Abstract
The involvement of IL-4 in liver regeneration has not yet been recognized. Here, we show that IL-4, produced by natural killer T (NKT) cells that accumulate in regenerating livers after partial hepatectomy, contributes to this process by regulating the activation of complement after liver resection in mice. The mechanism of this regulation was associated with the maintenance of an appropriate level of IgM in mouse blood, since IgM deposited in liver parenchyma most likely initiated complement activation during liver regeneration. By controlling complement activation, IL-4 regulated the induction of IL-6, thereby influencing a key pathway involved in regenerating liver cell proliferation and survival. Furthermore, the secretion of IL-4 was controlled by complement through the recruitment of NKT cells to regenerating livers. Our study thus reveals the existence of a regulatory feedback mechanism involving complement and IL-4 that controls liver regeneration.
Introduction
Liver regeneration is a homeostatic mechanism which assures the preservation of liver functions in cases of hepatocyte injury (1). The importance of this process is underscored by the constant exposure of the liver to various harmful factors that are present in blood it filters, such as exogenous toxins from the gut and pathogens circulating in the blood. Although bacteremia is rather uncommon, any type of viral infection can produce viremia and subsequent hepatitis, which is most often subclinical. In addition, hepatotropic viruses cause even more severe and clinically overt liver disease. Thus, liver regeneration is constantly required to maintain metabolic liver function (2). Regeneration is also triggered by liver resection and transplantation, when the transplanted organ is too small to cope with metabolic demands (3). Finally, various liver pathologies such as chronic hepatitis and cirrhosis are associated with repeated cycles of injury and regeneration that can ultimately lead to hepatocellular carcinoma (4). Therefore, studies of liver regeneration can broaden our understanding of homeostatic mechanisms that are essential for the survival of humans and animals, as well as provide insights into the pathogenesis of major liver pathologies.
The contribution of the immune system to liver regeneration is still poorly understood. However, recent studies have demonstrated the importance of a small number of immune mediators for the regenerative process resulting from partial hepatectomy (PHx; ~70% liver resection), which has become a useful paradigm of regenerative organ growth (1). Although liver regeneration after PHx is traditionally perceived as a process which does not trigger an inflammatory response (1), innate immunity seems to be particularly involved in regulating the early phases of regeneration (5). For example, we have demonstrated that the complement anaphylatoxins C3a and C5a, which are potent inflammatory mediators (6), contribute to cell growth and survival after PHx (7, 8). Complement-dependent regulation of liver regeneration occurs through cross-talk with IL-6 and TNF, which provide essential signaling to initiate regeneration (7, 8). Importantly, IL-6 regulates pro-survival pathways in regenerating hepatocytes (9). Therefore, we hypothesized that liver regeneration is regulated by a complex innate immune response involving various cytokines interacting with the complement system.
We report here that PHx triggered the rapid release of various cytokines that have not yet been functionally linked to liver regeneration. We identified IL-4, which was produced by natural killer T (NKT) cells accumulating in the liver immediately after PHx, as the most promising candidate to be involved in the regenerative response, and demonstrated the requirement of IL-4 for hepatocyte proliferation and survival. IL-4 regulated regeneration by controlling complement activation and the subsequent induction of IL-6. The mechanism of this IL-4 function was associated with maintaining appropriate levels of plasma IgM, since complement activation during regeneration was most likely triggered by IgM deposited in regenerating liver. We also found that complement-IL-4 interactions during liver regeneration were reciprocal, as complement regulated the induction of IL-4 by contributing to the recruitment of NKT cells to regenerating livers.
Materials and Methods
Animal studies
Mice deficient in complement C3 (C3−/−) have been previously described (7, 10). Mice lacking IL-4 (IL-4−/−) were initially obtained from The Jackson Laboratory (Bar Harbor, ME). Deficient mice were backcrossed for at least 10 generations onto a C57BL/6 background and maintained in our facility at the University of Pennsylvania. Wild-type littermates (C3+/+) or C57BL/6J mice from The Jackson Laboratory were used as controls.
For PHx studies, male mice 12–16 weeks of age were anesthetized and subjected to midventral laparotomy with ~70% liver resection using a modified version of the technique originally described by Higgins and Anderson (11). Control animals underwent midventral laparotomy without manipulation of the liver (sham treatment). Histological slides from the resected liver lobes were analyzed to exclude pre-existing pathology. Mice that were sacrificed 24 h or later after surgery received a single dose (i.p., 50 mg/kg animal weight) of BrdU (Sigma Aldrich, St. Louis, MO) 1 h prior to sacrifice. At the time of sacrifice, clinical status was assessed, mice were anesthetized, blood was harvested, and the remaining liver lobes and other internal organs were removed, weighed, and processed for analysis. Histological slides prepared from lungs, kidneys, spleen, intestine, and pancreas were analyzed for the presence of any morphological abnormalities that could indicate infection. Mice with an inflammatory infiltrate in any of the internal organs were excluded from further analysis.
To assess complement activation or the levels of cytokines secreted during liver regeneration, 30–40 −l of blood was collected with EDTA (for plasma) or without anticoagulant (for serum) from the tail vein before and at 0.5, 1, 2, 3, 6, 12 and 24 h after surgery. Plasma and serum were separated by gentle centrifugation (1000 x g) at 4°C for 10 min and then stored at −70°C until analyzed.
The clinical status (activity and appearance) and pathological features (gross appearance of organs and tissues) of mice sacrificed 24 h after surgery or later was used to determine rates of morbidity and mortality. Mice showing normal activity with no evident injury (grossly and/or microscopically) to the liver and other organs, and with no discoloration of collected serum/plasma, were determined to be “healthy.” Those showing signs of significantly decreased activity and/or tissue injury or yellowish serum due to high bilirubin levels were counted in the “morbidity” group. Those dying at any time after surgery or requiring euthanasia prior to the predetermined time of sacrifice because of their poor clinical condition were included in the “mortality” group.
