Abstract
The immune system is designed to protect the host from infection and injury. However, when an adaptive immune response continues unchecked in the brain, the proinflammatory innate microglial response leads to the accumulation of neurotoxins and eventual neurodegeneration. What drives such responses are misfolded and nitrated proteins. Indeed, the antigen in Parkinson’s disease (PD) is an aberrant self-protein, although the adaptive immune responses are remarkably similar in a range of diseases. Ingress of lymphocytes and chronic activation of glial cells directly affect neurodegeneration. With this understanding, new therapies aimed at modulating the immune system’s response during PD could lead to decreased neuronal loss and improved clinical outcomes for disease.
Aberrant proteins (e.g., modified α-synuclein) activate microglia and engage the adaptive immune system, causing neuroinflammation and neurodegeneration in PD. Therapeutic strategies that transform T-cell functions may reverse this.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder affecting the elderly. Pathologically, the disease is characterized by the cytoplasmic accumulation of proteinaceous aggregates called Lewy bodies (LBs), which are mainly comprised of α-synuclein (α-syn) and ubiquitin (Spillantini et al. 1997, 1998). Progressive degeneration of dopaminergic neurons in the substantia nigra (SN) pars compacta and their projections into the caudate nucleus leads to substantial decreases in dopamine levels, which manifest as resting tremor, bradykinesia, rigidity, and gait dysfunction (Dauer and Przedborski 2003). Currently, no curative treatments or treatments that interdict disease progression exist. Although the etiology of PD remains unknown, abundant evidence implicates immune system abnormalities and central nervous system (CNS) inflammation in disease pathobiology (McGeer et al. 1988a; Stone et al. 2009; Kosloski et al. 2010). Harnessing inflammatory responses through targeted modulation of innate and adaptive immune responses has gained increasing interest in recent years as a potential therapeutic strategy. The interplay between innate and adaptive immunity in the pathobiology of PD, the evolution and change in such immune responses, and the means to alter it to the benefit of the diseased, is the focus of this article.
ADAPTIVE IMMUNITY AND THE CNS
William Hickey wrote, “vertebrates possess two bodily systems capable of learning and remembering: the nervous system and the immune system” (Hickey 2001; Weiner 2008). The CNS was once thought to be an “immune privileged” site, in which immune cells of the periphery could not enter or rarely entered, and thus the two systems had little to no interaction. This hypothesis was supported by the early observation that tissue grafts in the eye or brain survived longer than grafts in other areas of the body (Medawar 1948). However, today, evidence of an interactive adaptive immune system and the CNS abounds. Indeed, communication between the CNS and peripheral immune system is much more fluid than previously considered and, as such, may substantially affect disease progression in neurological disorders (Ferrari and Tarelli 2011). Peripheral immune responses can trigger inflammation and exacerbation of CNS degeneration in several neurodegenerative diseases such as Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), stroke, and prion-mediated diseases (Cunningham et al. 2005a,b; Kamer et al. 2008; Fiala and Veerhuis 2009; Holmes et al. 2009; Lee et al. 2009a; McColl et al. 2009; Reale et al. 2009; Stoll and Bendszus 2009; Teeling and Perry 2009; Heesen et al. 2010; Perry 2010), and particularly PD (Hasegawa et al. 2000; Arai et al. 2006). In those disorders, increasing inflammation and breakdown of the blood–brain barrier (BBB) forces increased communication between the CNS and peripheral immune systems as evidenced in several neurodegenerative diseases with increased leukocyte migration within the brain parenchyma (Stolp and Dziegielewska 2009). Under infectious or inflammatory conditions, peripheral immune cells have relatively unfettered access to the CNS. These immune cells influence neuroinflammation and neurodegeneration not only in a paracrine fashion, but also in an endocrine fashion. In turn, the CNS is capable of influencing the immune response to pathogens in the periphery through the neuroendocrine system. Thus, the immune system is not only charged with protecting the CNS from pathogens and injury, but is also capable of affecting the functions and homeostasis of resident CNS cells, for better or worse. Furthermore, researchers are beginning to harness the neurotrophic effects of the immune system to aid in repair and regeneration in the CNS.
Even under normal conditions, activated T and B lymphocytes patrol the CNS in low numbers, whereas naïve lymphocytes are excluded (Hickey 1999; Togo et al. 2002; Engelhardt and Ransohoff 2005). Although fewer activated T cells infiltrate the normal CNS than other tissues (Yeager et al. 2000), this may be owing to the low level of adhesion molecules expressed on endothelial cells under normal conditions (Hickey 2001), whereas increased expression of adhesion molecules leads to increased lymphocyte infiltration. When cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α are secreted by activated glia in the brain, or are present in circulating blood, permeability of the BBB is increased and the expression of cellular adhesion molecules (such as selectins) on microvascular endothelial cells are up-regulated (Wong et al. 1999). Activated T cells and B cells are then able to extravasate and migrate to the site of neuronal injury in increased numbers (Aloisi et al. 1999; McGeer and McGeer 2003; Olson and Miller 2004).
Indeed, abnormalities in the BBB have been shown where T-cell infiltration occurs in neuroAIDS (Petito and Cash 1992; Petito et al. 2003), AD (Rogers et al. 1988; Togo et al. 2002; Desai et al. 2007), and PD (Farkas et al. 2000). Furthermore, whereas the CNS lacks a defined lymphatic system, antigens do exit the CNS via arachnoid villi, cranial nerves, and spinal nerve root ganglia to lymph (Cserr and Knopf 1992). Once in the lymph, these antigens may be taken up by dendritic cells, processed, and presented to T and B cells to mobilize an adaptive immune response to the CNS. Whereas acute neuroinflammation is beneficial to regaining homeostasis and normal function of the CNS after injury or infection, chronic neuroinflammation is damaging to the CNS and may initiate or amplify neurodegeneration associated with HIV-1 encephalitis, AD, or PD.
Cross-Regulation of Adaptive and Innate Immunity in the CNS
Innate immunity consists of the immune mechanisms that are encoded in the germline and are possessed at birth, and work in a “nonspecific” manner, for immediate defense against microbial infection, notably sepsis (Perry 2011; Stearns-Kurosawa et al. 2011). A host’s first line of defense consists of physical barriers such as skin- and cell-regulated enzymes used to clear pathogens and debris, and serves to remove foreign substances by phagocytosis, to recruit immune cells to sites of infection, to activate the complement cascade, but most importantly, to process and present antigens for activation of and recognition by the adaptive immune response (Kim 2005; Filias et al. 2011; Sakaguchi 2011; Sly and Holt 2011; Veerhuis et al. 2011). Its conservation is matched only by its simplicity, except for a broad range of self–nonself pattern-recognition receptors. Such immune activation functions are through nonspecific, generic recognition of common cell signaling pathways shared through a host of endogenous and exogenous factors. These pathways are gaining considerable interest in therapeutic development (Goldman 2007; Basith et al. 2011). Cell debris and foreign matter within the CNS engage toll-like receptors (TLRs), which are expressed by microglia, astrocytes, oligodendrocytes, as well as by neurons (Lv et al. 2011; Zurolo et al. 2011). Engagement of TLRs activates signaling cascades that result in proinflammatory cytokine and chemokine production and in effects on the brain directly or indirectly through glial or BBB function (Franklin et al. 2011; Greenwood et al. 2011; Holman et al. 2011; Kacimi et al. 2011).
