Abstract
Anhedonia, or markedly diminished interest or pleasure, is a hallmark symptom of major depression, schizophrenia, and other neuropsychiatric disorders. Over the past three decades, the clinical definition of anhedonia has remained relatively unchanged, although cognitive psychology and behavioral neuroscience have expanded our understanding of other reward-related processes. Here, we review the neural bases of the construct of anhedonia that reflects deficits in hedonic capacity, and is also closely linked to the constructs of reward valuation, decision-making, anticipation, and motivation. The neural circuits subserving these reward-related processes include the ventral striatum, prefrontal cortical regions, and afferent and efferent projections. Understanding anhedonia and other reward-related constructs will facilitate diagnosis and treatment of disorders that include reward deficits as key symptoms.
Introduction
“For it is then that we have need of pleasure, when we feel pain owing to the absence of pleasure.” Epicurus (341-270 B.C.) [1]
Anhedonia, or loss of interest or pleasure in all or almost all activities, is a prominent symptom of many neuropsychiatric disorders, most notably major depressive disorder (MDD) and schizophrenia (Glossary) [2]. Greek philosophers, such as Epicurus, contemplated the nature of pleasure (and the absence of pleasure) over two thousand years ago. Today, however, reward-related deficits experienced by individuals with MDD, schizophrenia, and other neuropsychiatric disorders involve more than just an absence or loss of pleasure. Anhedonia is a core feature of reward deficits because the capacity to feel pleasure is a critical step during the normal processing of rewards. However, having the motivation to seek out pleasurable experiences and making appropriate decisions based on those previous experiences are important processes that are equally, if not more in some cases, disturbed in individuals with MDD or schizophrenia. Deficits in these reward processes are often inappropriately labeled under the umbrella of anhedonia. The current preclinical and clinical literature regarding the neural bases of the various aspects of reward processing, and the contribution of deficits in these reward processes to the clinical symptom of anhedonia, will be reviewed here.
A Brief History of Anhedonia
Anhedonia as a psychopathological symptom was first noted in the early 19th century. Haslam, who documented the first complete study of a psychiatric patient in 1809 (suffering from schizophrenia), noted a “neglect [of] those objects and pursuits which formerly proved sources of delight and instruction [3].” The term “anhedonie” was later introduced by the French psychologist Ribot in 1896 to describe the counterpart to analgesia in his patients, for which “it was impossible to find the least pleasure [4].” Nonetheless, the term anhedonia was subsequently seldom used to describe general signs of apathy or indifference that were often considered part of a myriad of behavioral abnormalities characterizing disorders, such as schizophrenia. In 1956, Rado offered a contradictory view, that the inability “to experience such pleasures [and] lacking the drive to pursue rewarding activities” was an inherited predisposition to schizophrenia [5]. Meehl corroborated this view and listed anhedonia as one of four cardinal features of schizophrenia [6]. By 1976, Chapman and colleagues devised the Chapman Physical and Social Anhedonia Scales (CPAS/CSAS) to test and confirm the hypotheses of Rado and Meehl that are widely used today in their revised forms as diagnostic tools [7].
With regard to depression, Klein is largely credited for providing a similarly influential hypothesis that “a sharp, unreactive pervasive impairment of the capacity to experience pleasure or to respond affectively to the anticipation of pleasure” is a central feature that predicted the prognosis and treatment of endogenomorphic depression [8]. By 1980, this definition of anhedonia was condensed to “loss of interest or pleasure in usual activities” and was designated as one of two essential features of MDD in the Diagnostic and Statistical Manual (DSM) III [9], while the term anhedonia was later introduced as a negative symptom of schizophrenia in the DSM-IV. The World Health Organization’s International Classification of Diseases (ICD) 10 does not list the term anhedonia, but rather “loss of interest and pleasurable feelings” as a nonessential symptom of depressive episodes [10]. In addition to mood disorders and schizophrenia, anhedonia is common among patients with Parkinson’s disease (PD) [11], substance use disorder (particularly during withdrawal) (reviewed in [12]), Alzheimer’s disease (AD) [13] and eating disorders [14].
Reward Deficits Beyond Anhedonia
There are numerous published studies documenting the neurobiological changes associated with MDD, schizophrenia, and other neuropsychiatric disorders characterized by anhedonia, but relatively few that specifically examined the presence or severity of anhedonia. Considering that MDD and schizophrenia are characterized by various symptoms, it is unlikely that the neural circuits mediating anhedonia are also involved in, for example, hallucinations or feelings of guilt. Thus, it is challenging to link single genetic and neural mechanisms to such complex behavioral disorders. Recently, there has been increased focus on understanding the neurobiology of specific behavioral dimensions, such as hedonic capacity, that are altered in psychiatric patients (reviewed in [15–20]). It is hypothesized that individual behavioral processes (or symptoms) are more likely than diagnostic categories to be linked to specific biological components, and that understanding the biological underpinnings of specific behavioral disruptions will facilitate the treatment of disorders that include such symptoms. This approach is consistent with research in experimental animals, where typically the neurobiology of specific behavioral processes is assessed [20].
Importantly, the term anhedonia does not adequately capture the complex and multifaceted reward-related deficits observed in neuropsychiatric disorders. Besides specific loss of ability to experience pleasure, deficits in other discrete reward-related processes can lead to behaviors that may be interpreted as loss of interest or pleasure. For example, individuals may lack the ability to: 1) anticipate or predict expected rewards; 2) associate relative values and costs with rewards; 3) determine the effort required to obtain rewards; 4) integrate this information to decide whether it is worthwhile to obtain rewards; or 5) become motivated to perform the necessary actions to obtain rewards. Deficits in any of these processes may preclude an individual from engaging in goal-directed actions for rewards, regardless of whether or not the reward is perceived as pleasant once obtained. Research in experimental animals has forged ahead in this regard, by exploring behaviors distinctly relating to pleasure, valuation, anticipation, motivation, and decision-making.
