Abstract
B cells contribute to the pathogenesis of chronic autoimmune disorders like systemic lupus erythematosus (SLE) via multiple effector functions. However, B cells are also implicated in regulating SLE and other autoimmune syndromes via release of IL-10. B cells secreting IL-10 have been termed “Breg” and have been proposed as a separate subset of cells, a concept that remains controversial. The balance between pro- and anti-inflammatory effects could determine the success of B cell targeted therapies for autoimmune disorders and it is therefore pivotal to understand the significance of B cell-secreted IL-10 in spontaneous autoimmunity. By lineage specific deletion of Il10 from B cells we demonstrate that B cell-derived IL-10 is ineffective in suppressing the spontaneous activation of self-reactive B and T cells during lupus. Correspondingly, severity of organ disease and survival rates in mice harboring Il10 deficient B cells are unaltered. Genetic marking of cells that transcribe Il10 illustrates that the pool of IL-10 competent cells is dominated by CD4 T cells and macrophages. IL-10 competent cells of the B lineage are rare in vivo and among them short-lived plasmablasts have the highest frequency, suggesting an activation rather than lineage-driven phenotype. Putative Breg phenotypic subsets such as CD1dhiCD5+ and CD21hiCD23hi B cells are not enriched in Il10 transcription. These genetic studies demonstrate that in a spontaneous model of murine lupus, IL-10 dependent B cell regulation does not restrain disease and thus the pathogenic effects of B cells are not detectably counterbalanced by their IL-10 dependent regulatory functions.
INTRODUCTION
B lymphocytes are pathogenic in autoimmune diseases such as rheumatoid arthritis, SLE, multiple sclerosis and type I diabetes and are a major clinical target for the treatment of these disorders (1). Notwithstanding their capacity to promote autoimmunity by auto-Ab secretion, antigen presentation, and proinflammatory cytokine production, it has become apparent that B cells also exert regulatory functions (2,3).
The documented mechanism by which B cells inhibit an immune response is through secretion of the anti-inflammatory cytokine IL-10. Using mixed bone marrow chimeras, Fillatreau et al. showed that mice did not recover from experimental autoimmune encephalomyelitis (EAE) when they lacked IL-10 specifically in B cells (4). Further, the adoptive transfer of IL-10 sufficient B cells but not IL-10 deficient B cells ameliorated disease in collagen induced arthritis (CIA) and an intestinal inflammation model (5,6).B cell subsets with phenotypes such as CD1dhiCD5+ (7), CD21hiCD23hi (akin to transitional type 2 B cells) (8) or CD23−CD21hi (marginal zone B cells) (9) have been found to be enriched in IL-10+ B cells. Because of the causal association between IL-10 secretion and B cell regulatory function CD1dhiCD5+ B cells even have been labeled as “B10” cells (7). Recently, expression of T cell Ig domain and mucin domain protein 1 (TIM-1) has been described to identify IL-10 producing B cells across diverse B cell phenotypes (10).
IL-10 secreting B cells have mainly been studied in infections and autoimmune syndromes induced by immunization, such as EAE, CIA and adjuvant-induced arthritis (AIA) (4,5,11). Recently, however, B10 cells have been suggested to be protective in NZB/W F1 mice, a mouse model of spontaneous lupus-like disease with polygenic inheritance (12,13). This is of particular importance because such disease models are strongly reflective of human autoimmune conditions and several B cell targeted therapies are currently being investigated for SLE, including the recently-approved anti-B cell activating factor (BAFF) Ab, belimumab. Importantly, in patients with autoimmune diseases, IL10+ B cells have been identified that can inhibit TNF-α production by monocytes in vitro (14). Hence, non-specific B cell directed therapies might be a double-edged sword.
However, as yet there is no direct evidence of a role for IL-10+ B cells in spontaneous autoimmune syndromes such as lupus. Rather, data supporting a role in spontaneous disease comes from therapeutic cell transfer studies. Infusion of anti-CD40 treated CD21hiCD23hi B cells into MRL.Faslpr mice, another mouse model of polygenic spontaneous lupus-like disease, ameliorated lupus (15). Analogous results were obtained by transferring wildtype B10 cells into CD19−/− NZB/W F1 mice (13). Although such transfer studies demonstrate that IL-10 competent B cells have the potential to regulate disease, it is uncertain whether endogenous IL-10+ B cells would naturally do so. Notably, depletion of B cells in 4 wk old NZB/W F1 mice accelerated the disease course (12). Yet, whether this was a consequence of eliminating IL-10+ B cells, as suggested by the authors, was not clear, because all B cells and not just IL-10+ B cells were depleted. Thus, the function of native IL-10+ B cells in context of this disease remains unknown.
In this study we sought to determine the effect of IL-10 secreted by B cells on murine lupus and what aspects of the disease it modulates. To answer these questions we deleted the Il10 gene in cells of the B lineage in the MRL.Faslpr model of lupus. IL-10 exerts a strong protective effect in this strain, as demonstrated by severely exacerbated disease in MRL.Faslpr mice globally lacking in IL-10 (16). The finding that transfer of IL-10-secreting CD21hiCD23hi B cells mitigates disease in MRL.Faslpr mice (15) further suggests that IL-10 derived from B cells restrains disease in this strain.
Surprisingly, despite efficient Il10 gene deletion in the B cell lineage, we discerned no appreciable effect of B cell-derived IL-10 on anti-self B and T cell responses and consequently organ manifestations. This work is the first direct genetic test of whether endogenous B cells via IL-10 really control a spontaneous chronic autoimmune disease. We conclude that while artificially generated and infused IL-10 secreting B cells may be a useful cellular therapy (13,15), the importance of endogenous Bregs may have been overestimated in lupus and possibly other spontaneous chronic autoimmune syndromes.