Mice were housed in an animal facility of the University of Pennsylvania, within a barrier, on a 12-h light/dark cycle. Before the assignment of mice to the experimental groups, sera and feces were tested for the most common rodent infections, including Helicobacter hepaticus. Water and standard rodent diet were provided ad libitum. All mice were used with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee and according to criteria outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Monitoring of the cytokine response in mouse sera
The multiplex bead-based assay system (Bio-Plex; Bio-Rad Laboratories, Inc., Hercules, CA) was used to determine the levels of 18 different cytokines in mouse sera before and after PHx and sham surgery. The cytokines tested were: granulocyte-colony stimulating factor, granulocyte-macrophage-colony stimulating factor, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12 (p40), IL-12 (p70), IL-17, CXCL1/keratinocyte chemoattractant, CCL3/MIP-1α, CCL5/RANTES, and TNF-α. Assays were carried out according to the manufacturer’s instructions (Bio-Rad Laboratories, Inc.).
Hierarchical clustering
The function clustergram of the Statistics/Bioinformatics toolbox of Matlab® (Mathworks, Inc., Nautic, MA) was used to perform hierarchical clustering in which cytokines served as variables and each time-point served as an observation. The ‘correlation’ (Pearson correlation) option was used to compute the distance between the variables. The ‘average’ linkage-method was used for constructing the dendogram (hierarchical cluster tree). The vectors consisted of the observations. Data was averaged at each time-point. The data passed to the function clustergram was first normalized (by subtracting the mean and dividing by standard deviation) and then t = 0 value was subtracted for comparing shapes of time-courses.
Liver histology and immunohistochemical and immunofluorescent staining
Liver morphology was assessed in a blinded fashion by light microscopy (Olympus BX 60) of H&E-stained 5-μm paraffin sections. BrdU staining and quantification and analysis of the presence of apoptotic cells in paraffin liver sections were performed as described previously (12, 13). For immunofluorescent staining to measure IgM and C3 deposition, slides of frozen liver tissue sections were dried, fixed in cold acetone for 5 min, and incubated with 1:50 goat anti-mouse IgM (1021-05; Southern Biotech, Birmingham, AL) and rat anti-mouse C3b/iC3b/C3c (HM 1065b; Hycult Biotech, Plymouth Meeting, PA), or goat and rat IgG (controls) for 2 h. Antibodies were detected by incubating slides for 2 h with 1:600 anti-goat Cy2 and 1:300 anti-rat Cy3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Fluorescence was evaluated by standard fluorescence microscopy (Zeiss Axiovert 40 microscope).
Isolation of liver cell populations
Samples were processed from resected liver tissue (considered 0 h) and from regenerating liver tissue collected 3 h after PHx. For mononuclear cells including NK, T and NKT subpopulations, tissue was cut into small pieces and mechanically disrupted by passage through a 70-μm cell strainer (BD Falcon, Bedford, MA) to obtain single-cell suspensions. Cells were washed with PBS and then centrifuged at 300 x g for 5 min. The cell pellet was resuspended in 35 ml PBS, underlaid with 10 ml Ficoll (GE Healthcare, Piscataway, NJ), and centrifuged at 500 x g for 30 min. The interphase layer containing mononuclear cells was collected, washed with PBS, and resuspended in RPMI-1640 medium/5% heat-inactivated fetal calf serum. Cells were stimulated with 10 ng/ml phorbol myristate acetate (Sigma-Aldrich, Inc., St. Louis, MO) and 200 ng/ml ionomycin (Sigma-Aldrich, Inc.) in the presence of GolgiStop protein transport inhibitor (BD Biosciences, San Jose, CA) for 11 h at 37°C. For macrophages, tissue was cut into small pieces in the presence of HBSS/1 mg/ml Collagenase D (Roche Diagnostics Corp., Indianapolis, IN) and incubated at 37°C for 30 min with occasional agitation. Samples were washed with HBSS, the pellet was re-dissolved in 40% Ficoll in RPMI medium, overlaid in 80% Ficoll/RPMI, and centrifuged at 900 x g for 25 min. The interphase layer containing macrophages was collected, washed with HBSS, and incubated in 1 μg/ml GolgiPlug (555029; BD Biosciences) for 2 h.
Flow cytometric analysis
Cells were washed and incubated for 15 min at 4°C with a monoclonal antibody (mAb) recognizing mouse cluster of differentiation (CD)16/CD32 (553142; BD Biosciences) to block Fcγ receptors. Cells were then incubated for 30 min at 4°C with a fluorochrome-conjugated mAb recognizing mouse CD45 (557659; BD Biosciences), as well as Viaprobe (555815; BD Biosciences) and fluorochrome-conjugated mAbs recognizing CD3 (552774; BD Biosciences) and NK1.1 (557391; BD Biosciences) to identify NK, T and NKT cell populations, or F4/80 (25-4801-82; BD Biosciences) without Viaprobe to identify macrophages. Antibodies and Viaprobe were used according to the manufacturer’s instructions. Stained cells were analyzed by six-color flow cytometry on a FACS Canto II (BD Biosciences) with FlowJo software (TreeStar, Inc., Ashland, OR).
Intracellular IL-4 measurements
Liver cells isolated as described above were sequentially treated with Cytofix/Cytoperm solution (BD Biosciences) and then incubated with Perm/wash buffer (BD Biosciences) in the presence of an Alexa Fluor 488-conjugated mAb against IL-4 (557728; BD Biosciences) or isotype-matched control antibody (557720; BD Biosciences). Stained cells were analyzed by flow cytometry as described above.
Enzyme-linked immunosorbent assay for measuring C3b/iC3b/C3c
The levels of C3 cleavage products, reflecting the activation of complement, were determined in mouse plasma as described (12).
Determination of serum IgM levels
IgM was measured in mouse serum and plasma taken from mice before or 2–3 h after PHx. Samples were analyzed either by Anilytics, Inc. (Gaithersburg, MD) or in-house using an ELISA kit (Bethyl Laboratories, Inc., Montgomery, TX) according to the manufacturer’s instructions. Three samples tested by Anilytics, Inc. were also tested by in-house ELISA to ensure similarity between the two methods. Values were found to differ by an average of only 1.57 ± 0.96; thus, the assays were considered similar, and the results were combined for final analysis.