Assessments of Anhedonia in Humans and Experimental Animals
Traditional, subjective self-report measures of anhedonia assess the ability to experience pleasurable events. With these assessments, individuals respond to questions such as “I would enjoy being with my family or close friends” [Snaith-Hamilton Pleasure Scale (SHAPS)]. The preclinical analogues to these anhedonia scales, and the most commonly used procedures to assess depression-like behavior in rodents, are the sucrose intake and preference tests, whereby decreased intake of, or preference, for a sweet sucrose solution (relative to water) is argued to reflect an anhedonic state (Table 1). There are concerns, however, with this interpretation because of confounding motivational effects due to food/water restriction during testing and unreliability of this procedure among laboratories [21]. Notably, individuals with MDD do not differ in their preference for sweet solutions over water compared to healthy controls [22, 23]. Further, self-report assessments of anhedonia are only moderately associated with depression severity [24]. These results suggest that either: a) sucrose intake/preference is not a valid procedure to model human anhedonia; and/or b) human anhedonia does not just involve decreased hedonic capacity.
Table 1.
Reward deficits and their neural mediators: from experimental animals to humans.
| Reward Deficits | Anhedonia Assessments in Animals |
Anhedonia Assessments in Humans |
Circuits/Neural Mediators |
|---|---|---|---|
| Consummatory | Sucrose intake/ preference |
CPAS/CSASa SHAPSa FCPCSa |
• NAc, ventral pallidum, OFC • µ opioid, GABAA, endocannabinoid receptors |
| Anticipatory | Positive/negative contrast | TEPSa | • ACC, OFC, mPFC, basal ganglia, thalamus, hypothalamus |
| Motivational | Effort-based tasks (e.g., progressive ratio, concurrent choice, ICSS) | EEfRTb | • VTA to NAc dopamine • Amygdala µ opioid receptors • vmPFC to NAc glutamate • ACC • Lateral hypothalamus |
| Learning | Response Bias Probabilistic Reward Taskb |
• Dorsal basal ganglia (caudate) • ACC |
self-report questionnaire
behavioral assessment
Indeed, in the case of schizophrenia, it has become apparent that reward deficits involve more than just anhedonia (reviewed in [25–27]). In some cases, the ability to experience pleasure may even remain intact. Rather, schizophrenia appears to be associated with inappropriate valuation of rewards, particularly when patients are required to generate and maintain internal representations of rewards not immediately available (reviewed in [28]). For example, schizophrenia patients heavily discount the value of future rewards compared to controls. Further, motivation to engage in goal-directed actions is impaired in schizophrenia patients (reviewed in [29]). Consequently, decision-making is compromised, because it is based on inappropriate reward representations and impaired motivation. Each of these reward-related processes is mediated by discrete neural circuits (discussed below). Thus, the neurobiological mechanisms of hedonic experience are not necessarily involved in other reward deficits displayed by individuals with MDD and schizophrenia, highlighting the need for clinical measures that assess multiple reward-related deficits (Box 1).
Box 1. Constructs relating to anhedonia: Novel clinical assessments of discrete reward-related processes.
Clinical measures have been developed to assess reward processes beyond hedonic capacity, such as anticipation, motivation, and reinforcement learning. The TEPS is a self-report measure designed to distinguish between consummatory and anticipatory anhedonia [129]. Consummatory probes include questions such as: “I appreciate the beauty of a fresh snowfall”; anticipatory probes include questions such as: “I look forward to a lot of things in my life”. In animals, anticipatory and consummatory effects can also be distinguished. For example, positive/negative contrast effects probe anticipation and reward valuation in rodents. When presented consistently with a sucrose solution of a set concentration, rodents display an exaggerated increased or decreased response when suddenly presented with a sucrose solution of higher or lower concentration, respectively, in anticipation of the original concentration (e.g., [70]).
Motivation and effort-based decision-making is assessed with a recently developed procedure by Zald and colleagues called Effort Expenditure for Rewards Task (EEfRT). This task probes motivational deficits using an objective, laboratory-based procedure [102]. Using EEfRT, trait anhedonia was negatively correlated with willingness to expend effort for rewards. Effort-based tasks are commonly used in animal studies. A concurrent choice task assesses whether animals choose a larger reward requiring more effort to obtain vs. a smaller reward requiring little effort. Operant responding on a progressive ratio schedule of reinforcement assesses motivation, whereby animals must emit exponentially greater effort to continue receiving rewards [58].
Pizzagalli and colleagues designed the Response Bias Probabilistic Reward Task, an objective, laboratory-based procedure to assess deficits in reinforcement learning [101, 130, 131]. Using this task, individuals with MDD or healthy individuals that either: a) have high trait anhedonia; b) are exposed to an acute stressor; or c) are administered a dopamine autoreceptor agonist to lower dopamine levels, fail to develop a biased response over time for the more frequently rewarded of two stimuli, reflecting an inability to allow reinforcement history to alter subsequent responding for rewards.
One particular advantage of the latter two tasks is that they do not rely on subjective verbal responses from participants, which can be limited by their ability to recall or relate to subjective experiences [132]. Rather, these tasks are based on existing rodent procedures or may be readily developed for use in animals.
Dissecting the Circuits of Anhedonia and Other Reward-Related Deficits
The majority of studies in humans assessing the neurobiology of anhedonia have involved MDD or schizophrenia patients, although one could extrapolate that the same circuits are likely to be involved in anhedonia exhibited in other neuropsychiatric disorders, such as PD and AD. While anhedonia in clinical populations is primarily defined by subjective responses to self-report questionnaires, these purported measures of anhedonia may also reflect deficits in other reward processes, such as motivation, valuation, and decision-making that are implicitly assessed by these scales. Studies in experimental animals have probed neural markers of these discrete reward processes, which can be compared with imaging studies of anhedonic humans. Thus, the neurobiology of anhedonia, as generally defined in clinical populations, may correspond to the neurobiology of not just anhedonia, but also of other discrete reward-related processes that are more clearly defined in experimental animal procedures.