The innate immune system also is linked to its adaptive arm through the abilities to provide required “signals” for antigen presentation and to act as final effectors by T-cell-mediated responses in the CNS. The interrelationships between innate and adaptive immunity permit the host to recognize environmental and exogenous cues and work in concert to protect and sustain the host. Central to the innate immune network are microglia (Perry 2011). They secrete both anti- and proinflammatory cytokines and chemokines together with other factors that regulate not only adaptive immunity, but also neural function and neural homeostasis. Those microglial factors found in the brain, cerebrospinal fluid (CSF), and peripheral blood include transforming growth factor beta (TGF-β), IL-1 alpha/beta (IL-1α/β), IL-6, IL-10, IL-12, IL-23, and TNF-α, many chemokines (RANTES/CCL5, MCP-1/CCL2, and IP-10/CXCL10), proteolytic enzymes, matrix metalloproteinases, complement, growth factors, and glutamate (Griffin et al. 1989; Dickson et al. 1993; Moore and Thanos 1996; Qiu et al. 1997). Moreover, COX-2 is present as increased levels of TRAF family member-associated NFκB activator (TANK) and NFKB1 in the SN, and IL-15, RANTES, and IL-10 levels are significantly elevated in brains and peripheral circulation in PD patients (Blum-Degen et al. 1995; Teismann et al. 2003; Rentzos et al. 2007, 2009; Reynolds et al. 2008a). Furthermore, innate immunity regulates lymphocyte infiltration into the CNS. Cytokines, such as IL-1β and TNF-α, secreted by activated glia or endothelial cells increase BBB permeability (Desai et al. 2007), and the expression of cellular adhesion molecules (such as E-selectin) on microvascular endothelial cells are up-regulated (Wong et al. 1999), together increase permeability of the BBB and increase homing, extravasation, and activation of lymphocytes.
Of the antiinflammatory cytokines produced by T cells, macrophages, and microglia, TGF-β modulates injurious responses to the brain (Finch et al. 1993) and suppresses proinflammatory microglia and T-cell responses (Sakaguchi 2004), and as such may represent a neuroprotective host response (Chao et al. 1994). However, TGF-β released from the damaged brain microvasculature contributes to inflammation by increasing expression of endothelial IL-1β and TNF-α (Grammas and Ovase 2001). The engagement of TLRs leads to translocation of nuclear factor-κ light-chain enhancer of activated B cells (NF-κB) and AP-1 to the nucleus where they induce transcription of a broad range of innate immune proteins. Once activated, microglia secrete both neurotrophic and neurotoxic factors (Zhang and Fedoroff 1996; Glezer et al. 2007); proinflammatory cytokines including IL-1α, IL-1β, and TNF-α (Giulian et al. 1986; Sawada et al. 1989); and neurotrophins including nerve growth factor (NGF) and neurotrophin 3 (NT-3) (Elkabes et al. 1996; Heese et al. 1998). However, during chronic inflammation, the neurotoxic effects of microglia are proposed to eventually out-compete the neurotrophic effects, thus increasing neurodegeneration (Rock et al. 2004). In turn, increased inflammation and BBB permeability allow naïve T cells greater entry and accessibility to activated microglia that in the acute phase induce proinflammatory T-cell responses, and can, under chronic conditions, perpetuate the inflammatory state by engaging and activating polarized proinflammatory effector T cells that have expanded in the periphery and ingressed to sites of neurodegeneration.
Induction of Innate and Adaptive Immune Activation by Misfolded Proteins
Evidence abounds for the involvement of misfolded proteins in the pathology of neurodegenerative diseases, as well as in the activation of microglia and antigen-presenting cells (APCs) that function to induce the adaptive immune arm. In PD, LBs are associated with activated microglia and dopaminergic neuronal death. LBs are comprised mostly of α-syn, ubiquitin, and neurofilament (Goldman et al. 1983; Spillantini et al. 1997, 1998; Jellinger 2007) and posttranslationally modified forms of α-syn have an increased propensity to aggregate (Uversky et al. 2005; Cavallarin et al. 2010). These α-syn species are created by ubiquitination (Shimura et al. 2001), phosphorylation (Fujiwara et al. 2002), or oxidation and nitration (Giasson et al. 2000), and are found in LB inclusions, extraneuronally in PD brains (Lee 2008), and in the periphery of PD patients (Beach et al. 2010). Abnormal species of α-syn found in PD patients are also present in LBs of other synucleinopathy-affected brains, including AD and multiple-system atrophy (MSA) (Duda et al. 2000; Giasson et al. 2000; Cavallarin et al. 2010). These observations are supported by in vitro data and animal models of PD in which overexpression of native or mutated forms of α-syn show that aberrant species have an increased propensity to aggregate (Parihar et al. 2009; Koprich et al. 2010). Aggregation of α-syn is also caused by genetic mutations. Although most cases of PD have no family history of disease, point mutations in the gene encoding α-syn (SNCA; OMIM 163890) are linked to autosomal-dominant parkinsonism (PARK1, OMIM 168601) (Polymeropoulos et al. 1996, 1997; Kruger et al. 1998; Zarranz et al. 2004), as are duplications and triplications of the SNCA gene (PARK4, OMIM 605543) (Singleton et al. 2003; Chartier-Harlin et al. 2004), all of which present increased aggregation of α-syn (Narhi et al. 1999; Li et al. 2001; Uversky 2007). Taken together, these observations led to the α-syn burden hypothesis, which posits that sporadic PD results from the inability to clear α-syn, whereas familial PD results from overproduction of normal α-syn, mutations in α-syn that prevent or slow clearance, or mutations in other proteins that normally assist in α-syn clearance (McGeer and McGeer 2008). McGeer further hypothesized that disease can be eliminated with the reduction of α-syn production or prevention of α-syn aggregation. Thus, vaccines currently being developed for PD, target α-syn in order to increase clearance of aggregated and aberrant forms of the protein. However, because evidence supports a nonneuronal cell autonomous theory for PD progression whereby neuroinflammation is strongly implicated (Dawson 2008), immunotherapeutic strategies also incorporate means by which to attenuate the neuroinflammatory component.
Furthermore, reactive oxygen species produced by activated microglia increases nitration of α-syn and neuronal cell death (Shavali et al. 2006); and in turn, immune T cells that recognize nitrated α-syn (N-α-syn) enhance the neurotoxic activities of microglia in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of nigrostriatal degeneration (Reynolds et al. 2008b). Activated T cells and B cells are then able to enter the CNS more readily and migrate to the site of neuronal injury (Aloisi et al. 1999; McGeer and McGeer 2003; Olson and Miller 2004). Indeed, increased BBB permeability is found in both AD and PD, allowing for increased lymphocytic ingress (Rogers et al. 1988; Farkas et al. 2000; Togo et al. 2002; Desai et al. 2007). In this way, activated innate immune cells of the CNS can affect the adaptive immune system in the periphery and recruit cells to the CNS. However, another way in which the adaptive immune system may be activated during neurodegeneration is through the escape of CNS proteins into the periphery. Indeed, aberrant species of disease-specific proteins, including phosphorylated α-syn, are present in tissues outside the CNS in PD patients (Beach et al. 2010). The occurrence of these aberrant forms of α-syn in the periphery, such as the gastrointestinal tract and draining cervical lymph nodes, presents a possible means for exposure to the protein and subsequent activation of the adaptive immune system.
