Abstract
Brain’s alpha activity and alpha responses belong to major electrical signals that are related to sensory/cognitive signal processing. The present study aims to analyze the spontaneous alpha activity and visual evoked alpha response in drug free euthymic bipolar patients. Eighteen DSM-IV euthymic bipolar patients (bipolar I n = 15, bipolar II n = 3) and 18 healthy controls were enrolled in the study. Patients needed to be euthymic at least for 4 weeks and psychotrop free for at least 2 weeks. Spontaneous EEG (4 min eyes closed, 4 min eyes open) and evoked alpha response upon application of simple visual stimuli were analyzed. EEG was recorded at 30 positions. The digital FFT-based power spectrum analysis was performed for spontaneous eyes closed and eyes open conditions and the response power spectrum was also analyzed for simple visual stimuli. In the analysis of spontaneous EEG, the ANOVA on alpha responses revealed significant results for groups (F(1,34) = 8.703; P < 0.007). Post-hoc comparisons showed that spontaneous EEG alpha power of healthy subjects was significantly higher than the spontaneous EEG alpha power of euthymic patients. Furthermore, visual evoked alpha power of healthy subjects was significantly higher than visual evoked alpha power of euthymic patients (F(1,34) = 4.981; P < 0.04). Decreased alpha activity in spontaneous EEG is an important pathological EEG finding in euthymic bipolar patients. Together with an evident decrease in evoked alpha responses, the findings may lead to a new pathway in search of biological correlates of cognitive impairment in bipolar disorder.
Keywords: EEG alpha activity, Bipolar disorder, Visual evoked alpha oscillations, Euthymia, Schizophrenia
Introduction
Since the 1980s, the concept of “oscillatory brain dynamics” has achieved prominent progress in neuroscience research. During last decade, applications of the oscillatory activity in clinical pathology have grown rapidly, as extensively explained in a recent review (Başar and Güntekin 2008). Our research group initiated research on brain oscillatory responses and cognitive processes in schizophrenia (Başar-Eroğlu et al. 2008), Alzheimer’s disease (Yener et al. 2007, 2008; Güntekin et al. 2008; Başar et al. 2010) and in two different states of bipolar disorders (Özerdem et al. 2008a, b).
The present study analyzes the spontaneous alpha activity and visual alpha responses in a group of drug-free euthymic bipolar patients, which was already shown in manic bipolar patients (Özerdem et al. 2008a). Further, it is noteworthy to examine the degree of change of alpha activity in bipolar euthymic disease in comparison to schizophrenia.
Alpha rhythm, which was first observed by Hans Berger (1929), was initially considered as the “Brain’s Idling Rhythm”. Later, several authors described that EEG is not noise and that selectively synchronized alpha oscillations in the mammalian and human brain are part of the fundamental functional signaling of CNS (Başar 1980; Lehmann 1989; Klimesch 1999; Başar et al. 2001; Nunez et al. 2001). The alpha response is a fundamental component in sensory/cognitive tasks of healthy subjects. Following the publication of papers in the alpha special issue (Başar et al. 1997) research on cognitive processes manifesting alpha oscillations is rapidly increasing.
Bipolar disorder is a chronic mental illness with a relapsing and remitting course. Relapses are manic or depressive in nature. Patients suffer from a wide range of cognitive deficits (Martinez-Aran et al. 2004; Clark et al. 2002; Wilder-Willis et al. 2001) even when they are euthymic. There have been a relatively limited number of earlier electrophysiology studies of bipolar disorder, both in symptomatic and euthymic states (Bruder et al. 1992; Muir et al. 1991; Souza et al. 1995; Salisbury et al. 1998, 1999; O’Donnell et al. 2004). Despite different stimulus modalities, mostly being auditory, the common finding was prolonged P300 latency and reduced P300 amplitude, which was equivocal, mostly found to be related to psychosis and suggested to have an association with underlying frontal lobe pathology (Salisbury et al. 1999). More recent studies showed disturbed resting EEG activity in euthymic bipolars (El-Badri et al. 2001) and abnormal high frequency synchronization1 in response to auditory stimuli (O’Donnell et al. 2004) in symptomatic bipolar patients. Özerdem et al. (2008a) observed increased occipital beta activity in manic states in response to visual oddball paradigm.