Pleasure: Ventral Striatum and Orbitofrontal Cortex
The ventral striatum and orbitofrontal cortex (OFC) contribute to experiences of pleasure. In particular, mu opioid and endocannabinoid receptors in the nucleus accumbens (NAc) and ventral pallidum mediate hedonic perception of rewards, such that activation of these receptors enhances the affective response for highly palatable rewards, like sucrose (reviewed in [30, 31]). Activation of GABAA receptors in the NAc is also known to regulate the affective response to sucrose [32]. Human neuroimaging studies suggest that subjective assessments of pleasure are also mediated by the OFC [33], though it is unclear whether the OFC mediates the perception of pleasure or rather codes for pleasure [e.g., by assessing relative reward value (see below)] [31]. Activity of the ventral striatum and OFC is decreased in anhedonic individuals with MDD [34] or schizophrenia [35], although it is debated whether schizophrenia is associated with impaired reward valuation and motivation rather than decreased hedonic capacity [25–28]. Importantly, the NAc and OFC are involved in other reward-related processes that are possibly disrupted in individuals broadly classified as anhedonic (see below).
Reward Valuation, Cost/Benefit Analysis, and Decision-Making: Prefrontal Cortex
Deficits in many areas of the prefrontal cortex (PFC) have been implicated in anhedonia. In schizophrenia patients, self-reported anhedonia severity negatively correlated with OFC, ventromedial (vm) PFC, and dorsolateral (dl) PFC activity [35, 36]. In healthy individuals, trait anhedonia negatively correlated with rostral anterior cingulate cortex (ACC) [35, 37] and dlPFC [36] resting activity, and vmPFC gray matter volume [38]. Similarly, opioid-dependent subjects and healthy individuals showed negative correlations between anhedonia severity and activity in the ventral ACC, dlPFC, and anterior regions of the PFC [39]. However, there is also evidence of increased vmPFC activity in individuals with high levels of trait anhedonia [34, 40]. Increased vmPFC activity was observed in healthy subjects, whereas decreased vmPFC is limited to schizophrenia patients [34–36, 40]. Thus, it is possible that vmPFC structure and function is altered after progression of schizophrenia. Furthermore, distinct vmPFC subregions may differentially regulate aspects of reward processing in different populations that cannot be discretely visualized with current imaging techniques. Indeed, multiple studies reported contradictory results reflecting increased [41, 42] and decreased [43, 44] activity of the subgenual PFC (corresponding to the ventral ACC) in depressed individuals. Interestingly, deep brain stimulation (DBS) of the subgenual PFC alleviated depression symptoms in treatment-resistant depressed individuals [45], although it is unclear whether anhedonia was affected by this treatment. It also remains unclear whether the mechanisms underlying DBS of this brain region involve inhibition or excitation of neural activity.
Research in experimental animals indicates that the OFC codes the absolute value of rewards, along with relative value compared to other rewards [31, 46]. Determination of reward value is based on the hedonic perception of the reward and associated costs and benefits of obtaining that reward. Thus, insufficient valuation of rewards may be misinterpreted as decreased hedonic capacity using traditional self-report measures. The ACC, that receives input from the OFC, determines the effort required to obtain rewards (reviewed in [47]). Dorsal ACC neurons encode previous reward outcomes that guide future decisions [48]. Accordingly, ACC lesions result in preferences for low cost/low reward compared to high cost/high reward choices [49–51]. Reward value and effort information are then processed by the anterior vmPFC and dlPFC, that are responsible for decision-making based on reward values and effort calculations of multiple choices to promote goal-directed behavior [47]. The decision to choose a particular reward based on cost/benefit analyses incorporating reward value, effort requirement, and reinforcement history leads to goal-directed action. Impairment of this circuit would result in abnormal reward valuation, abnormal calculation of required effort, or deficits in decision-making for optimal reward-based actions, each of which could be mistaken for anhedonia, or loss of pleasure.
Prediction, Anticipation, and Motivation: Ventral Tegmental Area, Amygdala, and Ventral Striatum
Investigation of the neurobiological bases of anhedonia has traditionally centered on the neurotransmitter dopamine and the mesolimbic circuit consisting of dopaminergic projections from the ventral tegmental area (VTA) to the ventral striatum, including the NAc. In addition, there are dopaminergic projections from the substantia nigra to areas such as the dorsal striatum, also called caudateputamen. The latter dopaminergic projections may also be involved in anhedonic responses, particularly in individuals suffering from Parkisonism that is characterized by gradual degeneration of the substantia nigra dopaminergic projections. In humans, anhedonia severity, but not depression severity per se, negatively correlated with ventral striatal activity in response to pleasant stimuli [34, 35, 52], monetary rewards [37], and positive words [53]. Further, selfreported anhedonia negatively correlated with ventral striatal gray matter and caudate volume [40, 54], but not with NAc volume in unmedicated MDD subjects [54]. Further, severity of Parkinsonian symptomatology is negatively correlated with motivational arousal responses to appetitive food images [55]. Additionally, microstructural abnormalities were found in the VTA of MDD patients compared to controls [56]. The severity of abnormalities did not correlate with anhedonia, though anhedonia may have been inadequately probed with one question on the Inventory of Depressive Symptomatology – Self Report. Thus, decreased activity and/or volume of the ventral and dorsal striatum may contribute to anhedonia in affected and healthy individuals.
Research in experimental animals indicates that while dopamine does not mediate the perception of pleasure (reviewed in [57]), dopamine is associated with prediction/anticipation of and motivation to obtain rewards (reviewed in [58]). While administration of addictive drugs that increase synaptic dopamine levels leads to feelings of euphoria in humans [59], it is unclear that this dopamine release mediates hedonic arousal. It is well established that dopamine projections from the VTA to the ventral striatum fire in response to unpredicted rewards (reviewed in [60]). Subsequently, dopaminergic neurons fire in response to cues that predict rewards. Thus, it is hypothesized that one role of dopamine is to transfer positive incentive value from the reward to the cue that predicts the reward [57]. Conversely, when predicted rewards are not presented, dopamine firing is blunted (reviewed in [60]). Hence, ventral striatal dopamine regulates the prediction and anticipation of rewards, two mechanisms responsible for basic reinforcement learning (reviewed in [61]). With regard to motivation, studies have shown that dopamine depletion or antagonists in the NAc of rats decrease responding for large rewards requiring greater effort to obtain, while increasing responding for smaller rewards requiring less effort [62]. Similar dopamine lesions also decreased responding for rewards requiring five, but not one, successive responses [63], indicating that NAc dopamine is necessary to elicit responding for rewards when the required effort is increased (reviewed in [64]). Similarly, in human imaging studies, appearance of food that was unavailable for consumption elicited an increased striatal dopamine response in fasting subjects, implicating a role for dopamine in motivated behavior and anticipation of reward (reviewed in [65]).