Data analysis
Statistical analyses were performed using Student’s t-test (two-tailed), Fisher’s exact test, and two-way ANOVA (GraphPad Prism, GraphPad Software Inc., San Diego, CA). A p-value less than or equal to 0.05 was considered significant. For hierarchical clustering analysis, the Pearson Correlation Coefficient was calculated to determine similarity between cytokine time courses (8 time points) for each experimental set (wild-type PHx and wild-type sham), using the following methodology (14–16): Correlation value was thought of as the cosine of the angle between the normalized time course curves (z-scores). The two time courses were denoted as X and Y (both were row-vectors):
The two vectors were normalized individually by their means and standard deviations (constant time courses were removed or a small normal-random number of the order of the machine precision was added). The resultant was the z-score:
The correlation was the dot-product of the two z-scores, which was then normalized by (m – 1), where m = the length of the vectors:
An r-value of 0.2 to 0.5 was considered to indicate a minor difference for the time course of a particular cytokine in mice undergoing PHx versus sham surgery, while an r-value of less than 0.2 was considered to indicate a substantial difference.
Results
Liver regeneration is associated with a distinctive cytokine signature
Liver regeneration is thought to be regulated by interplay between cytokines and growth factors (17). However, to date only a few cytokines, such as IL-6, TNF, and TGF-β, have been clearly linked to this process (4). Since various innate and adaptive immune responses are regulated by complex interactions within a cytokine network (18–20), it is likely that other cytokines, in addition to the well-known regulators, play a role in liver regeneration. Therefore, we conducted a comprehensive analysis of the cytokine response to 70% liver resection (PHx), which induces robust regeneration of the remaining liver parenchyma. The cytokine signature associated with liver resection was significantly different from that after laparotomy alone (sham surgery; Fig. 1A, B) suggesting that liver resection and the resulting regeneration trigger a specific cytokine response through mechanisms that differ from those triggered by other types of surgery. Correlation analysis revealed that cytokines that differed significantly in terms of their secretion pattern after PHx and sham surgery included IL-1β, IL-2, IL-3, IL-4, IL-10, IL-12 (p40), IL-17, CCL5/RANTES, and TNF (Supplemental Table I). However, significant induction of these cytokines over baseline values was observed only for IL-1β, IL-4, IL-10, IL-17, and TNF. Increases in the induction of TNF after PHx, but not sham surgery, demonstrated the reliability of the assay, since TNF has previously been shown to be upregulated after PHx (4). Additionally, although the secretion pattern for IL-6 was similar after PHx and sham surgery, the magnitude of the induction of this cytokine was higher after PHx (Supplemental Fig. 1), consistent with its established role in liver regeneration (4, 21). The patterns of secretion for IL-4, IL-10, and IL-1β were similar to that for IL-6, according to cluster analysis (Fig. 1A). Thus, we hypothesize that these cytokines are potentially involved in regeneration. Furthermore, since a robust cytokine response was seen immediately after PHx, it is likely that liver regeneration-associated cytokine induction is a consequence of the activation of innate immunity, which includes macrophages and can occur rapidly. Simultaneous induction of IL-4 and IL-10, and the lack of an increase in IFN-γ, indicated that the cytokine response triggered by liver regeneration overlaps to some extent with the cytokine signature of alternatively activated macrophages (22, 23). Since IL-4 provides signaling essential for this activation (22, 23) and was induced by PHx, and since the pattern of its secretion resembled that of IL-6, we chose to focus our further studies on IL-4.
Figure 1. Cytokine profile following partial hepatectomy.
A) Heat map showing the induction of serum cytokines, based on multiplex bead-based analysis, over baseline levels (0 h) in wild-type (WT) mice after PHx. Colored blocks represent changes at each time point, with the color intensity indicating the magnitude of the change, as indicated in the legend to the right. Cytokines are clustered based on similarity of post-PHx induction. Branches to the left of the heat map indicate similar groups; branches closer to the left edge of the heat map connect cytokines with stronger similarities, as compared to those farther out (n = 8–18 mice per time point). B) Same cluster analysis as in (A), but for cytokine induction following sham surgery (n = 5–9 mice per time point).
IL-4 is required for liver regeneration
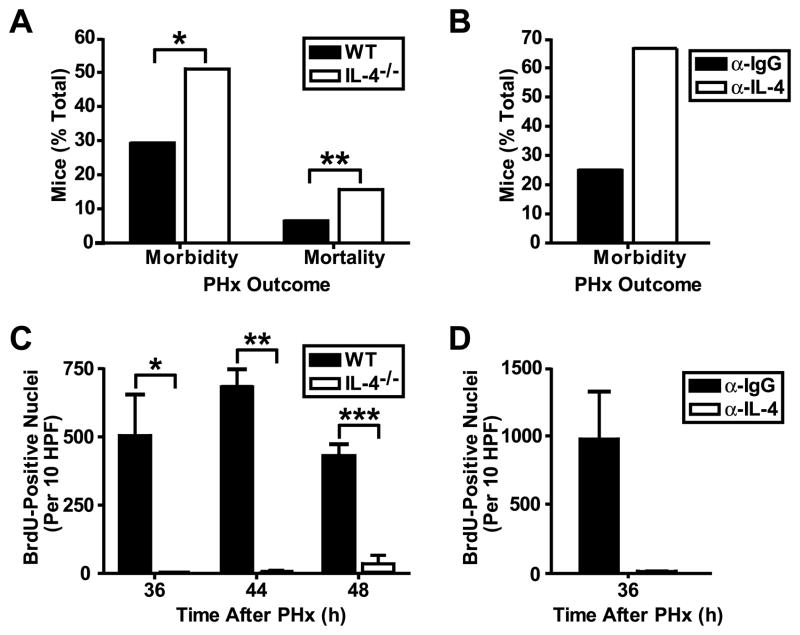
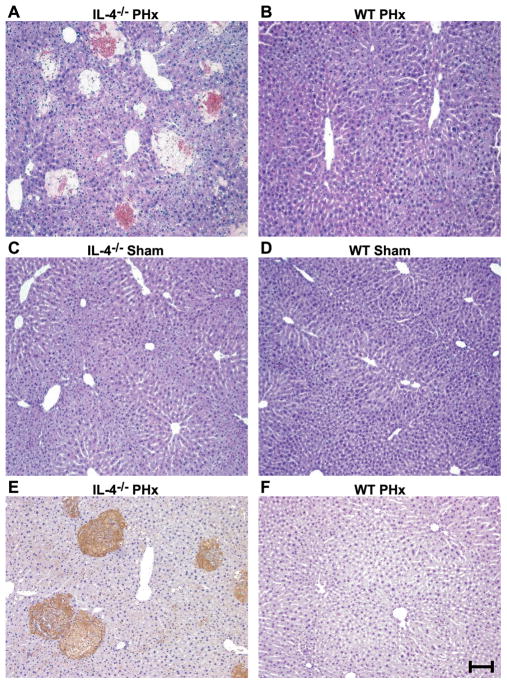