Cortical alpha rhythms are reduced in different pathologies as Schizophrenia, Mild Cognitive Impairment and Alzheimer’s disease. In most of the studies spontaneous EEG rhythms were analyzed. There are also studies that analyzes evoked/event related oscillations in different pathologies (Başar and Güntekin 2008). In the Schizophrenia studies auditory stimuli or visual steady inputs were mostly used (See reviews: Brenner et al. 2009; Krishnan et al. 2005; Kwon et al. 1999). Alzheimer’s disease (AD) patients have been characterized by low power of posterior alpha and/or beta (13–30 Hz) rhythms similar to healthy elderly subjects. Posterior alpha rhythms showed a power decrement in subjects with mild cognitive impairment compared with healthy elderly subjects that are in a resting-state condition (Zappoli et al. 1995; Huang et al. 2000; Jelic et al. 2000; Koenig et al. 2005; Babiloni et al. 2006, 2007; Rossini et al. 2007). EEG Alpha activity was also found to be decreased in schizophrenia (Alfimova and Uvarova 2008; Iacono 1982; Itil et al. 1972, 1974; Miyauchi et al. 1990; Sponheim et al. 1994, 2000). Başar-Eroğlu et al. (2009) observed significantly reduced alpha responses in the range of 15% and Ford et al. (2008) also reported reduced alpha response in schizophrenia.
To the best of our knowledge, there is only one prior study assessing the alpha activity in bipolar disorder (Clementz et al. 1994) where alpha activity was shown to be reduced in bipolar patients with psychotic characteristics and in patients with schizophrenia in comparison to healthy controls. To date, no published study has investigated the alpha activity in drug-free euthymic bipolar patients.
The aims of the present study are as follows:
The analysis of the spontaneous “eyes closed” and “eyes open” EEG alpha activity of drug-free euthymic bipolar patients. Although spontaneous EEG activity of Bipolar Patients have been studied before by several groups, there is no prior study investigating the spontaneous EEG activity of the bipolar patients who are at euthymic stage and who are also drug-free (Clementz et al. 1994; Dewan et al. 1988; El-Badri et al. 2001; Small et al. 1998).
The evaluation of EEG Dynamics may include many methods including analysis of spontaneous EEG, analysis of evoked and/or event related potentials, analysis of evoked and/or event related oscillations in different frequency windows. Since there is no previous study on drug free eutyhmic patients, the aim of the present study is the efficient analysis of spontaneous EEG alpha activity, as one of the most basic and important methods.
Another question is whether changes in the amplitude of spontaneous alpha activity in neuropsychiatric disorders, euthymic Bipolar Patients show also different spontaneous alpha activity than healthy controls.
Can the reduction of alpha activity be considered as a candidate biomarker in future, especially for differentiation between schizophrenia, bipolar and other psychiatric disorders, as a consequence of the foregoing question? Accordingly, one of the aims of the study is also to provide a database of alpha activity in euthymic bipolar disorders to open the possibility of comparison with schizophrenia patients in future research.
Material and method
Subjects
Eighteen DSM-IV (Diagnostic and Statistical Manual of Psychiatric Disorders, fourth edition) euthymic bipolar I (n = 15) patients, euthymic bipolar II (n = 3) patients (13 female, 5 male), aged between 28 and 44 years (mean age 31.66 ± 5.99 SD) and an equal number (n = 18) of sex, age (mean age 29.83 ± 7.77 SD) and educationally matched healthy controls were enrolled in the study. All subjects were followed by the psychiatrics of Bakirkoy State Hospital of Mental Health Research Training and Education Center, which has the largest psychiatric pathology population in Turkey. The possibility to recruit the 18 drug-free euthymic patients was possible, since the Bakirkoy State Hospital of Mental Health Research, Training and Education Center; Istanbul, Turkey is the largest psychiatric hospital in Turkey. This hospital has the possibility to recruit drug-free patients from all parts of Istanbul City, which has a 13 million population. All subjects were interviewed with SCID-I (Structured Interview for DSM-IV) (First et al. 1996). The study was approved by the local Ethics Committee of Bakirkoy State Hospital of Mental Health Research, Training and Education Center, Istanbul, Turkey. All participants provided written informed consent. Patients needed to be euthymic at least for 6 months, patients were psychotropic-free for at least 2 weeks prior to study enrollment and none of them were using benzodiazepines. Only one of the subjects was drug-free for 2 weeks prior to study. One of the subjects was drug-free for 3 weeks prior to study. All other 16 patients were drug-free for at least 6 weeks prior to study. Patients needed to score 7 or less on the reliable and validated Turkish versions of the Young Mania Rating Scale (YMRS) (Karadağ et al. 2002), Hamilton Depression Rating Scale (HAM-D 21) (Aydemir and Deveci 2003); to have no co-morbid axis I diagnosis, and be medically healthy, as shown through physical examination and routine laboratory tests. Volunteers who proved to have no present or past psychiatric condition and to be medically healthy on physical examination were enrolled as the control group.