ADAPTIVE IMMUNITY IN PD
From an earlier Perspectives in Biology and Medicine (Johns Hopkins University Press), Abramsky and colleagues broached the possibility that PD may arise from autoimmune blockade of striatal dopamine receptor function (Abramsky and Litvin 1978). Although clinical evidence has not sufficiently supported this possibility, many studies have implicated the adaptive immune system in PD progression. With high glia/neuron ratios of 3:1 in the brain (Lawson et al. 1990) and the density of microglia contained within the SN, the highest of any region in the brain (Kim et al. 2000), any increase in the inflammatory status of the patient may additively, if not synergistically amplify neuroinflammation. Since 1988, when McGeer and colleagues found HLA-DR-positive (activated) microglia phagocytosing-free neuromelanin in post mortem PD SN (McGeer et al. 1988a), activated microglial consistently have been observed in PD patients, whereas others have shown higher expression levels of polymorphic major histocompatibility complex (MHC) class II (MHC II) molecules, HLA-DR and HLA-DQ, expressed by monocytes in the CSF and peripheral blood of PD patients compared with controls (McGeer et al. 1988b; Fiszer et al. 1994a; Lampe et al. 2003). More recently, genome-wide association studies (GWASs) of PD patients (Hamza et al. 2010; Saiki et al. 2010; Nalls et al. 2011; Puschmann et al. 2011; Simon-Sanchez et al. 2011), including a meta-analysis of the GWASs (Nalls et al. 2011), verified an increased relative risk for PD and expression of HLA-DR or HLA-DQ MHC II molecules, leading to the designation of HLA-DRA as PARK18 (Hamza et al. 2010). Increased susceptibility to PD owing to MHC II molecules could reflect increased neuroinflammation associated with up-regulation of those molecules or alternatively, could represent immune responses to self- or weak nonself-antigens. Activated microglia and monocytes in PD brains and CFS secrete proinflammatory and neurotoxic cytokines and chemokines that disrupt the BBB and attract lymphocytes to the site of neuronal injury. Indeed, levels of IL-1β, IL-6, and TNF-α are elevated in the CFS of PD patients (Blum-Degen et al. 1995; Gonzalez-Scarano and Baltuch 1999), and intercellular adhesion molecule-1 (ICAM-1)-positive glia are also increased in the SN of PD brains (Miklossy et al. 2006). Together, these data support the hypothesis that activation of cells of the innate immune system, such as microglia and monocytes, directly contribute to the pathobiology of PD. Furthermore, it has been shown that these cells are activated by overexpression of α-syn or aberrant forms of α-syn. Aberrant posttranslational modifications of α-syn, such as nitration (N-α-syn), can be found in LB inclusions of PD brains (Giasson et al. 2000) and cause the protein to aggregate more readily (Uversky et al. 2005). Aggregated α-syn activates microglia (Zhang et al. 2005), which have been shown to produce nitric oxide and superoxide in mice and inducible nitric oxide synthase (iNOS) in humans, which increases nitration of α-syn and perpetuates the proinflammatory innate immune response in PD (Fig. 1) (Hunot et al. 1996; Gao et al. 2008). Nitrated α-syn in turn amplifies activation of microglia and antigen-presenting cells that correspondingly up-regulate both humoral and cell-mediated responses to nitrated α-syn in the MPTP model (Benner et al. 2008).
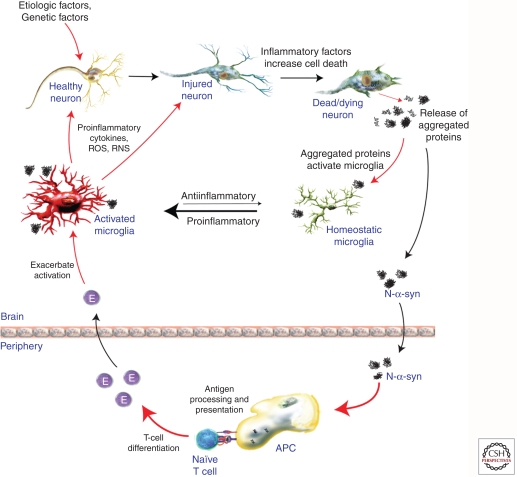
Figure 1.
Immune contributions to PD pathogenesis. Reactive microgliosis is a prominent feature of neurodegenerative diseases such as PD. Microglia are sensitive to changes in their microenvironment, which include factors released from damaged/dying neurons such as modified and aggregated proteins (i.e., α-synculein found in Lewy bodies). Activated microglia may respond to these factors by secreting proinflammatory factors and ROS/RNS that perpetuate neuronal injury and death. Furthermore, modified α-synuclein (e.g., N-α-synunclein) may drain to the periphery where an adaptive immune response is mounted against these antigens. Although increased numbers of lymphocytes are found in the SN of PD patients’ brains, their exact role is not yet known. However, experimental evidence suggests that effector T-cell (E) responses contribute to microglia activation and accelerate neurodegeneration. Taken together, the innate and adaptive immune responses operative in PD may accelerate neurodegeneration and are an active area of research.
Although autoantibodies against dopamine neuron antigens are present in sera and CSF of PD patients (McRae-Degueurce et al. 1988; Dahlstrom et al. 1990; Kunas et al. 1995), the role of the humoral adaptive immune system has only recently begun to be investigated in depth. In addition to a variety of antibodies directed against globally expressed tissue antigens such as heat shock protein (HSP)-65 and HSP-70 (Fiszer et al. 1996), PD patients also show brain-associated autoantibodies including those directed against, GM1, S100B, glial fibrillar acidic protein (GFAP), NGF, neurofilament, myelin basic protein, tau, Aβ, and neuronal calcium channels, as well as α-syn and its modified and fibriliary forms (Elizan et al. 1983; Karcher et al. 1986; Appel et al. 1994; Terryberry et al. 1998; Poletaev et al. 2000; Zappia et al. 2002; Papachroni et al. 2007; Gruden et al. 2011; Yanamandra et al. 2011). Immunohistochemical staining of tissues from idiopathic and familial PD patients show dopaminergic neurons within the SN bind IgG, but not IgM, whereas tissues from age-matched controls and nonnigral control tissues show no detectable bound immunoglobulins (Orr et al. 2005). In one study, IgG reacted with 30% of the dopaminergic neurons within the nigra, and yielded positive correlations with numbers of MHC II+ and CD64+ (FcγRI) reactive microglia, yet yielded a negative correlation with disease duration. In PD patients, approximately 4% of the pigmented neurons contained Lewy bodies and all pigmented, Lewy body-containing neurons showed detectable IgG and α-syn within inclusions. This pattern of antibody reactivity was consistent with activated microglia and destruction of dopaminergic neurons in PD. Together these data suggest that endogenous antibodies of unknown specificity have the capacity to cross the BBB and bind cognate antigens expressed by dopaminergic neurons. Of interest, deletion of the FcγR by genetic ablation inhibits microglial activation and dopaminergic cell death in animal models of PD (He et al. 2002). Moreover, levels of antibodies to α-syn and catecholamine-derived melanin (neuromelanin) are increased in PD patients with antineuromelanin immunoglobulin binding shown to be more active in early disease (Double et al. 2009). Opsonization of cells or damaged neurons with antibody, targets the cells for phagocytosis and degradation by phagocytic macrophages, and also can activate the complement system, a major mediator of immune/inflammatory reactions. Interestingly, activation of the complement system may also be involved in neuronal death. Microglia are the only cells within the SN that express the initial recognition component of complement, C1q (Depboylu et al. 2011). Moreover, compared with controls, PD patients show increased areas of C1q-opsonized extracellular depositions of neuromelanin within the parenchyma, and C1q-expressing phagocytic microglia surround those areas, as well as cells around the luminal surfaces of the vasculature that express neuromelanin and C1q, suggesting a role for antiself antibodies and C1q-mediated clearing pathways in PD.
Along with activated microglia and astrocytes, T cells may also comprise components of PD pathobiology, although the mechanism(s) by which they effect disease remains enigmatic. Early autopsy evidence within the SN of PD patients showed increased numbers of CD8+ T cells in close proximity to activated microglia and degenerating neurons (McGeer et al. 1988a). More recently, both CD4+ and CD8+ T cells have been discovered within the SN of PD patients (Brochard et al. 2009). Although a dysfunctional BBB in PD patients may show some leakiness (Kortekaas et al. 2005), this may not be sufficient to allow unrestricted lymphocyte infiltration because CD4/CD8 ratios were 1:4.8 (Brochard et al. 2009) compared with the typical 2:1 ratio expected for peripheral T cells performing surveillance functions. Thus, the mechanism by which these T cells gain access to the SN, their activation state, and their function are questions that remain to be answered.
