Abstract
Topiramate is an antiepileptic drug that has marked, treatment-limiting side effects on specific aspects of cognitive performance in both patients and healthy volunteers. As these severe side-effects occur only in certain individuals, identifying genetic or environmental variables that influence cognitive response would be of great utility in determining whether to administer this drug to a patient. We gave an acute 100 mg oral dose of topiramate to 158 healthy volunteers and measured how the drug changed their performance on a diverse battery of cognitive tests. We found a wide range of responses to topiramate and demonstrated that not all tests in the battery were equally affected. There was no correlation between the effect of topiramate and either education level or baseline cognitive performance. Interestingly, there was up to 55-fold variation in the topiramate plasma levels of the participants. Our genome-wide association study (GWAS) of cognitive response did not reveal any genome-wide significant associations; the study was powered to find variants explaining at least 25% of the variation in cognitive response. Combining the results of this GWAS with a retrospective study of cognitive complaints in 290 epilepsy patients who received topiramate as part of their treatment also did not result in a significant association. Our results support the need for additional genetic studies of topiramate that utilize larger sample sizes.
Keywords: topiramate, cognition, genome-wide association study, genetics, taste change
Introduction
While many antiepileptic drugs are associated with cognitive side effects, none have effects as severe, specific and well-characterized as topiramate(for example, (Greenwood 2000)). It seems that only specific areas of cognition, such as verbal fluency and working memory, are affected by topiramate, and the cognitive effect differs by person. No study has yet found a factor that can explain the difference in response, with the exception of some epilepsy-related traits such as hippocampal sclerosis and seizure type (Mula, et al. 2003). This general lack of impact of environmental variables suggests that genetic components may explain a portion of the variation in response; to our knowledge, however, no one has yet looked for genetic contributions to cognitive response following topiramate administration.
It has been demonstrated that topiramate affects cognition within three hours of taking the first dose, and it seems that the level of cognitive inhibition is similar to that experienced after long-term topiramate use(Martin, et al. 1999). Topiramate is therefore suited to a pharmacogenetic study in which healthy volunteers are given an acute dose. In the present study, we have administered a brief cognitive battery to a group of 158 healthy volunteers both at baseline and at approximately two hours following a single 100 mg oral dose of topiramate. We have characterized their change scores relative to the change scores of 240 healthy volunteers who took the battery twice without drug while controlling for relevant demographic variables impacting performance. Additionally, we have performed a genome-wide association study (GWAS) of cognitive response to acute topiramate exposure and compared the results to a GWAS of adverse cognitive effects in epilepsy patients taking topiramate.
Methods
The methods are described in detail in the Supplemental Data. In brief, a short neurocognitive test battery that utilized the 11 common, well-known measures shown in Table 1 was administered to 158 healthy volunteers both before and after the administration of a 100 mg acute dose of topiramate, and blood was taken to measure topiramate levels. The change scores of 240 controls who took the test twice without topiramate were used to calculate scaled, standardized regression based (SRB) change scores (Attix, et al. 2009) for the 158 cases. The SRB change score for each test was analyzed as its own phenotype, and in addition, the first principal component emerging from the 11 scores, ChangePC1, was used as a measure of the overall change. Additionally, the first principal component emerging from analysis of the baseline scores for the 11 cognitive tests, PC1, was taken as a representation of general cognitive ability at baseline.
Table 1. Effect of topiramate, topiramate plasma levels and other covariates on change in test score.
The regression model built to determine the percent of variation in test scores explained by whether the subject took topiramate included all 240 subjects who did not take topiramate and all 158 who did. The regression models built to determine the percent of variation in test score explained by topiramate plasma levels and other variables was restricted to the 135 subjects who took topiramate, had plasma levels measured and were included in the GWAS.