PHx was associated with higher morbidity and mortality in IL-4-deficient mice (Fig. 2A) and wild-type mice treated with IL-4-neutralizing antibody (Fig. 2B) when compared to IL-4 wild-type controls or IgG-treated wild-type mice, respectively (morbidity and mortality in control mice was due to complications arising from the surgery). This poor clinical outcome was associated with significantly impaired liver regeneration in morbid IL-4-deficient (Fig. 2C) and IL-4 antibody-treated wild-type mice (Fig. 2D), as demonstrated by an almost complete lack of proliferation of hepatocytes in all of these mice when compared to control animals. PHx also resulted in massive injury in the remaining liver parenchyma in IL-4-deficient mice (Fig. 3A) and IL-4 antibody-treated wild-type mice (data not shown), whereas wild-type livers after PHx showed no injury and displayed hepatocyte morphology typical of regenerating cells, including nuclear hypertrophy (Fig. 3B). Sham surgery did not cause any morphological abnormalities in livers from IL-4-deficient (Fig. 3C) or wild-type control mice (Fig. 3D), indicating that damage to liver parenchyma in mice lacking IL-4 is specifically associated with liver resection and failure of the liver to regenerate. Positive staining for the activated caspase 3 cleavage site on cytokeratin 18 seen in areas of injury in IL-4-deficient livers (Fig. 3E) indicated that hepatocytes die through apoptosis in the absence of IL-4. As expected, this staining was not observed in wild-type livers (Fig. 3F). Thus, these data demonstrate a requirement for IL-4 for both aspects of liver regeneration: proliferation of hepatocytes and hepatoprotection.
Figure 2. Poor clinical outcome and impaired liver cell proliferation after partial hepatectomy in mice lacking IL-4 signaling.
A) Graph representing the percentage of WT and IL-4-deficient (IL-4−/−) mice showing morbidity (clinically and/or pathologically) or mortality 24–72 h after PHx. Data are presented as means ± s.e.m. (n =92 [WT] and 51 [IL-4−/−] mice). *, p = 0.0023, Fisher’s exact test; **, p = 0.0005, Fisher’s exact test. B) Same analysis as in (A), but for WT mice treated with IL-4 antibody (α-IL-4) or control IgG antibody (α-IgG). Only morbidity is shown, as there were no differences in mortality rates (n = 8 [α-IgG] and 9 [α-IL-4] mice). C) Graph representing proliferating hepatocytes, expressed as the number of BrdU-positive nuclei stained per 10 high-powered fields per liver, in healthy WT and morbid IL-4−/− mice following PHx. Data are presented as means + s.e.m. (n = 4–27 [WT] and 3–4 [IL-4−/−] mice per time point). *, p = 0.0449, t-test; **, p = 0.0402, t-test; ***, p = 0.0145, t-test. D) Same analysis as in (C), but for WT mice treated with α-IL-4 and showing morbidity, or treated with α-IgG without subsequent morbidity or mortality (n = 4 [α-IgG] and 3 [α-IL-4] mice).
Figure 3. Liver injury after partial hepatectomy in IL-4-deficient mice.
A-B) H&E staining of IL-4−/− (A) and WT (B) liver sections 48 h after PHx. Necrotic foci are evident in the IL-4−/− liver (n = 33 [WT] and 24 [IL-4−/−] mice). C-D) Same analysis as in (A–B), but following sham surgery (n = 7 [WT] and 3 [IL-4−/−] mice). E–F) Staining for the activated caspase 3 site of cytokeratin 18, indicating areas of apoptotic injury (brown staining) in liver sections from IL-4−/− (E) and WT (F) mice analyzed 44 h after PHx (n = 3 mice per genotype). Scale bar = 100 μm.
NKT cells produce IL-4 during liver regeneration
The importance of NKT cells in the liver for regulating innate and adaptive immunity has been shown; however, the potential role of these cells in liver regeneration remains unclear (24). In wild-type mice, we observed the accumulation of CD45+CD3+NK1.1+ (NKT) cells in the remaining liver parenchyma 2–3 h after PHx, in accordance with previous reports (25). This increase was seen whether NKT cells were expressed as a percentage of CD45+ cells (Fig. 4A, black bars) or total counts for NKT cells were expressed as a ratio of all viable cells (data not shown). Since NKT cells have the ability to rapidly secrete IL-4 during an innate immune response (26), we hypothesized that these cells produce IL-4 during liver regeneration. Intracellular staining for IL-4 revealed that NKT cells accumulating in the regenerating liver produced IL-4 even without additional in vitro restimulation (Fig. 4B, black bars). Importantly, IL-4 production by NKT cells was induced by PHx, as cells isolated from livers before PHx did not show any significant accumulation of IL-4 in their cytoplasm (Fig. 4B, black bars). We also examined IL-4 production during regeneration in liver NK cells and T cells, given some of their shared characteristics with NKT cells, but no induction was observed (data not shown). Additionally, to rule out the possible contribution of Kupffer cells, we analyzed IL-4 production in macrophages. No macrophage IL-4 production was detected either before or after PHx (data not shown). Thus, we concluded that NKT cells are a primary source of IL-4 produced during liver regeneration.
Figure 4. Reduced recruitment of NKT cells to regenerating livers in C3-deficient mice and production of NKT cell IL-4 induced by partial hepatectomy.