Peripheral immune aberrations, particularly in lymphocyte subsets, are abundant in PD patients. Total numbers of lymphocytes have been shown to be diminished by 17%, whereas CD19+ B cells are diminished as much as 35% and CD3+ T cells are diminished by 22% (Bas et al. 2001). Among CD3+ T cells, numbers of CD4+ T cells have been shown to be diminished by 31%, whereas numbers of CD8+ T cells are not significantly changed. A greater loss of naïve helper CD4+ T cells (CD45RA+) and either unchanged or increased levels of effector/memory helper T-cell subset (CD29+ or CD45R0+) have also been observed. Selective loss of CD4+CD45RA+ cells are also detected in other neuoropathological-associated disorders such as MS and Down’s syndrome, suggesting a common immunological abnormality in neurological disorders (Fiszer et al. 1994a; Crucian et al. 1995). Increased frequencies of activated CD4+ T cells expressing Fas (Hisanaga et al. 2001) and increased IFN-γ-producing Th1 cells, decreased IL-4-producing Th2 cells, and a decrease in CD4+CD25+ T cells have been found in the peripheral blood of PD patients (Baba et al. 2005), whereas circulating IL-15, RANTES, and IL-10 are significantly elevated in PD patients compared with controls (Rentzos et al. 2007, 2009). Evidence of increased mutual coexpression of CD4 and CD8 by CD45R0+ T cells, increased expression of CD25 (α chain of the high-affinity IL-2 receptor) and TNF-α receptors, and diminished expression of IFN-γ receptors suggest that these T-cell subsets from PD patients are indeed activated. In addition to T cells that express α and β chains of the T-cell receptor (TCRαβ+ T cells), elevated frequencies of T-cell populations expressing γ and δ chains of the T-cell receptor (TCRγδ+T cells) also have been found in the CSF of PD patients (Fiszer et al. 1994b) and are thought to play a regulatory role in CNS inflammation (Ponomarev and Dittel 2005; Bennett and Stuve 2009; Blink and Miller 2009). Moreover, a large proportion of the TCRγδ+ T cells also express CD25, suggesting these CSF-obtained T cells are preferentially activated in PD patients (Fiszer et al. 1994b). One way in which the adaptive immune system could be mobilized to infiltrate the CNS during PD is through the drainage of aberrant forms of α-syn into the lymphatic system where the protein could activate lymphocytes. Indeed, in MPTP-intoxicated mice, α-syn drains to cervical lymph nodes where it activates antigen-presenting cells and T cells (Benner et al. 2008). An influx of α-syn-specific Th1 or Th17 effector T cells into the brain during PD could increase the inflammatory phenotype and neurotoxic response of microglia near dopaminergic neurons by increasing the concentration of proinflammatory molecules in the SN (Reynolds et al. 2010). Taken together, increased frequencies of memory and activated peripheral T-cell subsets, as well as those cells within the nigra of PD patients, suggest putative roles of T cells in disease progression, if not PD etiology. Although those roles have yet to be delineated, activated effector T cells (Teff) or regulatory T cells (Treg), the latter also showing an effector/memory T-cell phenotype, may migrate to the foci of inflammation in PD patients and either exacerbate or attenuate PD-associated neuroinflammatory responses and neurodegeneration. Thus, Treg have the capacity to keep the disorder in check during the asymptomatic phase, whereas Teff can accelerate disease progression (Fig. 1). Whether T-cell aberrations in PD patients reflect specifically activated effector or regulatory T-cell subsets and to what antigen(s) those T cells are induced, require answers to develop more precise immune-based therapeutic strategies.
ADAPTIVE IMMUNITY FOR THERAPEUTIC GAIN IN PD
It is likely that the adaptive immune system’s response to disease in the CNS is similar in a range of neurodegenerative diseases. Thus, therapeutic strategies aimed at modulating the immune response during disease may be applicable to several neurodegenerative diseases. Here we will discuss recent approaches taken to modulate the adaptive immune system for disease therapy.
Treg are an important subset of CD4+ T cells that are known to maintain self-tolerance, prevent autoimmunity, and regulate immune homeostasis by attenuating excessive inflammation caused by pathogens or injury (Sakaguchi et al. 1995; Cederbom et al. 2000; Kipnis et al. 2002; Hori et al. 2003; Sakaguchi 2004; Coombes et al. 2005; Kim et al. 2007; Bourreau et al. 2009). They are identified by the expression of CD4 and CD25 cell-surface markers and by the transcription factor forkhead box P3 (FoxP3) in mice (Hall et al. 1990; Fontenot et al. 2003; Hori et al. 2003), and the expression of FOXP3, CD4, CD25, CD39, CD49d, and a lack of CD127 in humans (Fletcher et al. 2009; Kleinewietfeld et al. 2009). Although naturally occurring Treg mature in the thymus, naïve CD4+-stimulated T cells in the periphery can be polarized into an inducible Treg (iTreg) phenotype under certain conditions. For example, TGF-β, IL-2, IL-10, and all-trans retinoic acid are known to polarize T cells to iTreg (Zheng et al. 2007, 2008; Khattar et al. 2009; Lee et al. 2009b), whereas histone deacetylase inhibitors are known to increase proliferation and suppressor activity of Treg (Tao et al. 2007; Johnson et al. 2008; Lucas et al. 2009; Saouaf et al. 2009). In vitro studies have shown that Treg can suppress Teff responses via cell-to-cell contact (Cederbom et al. 2000) and soluble factors (Wahl et al. 2004). Treg can also inhibit the adaptive immune system indirectly by suppressing antigen presentation by APCs (Maloy et al. 2003). In the brain, Treg promote neurotrophic support by inducing astrocytes to increase expression of brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) (Benner et al. 2004; Reynolds et al. 2007), and may promote glutamate clearance (Garg et al. 2008). Dysfunctional and reduced frequencies of Treg are associated with several autoimmune diseases (Costantino et al. 2008), including multiple MS (Venken et al. 2008; Fletcher et al. 2009; Royal et al. 2009; Koen 2010), type 1 diabetes (Glisic et al. 2009), inflammatory skin disorders (Fujimura et al. 2008), autoimmune myasthenia gravis (Mu et al. 2009), and rheumatoid arthritis (Cao et al. 2003) as well as chronic inflammatory diseases such as systemic lupus erythematosus (Horwitz 2008; Venigalla et al. 2008; Barreto et al. 2009), asthma (Xue et al. 2007), inflammatory bowel disease (Bourreau et al. 2009), and immune dysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome, which is caused by a genetic mutation in the transcription factor FOXP3 (Bennett et al. 2001). Furthermore, Treg are being investigated for therapeutic use in several of these diseases (Gonzalez-Rey et al. 2006; Brusko et al. 2008; Haas et al. 2009; Putnam et al. 2009; Vandenbark et al. 2009).
In our own works, Treg can migrate from the periphery to the site of HIV-1-induced neuroinflammation in a mouse model of HIV-1 encephalitis (unpubl.). Adoptive transfer of activated CD4+CD25+ Treg to HIV-1 encephalitic mice is neuroprotective (Liu et al. 2009). This study showed that adoptively transferred Treg attenuated microgliosis and astrogliosis, increased expression of BDNF and GDNF expression, and down-regulated proinflammatory cytokines, oxidative stress, and viral replication, whereas effector CD4+ T cells were not therapeutic (Liu et al. 2009). Treg inhibit release of viral particles from HIV-1-infected human mononuclear phagocytes (MP), kill infected cells, and induce phenotypic changes in MP (Huang et al. 2010). Up-regulation of the antiviral ubiquitin-like protein, interferon-stimulated gene 15 (ISG15) was concordant with the decrease in viral release from MP , and implicating caspase-3 and granzyme/perforin pathways in MP killing. Finally, it was shown that Treg induce the phenotypic switch of MP from the neurotoxic MI phenotype to the more neurotrophic M2 phenotype with the down-regulation of iNOS and up-regulation of arginase 1. In the MPTP mouse model, Treg control microglia function by suppressing reactive oxygen species production and NFκB activation via mechanisms that modulate redox enzymes, cell migration, and phagocytosis (Reynolds et al. 2007, 2009, 2010). Furthermore, the adoptive transfer of Treg leads to >90% protection of dopaminergic neurons within the nigrostriatal system, whereas adoptive transfer of Th1 or Th17 Teff exacerbate neuronal degeneration and cotransfer of Treg with Th1 or Th17 increases the protective effect (Reynolds et al. 2010). Together, these data suggest that Treg may be used to suppress the activity of the innate and adaptive immune responses operative in HIV-1 neurodegeneration and PD pathogenesis by transforming the neurotoxic phenotype of activated microglia, Th1, and Th17 cells.