Experimental procedure and stimuli
The subjects were sat in a dimly-lit isolated room. Two different experimental set ups were performed: (1) Spontaneous EEG of the subject were recorded for 4 min for “eyes open” and 4 min for “eyes closed” conditions. (2) The EEG was recorded upon application of simple light stimuli for analyzing simple evoked oscillations. A visual sensory paradigm was used in the experiments. Stimulation consisted of a white screen with 10 cd/cm2 luminance. A series of 60 stimulation signals (1,000 ms duration) were applied randomly, with the inter-stimulus intervals varying between 3 and 7 s.
EEG recording
EEG was recorded by using the BrainAmp EEG amplifier, Brain Vision Recorder software (Brainproducts, Munich, Germany), and the BrainCap electrode cap at 30 positions. The EEG was amplified by means of a BrainAmp with band limits of 0.01–250 Hz. The EEG was digitized on-line at a sampling rate of 500 Hz. All electrode impedances were less than 10 kΩ. In order to maintain a constant level of vigilance, a researcher controlled on-line the subject and the EEG traces; The subject was verbally alerted any time there were signs of behavioral and/or EEG drowsiness. Two linked earlobe electrodes (A1 + A2) served as references. The EOG from the medial upper and lateral orbital rim of the right eye was also registered. For the reference electrodes and EOG recordings, Ag–AgCl electrodes were used. The EEG and EOG signals were visually scored and portions of the data that contained aberrant eye movements, muscle movements or artifacts were removed.
EEG analysis
Spontaneous EEG analysis
The spontaneous EEG was recorded for 4 min eyes closed and 4 min eyes open conditions and subsequent analyses were performed separately for eyes closed and eyes open conditions. The recorded EEG data were analyzed and fragmented off-line in consecutive epochs of 1 s. The digital FFT-based power spectrum analysis was performed. (10% Hanning windowing function was evaluated in order to calculate alpha frequency peak). These power values were averaged across the epochs of a given trial. The standard frequency band of interest was alpha (8–13 Hz). The maximum individual alpha frequency value for each subject was included, for the purpose of statistical analysis, as the maximum individual alpha frequency value of that subject.
Visual evoked oscillations analysis
The epochs (between 0 and 1,000 ms) of each subject were averaged and then the digital FFT-based power spectrum analysis was performed. (10% Hanning windowing function was evaluated in order to calculate the alpha frequency peak). The standard frequency band of interest was alpha (8–13 Hz). The maximum individual alpha frequency value for each subject was included, for the purpose of statistical analysis, as the maximum individual alpha frequency value of that subject.
Statistics
SPSS was used for statistical analysis. A repeated measure ANOVA was used to determine the statistical significance of differential alpha responses over different conditions, locations; and between patients and controls. Two separate ANOVA were used for two different experimental set ups (Spontaneous EEG, Visual Evoked Oscillations): In the analysis of spontaneous EEG alpha power differences, repeated measures of ANOVA included the between-subjects factor as healthy subjects and euthymic patients; repeated measure ANOVA included the within-subject factors as condition (eyes open, eyes closed); location (Frontal, Central, Temporal 1 (T7–T8), Temporal 2 (TP7–TP8), Parietal, Occipital) and hemisphere (right, left). In the analysis of Visual Evoked alpha power differences, repeated measures of ANOVA included the between-subjects factor as healthy subjects and euthymic patients; repeated measure ANOVA included the within-subject factors as location (Frontal, Central, Temporal1 (T7–T8), Temporal 2 (TP7–TP8), Parietal, Occipital) and hemisphere (right, left). Post-hoc comparisons for between-subject effects and within-subject effects were analyzed using the t test. Greenhouse-Geisser corrected P values are reported, and the level of significance was set to P < 0.05 for post-hoc comparisons.