| Test | Cognitive areas |
Past studies show effect?* |
Effect in this study |
r2 for taking topiramate (n=364) |
r2for topiramate plasma levels (n=135) |
Additional significant covariates (n=135) |
r2 of full model (n=135) |
|---|---|---|---|---|---|---|---|
| Immediate Story Recall(Green 2005) |
Verbal Episodic Memory |
Uncertain | Yes | 0.14 | 0.14 | Ethnicity | 0.21 |
| Delayed Story Recall(Green 2005) |
Verbal Episodic Memory |
Uncertain | Yes | 0.21 | 0.18 | Ethnicity | 0.26 |
| TrailsA(USArmy 1944) | Attention, Processing Speed |
Uncertain | Yes | 0.23 | 0.11 | Third session | 0.19 |
| TrailsB(USArmy 1944) | Attention, Processing Speed, Executive Control |
Uncertain | Yes | 0.26 | 0.11 | 0.11 | |
| Digit Span Forward(Wechsler 1997) |
Working Memory |
Yes | Yes | 0.24 | 0.07 | Native language, chunking |
0.14 |
| Digit Span | Working | Uncertain | Yes | 0.18 | 0.10 | Chunking | 0.13 |
| Backward(Wechsler 1997) | Memory | ||||||
| COWA(Benton, et al. 1978) | Verbal Fluency, Executive Control |
Yes | Yes | 0.51 | 0.12 | BDI | 0.18 |
| Digit Symbol(Wechsler 1997) |
Processing Speed, Executive Control |
Yes | Yes | 0.13 | 0.16 | Days between tests | 0.19 |
| Animals | Semantic Fluency |
Yes | Yes | 0.34 | 0.05 | Time till testing, subcategorizing, grouping by habitat |
0.13 |
| Symbol Search(Wechsler 1997) |
Processing Speed, Executive Control |
Unknown | Yes | 0.50 | 0.23 | Weight, ethnicity, native language |
0.32 |
| Stroop Color- Word(Golden 1975) |
Response inhibition, Executive Control |
No | No | 0.01 | 0 | 0 | |
| ChangePC1 | Yes | 0.58 | 0.34 | Native language | 0.36 |
A list of past studies can be found in Table S2.
COW A; Controlled Oral Word Association, BDI; Beck Depression Inventory II, PC1; first principal component
We also utilized retrospective information from 290 epilepsy patients who had taken topiramate for seizure prevention. Patients were classified as cases if there was evidence that they had experienced word-finding difficulty or cognitive adverse effects with topiramate and as controls if they did not report any side effects with topiramate use.
A GWAS was performed on the 11 SRB change scores and ChangePC1 of the healthy volunteers and on the medical records of the epilepsy patients. In addition to the main analysis of all GWAS single nucleotide polymorphisms (SNPs), a focused analysis was performed on GWAS SNPs near genes considered to be good candidates for influencing topiramate response.
Results
Change after taking topiramate
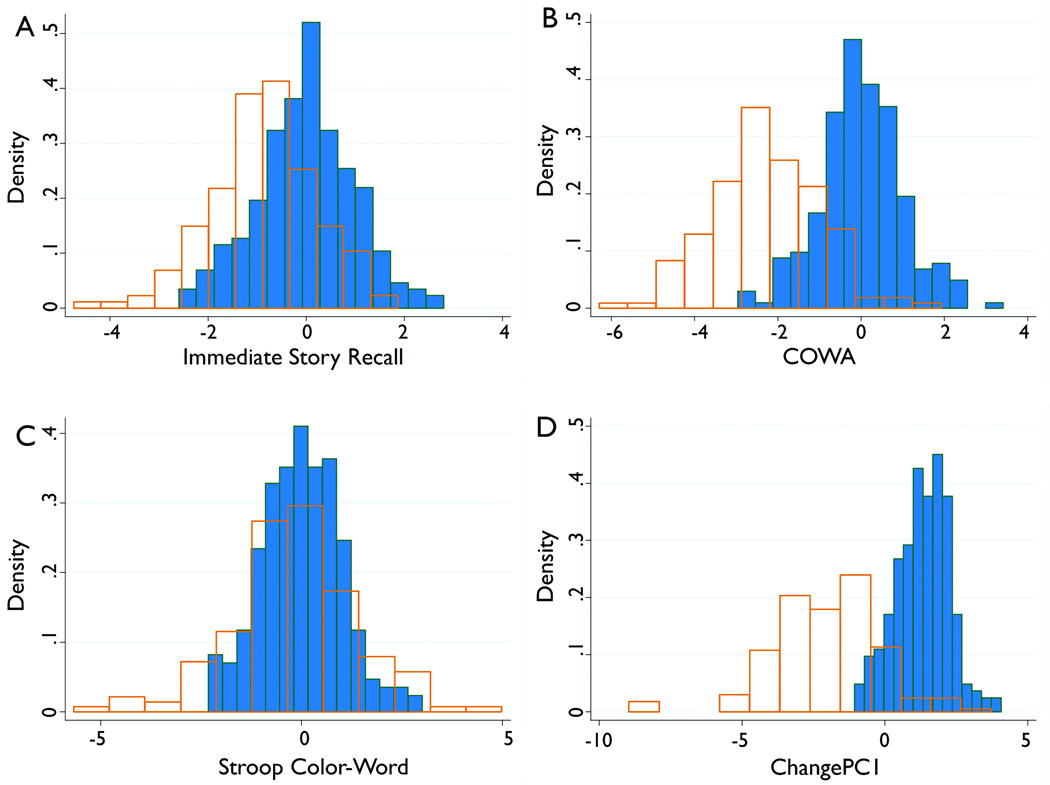
The only test that topiramate did not affect was Stroop Color-Word (Figure 1). All of the other tests in the battery were significantly affected by topiramate, and the percent of variation in change in test score explained by whether or not the subject took topiramate varied from 13 to 51%, with 58% of the variation in ChangePC1 explained by this variable (Figure 1, Table 1).
Figure 1. Change in test scores by whether or not took topiramate.
Here, the SRB change scores of all 158 individuals (orange outline, no fill) who took topiramate are compared to the 240 who did not (blue) for a subset of the tests. Immediate Story Recall, COWA and ChangePC1 all show clear effects of topiramate, while Color-Word does not.
