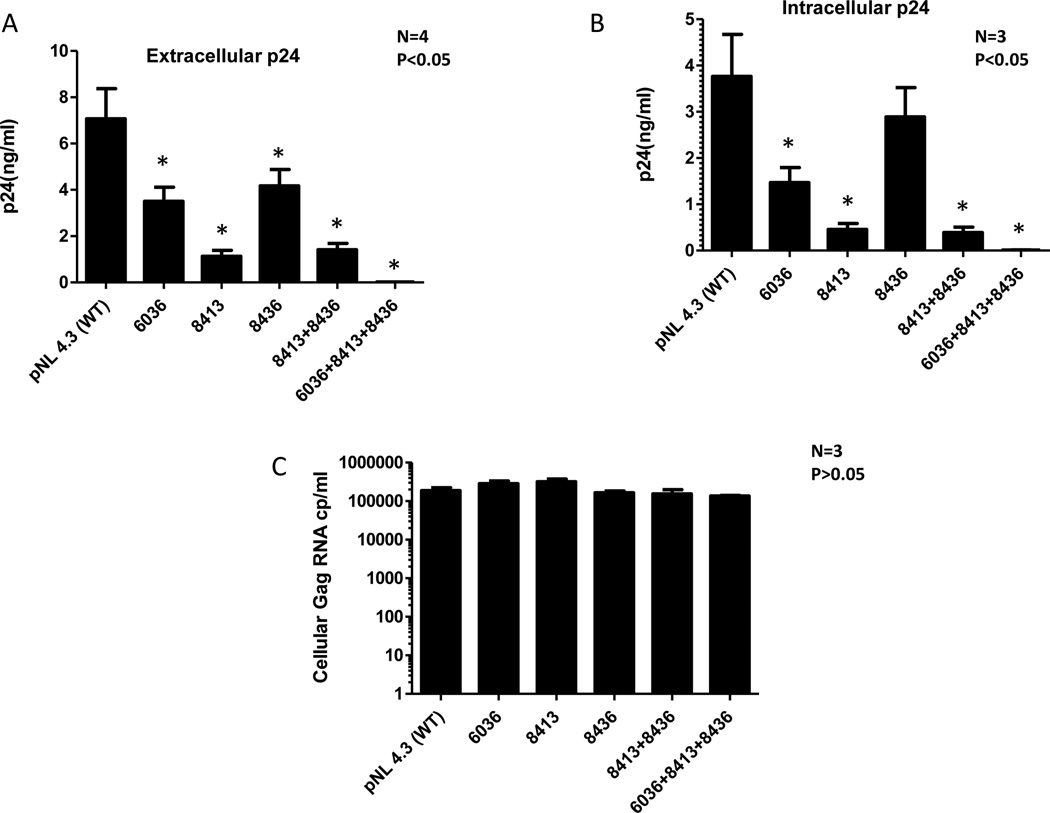
To confirm that A-to-G mutations at positions 6036, 8413 and 8438 in the RRE binding region of rev is responsible for inhibition of viral protein synthesis and not viral RNA synthesis, we introduced these mutations individually or together in the rev gene of an infectious proviral construct (pNL4.3) by site directed mutagenesis and measured their effect on HIV viral protein synthesis. As shown in Figure 9A a single A-to-G mutation in position 6036, 8413 and 8438 inhibited p24 production by 50%, 80% and 45% respectively as compared to synthesis of wild type HIV-1 viral protein. A-to-G mutation at both positions 8413 and 8438 inhibited virus production by 75% as compared to wild type virus. All three mutations were able to inhibit 98% of the viral protein synthesis. Similarly, intracellular HIV-1 p24 was also inhibited to similar levels in these rev mutants (Figure 9B). However, there was no change in intracellular viral gag RNA levels in the cells transfected with any of these mutants (Figure 9C).
Figure 9. A-to-G mutations in the proviral genome at position 6036, 8413 and 8438 affect the synthesis of viral protein.
A-to-G mutations were introduced at position 6036, 8413 and 8438 (single, double and triple) using site directed mutagenesis kit (Stratagene). 293T cells were transfected with 0.1µg mutant constructs and Renilla Luciferase plasmid. Viral protein synthesis was monitored by measuring HIV p24 in culture supernatant (Panel A) and intracellular HIV p24 production (Panel B). Viral gag RNA was quantitated in cells transfected with mutant constructs by Real-time RT-PCR (Panel C).