A) Graph showing the number of NKT cells (CD3+NK1.1+), expressed as a percentage of total CD45+ leukocytes, in WT and C3-deficient (C3−/−) mice before (resected livers, 0 h) and 3 h after PHx (remaining liver tissue from the same mice). Data are presented as means + s.e.m. (n = 19 mice per genotype). *, p < 0.0001, t-test; **, p = 0.0004, two-way ANOVA. B) Graph representing intracellular IL-4 in a portion of the NKT cell populations described in (A), expressed as the increase in mean fluorescence intensity over IgG control antibody staining (n = 9 mice per genotype). *, p = 0.0009, t-test; **, p = 0.0002, t-test.
IL-4 regulates liver regeneration through complement activation
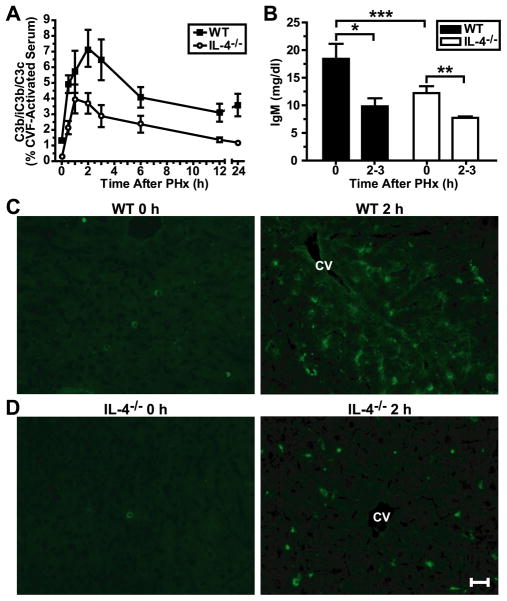
IL-4 induces rapid production and secretion of IgM in B cells in a model of contact sensitivity (27). These IgM antibodies bound to antigens activate complement, leading to vasodilation and the recruitment of immune cells (27). Therefore, we hypothesized that complement activation during liver regeneration is influenced by IL-4. Although a low grade of complement activation, as demonstrated by an increase in the plasma levels of C3 cleavage products, was observed in hepatectomized IL-4-deficient mice, the magnitude of this activation was significantly lower than in wild-type controls subjected to PHx, with about half as much activation at the wild-type peak time of 2 h (Fig. 5A, 3.7% vs. 7.2%, respectively), confirming our initial hypothesis. Furthermore, PHx decreased plasma levels of IgM in wild-type and IL-4-deficient mice (Fig. 5B), suggesting that these antibodies were utilized elsewhere, presumably in the liver. This hypothesis was confirmed by demonstrating IgM deposition in the regenerating liver parenchyma of wild-type mice (Fig. 5C). Importantly, IgM deposition was remarkably attenuated in IL-4-deficient livers after resection (Fig. 5D) as compared to wild-type mice. This can be attributed to the lower baseline levels of plasma IgM in IL-4-deficient mice than in wild-type controls (Fig. 5B), and is emphasized by the similar levels seen 2–3 h after PHx in both groups (Fig. 5B), suggesting that there is less IgM being utilized in the liver in IL-4-deficient animals. Although a decrease in IgM plasma levels was observed in both wild-type and IL-4-deficient mice after PHx, the limited deposition of this immunoglobulin in regenerating IL-4-deficient livers suggested that the decreased amounts of IgM in these mice affected complement activation induced by PHx. This was further suggested by co-localization of IgM and C3 in 3 h post-PHx wild-type livers, indicating that complement is being activated by IgM during regeneration (Supplemental Fig. 2). Thus, IL-4 is required for liver regeneration because it regulates the activation of complement by maintaining appropriate levels of IgM in the plasma.
Figure 5. Decreased complement activation, IgM levels and IgM deposition in IL-4-deficient mice.
A) Graph showing the levels of complement cleavage products (C3b/iC3b/C3c) in the plasma of WT and IL4−/− mice after PHx. Values represent percentages of values for cobra venom factor (CVF)-activated serum, which was considered to cause 100% complement activation. Data are presented as means ± s.e.m. (n = 4–6 [WT] and 4–5 [IL-4−/−] mice per time point). p < 0.0001, two-way ANOVA. B) Graph of IgM levels in the plasma of WT and IL-4−/− mice before (0 h) and 2–3 h after PHx. Data are presented as means + s.e.m. (n = 11–16 [WT] and 5–9 [IL-4−/−] mice per time point). *, p = 0.0022, t-test; **, p = 0.0326, t-test; *** p = 0.0316, t-test. C) Immunofluorescent staining for IgM deposition in liver sections from WT mice before (0 h) or 2 h after PHx. Bright green staining indicates the presence of deposited IgM. Immunofluorescence at 2 h is representative of staining seen at 2 and 3 h after PHx (n = 3–4 mice per time point). D) Same staining as in (C), but for IL-4−/− mice (n = 3–4 mice per time point). Scale bar = 100 μm. CV, central vein.
The lack of IL-4 disrupts a key regulatory pathway required for liver regeneration
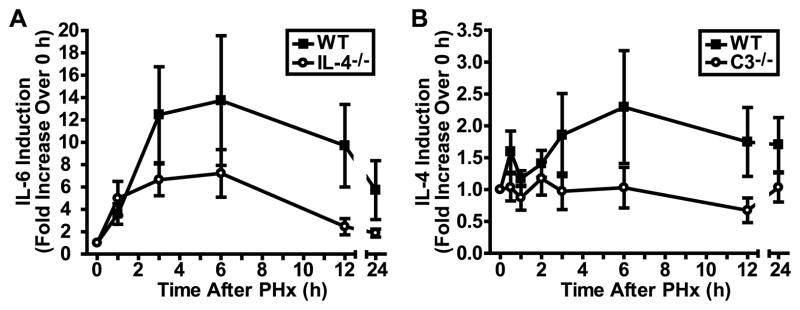
Complement anaphylatoxins C3a and C5a, generated as a result of complement activation (28), control proliferative and pro-survival signaling during liver regeneration by regulating IL-6 (8). Since IL-4 is required to sustain wild-type levels of complement activation and, consequently, anaphylatoxin production in the course of regeneration (Fig. 5A), we hypothesized that a lack of IL-4 would affect the levels of IL-6 secreted/produced during this process, thereby influencing the most critical pathway regulating regeneration. Indeed, the induction of IL-6 in the response to PHx was reduced as a result of IL-4 deficiency (Fig. 6A).