PROSPECTS TOWARD EFFECTIVE IMMUNOTHERAPEUTICS FOR PD
Over the past decade, immunization strategies have been proposed to combat disease progression for neurodegenerative disorders, but principally for AD. Such strategies readily induce humoral immune responses against misfolded protein aggregates to facilitate their clearance in diseased brain tissue. However, such activities also induce robust adaptive immunity against the same misfolded proteins and serve to accelerate disease progression. This is precipitated by induced effector T-cell responses that can lead to encephalitis and profound neural injuries, and has led investigators to search for mechanisms that attenuate such adaptive neurotoxic immune responses. We posit that the Treg responses in PD are dysfunctional during advanced disease states. As such, this serves to shift the balance from regulatory to effector T-cell activities, and yields an inability to attenuate ongoing neurotoxic inflammatory events (Fig. 2). If clearance of misfolded proteins can be achieved with subsequent immune modulation and restoration of Treg responses, improved therapeutic outcomes for neuronal protection would be realized. This may be achieved through advances in immune regulation used to achieve a homeostatic glial response for therapeutic gain.
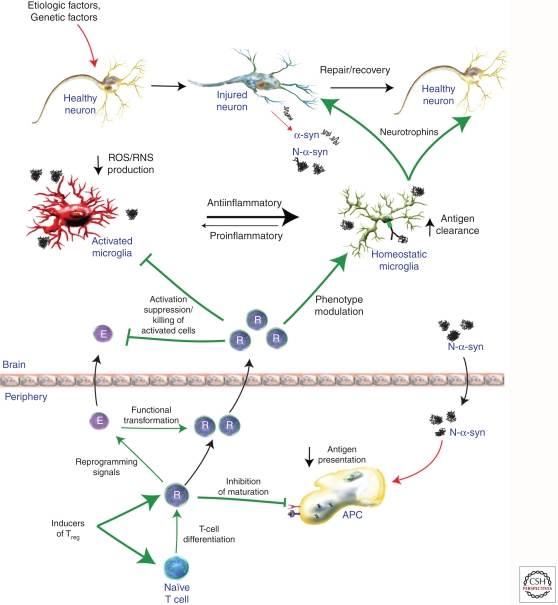
Figure 2.
Adaptive immunity for therapeutic gain. Regulatory T cells (Treg, R) work to control excessive inflammation and may be harnessed to control neuroinflammation in PD and other neurodegenerative diseases. Treg can modulate microglia and other immune cells away from proinflammatory responses to anti-inflammatory/homeostatic functions. Therefore, strategies aimed at inducing, boosting, or reprogramming Treg responses (e.g., functional transformation of Teff [E] to Treg, increasing nTreg numbers, or activating Treg in an antigen-specific manner) show promise as possible disease-modifying therapies. Treg can modulate immune responses via several mechanisms, including suppressing microglia activation, inducing microglia phenotypic switching (toward anti-inflammatory), killing activated cells (e.g., Teff and microglia), and inhibition of APC maturation and antigen presentation. All together, Treg may suppress innate and adaptive proinflammatory, neurotoxic immune responses and boost homeostatic, neurotrophic immune responses resulting in slowing of neurodegeneration and allowing repair of damaged neurons. Such strategies may help to slow or halt the progression of PD.
SUMMARY AND CONCLUSIONS
No doubt, the role of inflammation in PD is now well appreciated. This is supported by data seen in a growing number of laboratory and animal investigations as well as in human studies. Inflammation is linked to the extracellular appearance of brain protein modifications and aberrant protein misfolding, which remain prominent hallmarks of PD-associated dopaminergic neurodegeneration. During disease, dopaminergic neurons accumulate α-synuclein together with other misfolded proteins seen as intracellular Lewy body inclusions. On injury or death, neurons release these proteins to the surrounding neuroenvironment and the modified proteins find their way to the peripheral lymphatic system. In an attempt to clear and digest cellular debris, microglia and blood-borne macrophages infiltrate sites of neuronal injury and death and convert to a proinflammatory activated state. Misfolded and modified α-synuclein species draining from these sites also have the capability to activate antigen-presenting cells in peripheral lymphoid tissues inducing effector neurotoxic T-cell responses. This normally serves to facilitate debris clearance and repair functions. Indeed, under steady-state conditions and early in disease, activated microglia and adaptive immune responses are limited. Nonetheless, as disease becomes more robust, activated microglia are seen in abundant numbers removed from proximate areas of neuronal death. This may be attributable to the failure of activated microglia to return to a homeostatic state. Thus, disease-affected microglia are in a constant activation flux, oscillating between homeostatic and activation states. Interestingly, whereas this cell maintains an activated amoeboid phenotype, microglial functional profiles can encompass a broad range of responses that range from an M1 (proinflammatory neurotoxic) to M2 (anti-inflammatory neuroprotective) activities. Such evolution in cell function may be driven by the deposition and release of nondegradable modified proteins or by infiltrating T lymphocytes. Once modified protein species enters the peripheral lymphoid tissues, activate antigen-presenting cells, and are presented as neoepitopes, adaptive immune responses are induced. Subsequent induction of effector T-cell-mediated responses may profoundly affect microglial states or directly kill neurons. As disease evolves, these responses emerge from a well-regulated neuroprotective state to loss of regulatory function and disease. In this scenario, the engagement of therapeutic strategies that dampen inflammatory and neurotoxic profiles by induction or repair of aberrant regulatory T-cell responses could provide a means to reverse the neurodegenerative process. Thus, modulation of inflammatory responses for therapeutic gain remains an ever-pressing directive to improve disease outcomes.