A catalytic domain of ADAR1 is critical for inhibition of HIV-1protein synthesis and production of infectious virus
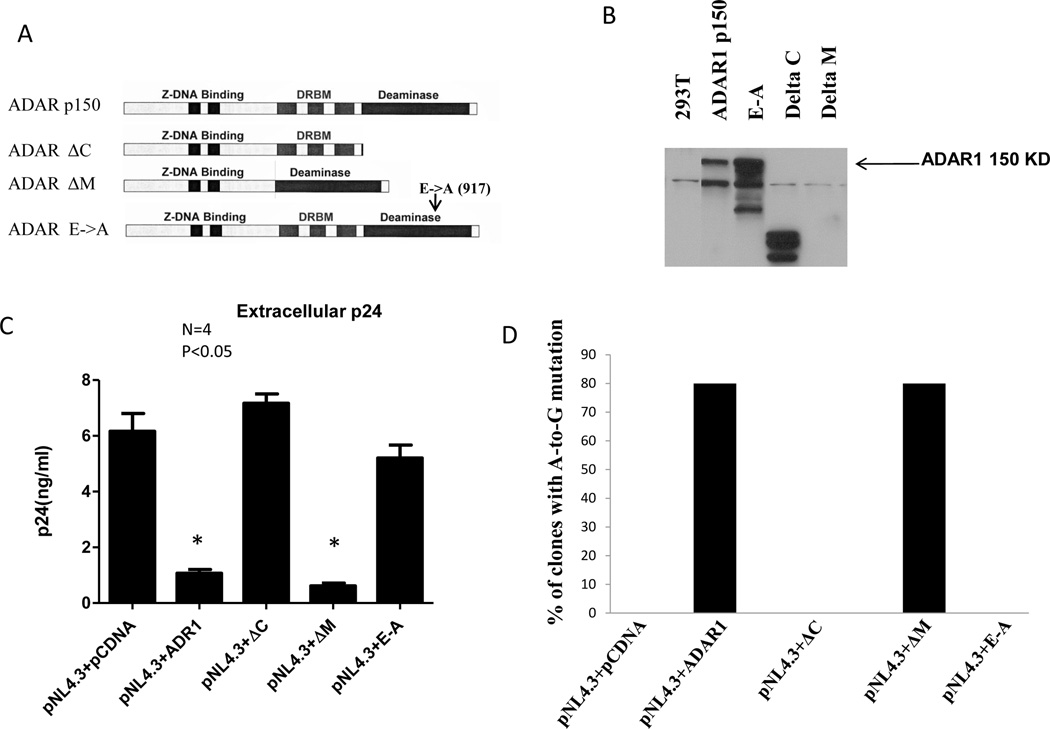
Human ADAR1 has a Z-DNA binding domain, three dsRNA binding domains and a conserved deaminase domain (Eckmann et al., 2001; Ryter and Schultz, 1998). Figure 11A shows a schematic diagram of ADAR1 and ADAR1 mutant constructs used to determine which domains of ADAR1 that are critical for antiviral activity (Figure 11A) (Eckmann et al., 2001; Ryter and Schultz, 1998). Figure 11B shows the expression of full length ADAR1, ADAR1ΔC (catalytic domain deletion mutant) and ADAR1 E-to-A (single amino acid change from glutamic acid to alanine in catalytic domain) mutant clones. ADAR1ΔM construct (double stranded RNA binding domain) did not show expression as the ADAR1 antibody used was targeted to the DRBM domain. To determine which domains are involved in inhibiting viral protein synthesis and infectivity, 293T cells were co-transfected with either full length ADAR1 or ADAR1 deletion constructs in the presence of pNL4.3 DNA. Virus-containing supernatants were collected 48 h post-transfection, and virus production was quantified. As shown in Figure 10C, ADAR1 with a deletion in the deaminase domain (ADAR1ΔC) or a catalytic domain mutation (ADAR1 E-to-A) significantly diminished inhibition of extracellular p24 production, (Figure 10C) intracellular p24 level (data not shown) and infectivity of HIV-1 produced from the cells (data not shown). In contrast, ADAR1 ΔM, which has a deletion in double stranded RNA binding domain, showed inhibition of extracellular (Figure 11C) and intracellular p24 synthesis, (data not shown) as well as reduced infectivity of HIV-1 produced from the cells (data not shown). These mutants behave similar to wild type ADAR1.
Figure 10. Evaluation of domain of ADAR1 critical for inhibition of HIV-1 protein synthesis and production of infectious virus.
Schematic representation of full length human ADAR1 and ADAR1 mutant constructs (Panel A) and their expression (Panel B). 0.1µg of pNL4.3 plasmid was co-transfected with 1µg of ADAR1 and different domain mutant constructs of ADAR1. The Renilla Luciferase expression was used as transfection normalization control (data not shown). Extracellular p24 production in presence and absence of ADAR1 and its mutant constructs (Panel C). Mutational analysis of the rev region of HIV-1 in presence of wild type and ADAR1 mutant constructs (Panel D).