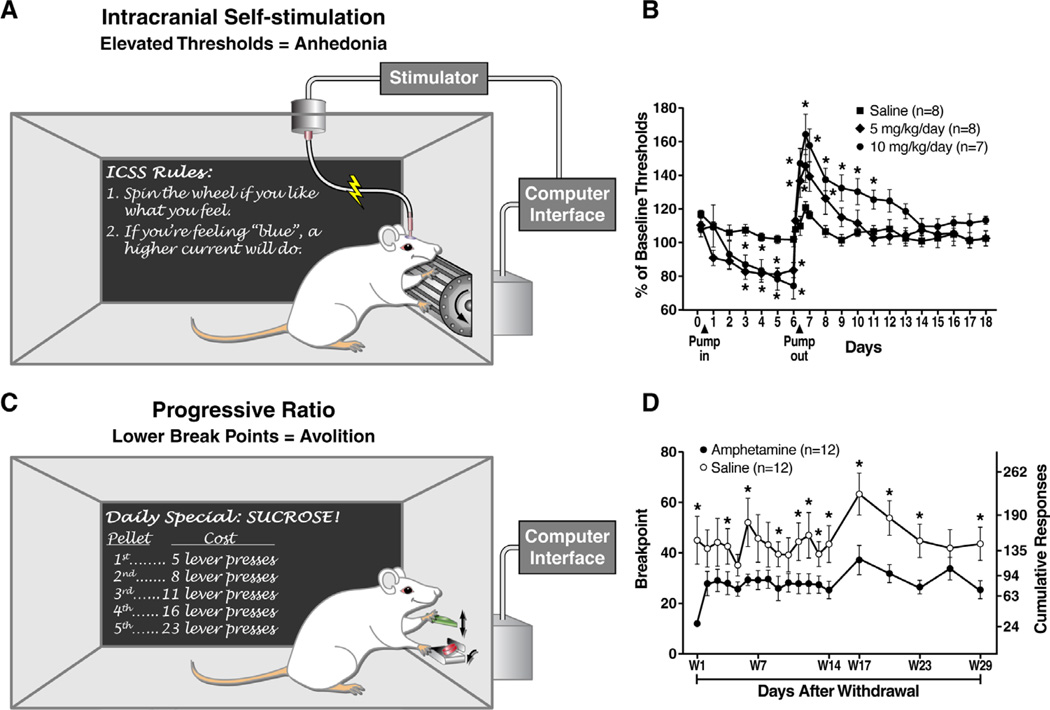
Psychostimulant withdrawal results in numerous symptoms of MDD, including anhedonia [12]. Incidentally, psychostimulant withdrawal decreases NAc dopamine levels in rodents [66] and induces reward deficits as measured by: (i) elevations in reward thresholds using intracranial self-stimulation (ICSS) of the posterior lateral hypothalamus (Figure 1 A,B; [67, 68]); (ii) increased avolition using the progressive ratio test (Figure 1 C,D; [69, 70]); and (iii) increased successive negative contrast effects, where withdrawing rats respond significantly less than controls when presented with a sucrose solution of a lower concentration than anticipated [71]. Treatment with the norepinephrine/dopamine reuptake inhibitor bupropion, an atypical antidepressant, attenuated withdrawal-induced ICSS threshold elevations in rats [72]. Thus, deficits in dopamine neurotransmission in the ventral striatum may impair reinforcement learning and increase avolition, whereby unconditioned or conditioned rewards fail to stimulate responding for those rewards. Again, this may be incorrectly interpreted as decreased hedonic capacity if anticipation or motivation is not specifically assessed. In this regard, it should be noted that while bupropion increases striatal dopamine in rats [73], clinically therapeutic doses do not increase striatal dopamine or dopamine transporter occupancy in humans [74, 75]. Thus, it is important to determine whether bupropion indeed improves motivation or anticipation of rewards, irrespective of its general antidepressant properties, in depressed patients. Two separate groups used DBS of the ventral striatum/NAc to relieve patients with otherwise treatment-resistant depression [76, 77]. DBS of this region attenuated depression severity in half of the patients, with treatment responders showing a corresponding decrease in anhedonia [77]. Again, it is unclear whether DBS altered ventral striatal dopamine activity or whether stimulation of this region indirectly affected function of other regions that mediated the change in anhedonic symptoms.
Figure 1. Examples of contemporary measures of anhedonia and reward-related deficits in experimental animals.
A. ICSS can be used to assess brain reward function. Self-stimulation of parts of the brain reward circuitries (e.g., lateral hypothalamus) is positively reinforcing at certain current intensities. The current intensity is systematically increased and decreased to determine the threshold that will support self-stimulation. Elevations of this reward threshold indicate that an increased current intensity is required to elicit a behavioral response, reflecting anhedonia. B. Withdrawal from chronic amphetamine exposure elevates reward thresholds (i.e., anhedonia) for up to five days in rats [103]. “Pump in/out” indicates initiation/termination of amphetamine exposure using subcutaneous osmotic minipumps. C. Responding for a palatable reward (e.g., sucrose pellets) on a progressive ratio schedule of reinforcement can be used to assess motivation to obtain rewards. Animals must perform an operant response (e.g., lever press) at an exponentially increasing rate to obtain each subsequent sucrose pellet. Eventually, animals will cease to respond as the response requirement to obtain a single pellet demands too much effort, termed the break point. A decrease in break points is an indication of avolition, or decreased motivation to obtain the reward. D. Withdrawal from chronic amphetamine exposure (10 mg/kg/day for 7 days) decreases break points for a sucrose pellet for up to 29 days in rats [70]. Reproduced, with permission from [103] (B) and [70] (D).