MATERIALS AND METHODS
Mice
CD19-Cre C57BL/6 mice (17) were backcrossed to the MRL-MpJ-Faslpr/2J strain for 10 generations. Il10fl (18) and 10BiT (19) C57BL/6 mice were backcrossed to MRL-MpJ-Faslpr/J 8 times. MRL-MpJ-Faslpr/2J and MRL-MpJ-Faslpr/J mice were obtained from Jackson Laboratory. Homozygosity for the lpr mutation was verified by PCR. CD19-Cre MRL.Faslpr were intercrossed with Il10fl/wt MRL.Faslpr mice. CD19-Cre Il10fl/wt MRL.Faslpr mice were then crossed with Il10fl/wt MRL.Faslpr animals. To generate mice for the experiments, offspring CD19-Cre Il10fl/fl and Il10fl/fl MRL.Faslpr mice were interbred. Thus, mice in those two groups were littermates. Analogously, offspring CD19-Cre and wt mice were used to expand those two groups. Animals were analyzed at 16 wks of age if not stated otherwise. Animals were maintained under specific-pathogen-free (SPF) conditions and handled according to protocols approved by the Yale Institutional Animal Care and Use Committee (IACUC).
Quantitative PCR
For quantitation of genomic Il10 exon 1, DNA was extracted from FACS purified cells and qPCR was performed with the Agilent Brilliant II SYBR Green QPCR kit. Il10 primers were: forward 5'-GCTCTTACTGACTGGCATGAG-3'; and reverse 5'-CGCAGCTCTAGGAGCATGTG-3'. The amount of Il10 in each sample was normalized to the unaffected gene Tlr9 (forward 5'-ACTCCGACTTCGTCCACCT-3'; and reverse 5'-GGCTCAATGGTCATGTGGCA-3'). To calculate the amount of residual Il10 in various cell types of CD19-Cre Il10fl/fl mice, genomic DNA of the same cell type from Il10fl/fl mice was used as undeleted control. Mice were 10 wks of age. Samples were run on a Stratagene Mx3000P instrument.
Flow cytometry
Surface staining was performed in ice-cold PBS with 3% calf serum in the presence of FcR blocking Ab 2.4G2. Ab clones used for surface staining were: anti-BST2 (927), anti-CD1d (1B1), anti-CD4 (GK1.5), anti-CD5 (53-7.3), anti-CD8 (TIB 105), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD19 (1D3), anti-CD21/35 (7G6), anti-CD22 (Cy34.1), anti-CD23 (B3B4), anti-CD25 (PC61), anti-CD44 (1M7), anti-CD90.1 (1A14), anti-CD93 (AA4.1), anti-CD138 (281-2), anti-F4/80 (BM8), anti-I-A/I-E (M5/114), anti-IgDa (AMS15), anti-IgMa (RS3.1), anti-Ly6G/Ly6C (RB6-8C5) and anti-TCRβ (H57-597). Intracellular staining was performed using the BD Cytofix/Cytoperm and Perm/Wash buffers or, for intracellular FoxP3 staining, the eBioscience FoxP3 staining buffer set. For intracellular cytokine staining, 4 × 106 splenocytes were cultured for 4 hr at 37°C in 24-well plates in 2 ml culture medium containing ionomycin (750 ng/ml) and PMA (20 ng/ml). For the last 2 hr brefeldin A (10 μg/ml) was added to the cultures. Ab clones used for intracellular staining were: anti-FoxP3 (FJK-16), anti-kappa (187.1) and anti-IFN-γ (XMG1.2). Ethidium monoazide (EMA) was used for live-dead discrimination. Cells were analyzed on a LSRII instrument (BD).
Auto-Abs
HEp-2 immunofluorescence assays (Antibodies Inc.) were performed as previously described (20) with serum dilutions of 1:100. Stained slides were read on a Olympus BX-40 microscope. Anti-IgG2a rheumatoid factor and anti-nucleosome IgG serum concentrations were determined by ELISA as previously described (21). The monoclonal Abs 400tμ23 (IgM rheumatoid factor) and PL2-3 (IgG2a anti-nucleosome) were used as standards.
Luminex
IL-10 concentrations in the supernatants of B cell cultures were measured by Luminex assay (Bio-Rad) according to the manufacturer's protocol.
Evaluation of clinical disease
To assess kidney disease, formalin-fixed kidneys were paraffin embedded and sectioned. Sections were then stained with H&E or PAS and scored for glomerular and interstitial nephritis by a pathologist (M.K.), who was blinded to the genotype of the mice. Proteinuria was measured with Bayer Albustix reagent strips. For dermatitis, the size of lesions on the dorsum of the neck and back was scored from 0–4; additionally, 0.5 points were given for dermatitis of each ear and the face. For survival analysis, mice were aged until they succumbed to terminal autoimmune disease or deemed moribund by Yale Veterinary Clinical Services.