Footnotes
Editor: Serge Przedborski
Additional Perspectives on Parkinson’s Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abramsky O, Litvin Y 1978. Autoimmune response to dopamine-receptor as a possible mechanism in the pathogenesis of Parkinson’s disease and schizophrenia. Perspect Biol Med 22: 104–114 [PubMed] [Google Scholar]
- Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L 1999. Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur J Immunol 29: 2705–2714 [DOI] [PubMed] [Google Scholar]
- Appel SH, Smith RG, Alexianu M, Engelhardt J, Mosier D, Colom L, Stefani E 1994. Neurodegenerative disease: Autoimmunity involving calcium channels. Ann NY Acad Sci 747: 183–194 [DOI] [PubMed] [Google Scholar]
- Arai H, Furuya T, Mizuno Y, Mochizuki H 2006. Inflammation and infection in Parkinson’s disease. Histol Histopathol 21: 673–678 [DOI] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T 2005. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord 11: 493–498 [DOI] [PubMed] [Google Scholar]
- Barreto M, Ferreira RC, Lourenço L, Moraes-Fontes MF, Santos E, Alves M, Carvalho C, Martins B, Andreia R, Viana JF, et al. 2009. Low frequency of CD4+CD25+ Treg in SLE patients: A heritable trait associated with CTLA4 and TGFβ gene variants. BMC Immunol 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, Buendia E 2001. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol 113: 146–152 [DOI] [PubMed] [Google Scholar]
- Basith S, Manavalan B, Lee G, Kim SG, Choi S 2011. Toll-like receptor modulators: A patent review (2006–2010). Expert Opin Ther Pat 21: 927–944 [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, et al. 2010. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE 2004. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci 101: 9435–9440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, et al. 2008. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One 3: e1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Stuve O 2009. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: Therapeutic implications. Clin Neuropharmacol 32: 121–132 [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Gen 27: 20–21 [DOI] [PubMed] [Google Scholar]
- Blink SE, Miller SD 2009. The contribution of γδ T cells to the pathogenesis of EAE and MS. Curr Mol Med 9: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P 1995. Interleukin-1 βand interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202: 17–20 [DOI] [PubMed] [Google Scholar]
- Bourreau E, Ronet C, Darcissac E, Lise MC, Sainte Marie D, Clity E, Tacchini-Cottier F, Couppie P, Launois P 2009. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect Immun 77: 1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119: 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA 2008. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev 223: 371–390 [DOI] [PubMed] [Google Scholar]
- Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C 2003. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 33: 215–223 [DOI] [PubMed] [Google Scholar]
- Cavallarin N, Vicario M, Negro A 2010. The role of phosphorylation in synucleinopathies: Focus on Parkinson’s disease. CNS Neurol Disord Drug Targets 9: 471–481 [DOI] [PubMed] [Google Scholar]
- Cederbom L, Hall H, Ivars F 2000. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 30: 1538–1543 [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Kravitz FH, Tsang M, Anderson WR, Peterson PK 1994. Transforming growth factor-β protects human neurons against β-amyloid-induced injury. Molec Chem Neuropathol 23: 159–178 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. 2004. α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: 1167–1169 [DOI] [PubMed] [Google Scholar]
- Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F 2005. Regulatory T cells and intestinal homeostasis. Immunol Rev 204: 184–194 [DOI] [PubMed] [Google Scholar]
- Costantino CM, Baecher-Allan CM, Hafler DA 2008. Human regulatory T cells and autoimmunity. Eur J Immunol 38: 921–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucian B, Dunne P, Friedman H, Ragsdale R, Pross S, Widen R 1995. Alterations in levels of CD28−/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol 2: 249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr HF, Knopf PM 1992. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: A new view. Immunol Today 13: 507–512 [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Boche D, Perry VH 2005a. Comparison of inflammatory and acute-phase responses in the brain and peripheral organs of the ME7 model of prion disease. J Virol 79: 5174–5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH 2005b. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25: 9275–9284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Wigander A, Lundmark K, Gottfries CG, Carvey PM, McRae A 1990. Investigations on auto-antibodies in Alzheimer’s and Parkinson’s diseases, using defined neuronal cultures. J Neural Transm Suppl 29: 195–206 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S 2003. Parkinson’s disease: Mechanisms and models. Neuron 39: 889–909 [DOI] [PubMed] [Google Scholar]
- Dawson TM 2008. Non-autonomous cell death in Parkinson’s disease. Lancet Neurol 7: 474–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C, Schafer MK, Arias-Carrion O, Oertel WH, Weihe E, Hoglinger GU 2011. Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol 70: 125–132 [DOI] [PubMed] [Google Scholar]
- Desai BS, Monahan AJ, Carvey PM, Hendey B 2007. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: Implications for drug therapy. Cell Transplant 16: 285–299 [DOI] [PubMed] [Google Scholar]
- Dickson D, Lee S, Mattiace L, Yen S, Brosnan C 1993. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia 7: 75–83 [DOI] [PubMed] [Google Scholar]
- Double KL, Rowe DB, Carew-Jones FM, Hayes M, Chan DK, Blackie J, Corbett A, Joffe R, Fung VS, Morris J, et al. 2009. Anti-melanin antibodies are increased in sera in Parkinson’s disease. Exp Neurol 217: 297–301 [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ 2000. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol 157: 1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizan TS, Casals J, Yahr MD 1983. Antineurofilament antibodies in postencephalitic and idiopathic Parkinson’s disease. J Neurol Sci 59: 341–347 [DOI] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB 1996. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci 16: 2508–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM 2005. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol 26: 485–495 [DOI] [PubMed] [Google Scholar]
- Farkas E, De Jong GI, Apro E, De Vos RA, Steur EN, Luiten PG 2000. Similar ultrastructural breakdown of cerebrocortical capillaries in Alzheimer’s disease, Parkinson’s disease, and experimental hypertension. What is the functional link? Ann NY Acad Sci 903: 72–82 [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Tarelli R 2011. Parkinson’s disease and systemic inflammation. Parkinsons Dis 2011: 436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Veerhuis R 2009. Biomarkers of inflammation and amyloid-βphagocytosis in patients at risk of Alzheimer disease. Exp Gerontol 45: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M 2011. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM 1993. TGF-β1 is an organizer of responses to neurodegeneration. J Cell Biochem 53: 314–322 [DOI] [PubMed] [Google Scholar]
- Fiszer U, Mix E, Fredrikson S, Kostulas V, Link H 1994a. Parkinson’s disease and immunological abnormalities: Increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand 90: 160–166 [DOI] [PubMed] [Google Scholar]
- Fiszer U, Mix E, Fredrikson S, Kostulas V, Olsson T, Link H 1994b. γδ+ T cells are increased in patients with Parkinson’s disease. J Neurol Sci 121: 39–45 [DOI] [PubMed] [Google Scholar]
- Fiszer U, Fredrikson S, Czlonkowska A 1996. Humoral response to hsp 65 and hsp 70 in cerebrospinal fluid in Parkinson’s disease. J Neurol Sci 139: 66–70 [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KHG 2009. CD39+ Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183: 7602–7610 [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336 [DOI] [PubMed] [Google Scholar]
- Franklin BS, Ishizaka ST, Lamphier M, Gusovsky F, Hansen H, Rose J, Zheng W, Ataide MA, de Oliveira RB, Golenbock DT, et al. 2011. Therapeutical targeting of nucleic acid-sensing Toll-like receptors prevents experimental cerebral malaria. Proc Natl Acad Sci 108: 3689–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Okuyama R, Ito Y, Aiba S 2008. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br J Dermatol 158: 1256–1263 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T 2002. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: 160–164 [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM 2008. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28: 7687–7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Banerjee R, Kipnis J 2008. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol 180: 3866–3873 [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM 2000. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290: 985–989 [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB 1986. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med 164: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S 2007. Neuroprotective role of the innate immune system by microglia. Neuroscience 147: 867–883 [DOI] [PubMed] [Google Scholar]
- Glisic S, Klinker M, Waukau J, Jailwala P, Jana S, Basken J, Wang T, Alemzadeh R, Hagopian W, Ghosh S 2009. Genetic association of HLA DQB1 with CD4+CD25+(high) T-cell apoptosis in type 1 diabetes. Genes Immun 10: 334–340 [DOI] [PubMed] [Google Scholar]