Figure 6. Changes in specific cytokine induction after liver resection in IL-4- and C3-deficient mice.
A) Graph showing the induction of IL-6 in sera of WT and IL-4−/− mice after PHx. Baseline (0 h) levels were set to 1, and subsequent levels measured in the same mice were adjusted to show increases over baseline. Data are presented as means ± s.e.m. (n = 5 mice per time point per genotype). p = 0.0195, two-way ANOVA. B) Graph showing the induction of IL-4 in sera of WT and C3−/− mice after PHx. Data are presented as means ± s.e.m. (n = 13–14 [WT] and 8–9 [C3−/−] mice per time point). p = 0.0067, two-way ANOVA.
Complement is required for IL-4 induction during liver regeneration
We have shown here that IL-4 regulates complement activation during liver regeneration. However, since complement is often an upstream regulator of cytokine responses (29), we asked whether complement also regulates IL-4 induction. To address this question, we monitored IL-4 induction in C3-deficient mice, since this deficiency eliminates the majority of complement effector functions. We found that the lack of C3 almost completely abrogated IL-4 induction by PHx (Fig. 6B). This observation suggests the existence of a regulatory circuit in which complement is essential for IL-4 induction, and which in turn potentiates complement activation by IL-4.
Complement regulates IL-4 production by recruiting NKT cells to regenerating livers
Since we identified NKT cells accumulating in regenerating livers as a major source of the IL-4 secreted during regeneration, and since complement effectors are potent chemoattractants that recruit leukocytes to sites of inflammation (29), we hypothesized that complement controls IL-4 production by recruiting NKT cells to regenerating livers. This hypothesis was confirmed by the attenuated recruitment of NKT cells to livers after PHx in C3-deficient mice (Fig. 4A, white bars vs. black bars). The amount of NKT cells present in regenerating livers of both C3a receptor (C3aR)- and C5a receptor (C5aR)-deficient mice was similar to that seen in wild-type livers, suggesting that the anaphylatoxins were not responsible for the infiltration of these cells, despite the fact that they were found to express C5aR mRNA (data not shown). We also examined liver mRNA levels for several chemokines thought to be involved in NKT cell migration (30), but their patterns of induction were similar in wild-type and C3-deficient livers (data not shown), indicating they also were not responsible for the differences observed in NKT cells numbers after PHx. Though C3 deficiency did not affect the amount of IL-4 produced by NKT cells after PHx (Fig. 4B, white bars vs. black bars), we concluded that limited recruitment of NKT cells to regenerating livers was sufficient to cause decreased production of IL-4 in mice lacking C3, given that only NKT cells obtained after PHx were capable of producing IL-4, in contrast to NKT cells residing in quiescent livers (Fig. 4B).
Discussion
Although the role of inflammation and innate immunity in liver regeneration has long been neglected, since regenerating liver parenchyma lacks an overt inflammatory infiltrate (1), recent studies have clearly established the role of several cytokines and complement anaphylatoxins in the early phases of this process (2, 4). Furthermore, lipopolysaccharide released from enteric bacteria seems to be a logical candidate for a master upstream regulator of the innate immune response induced during regeneration (31, 32). However, the list of cytokines and other innate immunity mediators that could be involved in this process is surprisingly short. Here, we provide evidence that liver regeneration is associated with the immediate and robust activation of innate immunity and involves the secretion of numerous cytokines that have not yet been linked to this process. The differences in cytokine secretion patterns after PHx and sham surgery suggested that the cytokine response after PHx is specific for the regenerative process and is not simply a response to trauma induced by the surgical procedure. Similarities between the cytokine response to liver resection and the cytokine milieu associated with the alternative activation of macrophages led us to the conclusion that IL-4, which regulates alternative macrophage polarization, may be a key regulator of liver regeneration and the associated cytokine response after PHx. Our data shown here supports this conclusion, and demonstrates a mechanism of both IL-4 regulation of complement activation and complement-induced induction of IL-4.
The mechanisms by which IL-4 contributes to complement activation apparently involved the maintenance of the appropriate levels of IgM in mouse plasma, since we found that IgM, a potent activator of complement (33), was deposited in liver tissue after PHx and co-localized with C3, and also that IgM plasma levels and IgM deposition in regenerating liver tissue were reduced in IL-4-deficient mice. Thus, in the absence of IL-4, there is not enough IgM to activate complement at the wild-type level needed for proper regeneration. Importantly, though the role of natural IgM in activating complement is well-known (through the binding of C1q to IgM complexes (33)) and has been established for models of ischemia and reperfusion injury (34, 35), the contribution of these antibodies to complement activation during liver regeneration has not previously been demonstrated. We found that IgM and C3 were deposited in wild-type livers shortly after surgery, at times corresponding to the peak of complement activation measured in plasma. The fact that IL-4 contributes to IgM production is known (27), but the importance of this regulatory mechanism for liver regeneration has not yet been explored.
The similarity of the phenotypes for C3aR- and C5aR-deficient mice subjected to PHx (8) to that observed for mice lacking IL-4 signaling further strengthens the hypothesis that IL-4 contributes to regeneration through the regulation of complement activation. In addition, we found that IL-4-deficient mice showed reduced induction of IL-6 after PHx. IL-6, shown to be produced in the liver during regeneration, has recently emerged as a key regulator of pro-proliferative and pro-survival pathways that are essential for this process (4, 9, 17). Though there has been some discrepancy in the literature as to the necessity of IL-6 for proper liver regeneration, it has been suggested that this may reflect that the levels of IL-6 are the critical factor, whereas most studies simply analyze regeneration in its presence or absence (4). Regardless, overall the involvement of this cytokine in the restoration of liver mass is widely accepted. Since complement regulates the induction of IL-6 (7, 8), our finding confirms that reduced complement activation in IL-4-deficient mice affects a major regulatory pathway involved in the priming phase of liver regeneration (17), and the lack of hepatocyte proliferation and the presence of massive injury to the liver parenchyma that we observed in IL-4-deficient mice can be primarily attributed to decreased complement activation and consequently lower IL-6 induction.