RESULTS
B cell specific Il10 deletion in lupus prone mice
We deleted the Il10 gene in B cells by intercrossing MRL.Faslpr mice with an Il10fl allele (18) with MRL.Faslpr mice carrying a CD19-Cre knock-in (17). Deletion of the Il10fl alleles in CD19-Cre IL10fl/fl mice (called B-IL10−/− hereafter) was measured by quantitative PCR on genomic DNA. We found that >90% of the Il10fl alleles were deleted in splenic B cells of the marginal zone (MZ), follicular I (FOL I) and transitional 2 (T2) type and B10 cells (Table I). Deletion in peritoneal B1a, B1b and B2 cells was similarly effective. Purity of sorted B cell populations was about 97% leading to a slight underestimation of Il10 deletion efficiency. No deletion was observed in T cells, macrophages, neutrophils, or conventional and plasmacytoid dendritic cells. Consistent with this, supernatants of sorted B cells from B-IL10−/− and IL10fl/fl mice cultured in the presence of TLR agonists had 10-fold lower IL-10 concentrations than those from control mice (Supplemental Fig. 1). Thus, B-IL10−/− MRL.Faslpr mice are a suitable tool to investigate the function of IL-10 secreting B cells in systemic autoimmunity.
Table I.
Deletion efficiency in B-IL10−/− mice.
| Cell population | n | % Deletion |
|---|---|---|
| MZ B | 3 | 94.1 |
| FOL I B | 3 | 93.2 |
| T2 B | 3 | 94.0 |
| B10 | 3 | 91.1 |
| T | 2 | 0 |
| MΠ | 2 | 0 |
| Neutrophils | 3 | 0 |
| cDC | 2 | 0 |
| pDC | 2 | 0 |
| B1a* | 2 | 95.0 |
| B1b* | 3 | 90.9 |
| B2* | 3 | 88.6 |
| PerMΠ* | 3 | 1.4 |
Measurement of the amount of residual Il10 by quantitative PCR in various cell types of B-IL10−/− mice in the spleen and peritoneal lavage.
Cell populations were purified by FACS. MZ, FOL I and T2 B cells were gated as illustrated in Fig. 2A. B10 cells were identified as shown in Fig. 2D. T cells, TCRβ+CD19−; macrophages (MΠ), CD11b+F4/80hiGr1lo-int; neutrophils, CD11b+Gr1hi; c D C s, C D 1 1 chiIA/IE+CD19−; pDCs, CD11cintBst2+; B1a cells, CD19+CD11b+CD5+; B1b cells, CD19+CD11b+CD5−; B2 cells, CD19+CD11b−. peritoneal MΠ: CD11b+F4/80hi. To calculate % deletion values the equation (1 − residual Il10) × 100 was used. Residual Il10 values were calculated as 2−ΔΔCt comparing B-IL10−/− and IL10fl/fl mice. Negative % deletion values were set to 0.
Deficiency for IL-10 in B cells does not exacerbate organ disease
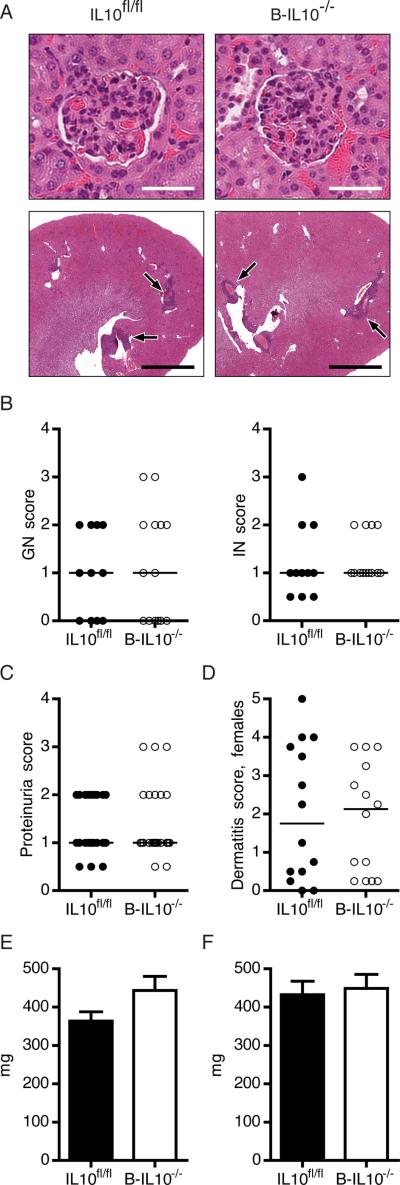
Glomerulonephritis (GN) and interstitial nephritis (IN) in MRL.Faslpr mice is greatly enhanced by global deficiency for IL-10 (16). Severity of GN and IN in 16 week old B-IL10−/− and IL10fl/fl mice was similar, with glomeruli showing hypercellularity and collapsed capillary loops (Fig. 1A, 1B). Interstitial infiltrates were present in the perivascular, peritubular and occasionally periglomerular region in kidneys of all mice (Fig. 1A). Accordingly, B-IL10−/− mice did not have more proteinuria than IL10fl/fl mice (Fig. 1C).
FIGURE 1.
Deletion of Il10 in B cells does not exacerbate organ disease. A, Representative H&E stained kidney sections showing glomeruli (upper images) and perivascular infiltrates (lower images, black arrows). Scale bars represent 50 μm in upper and 2 mm in lower images. B–D, Glomerular and interstitial renal disease (B, n ≥ 12), proteinuria (C, n ≥ 25) and dermatitis severity (D, n = 14) were scored for B-IL10−/− and IL10fl/fl mice. Each dot represents an individu al mouse. Horizontal lines indicate the median. E and F, Weight of spleens (E) and the two largest axillary lymph nodes (F) (n ≥ 25). Data are represented as mean ± SEM in bar graphs. Data shown are combined from 5 experiments.