Results
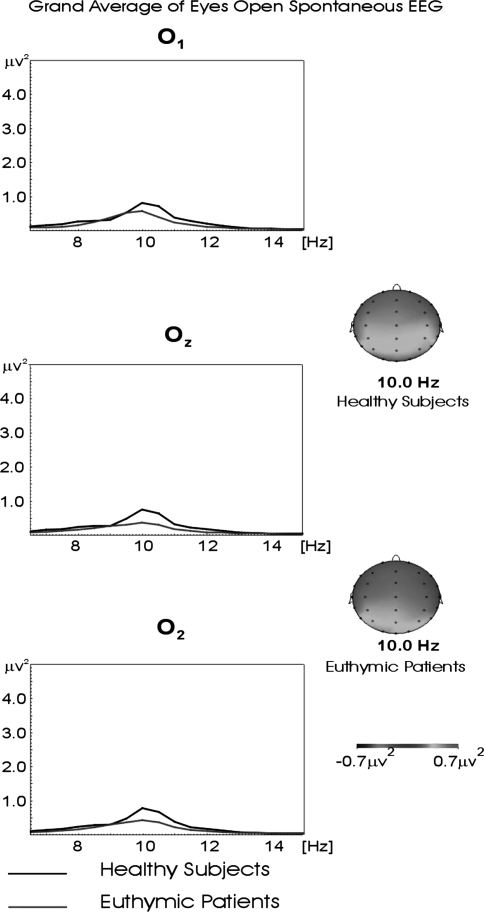
Figure 1 shows the grand averages of power spectrum of the alpha frequency range in occipital locations (O1, Oz, and O2) in 18 healthy and 18 euthymic bipolar participants for the eyes open condition. Here, the alpha frequency range power spectrum of healthy controls can be as high as 0.90 μV2 for all occipital electrodes, while the power spectrum of the euthymic patients reaches 0.40 μV2.
Fig. 1.
Grand averages of power spectra of 18 healthy and 18 euthymic subjects for the eyes open recording session for occipital locations. The black line represents the grand average of power spectra of evoked response in healthy subjects. The red line represents the grand average of power spectra of evoked response in euthymic subjects
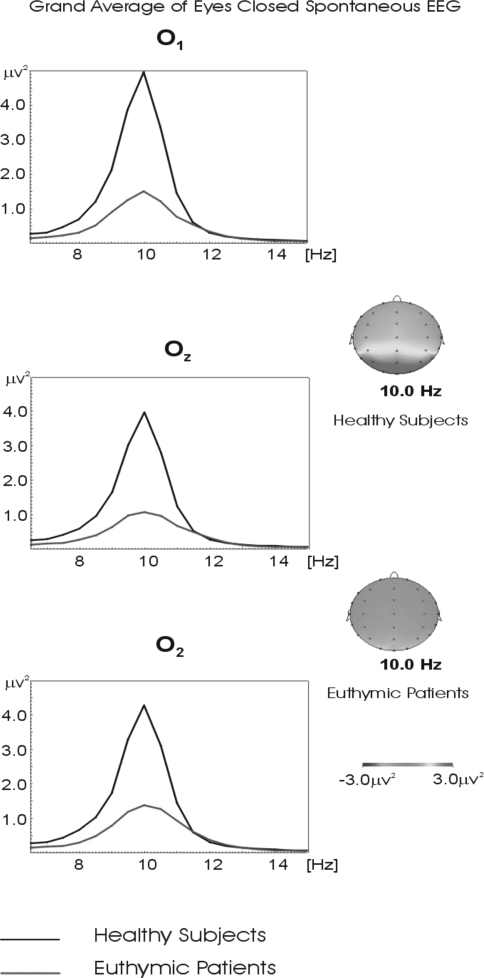
Figure 2 represents the grand averages of power spectra of 18 healthy and 18 euthymic subjects in the alpha frequency range for the eyes closed recording session for occipital locations (O1, Oz, and O2). While the power spectrum of the alpha frequency range reached 4.80 μV2 for O1; 4.0 μV2 for Oz and 4.50 μV2 for O2 electrode in healthy controls, it remained at 1.0 μV2 across all occipital electrodes in the euthymic patients.
Fig. 2.