We identified NKT cells accumulating in regenerating liver shortly after PHx as a major source of IL-4 secreted during regeneration. Furthermore, since NKT cells residing in quiescent livers did not produce detectable amounts of IL-4, we concluded that PHx, and consequently regeneration, primed NKT cells to produce this cytokine. NKT cells have been shown to regulate innate and adaptive immune responses through the production of a variety cytokines, including IFN-γ and IL-4 (26, 36, 37). Importantly, liver lymphocytes are enriched in NK and NKT cells that contribute to antiviral and antitumor defense (24). These cell populations are also involved in the pathogenesis of chronic liver diseases (24). However, the role of hepatic NKT cells in liver regeneration remains controversial, since it has been reported that NKT cells play a minor role in regeneration (24), inhibit this process (38, 39), or promote it (40). Our observation that NKT cells accumulate in regenerating livers is in agreement with previously published reports (24, 25, 41). The fact that these cells produce IL-4, which we found to be required for regeneration, suggests that they play a role in a regulatory network that is mandatory for successful regeneration. The mechanisms by which NKT cells are recruited to regenerating liver require further clarification, although a role for sympathetic nerve activation in this process has been suggested (25). The reduced migration of NKT cells observed in C3-deficient mice shortly after PHx highlights the role of complement in the recruitment of these cells to regenerating liver. The chemoattractant abilities of the anaphylatoxins C3a and C5a make them natural candidates responsible for this recruitment, but we did not detect any changes in the amount of NKT cells in the regenerating livers of C3aR- or C5aR-deficient mice when compared to wild-type animals.
Additionally, the recruitment mechanism does not appear to be related to any of the chemokines currently thought to be involved in NKT cell migration (30), as there were no significant alterations in the expression of these chemokines when comparing wild-type and C3-deficient livers. However, the CR3 (CD11b/CD18) receptor can bind to deposited iC3b resulting from complement activation (6) and may therefore help increase adhesion of NKT cells in the liver. We have seen C3 deposition in livers after PHx using an antibody that detects C3 cleavage products, including iC3b, and our preliminary experiments have detected CD11b expression on liver NKT cells. Thus, C3 cleavage products may form a bridge between endothelial and NKT cells to promote their accumulation during liver regeneration. Regardless of the mechanism of NKT cell recruitment, since the amount of IL-4 measured in NKT cells was not affected by C3 deficiency and we did not see IL-4 production by other liver leukocyte populations, including macrophages, NK cells and T cells, the reduced induction of IL-4 in C3-deficient mice after PHx appears to be a result of the decreased migration of NKT cells to regenerating livers in the absence of functionally active complement.
As mentioned, the cytokine profile observed after PHx was similar to that associated with the alternative activation of macrophages. The importance of IL-4 for liver regeneration, as demonstrated here, further suggests a possible role of alternatively-activated macrophages in this process. The potential role of Kupffer cells in liver regeneration has previously been studied (17, 42–44), with some work emphasizing the importance of their cytokine profile (45, 46). Thus, it is very likely that the polarization state of macrophages in the liver has a significant effect on the course of regeneration. This important concept requires further thorough investigation, which was beyond the scope of the current study.
Our conclusion that IL-4 is an important regulator necessary for proper liver regeneration may seem to contradict several studies that have shown IL-4 to be a promoter of hepatocyte apoptosis. However, at least one of these studies analyzed the expression of IL-4 from an injected adenovirus vector (47), which likely resulted in IL-4 levels higher than those seen in physiological processes. Other studies have utilized different models, such as drug-induced injury or infection (48–50), which involve mechanisms related to the repair of injured tissue, as opposed to regeneration after PHx, which is considered to be more of a growth response (i.e., the remaining parenchyma is not damaged). Additionally, IL-4 has in fact been shown to promote hepatocyte proliferation and/or protect cells from injury during schistosomiasis (51) and in ischemia/reperfusion (52), again demonstrating the context specificity of its actions.
Morbidity and mortality rates in IL-4-deficient mice subjected to PHx were significantly higher than those in wild-type controls. However, some IL-4-deficient mice exhibited normal regeneration and a good clinical outcome after liver resection. These results point to the high redundancy among constituents of regulatory networks that control liver regeneration, as it has been repeatedly demonstrated that no single genetically modified mouse model produces 100% mortality and a complete blockage of DNA replication and cell proliferation after PHx (4).
The restoration of liver mass after PHx is achieved by hyperplasia and hypertrophy of mature cells. Nevertheless, several mechanisms triggered during liver regeneration, especially those responsible for the survival of the regenerating liver cells, are applicable to the regenerative process induced by stem cell therapies (1, 53). This commonality is particularly important because maintaining the viability of transplanted stem cells is one of the major challenges faced by those in the field of regenerative medicine (54). Additionally, the mobilization of stem cells is regulated by innate immune factors, including complement activation, suggesting similarities in the early stages of stem cell-induced regenerative processes and liver regeneration (55). Therefore, we anticipate that our results can be translated to several other fields of regenerative medicine.
In summary, the results presented here demonstrate the existence of a regulatory feedback mechanism involving major immune system players, including complement, cytokines, NKT cells, and possibly macrophages, which contributes to the maintenance of liver cell proliferation and survival during the regenerative response to liver resection (Fig. 7). The continuing discovery of new mechanisms that regulate liver regeneration has resulted in a transformation of our understanding of this process, from the original idea that a single humoral agent initiates and controls regeneration to the recent concept that multiple pathways, interacting with each other on various levels, are required to initiate and coordinate the cellular processes required for successful regeneration (4). This new concept is fully supported by our current demonstration of the importance of the regulatory circuit involving complement and IL-4 for this crucial physiological process.
Figure 7. Suggested regulatory network demonstrating the involvement of IL-4 in liver regeneration.