Cutaneous lupus manifestations in the MRL.Faslpr strain include facial rash, ulceration of the ears and lesions of the back and neck. In female mice dermatitis occurs more frequently than in males. The extent of dermatitis was not different between female B-IL10−/− and IL10fl/fl mice (Fig. 1D). Likewise, male mice in both groups had equally severe dermatitis (Supplemental Fig. 2).
MRL mice spontaneously develop splenomegaly and lymphadenopathy, as do many SLE patients during active disease. Measurement of spleen (Fig. 1E) and axillary lymph node (Fig. 1F) weight revealed no differences between B-IL10−/− and IL10fl/fl mice.
The inability of B cell-secreted IL-10 to modulate lupus-like organ manifestations prompted us to ask whether disruption of one copy of the CD19 gene by the CD19-Cre allele, might influence disease expression, countering the effect of Il10 deletion in B cells. Hemizygosity of CD19 results in lower expression levels on B cells, possibly changing the threshold for B cell receptor signaling. We therefore generated a cohort of CD19-Cre and wildtype MRL.Faslpr mice and performed a similar analysis as for B-IL10−/− and IL10fl/fl MRL.Faslpr mice. We observed no significant alterations in nephritis, dermatitis, splenomegaly or lymphadenopathy in CD19-Cre mice compared to wild type animals (Supplemental Fig. 2). Thus, CD19 hemizygosity was not a confounding factor in our study. Taken together, the analysis demonstrates that B cell specific Il10 deletion does not aggravate end-organ disease in lupus.
B cell homeostasis is unperturbed in B-IL10−/− mice
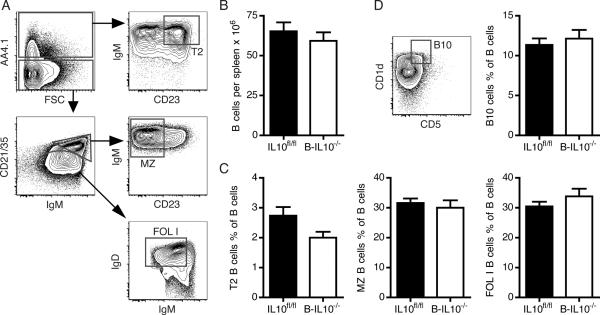
It has been proposed that IL-10 regulates B cell differentiation and survival (22). We examined whether IL-10 secreted by B cells influences B cell homeostasis in an autocrine or paracrine manner. Splenic B cell numbers in B-IL10−/− mice were not different from those in IL10fl/fl mice (Fig. 2B). Absence of B cell-secreted IL-10 did not affect B cell subset frequencies (Fig 2A, C, D). In particular we did not observe significant changes in frequencies of transitional type 2 B cells (Fig. 2C) and B10 cells (Fig. 2D), both of which have been ascribed regulatory functions (7,8). For transitional type 2 B cells there was a downtrend in frequency in B-IL10−/− mice (p = 0.11), which was also evident in absolute numbers per spleen but this did not reach significance. Splenic transitional type 2 B cell numbers were 1.63×106 ± 0.23×106 (mean ± SEM) for IL10fl/fl mice and 1.15×106 ± 0.17×106 for B-IL10−/− mice (p = 0.10).
FIGURE 2.
B cell-derived IL-10 does not affect B cell homeostasis. A, Contour plots show gating of T2, MZ and FOL I B cells after exclusion of IgM−IgD− cells and gating on CD19+ cells. B, Numbers of B cells (CD19+CD22+) per spleen (n ≥ 15). C, Frequencies of T2, MZ and FOL I B cells as a percentage of total B cells (n ≥ 8). B cell subsets were gated as depicted in (A). D, Frequency of B10 cells as a percentage of total B cells (n ≥ 11). The contour plot illustrates how B10 cells were identified after gating on CD19+TCR− cells. Data shown are combined from 3 experiments (mean ± SEM).
Auto-Ab formation is not enhanced in mice lacking IL-10 in B cells
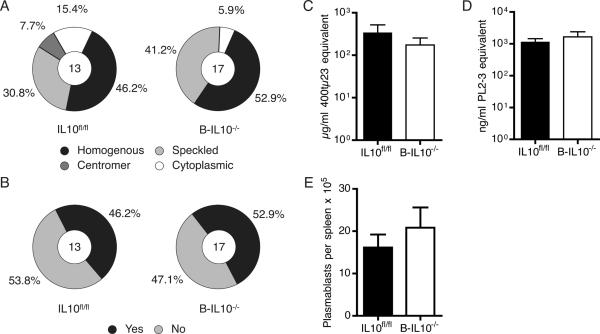
To ask whether Il10 expression in B cells regulates the humoral response against self in lupus, we used the HEp-2 cell-based immunofluorescent microscopy assay to detect serum IgG antinuclear and anticytoplasmic Abs. 9 of 17 (52.9%) sera from B-IL10−/− mice demonstrated homogenous nuclear staining and equatorial staining of mitotic chromatin, corresponding to anti-chromatin (Fig. 3A, B). A similar fraction of sera from IL10fl/fl mice (6 of 13, 46.2%) produced these same staining patterns (Fig. 3A, B). A speckled nuclear staining pattern, corresponding to Abs that recognize RNA or RNA-associated proteins, was observed for 41.2% of sera from B-IL10−/− and 30.8% of sera from IL10fl/fl animals. ELISAs for serum rheumatoid factor (Fig. 3C) and anti-nucleosome IgG (Fig. 3D) demonstrated similar concentrations in B-IL10−/− and IL10fl/fl mice.