Grand averages of power spectra of 18 healthy and 18 euthymic subjects for the eyes closed recording session for occipital locations. The black line represents the grand average of power spectra of evoked response in healthy subjects. The red line represents the grand average of power spectra of evoked response in euthymic subjects
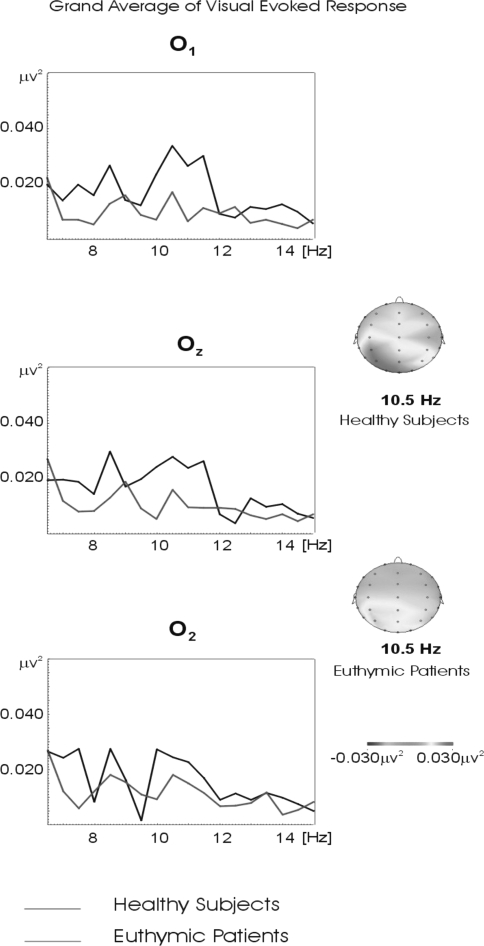
Figure 3 represents the grand average of the evoked response power spectra for 18 healthy and 18 euthymic subjects in the alpha frequency range upon application of simple light stimuli (for O1, Oz, and O2 electrodes). The alpha frequency power spectrum of evoked response reached 0.04 μV2 in healthy controls, whereas the power spectrum of evoked response of euthymic patients only reached 0.015 μV2.
Fig. 3.
Grand averages of power spectra of evoked response in 18 healthy and 18 euthymic subjects in the alpha frequency range upon application of simple light stimuli for occipital locations. The black line represents the grand average of power spectra of evoked response in healthy subjects. The red line represents the grand average of power spectra of evoked response in euthymic subjects
The observed differences between healthy subjects and euthymic patients are in accordance with the statistical findings described below.
Statistical description: spontaneous EEG
The ANOVA of alpha responses revealed significant differences between groups (F(1,34) = 8.703; P < 0.007). Post-hoc comparisons showed that the spontaneous EEG alpha power of healthy subjects was significantly higher than that of euthymic patients (P < 0.0001). The ANOVA of alpha responses revealed significant differences between experimental conditions (eyes open, eyes closed; (F(1,34) = 33.043; P < 0.0001). Post-hoc comparisons showed that spontaneous EEG alpha power for the eyes closed condition were significantly higher than for the eyes open condition (P < 0.0001). The ANOVA of alpha responses revealed significant results for condition × group (F(1,34) = 5.726; P < 0.03). Post-hoc comparisons showed that spontaneous EEG alpha power of healthy subjects was significantly higher than euthymic patients for the eyes closed condition (P < 0.0001) and eyes open condition (P < 0.002). The ANOVA of alpha responses revealed significant differences between locations (F(5,17) = 17.129; P < 0.0001). Post-hoc comparisons showed that spontaneous EEG alpha power at occipital electrodes was higher than that of frontal, central, temporal and parietal electrodes (P < 0.001 for all electrode sites). Furthermore, spontaneous EEG alpha power at parietal electrodes was higher than that of frontal, central and temporal electrodes (P < 0.001 for all electrode sites). The ANOVA of alpha responses revealed significant results for condition (eyes closed, eyes open) × location (F(5,17) = 14.644; P < 0.0001). The post hoc comparisons showed that the eyes closed alpha power means at frontal, central, temporal, parietal and occipital electrodes were higher than the corresponding eyes open alpha mean for these electrodes (P < 0.001 for all electrode sides).