Among other functions, the amygdala is involved in the evaluation of rewards (reviewed in [78–81]). Opioids in the basolateral amygdala (BLA) partly mediate the incentive properties of rewards. Infusion of the mu opioid antagonist naloxone into the BLA attenuated the increased response for sucrose by food deprivation, without affecting palatability for sucrose [82]. In addition, optogenetic inhibition of glutamatergic projections from the BLA to NAc decreased motivated responding for sucrose [83]. These observations suggest that, along with NAc dopamine, opioid and glutamatergic activity in the BLA is necessary for motivated behavior. In schizophrenia patients, increased self-reported anhedonia severity was associated with decreased bilateral amygdala activation in response to stimuli with a positive emotional valence [52]. Again, anhedonia was assessed using the CPAS, and thus it is unknown whether changes in amygdala activity specifically represent avolition.
Constructing the Circuits of Anhedonia, Anticipation, Valuation, Decision-Making, and Avolition
Although distinct neural regions code for separate reward processes, the circuits connecting these regions allow an individual to: 1) sense a pleasant stimulus; 2) compute reward value and associated costs; 3) determine effort requirements to obtain that stimulus; 4) decide to obtain that stimulus; and 5) anticipate and increase motivation to obtain that stimulus (Figure 2). The hedonic perception of rewards is mediated primarily by endogenous opioid, GABA, and endocannabinoid systems in the NAc, ventral pallidum, and OFC. The OFC and ventral striatum receive inputs from sensory cortices and calculate the reward values. The OFC then projects reward value information to the ACC to incorporate costs, benefits, and reinforcement history to determine the effort required for different possible actions. The ACC sends projections to the anterior vmPFC and dlPFC, that are involved in decision-making based on reward value, effort, and reinforcement history regarding future actions. Glutamatergic efferents relay this information to the NAc, that receives dopaminergic and glutamatergic input from the VTA and amygdala, respectively, providing incentive salience properties and increasing motivation to carry out the goal-directed action planned in the PFC. Indeed, there is focus on glutamate for its putative antidepressant properties (reviewed in [84, 85]). Ketamine, a NMDA receptor antagonist, produces rapid antidepressant effects hypothesized to be partly mediated by increased glutamatergic signaling via AMPA receptors [86] (see also [87] in this Issue). In animal studies, disruption of glutamatergic signaling between the mPFC and NAc or administration of an AMPA receptor antagonist in the NAc shell resulted in avolition for rewards [32]. Further, nicotine withdrawal-induced elevations of ICSS thresholds were exacerbated by decreasing glutamatergic activity, and conversely, attenuated by increasing glutamatergic activity [88]. Disruption in any of these circuits can lead to different types of reward deficits. For example, blocking (i) dopaminergic transmission from the VTA to NAc; (ii) opioid signaling in the BLA; (iii) ACC activity; or (iv) glutamate transmission from the vmPFC to NAc each decreased motivation for a large reward.
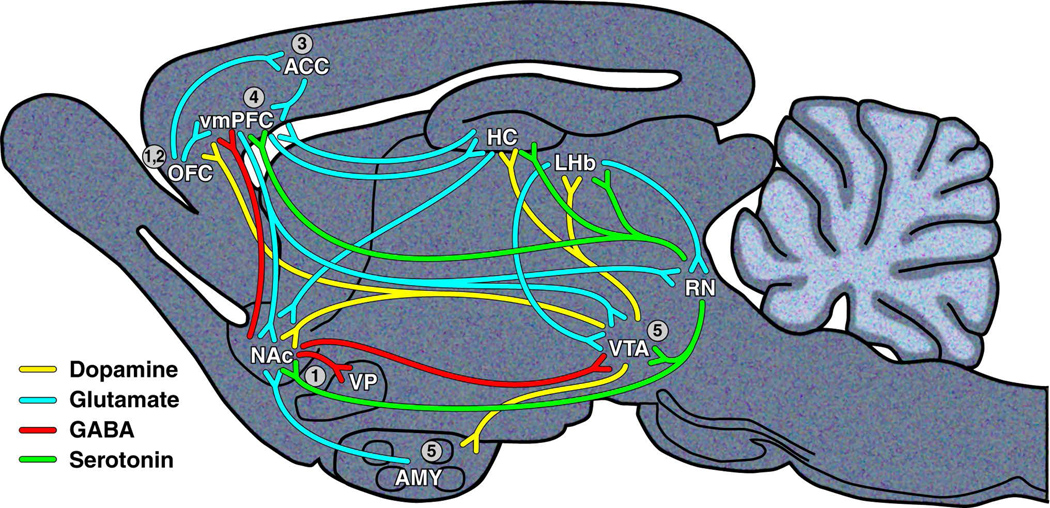
Figure 2. Simplified model of a rodent brain illustrating the neural circuitry of anhedonia and other reward-related deficits.
1) The nucleus accumbens (NAc), ventral pallidum (VP), and orbitofrontal cortex (OFC) mediate the perception of pleasure. 2) The relative reward value of the pleasant stimulus is computed by the OFC. 3) The effort required to obtain the stimulus is computed by the anterior cingulate cortex (ACC). 4) The ventromedial prefrontal cortex (vmPFC) is involved in the decision to engage in goal-directed activity to obtain the pleasant stimulus. 5) The ventral tegmental area (VTA) and amygdala (AMY) are responsible for increasing anticipation and motivation to carry out the goal-directed activity. Additional brain areas that interact with these circuits also play critical roles in reward-related behaviors. Decreased neurogenesis in the hippocampus (HC) may lead to anhedonia and prevent the reversal of anhedonia by SSRI treatment. SSRI treatment, which purportedly has antidepressant effects by increasing 5-HT activity from the raphe nuclei (RN), increases striatal activity and sucrose preference, while reversing drug-withdrawal-induced anhedonia. Deep brain stimulation of the LHb,, which purportedly leads to disinhibition of mesolimbic dopamine and RN 5-HT circuits, has been shown to have antidepressant effects.