From these experiments, it is concluded that catalytic motif of ADAR1 deaminase domain, but not the ds RNA binding domain is critical in mediating inhibition of HIV-1 protein synthesis and production of infectious virus. To examine the effect of these ADAR1 deletion construct on viral RNA synthesis, intracellular levels of HIV-1 viral gag, pol and env RNA were measured in cells transfected with pNL4.3 and ADAR1, ADAR1ΔC, ADAR1ΔM and ADAR1 E-to-A DNA or control DNA by the Real-time RT-PCR. As shown in supplemental Figure 5, no significant changes in the level of gag, pol and env RNA were observed in the presence of ADAR1 and its mutant constructs (compared to pcDNA vector control). These results reconfirm our previous data described above that ADAR1 acts at a post transcriptional step of viral replication.
Since the catalytic domain of ADAR1 appears to be involved in inhibition of viral protein synthesis we investigated whether this domain is also responsible for A-to-G mutation in rev. Therefore we analyzed mutations in rev RNA isolated from cells transfected with wild type ADAR1or the various deletion constructs described in Figure 10A. No mutations were observed when ADAR1ΔC, ADAR1 E-to-A, or the control was expressed. However, mutations were observed in the presence of wild type ADAR1 or ADAR1ΔM expression, presumably due to the presence of a functional catalytic domain in both (Figure 10E).
Discussion
In our current study, we provide evidence for the inhibitory effect of ADAR1 on HIV-1 protein synthesis and infectivity using a transfection system. Our data show that ADAR1 inhibits HIV-1 protein synthesis irrespective of viral tropisms or clades. ADAR1 mediated HIV-1 inhibition was observed in different cell lines, like 293T, HeLa and Jurkat T cell line and primary CD4+ T cells isolated from peripheral blood. Furthermore we have shown that antiviral activity of ADAR1 was not only against transfected HIV-1, but it also affected HIV-1infectivity. Such inhibition of viral protein synthesis was observed with as little as 0.2 µg of ADAR1 DNA in 293T cells. Additionally, we have shown that, antiviral activity of ADAR1 can be blocked by shRNA against ADAR1 p150, demonstrating specificity of ADAR1 induced inhibition of HIV-1 protein synthesis.
Our results indicate that ADAR1 mediated inhibition of viral protein synthesis occurs at a posttranscriptional step of viral replication, namely at the step of nuclear export of viral gag, pol and env mRNA. This effect of ADAR1 on the nuclear export was seen to be due to ADAR1-induced A-to-G mutations on rev and RRE region on env. These ADAR1 induced A-to-G mutations on HIV-1 rev and env mRNA correlated with inhibition of virus replication, production and infectivity. HIV-1 env overlapping with rev, had the same A-to-G mutations which were observed in case of rev. However, no such ADAR1 induced mutation was observed in LTR, vif and tat regions. It is known that ADAR1 prefers certain secondary structure for its enzymatic action. Therefore differential susceptibility of certain region for mutation could be due to some structural specificity of the RNA in that region. It is especially puzzling why the sequences in tat that overlaps with rev did not show any mutation. It is possible that the overall secondary structure of tat interfered with the editing function of ADAR1 in the overlapping region due to some steric hindrance. Lack of ADAR1 induced mutation in LTR and tat is consistent with our observation of no change in viral RNA in the presence of ADAR1.
Since ADAR1 inhibits HIV-1 viral protein synthesis and concomitantly induces mutation in viral rev RNA, we examined the mechanisms by which these mutations could perturb viral protein synthesis. It is known that the Rev protein is involved in controlling synthesis of the structural proteins by facilitating transport of their mRNA from nucleus to cytoplasm (Felber et al., 1989; Malim et al., 1989b) through binding of RNA binding domain in rev with Rev Responsive Element (RRE) which is present in all unspliced viral transcripts of structural proteins (Bohnlein, Berger, and Hauber, 1991; Malim et al., 1989a; Malim et al., 1991).
In this report we have shown that ADAR1-induced mutations in the first exon and NLS/RRE binding region of rev resulted in a decrease in binding of rev to RRE and reduced transport of singly and multiple spliced HIV-1 gag, pol and env mRNA from nucleus to cytoplasm. Importance of these mutations have been further demonstrated by introduction into an infectious molecular clone of HIV-1 (pNL4.3 construct) resulting in a decrease in viral protein synthesis with no change in viral RNA synthesis. These data together indicate that ADAR1-induced mutations in rev cause inefficient binding of Rev to the RRE region of HIV-1 which leads to reduced transport of unspliced and single spliced HIV-1 mRNA from nucleus to cytoplasm, resulting in inhibition of viral protein synthesis without any effect on viral RNA synthesis.
In addition to mutations in the rev RNA, we also observed ADAR1-induced mutations in the sequence encoding the V2 and V4 regions of gp120. This supports our observation that ADAR1 inhibits synthesis of structural proteins without inhibiting synthesis of their mRNA. It is known that constant loop 4 (C4) and variable loop 3 (V3) along with V2 loop are important for viral entry process and therefore infectivity of the virus (Kowalski et al., 1987). Therefore, these ADAR1-induced mutations in HIV-1 env probably resulted in production of virions with defective Env proteins which decreased the infectivity of the virions. This supports our observation that virions secreted in the presence of ADAR1 were non-infectious.