When analyzing healthy individuals who took topiramate, the biggest factor influencing change scores was the topiramate plasma levels (r2 between 0.07 and 0.23 depending on the test; r2=0.34 for ChangePC1). Drug plasma levels showed up to 55-fold differences between individuals, were normally distributed, and had 39% of their variation explained by weight and the time from topiramate administration to testing (Figure S1).
After accounting for weight and time till blood draw, linear regression showed that there was a trend for males to have lower topiramate levels than females (p=.009). There was no significant effect of age or ethnicity on plasma levels.
In addition to plasma levels, other variables influenced the SRB change scores of those who took topiramate. Ethnicity affected both Immediate and Delayed Story Recall and Symbol Search; whether it was the third testing session (see Supplementary Data) affected TrailsA; use of the chunking strategy affected Digit Span Forward and Backward; native language affected Digit Span Forward, Symbol Search, and ChangePC1; Beck Depression Inventory-II (BDI, a measure of depressive symptomology)(Beck and Steer 1993) affected COWA; the number of days between testing sessions affected Digit Symbol; and ethnicity, time till testing, and use of the subcategorizing and grouping by habitat strategies affected Animals (Table 1).
Additional data about the effects of topiramate on the healthy volunteers, including taste change and subjective ratings of response, can be found in the Supplemental Data.
GWAS
A GWAS of response of healthy volunteers to topiramate yielded no significant associations, even when restricted to candidate genes. Combining the p-values for topiramate response in healthy volunteers with those from a GWAS of epilepsy patients taking topiramate also did not reveal any significant associations (see Supplemental Data and Supplemental Results).
Discussion
In this study, we have administered topiramate to a cohort of healthy controls and measured how it affects their cognition. We have found that topiramate plasma concentrations vary widely among individuals after an acute oral dose and that these plasma levels explain much of the variation in cognitive response to topiramate. However, no genetic variants were significantly associated with the phenotypes studied.
We used retest data from controls to establish the expected changes in the test scores of those who took topiramate; we then calculated cognitive response outcomes for each measure in our cognitive battery based on this expectation, controlling for practice effects and the influence of demographics on change. We found that all tests in this battery were significantly affected by having taken topiramate except for Stroop Color-Word. Previous studies had shown that Stroop Color-Word was not affected by topiramate (for example, (Salinsky, et al. 2005)), so this result was expected. For the tests that were affected by topiramate, plasma topiramate levels were always the most important predictor of the magnitude of the effect. This result was interesting as it is known that the majority of topiramate is excreted unmetabolized, and previous studies have not reported large differences in absorption rates or drug distribution (for example, (Perucca 1997)).