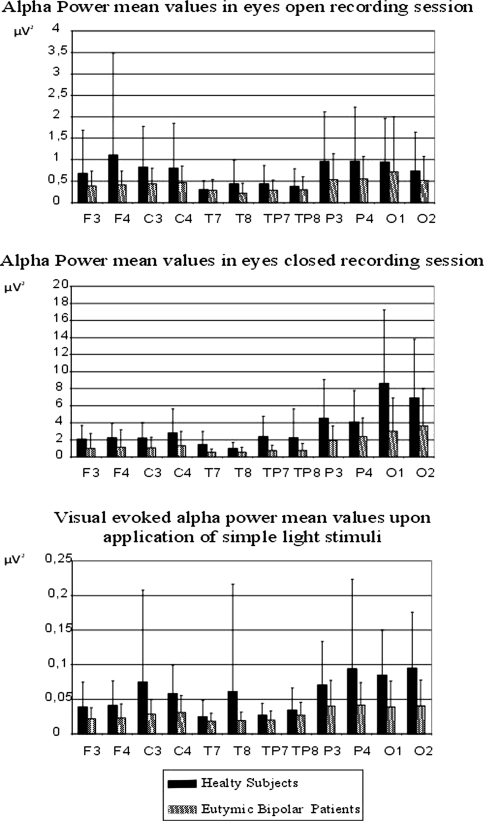
Figure 4a presents bar graphs of mean maximum alpha power value of healthy and euthymic subjects for the eyes open recording session for all electrode pairs. Figure 4b presents bar graphs of mean maximum alpha power values of healthy and euthymic subjects for the eyes closed recording session for all electrode pairs. As seen from Fig. 4a, during the eyes open recording session, the mean power spectrum of the healthy subjects varies between 0.29 and 1.10 μV2, while the mean power spectrum of the euthymic subjects is within the range 0.21–0.72 μV2. As seen from Fig. 4b, during the eyes closed recording session, the mean power spectrum of the healthy subjects is between 0.95 and 8.60 μV2, while that for the euthymic subjects is only between 0.51 and 3.63 μV2.
Fig. 4.
a Mean alpha power values of 18 healthy and 18 euthymic patients in eyes open recording session. b Mean alpha power values of 18 healthy and 18 euthymic patients in eyes closed recording session. c Mean visual evoked alpha power values of 18 healthy and 18 euthymic patients upon application of simple light stimuli. The mean alpha power spectrum values of healthy subjects are represented by black bars; the mean alpha power spectrum values of eutyhmic subjects are represented by gray bars
Visual evoked oscillations
ANOVA of the alpha responses revealed significant differences between groups (F(1,34) = 4.981; P < 0.04). Post-hoc comparisons showed that visual evoked alpha power of healthy subjects was significantly higher than that of euthymic patients (P < 0.0001). The ANOVA of alpha responses revealed significant results for location (F(5,170) = 8.966; P < 0.0001). Post-hoc comparisons showed that the visual evoked alpha power of occipital and parietal electrodes were higher than for frontal and temporal electrodes (P < 0.001 for all electrode sites).
Figure 4c presents bar graphs of mean maximum evoked alpha power response value of healthy and euthymic subjects for all electrode pairs. As seen from Fig. 4c, the mean evoked power spectra of the healthy subjects are between 0.024 and 0.095 μV2, while those of the euthymic subjects are only between 0.017 and 0.040 μV2.
Discussion
Electrophysiology in bipolar disorder, especially in alpha oscillations
The literature includes several previous investigations of spontaneous EEG in bipolar patients. (Clementz et al. 1994; Cook et al. 1986; Dewan et al. 1988; Gerez and Tello 1992; Kano et al. 1992; Koles et al. 1994; Souza et al. 1995; Small et al. 1989, 1998; Schulz et al. 2000; El-Badri et al. 2001; Ikeda et al. 2002). The present study differs from other studies since none of the other groups analyzed the spontaneous EEG jointly with visual evoked oscillations in groups of drug-free euthymic patients. Clementz et al. (1994) investigated alpha activity in a group of bipolar psychosis patients, schizophrenia patients and their first-degree relatives. EEG data obtained from patients and their first-degree relatives showed that patients with schizophrenia and bipolar disorder had reduced alpha in comparison to healthy subjects. Our results indicated a reduction in alpha activity in the range of 70% within a group of euthymic patients, compared with healthy controls. This was not observed in earlier studies and can even be considered a breakdown of alpha activity and visual alpha response.