Phuphuakrat et al. (Phuphuakrat et al., 2008) and Doria et al. (Doria et al., 2009) reported that ADAR1 enhanced the expression of HIV-1 proteins. However, Phuphuakrat et al. (Phuphuakrat et al., 2008) found inhibition of production of infectious virus in the presence of ADAR1, while Doria et al. (Doria et al., 2009) showed ADAR1 induced enhancement of infectious virus production. Recently, Clerzius et al. (Clerzius et al., 2009) have reported that ADAR1 interacts with the interferon-induced protein kinase during HIV-1 infection. It is unclear as to why our results are discrepant with those of Doria et al. (Doria et al., 2009) and Phuphuakrat et al. (Phuphuakrat et al., 2008).
Phuphuakrat et al (Phuphuakrat et al., 2008) and Doria et al. (Doria et al., 2009) did not provide any adequate explanation for the observed ADAR1 induced enhancement of viral protein synthesis. Phuphuakrat et al. (Phuphuakrat et al., 2008) did not explain clearly the reason of the enhancement of viral replication. They stated that a decrease in unspliced RNA in nucleus may have resulted in increased nuclear RNA splicing. Furthermore, they commented that sequencing around the RRE region in the ADAR1-overexpressing cells showed some site-specific A-to-G editing at nucleotides 8164 to 8166, which could provide an advantage in viral protein expression via an unknown cis-acting element. However they did not provide evidence in support of these hypotheses. Doria et al. (Doria et al., 2009) have explained their observation of ADAR1 induced enhancement by citing the paper by Clerzius et al. (Clerzius et al., 2009) which showed that ADAR1 inhibits the PKR kinase and thereby suppresses protein synthesis by phosphorylating eIF-2a (Clerzius et al., 2009). If that would have been the case we should have observed protein synthesis of all viral proteins. However our data show that ADAR1 had no effect on Nef or Tat protein synthesis.
Antiviral effect of ADAR1, on the other hand, makes sense from a biological and a mechanistic point of view. ADAR1 is an interferon-inducible protein involved in antiviral activity against a number of RNA viruses (Patterson and Samuel, 1995), therefore, it is expected that ADAR1 will have antiviral activity rather than enhancing activity. Viperin, another interferon-inducible protein, has been shown to have anti-viral activity against HCV and influenza virus (Helbig et al., 2005; Hinson and Cresswell, 2009; Jiang et al., 2008). As mentioned earlier, ADAR1 has been shown to have antiviral activity against a variety of RNA viruses (Horikami and Moyer, 1995; Patterson and Samuel, 1995; Polson, Bass, and Casey, 1996; Sato, Wong, and Lazinski, 2001). The hydrolytic deamination of adenosine to inosine in completely or partially double-stranded RNA by ADAR1 affects pre mRNA splicing, incorporates incorrect amino acid during translation, and decreases the binding of RNA to protein. All of these changes are expected to have deleterious effect on the function of viral protein and hence viral protein synthesis. In this report we have conclusively proven that ADAR1 introduced mutations in the Rev Response Element (RRE) binding regions of rev RNA inhibits binding of rev to the RRE region in the env and inhibits transport of primary transcripts like gag, pol and env from the nucleus to cytoplasm resulting in the inhibition of viral protein synthesis without any effect on viral RNA synthesis. This result was further confirmed by introducing same mutations in rev of a proviral DNA producing similar phenotype of viral inhibition, i,e suppression of viral protein synthesis without affecting viral RNA synthesis.
In summary, our data demonstrate that ADAR1 is an important cellular protein which inhibits HIV-1 protein synthesis by editing viral rev RNA, thereby disabling transport of unspliced and singly spliced viral mRNA from nucleus to cytoplasm. ADAR1 also inhibits infectivity of the virus by editing viral env RNA. Therefore, ADAR1 constitutes a novel multi-targeted cellular protein against HIV-1 which can have therapeutic implications.
Material and Methods
Cell Lines and DNA constructs
293T (ATCC CRL-11268), TZM-bl (NIHAIDS research and reagent program Catalog number 8129) and HeLa (NIHAIDS research and reagent program Catalog number 153) cell lines were cultured in DMEM (Invitrogen Life Technologies) supplemented with 10% FBS (HyClone), 1% penicillin, and 10µg/ml streptomycin. The Jurkat (ATCC®TIB-152TM) cell line was grown in RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, 1% penicillin, and 10µg/ml streptomycin. CD8 depleted PBMC was isolated by immune-magnetic beads coated with antibody to CD8 as described previously (Chen et al., 2000; Chen, Rinaldo, and Gupta, 1997).