The circuit presented here likely represents an incomplete view of the neurobiology mediating different aspects of anhedonia and other reward-related deficits. Indeed, additional neurotransmitter systems regulate various reward processes (Table 2). The multiple reciprocal connections between different PFC subregions, as well as reciprocal connections with the NAc, VTA, amygdala, and hippocampus likely play an important role in regulating the behavioral response to rewards. For example, decreased neurogenesis in the dentate gyrus reduced sucrose preference in mice [89] and reversed the therapeutic effects of fluoxetine, a selective serotonin reuptake inhibitor (SSRI), on separation stress-induced anhedonia in non-human primates [90]. Serotonin (5-HT) originating from the midbrain raphe nuclei (RN) also regulates reward processing and anhedonic behaviors. For example, chronic treatment with SSRI antidepressants increased ventral striatal activity in humans [91] and sucrose preference in mice [92]. Interestingly, agomelatine, an antagonist of 5-HT2C receptors, also increased sucrose preference in mice [92] and improved SHAPS anhedonia scores in MDD patients [93]. 5-HT2C receptors inhibit NAc dopamine release, and antidepressant treatment increases striatal dopamine levels by decreasing 5-HT2C mediated dopamine inhibition [94]. Furthermore, fluoxetine treatment combined with a 5-HT1A antagonist, that rapidly elevates 5-HT levels in forebrain structures, reversed psychostimulant withdrawal-induced anhedonia in rats [67].
Table 2.
Effects of gene knockouts in mice on measures of natural reward.
| Knockout1 | Increased Anhedonia | Decreased Anhedonia | No Effect |
|---|---|---|---|
| DOPAMINE | |||
| Tyrosine Hydroxylase | Decreased initiation of sucrose intake.104 | No effect on sucrose preference.104 | |
| D1 dopamine receptor | Decreased ICSS responding, and decreased fixed and progressive ratio responding for sucrose.105,106 | No effect on sucrose intake or preference.105 | |
| D2 dopamine receptor | Decreased progressive ratio responding for food.107 | No effect on ICSS responding.108 | |
| D3 dopamine receptor | No effect on sucrose intake.109 | ||
| Dopamine transporter | Increased intake and positive bias for sucrose.110,111 | ||
| SEROTONIN | |||
| Serotonin transporter | Decreased sucrose intake.112 | No effect on sucrose intake or preference.110,113 | |
| Serotonin 1A receptor | Increased sucrose preference.114 | ||
| NOREPINEPHRINE | |||
| Norepinephrine transporter | No effect on sucrose intake.110 | ||
| GLUTAMATE | |||
| Glutamate transporter EAAT1; AMPA GluA1 subunit | No effect on sucrose preference.115,116 | ||
| GABA | |||
| GABAA alpha 1 subunit | Decreased sucrose intake.117 | ||
| GABAA alpha 3 subunit | Increased negative contrast.118 | No effect on sucrose preference.118 | |
| GABAA alpha 5 subunit | No effect on sucrose intake.119 | ||
| GABAA gamma 2 subunit | Decreased sucrose intake.120 | ||
| OPIOID | |||
| µ opioid receptor | Decreased progressive ratio responding for food or sucrose.121 | ||
| Beta-endorphin/Enkephalin | Decreased progressive ratio responding for food.122 | No effect on sucrose or saccharin preference.122 | |
| Preprodynorphin | Decreased saccharin preference.123 | ||
| CANNABINOID | |||
| Cannabinoid 1 receptor | Decreased sucrose intake and preference.124 | ||
| OXYTOCIN | |||
| Oxytocin | Increased sucrose intake.125 | ||
| Oxytocin receptor | No effect on sucrose intake.126 | ||
| CYTOKINE | |||
| Interleukin 6 | Increased sucrose preference.127 | ||
| TNF alpha receptor 2 | Increased sucrose intake.128 | ||
Abbreviations: EAAT, excitatory amino acid transporter; TNF, tumor necrosis factor
The lateral habenula (LHb) may also play an important role in reward processes given its reciprocal connections with the VTA and RN. LHb neurons inhibit dopaminergic and 5-HT cells in the VTA and RN, respectively (reviewed in [95]). Consequently, DBS of the LHb, purported to inhibit LHb activity and disinhibit dopamine and 5-HT activity, has been reported to have antidepressant effects, although it should be noted this is a single case study [96]. Other regions have been implicated also, such as the insula and precuneus/medial parietal cortex, based on imaging studies of anhedonic individuals [35, 36, 39, 40]. The anterior insula encodes the subjective value of rewards [97] and representations of interoceptive effects of rewards (reviewed in [98]). Furthermore, the parietal cortex encodes reward values relative to other available options (reviewed in [99]). Future integration of these reciprocal connections and identification of additional structures will provide a more comprehensive neural framework by which to investigate reward-related deficits.
In particular, the advent of DBS has provided a new and promising treatment for otherwise refractory depression and has promoted our understanding of the neurobiology of depression and other neuropsychiatric disorders. For example, DBS of the subgenual PFC [45], ventral striatum [76, 77], inferior thalamic peduncle [100], and LHb [96] each have antidepressant effects. Unfortunately, with the exception of one study [77], anhedonia was not specifically assessed and one can only speculate whether these brain regions are involved in anhedonia or depression per se based on current DBS studies.
Concluding Remarks and Future Considerations
The focus on neurobiological markers of specific behaviors, rather than entire disorders, has led to significant advances in the understanding of anhedonia and related reward deficits in neuropsychiatric disorders. One advantage for clinical researchers is that preclinical research has provided a wealth of information regarding the neurobiology of reward-related processes, from perception of pleasure, to coding of reward value, assessing costs and benefits, learning from prior reinforcement, evaluating effort, and making decisions that lead to action. Each process is regulated by specific neural circuits. Therefore, an improved understanding of which processes are disrupted in individuals with MDD, schizophrenia, or PD may offer guidance regarding which neural processes to target with treatments. To this end, translational measures incorporating different aspects of reward processes are needed from clinical and preclinical researchers. Two excellent examples are from the laboratories of Pizzagalli [101] and Zald [102], both of whom developed objective, laboratory-based procedures that are used to detect the influence of reinforcement history and motivation, respectively, in human subjects (Box 1). That these tasks are objective and not based on verbal reports make them ideal to adapt for experimentation in laboratory animals.
Given what is known about the sophisticated nature of reward deficits in neuropsychiatric disorders, it would be beneficial to limit the term anhedonia to describe only deficits in hedonic capacity, while incorporating additional terms to describe other aspects of reward-related processes that are compromised in neuropsychiatric disorders (i.e., avolition; deficits in anticipation/prediction, valuation, reinforcement learning, and decision-making). Consequently, it is necessary to expand the clinical and preclinical tools used to investigate discrete reward-related processes and their underlying neurobiology.