Although previous studies have shown that topiramate affects some of the tests studied here, few have examined the variables that might influence such a response. We have shown that after accounting for the effect of plamsa levels, there are some variables that impact change scores: ethnicity, native language, BDI, weight, certain testing strategies, days between test sessions, whether it was the third testing session, and time between drug administration and testing (Table 1). However, the effects of these variables were small and test-specific. Interestingly, we found no variables with a consistent effect on the change scores for all or even most tests, and we found no effect of age, gender or education. There has been some evidence that individuals with learning disabilities are less likely to complain of adverse side effects of topiramate(Lhatoo, et al. 2000). In our study, we did not include individuals with learning disabilities, but a linear regression that corrected for native language and topiramate plasma levels showed no relationship between initial PC1, our proxy for baseline cognition, and ChangePC1 (p=0.31).
A study design such as the one utilized here allows for the collection of a large cohort of subjects that have been given both topiramate and a battery of cognitive tests in a standardized manner. The current study was under-powered to detect the effects of genetic variants, with only 80% power to detect a common variant explaining 25% of the variation in ChangePC1 (19% of the variation if restricted to candidate genes). However, the quantitative cognitive phenotype assessed here, unclouded by disease conditions, would allow for a powerful study if a larger number of subjects were assessed. Such a study would facilitate the identification of genetic variants that influence one’s cognitive response to topiramate. This would immediately be of practical importance, as doctors could selectively prescribe topiramate to patients who are unlikely to have cognitive side effects. More broadly, understanding what genes mediate the specific cognitive effects of topiramate would also allow a deeper understanding of how those specific cognitive processes are controlled.
Supplementary Material
Acknowledgements
We acknowledge J. McEvoy and V. Dixon for testing of subjects, A. Husain, M. Mikati, W. Gallentine, S. Sinha, N. Walley, B. Legros, W. Van Paesschen and P. Tugendhaft for recruitment of subjects, and E. Heinzen for recruitment of subjects and pharmacological advice. The collection of the Belgian patients was supported by the Fonds National de la Recherche Scientifique, grant n. FC 63574 / 3.4.620.06 F, and the Fonds Erasme, Université Libre de Bruxelles. Part of this study was also supported by a K01 (NINDS K 01 NS050309) to S. E. Marino.
Footnotes
Ethical publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure
None of the authors has any conflict of interest to disclose.
References
- Attix DK, Story TJ, Chelune GJ, Ball JD, Stutts ML, Hart RP, Barth JT. The prediction of change: normative neuropsychological trajectories. Clin Neuropsychol. 2009;23:21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination: Manual of Instructions. Iowa City, IA: AJA Associates Inc.; 1978. [Google Scholar]
- Golden C. The measurement of creativity by the Stroop Color and Word Test. Journal of personality assessment. 1975;39:502–506. doi: 10.1207/s15327752jpa3905_9. [DOI] [PubMed] [Google Scholar]
- Green P. Story Recall Test. Edmonton, CA: Green's Publishing; 2005. [Google Scholar]
- Greenwood RS. Adverse effects of antiepileptic drugs. Epilepsia. 2000;41 Suppl 2:S42–S52. doi: 10.1111/j.1528-1157.2000.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang XQ. Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:123–130. doi: 10.1016/j.pnpbp.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo SD, Wong IC, Sander JW. Prognostic factors affecting long-term retention of topiramate in patients with chronic epilepsy. Epilepsia. 2000;41:338–341. doi: 10.1111/j.1528-1157.2000.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, Gilliam F, Faught E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- Mula M, Trimble MR, Sander JW. The role of hippocampal sclerosis in topiramate-related depression and cognitive deficits in people with epilepsy. Epilepsia. 2003;44:1573–1577. doi: 10.1111/j.0013-9580.2003.19103.x. [DOI] [PubMed] [Google Scholar]
- Perucca E. A pharmacological and clinical review on topiramate, a new antiepileptic drug. Pharmacol Res. 1997;35:241–256. doi: 10.1006/phrs.1997.0124. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- USArmy. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. Army Individual Test Battery. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scales. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.