Initial experiments were done using ADAR1 and ADAR1 mutant constructs as described (Lai, Drakas, and Nishikura, 1995). Subsequently a new ADAR1 construct made in pcDNA 3.1 vector was used. Sequence of the ADAR1 was confirmed by sequencing and found to be identical to that described originally by Nishikura group (Lai, Drakas, and Nishikura, 1995). ADAR1 ΔC is a deletion mutant construct where the catalytic domain (nucleotide 839bp to 1222bp of ADAR1) is deleted. In case of ΔM ADAR1, the double stranded RNA binding domain (from nucleotide 1689bp to 2376bp of ADAR1) was deleted. In E-to-A mutant construct, a single point mutation in the catalytic domain at the position 917, from glutamic acid to alanine was made which abolishes the catalytic activity of ADAR1 and was used for the experiment. Renilla Luciferase (RLuc) plasmid (Promega) was used as a transfection control (Matthews, Hori, and Cormier, 1977). Infectious molecular clone pNL4.3 plasmid, pNLAd8 and p89.6 were obtained from the NIHAIDS Research and Reagent Program. pIndie C plasmid was obtained from Dr. Masashi Tatsumi (Tokyo, Japan)(Mochizuki et al., 1999). pRev(−) construct was obtained from NIH AIDS Research and Reagent Program (catalog number 2086). pDM-128 and control plasmids were obtained from Dr. Thomas Hope (Hope et al., 1990).
Transfection and P24 ELISA
For transfection in 293T cells, media containing plasmids (pNL4.3/pNLAd8/p89.6, ADAR1 or control RLuc DNAs) and Lipofectamine 2000 (2× of the amount of plasmid) were incubated together at room temperature for 15–20 min, and added slowly to the wells containing 1×106 million 293T cells. After 4 h the media was replaced with fresh media containing 10% fetal bovine serum. 5×106 million HeLa cells were nucleofected with the Nucleofector Kit R (Amaxa) in program I-13 when cells reached 70–80% confluency (0.5–1×106 cells). Jurkat cells were also nucleofected with the same kit, using program A-017. Five million CD8 T depleted PBMC were nucleofected with the Human T cell Nucleofector solution (Amaxa) in program V-024.
To measure the production of intracellular and extracellular virus particles in the presence of ADAR1, 293T cell was co-transfected with cocktail comprising of pNL4.3 plasmid, different amount of ADAR1, pcDNA 3.1 empty vector and 0.1µg of RLuc plasmid. Virus-containing supernatants were collected 48 h post-transfection, and virus production was quantified by analyzing p24 production using ELISA (Perklin Elmer p24 ELISA kit). To evaluate the Renilla luciferase activity, cells were centrifuged at 5000 × g for 10 min and lysed by incubating with 100µl 1X lysis buffer (Promega Duel luciferase reporter assay kit, E1910) for 30 min. Thirty microliter cell lysate was mixed with 100µl Stop and Glo buffer and the luciferase activity was measured using Turner BioSystems Veritas™ MicroplateLuminometer. To determine the intracellular virus production, 48 h post-transfection, the cell pellet was washed with PBS twice, resuspended in PBS containing 0.5% NP-40 and was incubated in ice for 10min. The mixture was centrifuged and supernatant used for p24 ELISA.
Infectivity Assay
Infectivity was determined in TZM-bl cell line as described previously (Derdeyn et al., 2000; Platt et al., 1998).
MTT assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay was performed to determine cell viability as previously described (Mosmann, 1983).
Western Blot
Cell lysates were prepared by lysing of 293T cells with a lysis buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 0.5% Nonidet P40, 50mM NaF, 1mM Na3VO4, 5mM β-glycerophosphate, 1mM dithiothreitol, 1mM phenylmethylsulfonyl fluoride) supplemented with a protease inhibitor mixture (Sigma) on ice. Lysate was clarified by centrifuging at 14,000 × g for 20 min and subjected to Western Blot analysis using ADAR1 monoclonal antibody (1:2000 dilution from Santa Cruz (1: 500 dilution), anti-actin antibody (Sigma, 1:1000 dilution) and rabbit anti-gp120 antibody (1:1000 dilution, Advanced Biotechnologies Inc) or HIV-1 infected patient’s antisera. Western Blot analysis was also done using Rev antibody (1:1000 dilutions), and anti-Histone H3 antibody (Santa Cruz, 1:500 dilutions).
CAT assay
293T cells were grown to 90% confluency and were transfected with 0.9µg pDM-128 construct and 8ug of rev mutant construct. Cells were lysed, and the amount of CAT protein was measured using a CAT ELISA (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions.
Site directed mutagenesis
Single nucleotide substitution (A-to-G) was introduced into rev region of pNL4.3 using Stratagene (La Jolla, CA) quick-change site directed mutagenesis kit in pNL4.3 proviral backbone.