Acknowledgments
This work was supported by National Institutes of Health grants R01MH62527 and R01MH087989 (to A.M.), and National Research Service Award Individual Postdoctoral fellowship F32MH080585 (to A.D.) from the National Institute of Mental Health. The authors would like to thank Ms. Janet Hightower for her assistance with graphics.
GLOSSARY
- Anhedonia
Markedly diminished interest or pleasure in all, or almost all, activities
- Avolition
The reduction, difficulty, or inability to initiate and persist in goaldirected behavior; lack of motivation
- Chapman Physical and Social Anhedonia Scales (CPAS/CSAS)
Self-report anhedonia scales that differentiate between physical (i.e., eating, sex) and social (i.e., expressing feelings and interacting with people) pleasures
- Deep brain stimulation (DBS)
Clinical procedure, involving surgical implantation of stimulating electrodes in discrete brain sites in humans, such as the subgenual cingulate cortex, ventral striatum, inferior thalamic peduncle, and lateral habenula. Subsequently, continuous stimulation of these brain sites is used to treat depression, particularly treatment-resistant depression.
- Diagnostic and Statistical Manual of Mental Disorders (DSM)
The standard classification of mental disorders published by the American Psychiatric Association and used by mental health professionals, including clinicians and researchers, in the United States
- Effort Expenditure for Rewards Task (EEfRT)
Human experimental task used to assess motivation and effort-based decision-making
- Fawcett-Clark Pleasure Capacity Scale (FCPS)
Self-report anhedonia scale that measures the intensity of pleasurable responses with some components of anticipatory pleasure
- International Classification of Diseases (ICD)
The international standard diagnostic classification of diseases and other health problems published by the World Health Organization for epidemiological and health management purposes
- Intracranial self-stimulation (ICSS)
Experimental animal procedure used to assess brain reward function. This procedure involves the surgical implantation of stimulating electrodes into discrete brain sites that are part of brain reward systems. Brief electrical stimulation of these brain reward sites is extremely rewarding for rats and allows the direct assessment of brain reward function. Please note that ICSS in experimental animals is different than DBS in humans in that (i) ICSS involves the delivery of brief (msec) electrical pulses while DBS is on continuously for months or longer; (ii) ICSS is delivered upon performance of an operant response by the experimental animal subject (and thus, the term self-stimulation), while DBS does not involve a discrete operant response by the patient; (iii) based on historical and anecdotal reports, ICSS involves brief stimulation of brain sites that lead to intense feelings of pleasure in humans, while DBS may alleviate depressive symptoms but is not associated with intense feelings of pleasure and euphoria. The latter may be due to the discrete versus continuous nature of ICSS vs. DBS, respectively.
- Snaith-Hamilton Pleasure Scale (SHAPS)
Self-report anhedonia scale that measures hedonic responses across four domains interests/pastimes, social interaction, sensory experience, and food/drink.
- Temporal Experience of Pleasure Scale (TEPS)
Self-report anhedonia scale that distinguishes between consummatory and anticipatory anhedonia
- Trait anhedonia
Anhedonia that is present in non-clinical populations or precedes the onset of psychiatric illness. Assessment of trait anhedonia in human subjects without a neuropsychiatric illness allows one to dissociate neural processes that are specific to anhedonia from confounding factors associated with the onset and progression of neuropsychiatric illnesses that include anhedonia as a symptom.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Epicurus, Bailey C. Epicurus, the extant remains. Clarendon press; 1926. [Google Scholar]
- 2.American Psychiatric Association. and American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; 1994. Task Force on DSM-IV. [Google Scholar]
- 3.Haslam J. Observations on madness and melancholy; including practical remarks on those diseases, together with cases, and an account of the morbid appearances on dissection. Callow; 1809. [Google Scholar]
- 4.Ribot T. La psychologie des sentiments. F Alcan; 1896. [Google Scholar]
- 5.Rado S. Psychoanalysis of behavior; collected papers. Grune and Stratton; 1956. [Google Scholar]
- 6.Meehl PE. Schizotaxia, Schizotypy, Schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 7.Chapman LJ, et al. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 8.Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Task Force on Nomenclature and Statistics. and American Psychiatric Association. Committee on Nomenclature and Statistics. Diagnostic and statistical manual of mental disorders. American Psychiatric Association; 1980. [Google Scholar]
- 10.World Health Organization. ICD 10 the ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. World Health Organization; 1992. [Google Scholar]
- 11.Isella V, et al. Physical anhedonia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:1308–1311. doi: 10.1136/jnnp.74.9.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markou A, et al. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, et al. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry. 2005;162:2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 14.Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry. 2002;43:189–194. doi: 10.1053/comp.2002.32356. [DOI] [PubMed] [Google Scholar]
- 15.Leboyer M, et al. Psychiatric genetics: search for phenotypes. Trends Neurosci. 1998;21:102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- 16.Hyman SE, Fenton WS. Medicine. What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 18.Insel T, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 19.Markou A, et al. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer MA, Markou A. The Role of Preclinical Models in the Development of Psychotropic Drugs. In: Davis KL, et al., editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; 2002. pp. 445–455. [Google Scholar]
- 21.Matthews K, et al. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 22.Berlin I, et al. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 23.Dichter GS, et al. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leventhal AM, et al. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 25.Wolf DH. Anhedonia in schizophrenia. Curr Psychiatry Rep. 2006;8:322–328. doi: 10.1007/s11920-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 26.Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr Bull. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold JM, et al. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley AE, et al. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 31.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faure A, et al. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011223. e11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolls ET, et al. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 34.Keedwell PA, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Harvey PO, et al. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J Psychiatr Res. 2010;44:707–716. doi: 10.1016/j.jpsychires.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Park IH, et al. Medial prefrontal default-mode hypoactivity affecting trait physical anhedonia in schizophrenia. Psychiatry Res. 2009;171:155–165. doi: 10.1016/j.pscychresns.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Wacker J, et al. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosso IM, et al. Regional prefrontal cortex gray matter volumes in youth at familial risk for schizophrenia from the Harvard Adolescent High Risk Study. Schizophr Res. 2010;123:15–21. doi: 10.1016/j.schres.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zijlstra F, et al. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Harvey PO, et al. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12(703):767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 41.Keedwell P, et al. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol. 2009;23:775–788. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- 42.Mayberg HS, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 43.Drevets WC, et al. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psychiatry. 1998;3:190–191. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- 44.Pizzagalli DA, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(325):393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 45.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi S, et al. Adaptation of reward sensitivity in orbitofrontal neurons. J Neurosci. 2010;30:534–544. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Seo H, Lee D. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 2007;27:8366–8377. doi: 10.1523/JNEUROSCI.2369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennerley SW, et al. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 50.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 51.Rudebeck PH, et al. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 52.Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epstein J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 54.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shore DM, et al. Appetitive motivational deficits in individuals with Parkinson's Disease. Mov Disord. 2011 doi: 10.1002/mds.23736. [DOI] [PubMed] [Google Scholar]