FIGURE 3.
Auto-Ab formation is not enhanced in mice lacking IL-10 in B cells. A and B, Hep-2 ANA staining patterns classified as homogenous, speckled, centromere, or cytoplasmic (A) and mitotic chromatin staining classified as positive or negative (B) produced by sera from B-IL10−/− and IL10fl/fl mice. The numbers in the circles indicate the numbers of mice analyzed in each group. C and D, ELISAs showing serum concentrations of anti-IgG2a rheumatoid factor (C) and anti-nucleosome IgG (D) (n ≥ 13). E, Plasmablasts were enumerated in spleens (n ≥ 15). After exclusion of T cells plasmablasts were gated as CD19loCD22loCD44hiCD138hiSSCint cells. Plasmablast data are pooled from 3 experiments. Hep-2 assay and ELISAs were performed once with mouse sera prepared in 3 experiments. Data are represented as mean ± SEM in bar graphs.
In MRL.Faslpr mice and other lupus-prone mouse strains, auto-Abs derive in large part from short-lived plasmablasts in the spleen (23). As expected, B cell specific IL-10 deficiency did not alter splenic plasmablast numbers as determined by flow cytometry (Fig. 3E). Similar evaluation of CD19-Cre and wild type MRL.Faslpr animals revealed no confounding effect of the CD19-Cre knock-in per se (Supplemental Fig. 3). We conclude that B cell-secreted IL-10 plays no role in B cell homeostasis, activation, plasmablast differentiation, and auto-Ab formation.
B cell-derived IL-10 does not affect T cell activation or differentiation
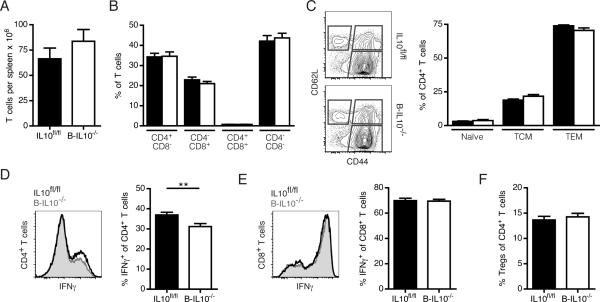
T cells contribute considerably to the pathogenesis of SLE (24). B cells affect T cells in autoimmunity in Ab-independent ways that probably involve both antigen presentation and cytokine secretion (25,26). We explored whether T cell autoimmunity is tempered by B cell-derived IL-10. T cell numbers in the spleen were unaltered in B-IL10−/− mice compared to IL10fl/fl mice (Fig. 4A). CD4 and CD8 staining did not reveal any changes in T cell composition in the absence of B cell-secreted IL-10 (Fig. 4B), including CD4−CD8− cells that typically accumulate in Faslpr animals. In MRL.Faslpr mice there is a paucity of phenotypically naïve T cells; this compartment amounted to less than 4% of all CD4+ T cells in both B-IL10−/− and IL10fl/fl mice (Fig. 4C).
FIGURE 4.
B cell-derived IL-10 does not constrain T cell activation, expansion or differentiation into effectors. A, T cell numbers in the spleen. B, Frequencies of CD4+, CD8+, CD4+CD8+ and double-negative T cells as a percentage of total T cells of IL10fl/fl (black bars) and B-IL10−/− (white bars) mice. C, CD44 and CD62L staining of CD4+ T cells of IL10fl/fl (black bars) and BIL10−/− (white bars) mice to identify naïve (CD44−CD62L+), central-memory (CD44+CD62L+, TCM) and effector memory (CD44+CD62L−, TEM) populations. Representative flow cytometric data on gated CD4+ T cells is shown on the left. D and E, Intracellular IFN-γ staining of PMA/ionomycin-stimulated splenocytes gated on CD4+ (D) or CD8+ E, T cells. Histograms show representative flow cytometric data. Statistics were calculated by two-tailed Mann-Whitney U test. **P < 0.01. (F) Frequency of Tregs (FoxP3+CD25+) as a percentage of CD4+ T cells. n ≥ 15. Data shown are combined from 3 experiments (mean ± SEM).
In myeloid cells IL-10 inhibits the transcription of p35 and p40, the subunits of IL-12 (27). IL-12 release by dendritic cells and macrophages induces differentiation of T helper and cytotoxic cells into IFN-γ secreting effectors. Excessive production of IFN-γ has been linked to SLE pathogenesis (28). In MRL.Faslpr mice deletion of Ifng or Ifngr1 dramatically ameliorates disease (29,30). To determine whether B cell-derived IL-10 suppresses differentiation of CD4+ and CD8+ T cells into IFN-γ secreting effectors we stained splenocytes for intracellular IFN-γ after 4 hr culture with phorbol myristate acetate (PMA) and ionomycin. The frequency of IFN-γ producing cells was decreased among CD4+ (Fig. 4D) but not CD8+ (Fig. 4E) T cells in B-IL10−/− mice. However, the effect was small. B cells can expand Tregs (31–33), prompting us to test whether B cells promote differentiation into Tregs via IL-10 secretion. We found similar percentages of CD4+ T cells from B-IL10−/− and IL10fl/fl mice to be FoxP3+CD25+ (Fig. 4F). The CD19-Cre knock-in itself did not affect any of these T cell phenotypes (Supplemental Fig. 4). Thus, Il10 deletion in B cells has no effect on the activation, expansion or differentiation of T cells.