RNA extraction, Cloning and Sequencing
RNA was isolated from transfected cells using RNA Bee (TEL-TEST, INC) according to the manufacturer's instructions. The extracted RNA was treated with RNase free DNase (Roche applied science) for 30 min followed by heat inactivation and subjected to RT-PCR. The cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) and random hexamar (IDT) in the presence of RNase inhibitor (Roche applied science). The rev and env PCR was done using primers listed in Table 1. The PCR product was cloned in pcDNA 3.1 Vector (Invitrogen) between the restriction sites KpnI and XhoI for rev and between Mlu1 and Xho1 for env. 12–25 clones were picked from each plate and sequenced using ABI PRISM 3730 automated DNA sequencer. For studies on rev 24 clones were sequenced and they were found to grouped into 4 identical patterns of mutations, whereas for env 24 clones were sequenced and they were found to fall into 11 groups of identical patterns of mutations.
Table 1.
HIV-1 rev and env PCR primers.
| Region | Primer Sequence |
|---|---|
| Rev | Forward: ttggtaccatggcaggaagaagcggagaca Reverse: tctcgagctattctttagttcctgactccaatactgtaggag |
| Env (1–652) | Forward: cca cgc gta tga gag tga agg aga agt atc agc act Reverse: cctcgaggggcacaataatgtatgggaattggc |
| Env (651–1300) | Forward: ata cgc gtc ccg gct ggt ttt gcg att cta Reverse: cctcgagcatacattgcttttcctacttcctgcc |
| Env (1301–1950) | Forward: ata cgc gtc ccc tcc cat cag tgg aca aat Reverse: cctcgaggctggttttgcgattcttca |
| Env (1951–2566) | Forward: cca cgc gtc cag caa gaa aag aat gaa caa g Reverse: gctcgagcttatagcaaaatcctttccaagccctgtc |
Real time RT-PCR analysis for Quantification of HIV-1 RNA from Cells
20ng of RNA was applied for reverse transcription using TaqMan® Reverse Transcription Reagents (Applied Biosystems) according to the manufacture’s protocol. A TaqMan® PCR was performed in a 30ul reaction mixture consisting of 5µl cDNA with TaqMan® Universal PCR Master Mix (Applied Biosystems), 900nM each of forward and reverse primers and 250nM FAM/TAMRA or FAM/MGB labeled probe. Real-Time RT PCR for the endogenous gene was performed using Eukaryotic 18S rRNA Endogenous Control Reagents (Applied Biosystems). ABI Prism 7000 Sequence Detection System was used to carry out Real-Time PCR using the following cycling condition: 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 sec and 60 °C for 1 min. The primers and probes used for the Real-Time PCR were based on HIV-1 pNL4.3 sequence encoding the pol, gag and env regions respectively (Supplemental Figure 3). A serial dilution of pNL4.3 plasmid DNA ranging from 10 to 106 copies was applied to each PCR assay serving as HIV standard curve. An endogenous gene standard curve containing serial diluted cellular RNA ranging from 100to 105 pg was included in each endogenous gene test. Both no RT control and no template control were included in each assay to monitor the genomic DNA contamination and guard against PCR cross contamination, respectively. Each sample was run in triplicate. ABS Prism 7000 SDS Software (Applied Biosystems) was used for PCR data analysis and copy number estimation. To rule out the sample input and amplification variations, HIV quantity in each sample was normalized by the endogenous control, and the final result was expressed as HIV-1 RNA copies per microgram of total RNA.
Supplementary Material
293T cells were co-transfected with 0.1µg pNL4.3 plasmid and 0.2µg, 0.4µg, 0.5µg, 0.6µg, 0.7 µg of ADAR1 plasmid. The expression of ADAR1 in the transfected cells was determined by Western blot and normalized against β-actin loading control to show the relative intensity of ADAR1 expression (Panel A). To show antiviral activity of endogenous ADAR1, 293T cells were transfected with 0.5µg of ADAR1 with 1µg (1:2 ratio), 2µg (1:4 ratio), 3µg (1:6 ratio) of shRNA constructs 1724 and 228 against ADAR1 and 3 µg of control scrambled shRNA 526 (1:6 ratio). Western blot was performed using ADAR1 monoclonal antibody to check the expression of ADAR1. β-actin was used as a control (Panel B).
293T cells were transfected with 89.6, AD8, pIndie C or pNL4.3 constructs in the absence and presence of ADAR1. Equal amount of viruses (3ng p24 equivalent) (from main Figure Panel 1F) were used to infect TZM-bl cell line and productive infection was monitored by measurement of luciferase activity.
Primer probes of gag, pol and env gene of HIV-1for Real-time PCR.
293T cells were transfected with 0.2µg pMrev(−) construct along with 0.3µg wild type rev and rev mutant constructs 1, 2, 3 and 4. Virus-containing supernatants were collected 48 h post transfection, and virus production was quantified by analyzing p24 production by ELISA; the mean +s.d. values for three independent experiments are shown.