Partial hepatectomy (removal of the two largest lobes of the liver) induces a regenerative response in the remaining liver tissue. A regulatory feedback loop exists in which complement activation, involving the cleavage of C3 and C5 to produce the C3a and C5a anaphylatoxins, leads to the recruitment of NKT cells to the liver (independently of anaphylatoxin activity), and the subsequent production of IL-4 by these cells. IL-4 maintains IgM levels, and increased IgM deposition in the liver induces further complement activation. C3a and C5a are thought to act on macrophages, leading to production of IL-6, a cytokine important for the hepatocyte proliferation and protection that is necessary for proper liver regeneration.
Supplementary Material
Acknowledgments
The authors thank Dr. E. Reis for technical support and D. McClellan for editorial assistance.
Abbreviations
- NKT
natural killer T
- PHx
partial hepatectomy
- CD
cluster of differentiation
- C3aR
complement component 3a receptor
- C5aR
complement component 5a receptor
Footnotes
This work was supported by P01-AI068730 (JDL), GM69338-04 (SS) and P01-DK074868 (SS). The authors thank the Morphology Core of the Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) for technical support.
References
- 1.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markiewski MM, DeAngelis RA, Lambris JD. Liver inflammation and regeneration: Two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol. 2006;43:45–56. doi: 10.1016/j.molimm.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Lakkis FG. The unfinished legacy of liver transplantation: emphasis on immunology. Hepatology. 2006;43:S151–S163. doi: 10.1002/hep.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 5.DeAngelis RA, Markiewski MM, Lambris JD. Liver regeneration: a link to inflammation through complement. Adv Exp Med Biol. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- 6.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, Wetsel RA, Lambris JD. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978–980. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai AJ, Briles DE, Volanakis JE, Wetsel RA, Colten HR. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 11.Higgins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 12.Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol. 2004;173:747–754. doi: 10.4049/jimmunol.173.2.747. [DOI] [PubMed] [Google Scholar]
- 13.Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson TW. An introduction to multivariate statistical analysis. Wiley; New York: 1984. [Google Scholar]
- 15.Egghe L, Leydesdorff L. The Relation Between Pearson’s Correlation Coefficient r and Salton’s Cosine Measure. J Am Soc Inf Sci Technol. 2009;60:1027–1036. [Google Scholar]
- 16.Fisher RA. The mathematics of experimentation. Nature. 1938;142:442–443. [Google Scholar]
- 17.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 18.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 19.Brown KL, Cosseau C, Gardy JL, Hancock RE. Complexities of targeting innate immunity to treat infection. Trends Immunol. 2007;28:260–266. doi: 10.1016/j.it.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Chabalgoity JA, Baz A, Rial A, Grille S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007;18:195–207. doi: 10.1016/j.cytogfr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 26.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 27.Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 31.Cornell RP. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am J Physiol. 1985;249:R551–R562. doi: 10.1152/ajpregu.1985.249.5.R551. [DOI] [PubMed] [Google Scholar]
- 32.Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916–922. doi: 10.1002/hep.1840110603. [DOI] [PubMed] [Google Scholar]
- 33.Daha NA, Banda NK, Roos A, Beurskens FJ, Bakker JM, Daha MR, Trouw LA. Complement activation by (auto-) antibodies. Mol Immunol. 2011;48:1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 37.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 38.Dong Z, Zhang J, Sun R, Wei H, Tian Z. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology. 2007;45:1400–1412. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- 39.Ito H, Ando K, Nakayama T, Taniguchi M, Ezaki T, Saito K, Takemura M, Sekikawa K, Imawari M, Seishima M, Moriwaki H. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima H, Inui T, Habu Y, Kinoshita M, Nagao S, Kawaguchi A, Miura S, Shinomiya N, Yagita H, Seki S. Activation of mouse natural killer T cells accelerates liver regeneration after partial hepatectomy. Gastroenterology. 2006;131:1573–1583. doi: 10.1053/j.gastro.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Kato T, Sato Y, Takahashi S, Kawamura H, Hatakeyama K, Abo T. Involvement of natural killer T cells and granulocytes in the inflammation induced by partial hepatectomy. J Hepatol. 2004;40:285–290. doi: 10.1016/j.jhep.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Selzner N, Selzner M, Odermatt B, Tian Y, van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 43.Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- 44.Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996;270:G909–G918. doi: 10.1152/ajpgi.1996.270.6.G909. [DOI] [PubMed] [Google Scholar]
- 45.Rai RM, Loffreda S, Karp CL, Yang SQ, Lin HZ, Diehl AM. Kupffer cell depletion abolishes induction of interleukin-10 and permits sustained overexpression of tumor necrosis factor alpha messenger RNA in the regenerating rat liver. Hepatology. 1997;25:889–895. doi: 10.1002/hep.510250417. [DOI] [PubMed] [Google Scholar]
- 46.Rai RM, Zhang JX, Clemens MG, Diehl AM. Gadolinium chloride alters the acinar distribution of phagocytosis and balance between pro- and anti-inflammatory cytokines. SHOCK. 1996;6:243–247. doi: 10.1097/00024382-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Guillot C, Coathalem H, Chetritt J, David A, Lowenstein P, Gilbert E, Tesson L, van RN, Cuturi MC, Soulillou JP, Anegon I. Lethal hepatitis after gene transfer of IL-4 in the liver is independent of immune responses and dependent on apoptosis of hepatocytes: a rodent model of IL-4-induced hepatitis. J Immunol. 2001;166:5225–5235. doi: 10.4049/jimmunol.166.8.5225. [DOI] [PubMed] [Google Scholar]
- 48.Toyabe S, Seki S, Iiai T, Takeda K, Shirai K, Watanabe H, Hiraide H, Uchiyama M, Abo T. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 49.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 50.Dharancy S, Podevin P, Aoudjehane L, Batteux F, Rosenberg AR, Soubrane O, Calmus Y, Conti F. Elevated interleukin-4 expression in severe recurrent hepatitis C virus after liver transplantation. Transplantation. 2007;83:906–911. doi: 10.1097/01.tp.0000258729.68871.be. [DOI] [PubMed] [Google Scholar]
- 51.La Flamme AC, Patton EA, Bauman B, Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 52.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Reduced hepatic ischemia/reperfusion injury by IL-4: potential anti-inflammatory role of STAT6. Inflamm Res. 2000;49:275–279. doi: 10.1007/PL00000207. [DOI] [PubMed] [Google Scholar]
- 53.Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 54.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.