- 56.Blood AJ, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 58.Salamone JD, et al. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 59.Volkow ND, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 60.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Hazy TE, et al. Neural mechanisms of acquired phasic dopamine responses in learning. Neurosci Biobehav Rev. 2010;34:701–720. doi: 10.1016/j.neubiorev.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salamone JD, et al. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 63.Correa M, et al. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 64.Salamone JD, et al. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Wang GJ, et al. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 66.Weiss F, et al. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 67.Harrison AA, et al. Fluoxetine combined with a serotonin 1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 68.Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav Brain Res. 2011;223:176–181. doi: 10.1016/j.bbr.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- 70.Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barr AM, Phillips AG. Increased successive negative contrast in rats withdrawn from an escalating-dose schedule of D-amphetamine. Pharmacol Biochem Behav. 2002;71:293–299. doi: 10.1016/s0091-3057(01)00664-5. [DOI] [PubMed] [Google Scholar]
- 72.Paterson NE, et al. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- 73.Santamaria A, Arias HR. Neurochemical and behavioral effects elicited by bupropion and diethylpropion in rats. Behav Brain Res. 2010;211:132–139. doi: 10.1016/j.bbr.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 74.Egerton A, et al. Acute effect of the anti-addiction drug bupropion on extracellular dopamine concentrations in the human striatum: an [11C]raclopride PET study. Neuroimage. 2010;50:260–266. doi: 10.1016/j.neuroimage.2009.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer JH, et al. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- 76.Malone DA, Jr, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 80.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Everitt BJ, et al. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 82.Wassum KM, et al. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathew SJ, et al. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- 85.Skolnick P, et al. Glutamate-based antidepressants 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 87.Duman RS, Voleti B. Signaling Pathways Underlying the Pathophysiology and Treatment of Depression: Novel Mechanisms for Rapid Acting Agents. Trends Neurosci. doi: 10.1016/j.tins.2011.11.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perera TD, et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Snyder JS, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ossewaarde L, et al. Two-week administration of the combined serotonin-noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biol Psychiatry. 2011;70:568–574. doi: 10.1016/j.biopsych.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 92.El Yacoubi M, et al. Chronic agomelatine and fluoxetine induce antidepressant-like effects in H/Rouen mice, a genetic mouse model of depression. Pharmacol Biochem Behav. 2011;100:284–288. doi: 10.1016/j.pbb.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Di Giannantonio M, et al. Major depressive disorder, anhedonia and agomelatine: an open-label study. J Biol Regul Homeost Agents. 2011;25:109–114. [PubMed] [Google Scholar]
- 94.Dremencov E, et al. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology. 2005;48:34–42. doi: 10.1016/j.neuropharm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 95.Hikosaka O, et al. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 97.Sescousse G, et al. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jimenez F, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. discussion 585. [DOI] [PubMed] [Google Scholar]
- 101.Pizzagalli DA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treadway MT, et al. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paterson NE, et al. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 104.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Ghundi M, et al. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- 106.Tran AH, et al. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A. 2005;102:2117–2122. doi: 10.1073/pnas.0409726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kruzich PJ, et al. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- 108.Tran AH, et al. Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc Natl Acad Sci U S A. 2002;99:8986–8991. doi: 10.1073/pnas.132284599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chourbaji S, et al. Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacol Res. 2008;58:302–307. doi: 10.1016/j.phrs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Perona MT, et al. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19:566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Costa RM, et al. Dopamine levels modulate the updating of tastant values. Genes Brain Behav. 2007;6:314–320. doi: 10.1111/j.1601-183X.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- 112.Olivier JD, et al. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 113.Kalueff AV, et al. Are serotonin transporter knockout mice 'depressed'?: hypoactivity but no anhedonia. Neuroreport. 2006;17:1347–1351. doi: 10.1097/01.wnr.0000230514.08962.76. [DOI] [PubMed] [Google Scholar]
- 114.Bechtholt AJ, et al. Sucrose intake and fasting glucose levels in 5-HT(1A) and 5-HT(1B) receptor mutant mice. Physiol Behav. 2008;93:659–665. doi: 10.1016/j.physbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barkus C, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karlsson RM, et al. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.June HL, Sr, et al. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH-enhanced locomotor stimulation in the GABAA alpha1 subunit null mutant mice. Neuropsychopharmacology. 2007;32:137–152. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- 118.Fiorelli R, et al. Affective and cognitive effects of global deletion of alpha3-containing gamma-aminobutyric acid-A receptors. Behav Pharmacol. 2008;19:582–596. doi: 10.1097/FBP.0b013e32830dc0c7. [DOI] [PubMed] [Google Scholar]
- 119.Stephens DN, et al. Role of GABAA alpha5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. Eur J Pharmacol. 2005;526:240–250. doi: 10.1016/j.ejphar.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 120.Shen Q, et al. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Papaleo F, et al. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- 122.Hayward MD, et al. Selective reward deficit in mice lacking betaendorphin and enkephalin. J Neurosci. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blednov YA, et al. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanchis-Segura C, et al. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- 125.Amico JA, et al. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 126.Lee HJ, et al. A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chourbaji S, et al. IL 6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Simen BB, et al. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 129.Gard DE, et al. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. 2006;40:1086–1102. [Google Scholar]
- 130.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pizzagalli DA, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Horan WP, et al. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol. 2006;115:496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]