0.1µg of pNL4.3 plasmid was co-transfected with 1µg of ADAR1 and different domain mutant constructs of ADAR1. Level of HIV-1 gag, pol, and env RNA in presence and absence of ADAR1 and its mutant constructs was determined by the real time RT PCR assay.
ACKNOWLEDGMENTS
We are grateful to Dr. Ronald Montelaro for anti gp120 antibody, Dr. Michael Malim for Rev antibody and Dr Thomas Hope for pDM128 construct. We thank Mary White for performing p24 ELISA assays and Varsha Sridhar for editing the manuscript. The work was supported by the AIDS International training grant TW001038-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnlein E, Berger J, Hauber J. Functional mapping of the human immunodeficiency virus type 1 Rev RNA binding domain: new insights into the domain structure of Rev and Rex. J Virol. 1991;65(12):7051–7055. doi: 10.1128/jvi.65.12.7051-7055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder A. The envelope of HIV-1 as a key component to infectivity. Prog AIDS Pathol. 1990;2:1–11. [PubMed] [Google Scholar]
- Chen Y, Dampf D, Chen M, Kulka K, Volsky DJ, Saha K, Gupta P. Dependence of CD8+ T-cell-mediated suppression of HIV type 1 on viral phenotypes and mediation of phenotype-dependent suppression by viral envelope gene and not by beta-chemokines. AIDS Res Hum Retroviruses. 2000;16(2):117–124. doi: 10.1089/088922200309467. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rinaldo C, Gupta P. A semiquantitative assay for CD8+ T-cell-mediated suppression of human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1997;4(1):4–10. doi: 10.1128/cdli.4.1.4-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerzius G, Gelinas JF, Daher A, Bonnet M, Meurs EF, Gatignol A. ADAR1 interacts with PKR during human immunodeficiency virus infection of lymphocytes and contributes to viral replication. J Virol. 2009;83(19):10119–10128. doi: 10.1128/JVI.02457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers RC, Lifson JD, Gibbs JS, Czajak SC, Howe AY, Arthur LO, Johnson RP. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72(2):1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria M, Neri F, Gallo A, Farace MG, Michienzi A. Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection. Nucleic Acids Res. 2009;37(17):5848–5858. doi: 10.1093/nar/gkp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann CR, Neunteufl A, Pfaffstetter L, Jantsch MF. The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein. Mol Biol Cell. 2001;12(7):1911–1924. doi: 10.1091/mbc.12.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber BK, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis GN. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzopoulou-Cladaras M, Felber BK, Cladaras C, Athanassopoulos A, Tse A, Pavlakis GN. The rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. J Virol. 1989;63(3):1265–1274. doi: 10.1128/jvi.63.3.1265-1274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig KJ, Lau DT, Semendric L, Harley HA, Beard MR. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology. 2005;42(3):702–710. doi: 10.1002/hep.20844. [DOI] [PubMed] [Google Scholar]
- Hinson ER, Cresswell P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic alpha-helix. Proc Natl Acad Sci U S A. 2009;106(48):20452–20457. doi: 10.1073/pnas.0911679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikami SM, Moyer SA. Double-stranded RNA adenosine deaminase activity during measles virus infection. Virus Res. 1995;36(1):87–96. doi: 10.1016/0168-1702(94)00103-j. [DOI] [PubMed] [Google Scholar]
- Hough RF, Bass BL. Purification of the Xenopus laevis double-stranded RNA adenosine deaminase. J Biol Chem. 1994;269(13):9933–9939. [PubMed] [Google Scholar]
- Jiang D, Guo H, Xu C, Chang J, Gu B, Wang L, Block TM, Guo JT. Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J Virol. 2008;82(4):1665–1678. doi: 10.1128/JVI.02113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Garner TL, Sanford T, Speicher D, Murray JM, Nishikura K. Purification and characterization of double-stranded RNA adenosine deaminase from bovine nuclear extracts. J Biol Chem. 1994;269(18):13480–13489. [PubMed] [Google Scholar]
- Kowalski M, Potz J, Basiripour L, Dorfman T, Goh WC, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Lai F, Drakas R, Nishikura K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem. 1995;270(29):17098–17105. doi: 10.1074/jbc.270.29.17098. [DOI] [PubMed] [Google Scholar]