IL-10 produced by B cells confers no survival advantage
The question remained whether B cell produced IL-10 might have many subtle effects each of which individually is not detectable but together would have an impact on disease outcome. We therefore conducted a survival analysis. The median survival of B-IL10−/− and IL10fl/fl mice was 139 and 142 days, respectively (Fig. 5). A logrank test showed no difference between the survival curves of both groups (p = 0.57).
FIGURE 5.
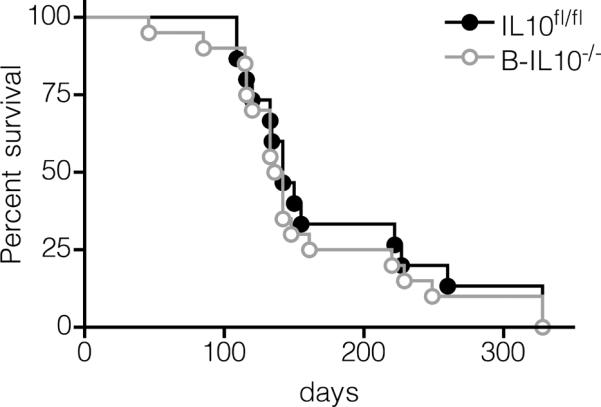
IL-10 produced by B cells confers no survival advantage. Kaplan-Meier survival curves of IL10fl/fl (n = 15, 9 females and 6 males) and B-IL10−/− (n = 20, 12 females and 8 males) mice.
B cells are only a minor source of IL-10 in MRL.Faslpr mice
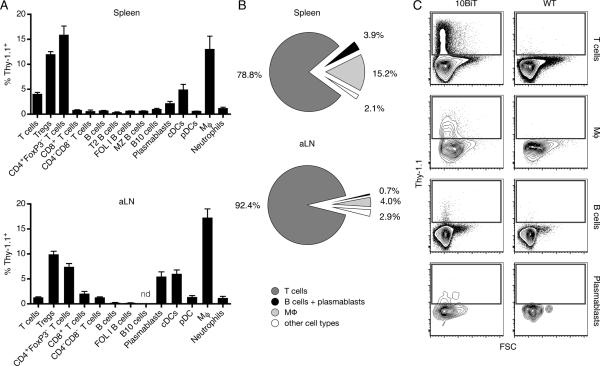
We sought to investigate why, in contrast to the proposed theory that IL-10 competent B cells have a protective role in lupus (12,13), Il10 deletion in B cells had no appreciable effect on disease expression. For this purpose we crossed the IL-10 reporter locus 10BiT (19) that encodes Thy-1.1 under the control of an Il10 promoter, thus marking cells that transcribe Il10, on the MRL.Faslpr background. In the spleen of aged 10BiT MRL.Faslpr mice the percentage of Thy-1.1+ cells was highest in CD4+FoxP3− T cells (15.8%), macrophages (12.7%) and Tregs (11.9%) (Fig. 6A, Upper). The same cell types most frequently upregulated Thy-1.1 in axillary lymph nodes (Fig. 6A, Lower). Only very few B cells were Thy-1.1+ (less than 1%) in both tissues. Importantly, we did not observe an enrichment of Thy-1.1 expressing B cells in putative Breg subsets such as B10 and T2. In fact, almost all splenic B cells in the FACS gates that delineate the B10 and T2 subsets lacked Thy-1.1 expression. In the axillary lymph nodes, B10 phenotype cells expressing Thy-1.1 were undetectable. Notably, a substantial fraction of CD138hiCD19loCD44+intracellular-kappahi cells (2.1% in the spleen and 5.5 % in the axillary lymph nodes), which are mainly short-lived plasmablasts (34), had detectable Thy-1.1 expression, indicating IL-10 mRNA synthesis. However, B cells and plasmablasts together constituted only 3.9% of the total Thy-1.1+ population in the spleen (Fig. 6B, Upper) and 0.7% in axillary lymph nodes (Fig. 6B, Lower). In contrast, 78.8% of Thy1.1+ cells in the spleen and 92.4% in axillary lymph nodes were T cells. Expression levels of Thy-1.1 were markedly higher in T cells than in all other cell types, likely reflecting a capacity of T cells to produce greater amounts of IL-10 (Fig. 6C). In young, pre-autoimmune 10BiT MRL.Faslpr mice, most analyzed cell subsets contained lower percentages of Thy-1.1+ cells than in aged animals (Table II) indicating that Il10 transcription is induced over the disease course. Induction was particularly strong in CD4+FoxP3− T cells and macrophages. The fraction of Thy-1.1+ cells in the Treg compartment remained essentially unchanged (11.9% in aged mice vs. 9.7% in young mice). In conclusion, T cells and macrophages are the predominant cell types that express Il10 in MRL.Faslpr mice. IL-10 competent B cells (Thy-1.1+), on the contrary, are rare and are not restricted to a specific B cell phenotypic subset, although they are more common among plasmablasts.
FIGURE 6.
T cells and macrophages are the main source of IL-10 in MRL.Faslpr mice. A and B, Flow cytometry of spleen and axillary lymph node single-cell suspensions of 16 wk old 10BiT MRL.Faslpr mice (n = 4). Bar graphs in (A) show the frequency of Thy-1.1+ cells in various cell populations. Pie charts in (B) illustrate the composition of the total Thy-1.1+ cell pool. C, Representative contour plots showing Thy-1.1 expression of T cells, macrophages, B cells and plasmablasts (CD138hiCD19loCD44+intracellular-kappahi). Data are combined from two experiments (mean ± SEM in bar graphs).