Clementz et al. (1994) included a mix patient group in their study and the bipolar patient group was not all in eutymic stage and was not all drug free. On the contrary, the patient group included in our analysis provides strongest advantages and makes the study unique in the literature. (2) On the other hand the mix subject groups included in Clementz’s study give the chance to the authors to compare different subject groups. In future, a possible comparison of drug free eutymic patients with drug free schizophrenic patients could be of major importance to detect the differences of alpha activity between these two groups. (2) Clementz et al. (1994) analyzed only the Central electrodes (C3, Cz, C4), we have analyzed Frontal (F3, F4), Central (C3, C4), Temporal 1 (T7–T8), Temporal 2 (TP7–TP8), Parietal (P3, P4), Occipital (O1, O2). (3) Clementz et al. (1994) recorded EEG during eyes closed recording session. In extension we have also recorded EEG during eyes closed, eyes open and upon application of basic visual stimuli.
To our knowledge the present study is the first one reporting decrease of alpha activity in drug-free euthymic patients. Clementz et al. (1994) reported that the bipolar patients had reduced alpha activity in central electrodes. Until now no other groups have reported such a difference between healthy subjects and bipolar patients. The present study further emphasizes the importance of including all electrodes and comparing different recording sessions.
Furthermore, increased occipital beta response and, in contrast to this, decreased alpha response in response to visual oddball paradigm was observed by Özerdem et al. (2008a, b) in manic state. According to Başar et al. (1997), if the ongoing activity has decreased within a specific frequency, then the evoked or event related responses within this frequency are also low; this was also the case in the present study.
A number of studies have shown significant differences in alpha asymmetry in bipolar individuals during an application of several paradigms (Kano et al. 1992; Allen et al. 1993; Harmon-Jones et al. 2008). In comparison with nonbipolar individuals, bipolar disorder patients showed greater relative left frontal cortical activation in preparation for the hard/win trials. In our study we did not find any lateralization effects as it is seen in Fig. 4. The present study clearly shows that drug-free euthymic subjects do not have any frontal alpha asymmetry during a spontaneous EEG recording. Application of different paradigms during the recordings may have changed the results. It is to note that in the present study there is no drug effect on the alpha activity. On the other hand in the studies by Kano et al. (1992), Allen et al. (1993) and Harmon-Jones et al. (2008) the patients were not all drug free. It is to note that drug applications have effects on oscillatory dynamics2 (Özerdem et al. 2008a; Yener et al. 2007; Başar and Güntekin 2008). Future studies are needed for tenable conclusions related to the alpha asymmetry by taking into consideration the application of different paradigms and different drug therapies.
Comparison of bipolar alpha band results with schizophrenia
According to several previous studies, schizophrenia patients display both reduced spontaneous alpha activity and event related alpha (Alfimova and Uvarova 2008; Başar-Eroğlu et al. 2009; Iacono 1982; Itil et al. 1972, 1974; Miyauchi et al. 1990; Sponheim et al. 1994, 2000). Few studies discuss the common and extinct parameters of schizophrenia and bipolar disorder by means of anatomical and genetically analysis (Lim et al. 1999; Roy et al. 1998; McDonald et al. 2004; Hoge et al. 1999; Campbell et al. 2004; Wright et al. 2000). According to Selemon (2004), there is a reduction of synaptic elements and neuronal connections in the cortex of schizophrenic patients without a decrease in total neuronal number (Table 1).
Table 1.
Subjects characteristics (Mean ± SD)
| Patients with bipolar disorder | Healthy controls | |
|---|---|---|
| Age | 31.66 ± 5.99 | 29.83 ± 7.77 |
| Education | 12.33 ± 3.41 | 14.05 ± 2.33 |
| Age at disease onset | 22.38 ± 6.52 | |
| Duration of euthymia | 48.85 ± 37.95 | |
| Duration of illness | 125.33 ± 42.42 | |
| Total episodes | 4.0 ± 2.9 | |
| Manic episodes | 2.20 ± 1.65 | |
| Depressive episodes | 0.90 ± 0.95 | |
| Hypomanic episodes | 0.60 ± 1.14 |
McDonald et al. (2004) performed a meta-analysis with 26 subjects and concluded that bipolar disorder is associated with mild ventricular enlargement. Sponheim et al. (2000) analyzed the brain alpha activity and its relation with brain morphology. These authors examined the power characteristics of resting electroencephalograms in 112 schizophrenic patients and seventy-eight non-schizophrenic psychosis patients (e.g. mood disorder patients, 33 bipolar) were included for comparison. Schizophrenic patients whose electroencephalograms were characterized by diminished alpha-band power had more negative symptoms, larger third ventricles, larger frontal horns of the lateral ventricles, increased cortical sulci widths, and greater ocular motor dysfunction compared with schizophrenic patients without these electroencephalogram characteristics. In non-schizophrenic psychosis patients, augmented low-frequency and diminished alpha band powers were not associated with any clinical or biological indices. In their study, psychosis bipolar patients were only one part of the control group. We will come back to the consequences of this comparison at the end of section “Future research toward determination of electrical biomarkers and need for standardization”.