- Malim MH, Bohnlein S, Hauber J, Cullen BR. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989a;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Malim MH, Cullen BR. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13(10):6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989b;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Malim MH, McCarn DF, Tiley LS, Cullen BR. Mutational definition of the human immunodeficiency virus type 1 Rev activation domain. J Virol. 1991;65(8):4248–4254. doi: 10.1128/jvi.65.8.4248-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Tiley LS, McCarn DF, Rusche JR, Hauber J, Cullen BR. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990;60(4):675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Hori K, Cormier MJ. Purification and properties of Renilla reniformis luciferase. Biochemistry. 1977;16(1):85–91. doi: 10.1021/bi00620a014. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Otsuka N, Matsuo K, Shiino T, Kojima A, Kurata T, Sakai K, Yamamoto N, Isomura S, Dhole TN, Takebe Y, Matsuda M, Tatsumi M. An infectious DNA clone of HIV type 1 subtype C. AIDS Res Hum Retroviruses. 1999;15(14):1321–1324. doi: 10.1089/088922299310223. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival - Application to Proliferation and Cyto-Toxicity Assays. Journal of Immunological Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- O'Connell MA, Keller W. Purification and properties of double-stranded RNA-specific adenosine deaminase from calf thymus. Proc Natl Acad Sci U S A. 1994;91(22):10596–10600. doi: 10.1073/pnas.91.22.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15(10):5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomis DC, Hans SL, Samuel CE. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons. Virology. 1995;210(2):508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- Phuphuakrat A, Kraiwong R, Boonarkart C, Lauhakirti D, Lee TH, Auewarakul P. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins. J Virol. 2008;82(21):10864–10872. doi: 10.1128/JVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Bass BL, Casey JL. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature. 1996;380(6573):454–456. doi: 10.1038/380454a0. [DOI] [PubMed] [Google Scholar]
- Rosen CA, Terwilliger E, Dayton A, Sodroski JG, Haseltine WA. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988;85(7):2071–2075. doi: 10.1073/pnas.85.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17(24):7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. RNA editing minireview series. J Biol Chem. 2003;278(3):1389–1390. doi: 10.1074/jbc.R200032200. [DOI] [PubMed] [Google Scholar]
- Sato S, Wong SK, Lazinski DW. Hepatitis delta virus minimal substrates competent for editing by ADAR1 and ADAR2. J Virol. 2001;75(18):8547–8555. doi: 10.1128/JVI.75.18.8547-8555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JH, Miller DL, Fouchier RA, Soares MA, Peden KW, Malim MH. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 1998;17(5):1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JH, Sheehy AM, Carpenter EA, Fouchier RA, Malim MH. Mutational analysis of the human immunodeficiency virus type 1 Vif protein. J Virol. 1999;73(4):2675–2681. doi: 10.1128/jvi.73.4.2675-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspene R, Petit V, Puyraimond-Zemmour D, Aynaud MM, Henry M, Guetard D, Rusniok C, Wain-Hobson S, Vartanian JP. Double-stranded RNA adenosine deaminase ADAR-1-induced hypermutated genomes among inactivated seasonal influenza and live attenuated measles vaccines. J Virol. doi: 10.1128/JVI.02138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Puig M, Darnell ME, Mihalik K, Feinstone SM. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J Virol. 2005;79(10):6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Luo X, Nie Y, Su Y, Zhao Q, Kabir K, Zhang D, Rabinovici R. Widespread inosine-containing mRNA in lymphocytes regulated by ADAR1 in response to inflammation. Immunology. 2003;109(1):15–23. doi: 10.1046/j.1365-2567.2003.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
293T cells were co-transfected with 0.1µg pNL4.3 plasmid and 0.2µg, 0.4µg, 0.5µg, 0.6µg, 0.7 µg of ADAR1 plasmid. The expression of ADAR1 in the transfected cells was determined by Western blot and normalized against β-actin loading control to show the relative intensity of ADAR1 expression (Panel A). To show antiviral activity of endogenous ADAR1, 293T cells were transfected with 0.5µg of ADAR1 with 1µg (1:2 ratio), 2µg (1:4 ratio), 3µg (1:6 ratio) of shRNA constructs 1724 and 228 against ADAR1 and 3 µg of control scrambled shRNA 526 (1:6 ratio). Western blot was performed using ADAR1 monoclonal antibody to check the expression of ADAR1. β-actin was used as a control (Panel B).
293T cells were transfected with 89.6, AD8, pIndie C or pNL4.3 constructs in the absence and presence of ADAR1. Equal amount of viruses (3ng p24 equivalent) (from main Figure Panel 1F) were used to infect TZM-bl cell line and productive infection was monitored by measurement of luciferase activity.
Primer probes of gag, pol and env gene of HIV-1for Real-time PCR.
293T cells were transfected with 0.2µg pMrev(−) construct along with 0.3µg wild type rev and rev mutant constructs 1, 2, 3 and 4. Virus-containing supernatants were collected 48 h post transfection, and virus production was quantified by analyzing p24 production by ELISA; the mean +s.d. values for three independent experiments are shown.
0.1µg of pNL4.3 plasmid was co-transfected with 1µg of ADAR1 and different domain mutant constructs of ADAR1. Level of HIV-1 gag, pol, and env RNA in presence and absence of ADAR1 and its mutant constructs was determined by the real time RT PCR assay.