Table II.
Il10 transcription is strongly induced in CD4+FoxP3− T cells and macrophages during lupus.
| Cell population | n | % Thy-1.1+ |
|
|---|---|---|---|
| 16 wk | 5 wk | ||
| T cells | 4 | 3.97 | 2.02 |
| Tregs | 4 | 11.92 | 9.74 |
| CD4+FoxP3− T cells | 4 | 15.81 | 0.89 |
| CD8+ T cells | 4 | 0.75 | 1.08 |
| CD4−CD8− T cells | 4 | 0.52 | 2.27 |
| B cells | 4 | 0.63 | 0.10 |
| T2 B cells | 4 | 0.30 | nd |
| FOL I B cells | 4 | 0.59 | 0.03 |
| MZ B cells | 4 | 0.61 | nd |
| B10 cells | 4 | 0.94 | 0.28 |
| Plasmablasts | 4 | 2.09 | |
| cDCs | 4 | 4.86 | 0.13 |
| pDCs | 4 | 0.55 | 0.87 |
| MΠ | 4 | 12.96 | 0.53 |
| Neutrophils | 4 | 1.13 | 0.10 |
Flow cytometry of spleen single cell suspensions of 16 and 5 wk old 10BiT MRL.Faslpr mice. The data for the 16 wk old mice is the same as in bar graph Fig. 6A and is shown here in tabular form for easier comparison to the results obtained from 5 wk old animals. nd, no detectable Thy-1.1 expression.
DISCUSSION
In this study, we have addressed the role of IL-10 derived from endogenous, unmanipulated B cells in spontaneous chronic autoimmune disease. By deleting Il10 specifically in B cells in lupus prone mice we demonstrate that B cell-secreted IL-10 has no protective effect in lupus. This was reflected in equally severe organ disease, similar degrees of immune system activation, and indistinguishable survival rates in MRL.Faslpr mice that lack IL-10 specifically in B cells. Consistent with those results, using reporter mice, we found that B cells were only a minor source of IL-10 in vivo. By deleting Il10 in B cells from birth in this model we maximized the opportunity for IL-10+ B cells to exert regulatory effects without making prior assumptions at what stage of disease those might ensue. The extent of deletion, though not 100%, as with every Cre-loxP system, was 10–20 fold, which we believe should have been more than enough to reveal a phenotype if B cell-secreted IL-10 were truly regulating spontaneous lupus in a biologically significant fashion. Thus, our work indicates that B cell-derived IL-10 is not a principal regulator of disease in murine lupus.
Based on the existing paradigm (12,13,15) and prior results in MRL.Faslpr mice (15,16), our findings are unexpected. Endogenous IL-10+ B cells do regulate the immune response in infections with bacteria, viruses and parasites (35–37). Directly pertinent to autoimmunity, IL-10-producing B cells contained disease in EAE (4) and AIA (11). An important difference between these diseases and lupus in MRL.Faslpr mice could be the nature and kinetics of the initiating stimulus. In infectious disease models, as well as induced “autoimmunity” models such as EAE and AIA, the immune response is incited by inoculation with a pathogen or an antigen in combination with an adjuvant, resulting in sudden onset of immune response and pathology. In contrast, spontaneous chronic autoimmune diseases like lupus have a gradual onset. B cells might require abrupt and strong stimulation for robust IL-10 production. Thus B cell-derived IL-10 could be critical in pathogen responses and induced models of autoimmunity whereas syndromes of chronic autoimmunity in humans—such as SLE, rheumatoid arthritis, type 1 diabetes and multiple sclerosis—and mice may not be regulated by B cell-secreted IL-10, even if IL-10+ B cells might be therapeutic when infused.
It has been described that CD24hiCD38hi B cells from healthy individuals suppress T helper 1 cell differentiation in vitro in a partially IL-10 dependent manner (38). In contrast, CD24hiCD38hi B cells from SLE patients lacked an equivalent suppressive capacity and expressed less IL-10 after stimulation. These results, although in vitro, are consistent with our findings that in lupus B cells do not bring their regulatory potential to bear.
To define the cells that produce IL-10 in the context of ongoing autoimmunity we used 10BiT reporter mice on the MRL.Faslpr background. B cells represented only a minor fraction of Il10 transcribing cells and had low expression levels. Importantly, the vast majority of B cells with a CD1dhiCD5+ or CD21hiCD23hi phenotype did not spontaneously synthesize IL-10 mRNA in vivo. From these data, we cannot confirm that there are bona fide, discrete, IL-10 producing, Breg subsets at least during active murine lupus. However, there was a considerable plasmablast population that transcribed Il10. This argues that the stimuli that lead to the acquisition of IL-10 competence frequently induce plasmablast differentiation at the same time. Similar findings were reported for Vert-X C57BL/6 mice, another IL-10 reporter mouse, after challenge with different immunogens (35). Whether the B cells that gave rise to IL-10 competent plasmablasts had a specific phenotype is unclear. Because plasmablasts are short-lived, our results imply that in lupus IL-10 producing B cell progeny represent a transient activation state and not a stable cell lineage with homeostatic regulation.