- Goldman M 2007. Translational mini-review series on Toll-like receptors: Toll-like receptor ligands as novel pharmaceuticals for allergic disorders. Clin Exp Immunol 147: 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JE, Yen SH, Chiu FC, Peress NS 1983. Lewy bodies of Parkinson’s disease contain neurofilament antigens. Science 221: 1082–1084 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M 2006. Vasoactive intestinal peptide induces CD4+, CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum 54: 864–876 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G 1999. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22: 219–240 [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R 2001. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 22: 837–842 [DOI] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B 2011. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol 37: 24–39 [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL III, Araoz C 1989. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci 86: 7611–7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden MA, Sewell RD, Yanamandra K, Davidova TV, Kucheryanu VG, Bocharov EV, Bocharova OR, Polyschuk VV, Sherstnev VV, Morozova-Roche LA 2011. Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J Neuroimmunol 233: 221–227 [DOI] [PubMed] [Google Scholar]
- Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B 2009. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4+CD25+ FOXP3+CD31+ T-cells in patients with multiple sclerosis. J Neuroimmunol 216: 113–117 [DOI] [PubMed] [Google Scholar]
- Hall BM, Pearce NW, Gurley KE, Dorsch SE 1990. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med 171: 141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, et al. 2010. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42: 781–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Inagaki T, Sawada M, Suzumura A 2000. Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macrophages in Parkinson’s disease. Acta Neurol Scand 101: 159–164 [DOI] [PubMed] [Google Scholar]
- He Y, Le WD, Appel SH 2002. Role of Fcγa receptors in nigral cell injury induced by Parkinson disease immunoglobulin injection into mouse substantia nigra. Exp Neurol 176: 322–327 [DOI] [PubMed] [Google Scholar]
- Heese K, Hock C, Otten U 1998. Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem 70: 699–707 [DOI] [PubMed] [Google Scholar]
- Heesen C, Schulz KH, Fiehler J, Von der Mark U, Otte C, Jung R, Poettgen J, Krieger T, Gold SM 2010. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav Immun 24: 1148–1155 [DOI] [PubMed] [Google Scholar]
- Hickey WF 1999. Leukocyte traffic in the central nervous system: The participants and their roles. Semin Immunol 11: 125–137 [DOI] [PubMed] [Google Scholar]
- Hickey WF 2001. Basic principles of immunological surveillance of the normal central nervous system. Glia 36: 118–124 [DOI] [PubMed] [Google Scholar]
- Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y 2001. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch Neurol 58: 1580–1583 [DOI] [PubMed] [Google Scholar]
- Holman DW, Klein RS, Ransohoff RM 2011. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta 1812: 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH 2009. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73: 768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061 [DOI] [PubMed] [Google Scholar]
- Horwitz DA 2008. Regulatory T cells in systemic lupus erythematosus: Past, present and future. Arthritis Res Ther 10: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Stone DK, Yu F, Zeng Y, Gendelman HE 2010. Functional proteomic analysis for regulatory T cell surveillance of the HIV-1 infected macrophage. J Proteome Res 9: 6759–6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC 1996. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 72: 355–363 [DOI] [PubMed] [Google Scholar]
- Jellinger KA 2007. More frequent Lewy bodies but less frequent Alzheimer-type lesions in multiple system atrophy as compared to age-matched control brains. Acta Neuropathol 114: 299–303 [DOI] [PubMed] [Google Scholar]
- Johnson J, Pahuja A, Graham M, Hering B, Hancock WW, Bansal-Pakala P 2008. Effects of histone deacetylase inhibitor SAHA on effector and FOXP3+regulatory T cells in rhesus macaques. Transpl Proc 40: 459–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacimi R, Giffard RG, Yenari MA 2011. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J Inflamm (Lond) 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ 2008. Alzheimer’s disease and peripheral infections: The possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis 13: 437–449 [DOI] [PubMed] [Google Scholar]
- Karcher D, Federsppiel BS, Lowenthal FD, Frank F, Lowenthal A 1986. Anti-neurofilament antibodies in blood of patients with neurological diseases. Acta Neuropathol 72: 82–85 [DOI] [PubMed] [Google Scholar]
- Khattar M, Chen W, Stepkowski SM 2009. Expanding and converting regulatory T cells: A horizon for immunotherapy. Arch Immunol Ther Exp 57: 199–204 [DOI] [PubMed] [Google Scholar]
- Kim J 2005. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 211: 193–198 [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS 2000. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J Neurosci 20: 6309–6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–197 [DOI] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M 2002. Neuroprotective autoimmunity: Naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci 99: 15620–15625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rötzschke O, Falk K 2009. CD49d provides access to “untouched” human Foxp3+ Treg free of contaminating effector cells. Blood 113: 827–836 [DOI] [PubMed] [Google Scholar]
- Koen V 2010. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med 16: 58. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM 2010. Expression of human A53T α-synuclein in the rat substantia nigra using a novel AAV1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol Neurodegener 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH 2005. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57: 176–179 [DOI] [PubMed] [Google Scholar]
- Kosloski LM, Ha DM, Hutter JA, Stone DK, Pichler MR, Reynolds AD, Gendelman HE, Mosley RL 2010. Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. J Neurochem 114: 1261–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O 1998. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18: 106–108 [DOI] [PubMed] [Google Scholar]
- Kunas RC, McRae A, Kesselring J, Villiger PM 1995. Antidopaminergic antibodies in a patient with a complex autoimmune disorder and rapidly progressing Parkinson’s disease. J Allergy Clin Immunol 96: 688–690 [DOI] [PubMed] [Google Scholar]
- Lampe JB, Gossrau G, Herting B, Kempe A, Sommer U, Fussel M, Weber M, Koch R, Reichmann H 2003. HLA typing and Parkinson’s disease. Eur Neurol 50: 64–68 [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S 1990. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39: 151–170 [DOI] [PubMed] [Google Scholar]
- Lee SJ 2008. Origins and effects of extracellular α-synuclein: Implications in Parkinson’s disease. J Molec Neurosci 34: 17–22 [DOI] [PubMed] [Google Scholar]
- Lee JK, Tran T, Tansey MG 2009a. Neuroinflammation in Parkinson’s disease. J Neuroimmune Pharmacol 4: 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mukasa R, Hatton RD, Weaver CT 2009b. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 21: 274–280 [DOI] [PubMed] [Google Scholar]
- Li J, Uversky VN, Fink AL 2001. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry 40: 11604–11613 [DOI] [PubMed] [Google Scholar]
- Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE 2009. Neuromodulatory activities of CD4+ CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol 182: 3855–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP 2009. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol 257: 97–104 [DOI] [PubMed] [Google Scholar]
- Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z 2011. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience 176: 162–172 [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ 2009. Systemic infection, inflammation and acute ischemic stroke. Neuroscience 158: 1049–1061 [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL 2003. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27: 741–749 [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG 2008. Glial reactions in Parkinson’s disease. Mov Disord 23: 474–483 [DOI] [PubMed] [Google Scholar]
- McGeer EG, Singh EA, McGeer PL 1988a. Peripheral-type benzodiazepine binding in Alzheimer disease. Alzheimer Dis Assoc Disord 2: 331–336 [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG 1988b. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38: 1285–1291 [DOI] [PubMed] [Google Scholar]
- McRae-Degueurce A, Rosengren L, Haglid K, Booj S, Gottfries CG, Granerus AC, Dahlstrom A 1988. Immunocytochemical investigations on the presence of neuron-specific antibodies in the CSF of Parkinson’s disease cases. Neurochem Res 13: 679–684 [DOI] [PubMed] [Google Scholar]
- Medawar PB 1948. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29: 58–69 [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL 2006. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 197: 275–283 [DOI] [PubMed] [Google Scholar]
- Moore S, Thanos S 1996. The concept of microglia in relation to central nervous system disease and regeneration. Progr Neurobiol 48: 441–460 [DOI] [PubMed] [Google Scholar]
- Mu L, Sun B, Kong Q, Wang J, Wang G, Zhang S, Wang D, Liu Y, Liu Y, An H, et al. 2009. Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 128: e826–e836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, et al. 2011. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet 377: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, et al. 1999. Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem 274: 9843–9846 [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173: 3916–3924 [DOI] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM 2005. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 128: 2665–2674 [DOI] [PubMed] [Google Scholar]
- Papachroni KK, Ninkina N, Papapanagiotou A, Hadjigeorgiou GM, Xiromerisiou G, Papadimitriou A, Kalofoutis A, Buchman VL 2007. Autoantibodies to α-synuclein in inherited Parkinson’s disease. J Neurochem 101: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P 2009. α-Synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol 41: 2015–2024 [DOI] [PubMed] [Google Scholar]
- Perry VH 2010. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol 120: 277–286 [DOI] [PubMed] [Google Scholar]
- *.Perry VH 2011. Innate inflammation in Parkinson’s disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a009373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cash KS 1992. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: Immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol 32: 658–666 [DOI] [PubMed] [Google Scholar]
- Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I 2003. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. J Neurovirol 9: 36–44 [DOI] [PubMed] [Google Scholar]
- Poletaev AB, Morozov SG, Gnedenko BB, Zlunikin VM, Korzhenevskey DA 2000. Serum anti-S100b, anti-GFAP and anti-NGF autoantibodies of IgG class in healthy persons and patients with mental and neurological disorders. Autoimmunity 32: 33–38 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, Sanges G, Stenroos ES, Pho LT, Schaffer AA, et al. 1996. Mapping of a gene for Parkinson’s disease to chromosome 4q21–q23. Science 274: 1197–1199 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Dittel BN 2005. γδT cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol 174: 4678–4687 [DOI] [PubMed] [Google Scholar]
- Puschmann A, Verbeeck C, Heckman MG, Soto-Ortolaza AI, Lynch T, Jasinska-Myga B, Opala G, Krygowska-Wajs A, Barcikowska M, Uitti RJ, et al. 2011. Human leukocyte antigen variation and Parkinson’s disease. Parkinsonism Relat Disord 17: 376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA 2009. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58: 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ 1997. Degradation of amyloid β-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J Biol Chem 272: 6641–6646 [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M 2009. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23: 55–63 [DOI] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D 2007. Circulating interleukin-15 and RANTES chemokine in Parkinson’s disease. Acta Neurol Scand 116: 374–379 [DOI] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D 2009. Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol Scand 119: 332–337 [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL 2007. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leuk Biol 82: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, et al. 2008a. Nitrated α-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem 104: 1504–1525 [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Kadiu I, Garg SK, Glanzer JG, Nordgren T, Ciborowski P, Banerjee R, Gendelman HE 2008b. Nitrated α-synuclein and microglial neuroregulatory activities. J Neuroimmune Pharmacol 3: 59–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE 2009. Proteomic studies of nitrated α-synuclein microglia regulation by CD4+CD25+ T Cells. J Proteome Res 8: 3497–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE 2010. Regulatory T cells attenuate th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol 184: 2261–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK 2004. Role of microglia in central nervous system infections. Clin Microbiol Rev 17: 942–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH 1988. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol Aging 9: 339–349 [DOI] [PubMed] [Google Scholar]
- Royal W III, Mia Y, Li H, Naunton K 2009. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol 213: 135–141 [DOI] [PubMed] [Google Scholar]
- Saiki M, Baker A, Williams-Gray CH, Foltynie T, Goodman RS, Taylor CJ, Compston DA, Barker RA, Sawcer SJ, Goris A 2010. Association of the human leucocyte antigen region with susceptibility to Parkinson’s disease. J Neurol Neurosurg Psychiatry 81: 890–891 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann Rev Immunol 22: 531–562 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S 2011. Regulatory T cells: History and perspective. Methods Mol Biol 707: 3–17 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155: 1151–1164 [PubMed] [Google Scholar]
- Saouaf SJ, Li B, Zhang G, Shen Y, Furuuchi N, Hancock WW, Greene MI 2009. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol 87: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Kondo N, Suzumura A, Marunouchi T 1989. Production of tumor necrosis factor-αby microglia and astrocytes in culture. Brain Res 491: 394–397 [DOI] [PubMed] [Google Scholar]
- Shavali S, Combs CK, Ebadi M 2006. Reactive macrophages increase oxidative stress and α-synuclein nitration during death of dopaminergic neuronal cells in co-culture: Relevance to Parkinson’s disease. Neurochem Res 31: 85–94 [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ 2001. Ubiquitination of a new form of α-synuclein by parkin from human brain: Implications for Parkinson’s disease. Science 293: 263–269 [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, van Hilten JJ, van de Warrenburg B, Post B, Berendse HW, Arepalli S, Hernandez DG, de Bie RM, Velseboer D, Scheffer H, et al. 2011. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur J Hum Genet 19: 655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. 2003. α-Synuclein locus triplication causes Parkinson’s disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Sly PD, Holt PG 2011. Role of innate immunity in the development of allergy and asthma. Curr Opin Allergy Clin Immunol 11: 127–131 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M 1997. α-Synuclein in Lewy bodies. Nature 388: 839–840 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M 1998. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci 95: 6469–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG 2011. The pathogenesis of sepsis. Annu Rev Pathol 6: 19–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Bendszus M 2009. Imaging of inflammation in the peripheral and central nervous system by magnetic resonance imaging. Neuroscience 158: 1151–1160 [DOI] [PubMed] [Google Scholar]
- Stolp HB, Dziegielewska KM 2009. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol 35: 132–146 [DOI] [PubMed] [Google Scholar]
- Stone DK, Reynolds AD, Mosley RL, Gendelman HE 2009. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid Redox Signal 11: 2151–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Wang L, Li B, Greene MI, Wells AD, Hancock WW 2007. Histone deacetylase inhibitors and transplantation. Curr Opin Immunol 19: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Perry VH 2009. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: Underlying mechanisms. Neuroscience 158: 1062–1073 [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S 2003. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci 100: 5473–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terryberry JW, Thor G, Peter JB 1998. Autoantibodies in neurodegenerative diseases: Antigen-specific frequencies and intrathecal analysis. Neurobiol Aging 19: 205–216 [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K 2002. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124: 83–92 [DOI] [PubMed] [Google Scholar]
- Uversky VN 2007. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem 103: 17–37 [DOI] [PubMed] [Google Scholar]
- Uversky VN, Yamin G, Munishkina LA, Karymov MA, Millett IS, Doniach S, Lyubchenko YL, Fink AL 2005. Effects of nitration on the structure and aggregation of α-synuclein. Brain Res Molec Brain Res 134: 84–102 [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Huan J, Agotsch M, La Tocha D, Goelz S, Offner H, Lanker S, Bourdette D 2009. Interferon-β-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J Neuroimmunol 215: 125–128 [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, Tenner AJ 2011. Complement in the brain. Mol Immunol 48: 1592–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM 2008. Reduced CD4+, CD25− T cell sensitivity to the suppressive function of CD4+,CD25high,CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthr Rheum 58: 2120–2130 [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P 2008. Natural naive CD4+CD25+ CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: Recovery of memory Treg homeostasis during disease progression. J Immunol 180: 6411–6420 [DOI] [PubMed] [Google Scholar]
- Wahl SM, Swisher J, McCartney-Francis N, Chen W 2004. TGF-β: The perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leuk Biol 76: 15–24 [DOI] [PubMed] [Google Scholar]
- Weiner HL 2008. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J Neurol 255 (Suppl 1): 3–11 [DOI] [PubMed] [Google Scholar]
- Wong D, Prameya R, Dorovini-Zis K 1999. In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: Regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J Neuropathol Exp Neurol 58: 138–152 [DOI] [PubMed] [Google Scholar]
- Xue K, Zhou Y, Xiong S, Xiong W, Tang T 2007. Analysis of CD4+ CD25+ regulatory T cells and Foxp3 mRNA in the peripheral blood of patients with asthma. J Huazhong Univ Sci Technolog Med Sci 27: 31–33 [DOI] [PubMed] [Google Scholar]
- Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L, Morozova-Roche LA 2011. α-Synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson’s disease patients. PLoS One 6: e18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager MP, DeLeo JA, Hoopes PJ, Hartov A, Hildebrandt L, Hickey WF 2000. Trauma and inflammation modulate lymphocyte localization in vivo: Quantitation of tissue entry and retention using indium-111-labeled lymphocytes. Crit Care Med 28: 1477–1482 [DOI] [PubMed] [Google Scholar]
- Zappia M, Crescibene L, Bosco D, Arabia G, Nicoletti G, Bagala A, Bastone L, Napoli ID, Caracciolo M, Bonavita S, et al. 2002. Anti-GM1 ganglioside antibodies in Parkinson’s disease. Acta Neurol Scand 106: 54–57 [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al. 2004. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173 [DOI] [PubMed] [Google Scholar]
- Zhang SC, Fedoroff S 1996. Neuron-microglia interactions in vitro. Acta Neuropathol 91: 385–395 [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J 2005. Aggregated α-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J 19: 533–542 [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA 2007. IL-2 is essential for TGF-α to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178: 2018–2027 [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Horwitz DA 2008. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-βare resistant to Th17 conversion by IL-6. J Immunol 180: 7112–7116 [DOI] [PubMed] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A, et al. 2011. Activation of toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain 134: 1015–1032 [DOI] [PubMed] [Google Scholar]