Both resting (39,40) and activated (3) B cells can suppress immune responses. Signals that have repeatedly been found to induce IL-10 production in B cells are Toll-like receptor activation, particularly in combination with B cell receptor ligation, and CD40 stimulation (41). In MRL.Faslpr mice self-reactive B cells are spontaneously activated via TLR7/9 and B cell receptor cross-linking by immune complexes (42). Further CD40L deficient MRl.Faslpr mice do not develop nephritis or make rheumatoid factor and anti-dsDNA autoantibodies (43) arguing that CD40-CD40L interactions occur in this strain. Hence it is reasonable to assume that B cells receive signals in vivo that are known to induce IL-10. Chronic exposure to those signals, however, might have a different outcome than acute stimulation or other factors might impede a regulatory B cell phenotype in MRL.Faslpr mice.
Recently, it was reported that deletion of all mature B cells, including B10 cells, in young preautoimmune NZB/W F1 mice accelerates disease onset and decreases survival time (12). CD19−/−NZB/W F1 mice had exacerbated nephritis paralleled by a reduction of B10 cells (13). Both studies were interpreted to support a protective effect of B10 cells in lupus. However, in these studies the total mature B cell population was either depleted or genetically impaired, but it was not directly tested whether the observed effects were actually caused by the lack of B10 cells or any other IL-10 producing B cell population. Many other mechanisms could explain the observed effects. Altered activation state of macrophages after uptake of Ab coated B cells during the depletion process, indirect effects on the T cell compartment owing to lack of global B cell interactions, structural changes in lymphoid architecture or skewing of residual or regenerating B cell compartments could all potentially account for these earlier findings.
Hypothetically it is possible that B cells can employ IL-10 independent mechanisms to suppress an immune response that are potent enough to compensate for Il10 deficiency. However, in vivo evidence for such mechanisms has yet to be presented. Rather, in essentially all papers on Bregs in which a mechanism of regulation has been demonstrated, IL-10 is implicated (4–7). Of greatest relevance to the present data, in MRL.Faslpr mice regulatory effects of infused B cells are clearly IL-10 dependent (15). Even if Bregs were to possess inhibitory means apart from IL-10, published studies indicate that Il10 transcription would at least identify most regulatory B cells. Yet, the fraction of Il10 transcribing B cells in MRL.Faslpr mice was very small. Our data therefore does not favor the interpretation that other factors than IL-10 account for suppressive effects of endogenous Bregs in lupus. In any case, it is important to emphasize that the previously-implicated mechanism of B cell regulation in this instance was not validated when directly tested.
Our results, along with studies of B cell-targeted therapies in humans (1,44) and mice (45,46), suggest that B cells have a net pathogenic role in lupus that is not substantially counterbalanced by their IL-10 dependent regulatory functions. Using lupus prone mice bearing an IL-10 reporter transgenic locus we did not identify distinct B cell subsets that are enriched for Il10 transcription (other than plasmablasts), calling into question the existence of specific Breg populations, at least in the context of ongoing lupus. Further, our findings should precipitate a rethinking of whether endogenous B cells exist that regulate spontaneous chronic autoimmunity and emphasize the need to define the variables that govern B cell regulatory capacity in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Yale Animal Resources Center for outstanding animal husbandry.
This work was supported by National Institutes of Health grant R01-AR044077 (M.J.S.) and Deutsche Forschungsgemeinschaft (fellowship to L.L.T.).
Abbreviations used in this paper
- AIA
adjuvant-induced arthritis
- ANA
anti-nuclear Ab
- Breg
regulatory B cell
- CIA
collagen induced arthritis
- EAE
experimental autoimmune encephalomyelitis
- FOL 1
follicular 1 type
- MZ
marginal zone
- SLE
systemic lupus erythematosus
- T2
transitional 2 type
Footnotes
DISCLOSURES The authors have no financial conflicts of interest.
REFERENCES
- 1.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 2.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Lampropoulou V, Calderon-Gomez E, Roch T, Neves P, Shen P, Stervbo U, Boudinot P, Anderton SM, Fillatreau S. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol Rev. 2010;233:146–161. doi: 10.1111/j.0105-2896.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 4.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 5.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of Arthritis by Interleukin 10--producing B Cells. The Journal of experimental medicine. 2003;197:489. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 7.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 9.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. Journal of Clinical Immunology. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 10.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011 doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice Lacking Endogenous IL-10-Producing Regulatory B Cells Develop Exacerbated Disease and Present with an Increased Frequency of Th1/Th17 but a Decrease in Regulatory T Cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 12.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, McNiff J, Madaio MP, Craft J. IL-10 regulates murine lupus. J Immunol. 2002;169:2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 17.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated muta-genesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Müller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 20.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 Regulates TLR7- and MyD88-Dependent Autoantibody Production and Disease in a Murine Model of Lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 23.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 24.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 28.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 29.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 31.Zheng J, Liu Y, Lau YL, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4(+) regulatory T cells. Cell Mol Immunol. 2010;7:44–50. doi: 10.1038/cmi.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta3. Eur J Immunol. 2008;38:2488–2498. doi: 10.1002/eji.200838201. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Jensen PE. Cutting edge: primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 34.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 35.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Gröbe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Müller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 37.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kühl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderón-Gómez E, Lampropoulou V, Shen P, Neves P, Roch T, Stervbo U, Rutz S, Kühl AA, Heppner FL, Loddenkemper C, Anderton SM, Kanellopoulos JM, Charneau P, Fillatreau S. Reprogrammed quiescent B cells provide an effective cellular therapy against chronic experimental autoimmune encephalomyelitis. Eur J Immunol. 2011 doi: 10.1002/eji.201041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 42.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Xu J, Madaio MP, Peng Q, Zhang J, Grewal IS, Flavell RA, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- 44.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA, BLISS-52 Study Group Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 45.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 46.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.