Future research toward determination of electrical biomarkers and need for standardization
Degabriele and Lagopoulos (2009) published a review of EEG and ERP studies in bipolar disorder. They reported the lack of a systematic approach towards experimental design and medication status. Further to the comments of these authors, in the following other issues are to be discussed in studies of Bipolar disorder.
Electrophysiological recordings as EEG and ERPs are less studied in bipolar patient groups compared to other psychiatric disorders such as schizophrenia and depression.
Since three different mood states are involved in bipolar disorder, a comparative study of EEG and EROs in mania, euthymia and depression is subject to future electrophysiology research in bipolar disorder. Such studies should cover all other EEG frequency bands (EEGs and EROs) and coherence values between various recordings. To attain a complete picture for different electrophysiological behavior between bipolar patients and healthy subjects, all these mentioned steps should be achieved. Another useful extension will be analysis of event related oscillations of bipolar disorder, as initiated by O’Donnell et al. (2004) and Özerdem et al. (2008a, b). Final conclusions can be reached only by analysis of ensembles including power spectra, evoked power spectra, single trial analysis, phase-locking factors, coherence, phase coherence etc. (For further information, see Başar 1980, 1998, 1999, 2004). One of the psychiatric diseases, to which almost all of these methods were applied, is schizophrenia, although mostly in the gamma frequency band. Important conclusions were also reached in lower frequency bands, as studies by Başar-Eroğlu et al. (2007), Schmiedt et al. (2005) and Ford et al. (2008) showed.
Can the drastic breakdown of alpha be considered as a candidate biomarker in future, especially for differentiation between schizophrenia and other psychiatric disorders? Başar-Eroğlu et al. (2009) published results showing a 10–15% reduction in alpha responses among schizophrenic patients; Itil et al. (1972, 1974) also reported only a 10–15% reduction in EEG. This suggests that a very large alpha decrease in bipolar disease will possibly serve as a biomarker for differentiation from schizophrenia.
The dynamic time-window of 500 ms upon cognitive load can be measured only with the EEG and MEG techniques; other imaging methods do not cover this short time-window.
Although a break-down of alpha can be considered as a candidate biomarker in the dynamic time window, we emphasize the consideration of an ensemble of electrophysiological responses as “efficient collective biomarkers” and it is recommended to include within this group the huge increase of beta response and the 40% decrease of 40 Hz coherence in manic and euthymic patients (Özerdem et al. 2010a, b).
Concluding remarks
The described results indicated a huge decrease of the amplitude of alpha activity in the range of 70% in a drug-free group of euthymic bipolar patients in comparison to healthy subjects.
This reduction can be even considered as a breakdown of alpha activity and also of the visual alpha response.
It is suggested that breakdown of alpha activity can be considered as a biomarker for euthymic bipolar disorders in comparison to healthy subjects and also in comparison to other neuropsychiatric disorders as schizophrenia.
These findings can be in future used also as basis model to compare changes upon application of cognitive paradigms as the oddball analysis, since the cognitive processes of euthymic bipolar patients are not completely abolished. In turn, such extended studies could be relevant for understanding of the role of alpha activity in cognitive processes, in general.
Footnotes
Stabilization of frequency response in a narrow frequency window.
Brain’s temporal response within EEG frequency channels.
References
- Alfimova MV, Uvarova LG. Changes in EEG spectral power on perception of neutral and emotional words in patients with schizophrenia, their relatives, and healthy subjects from the general population. Neurosci Behav Physiol. 2008;38:533–540. doi: 10.1007/s11055-008-9013-6. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Iacono WG, Depue RA, Arbisi P. Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biol Psychiatry. 1993;33:642–646. doi: 10.1016/0006-3223(93)90104-L. [DOI] [PubMed] [Google Scholar]
- Aydemir Ö, Deveci A (2003) Validity and reliability of structured inter view for Hamilton depression rating scale seasonal affective disorders. Annual Spring Symposium of Psychiatric Association of abstract book, pp 187
- Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Romani GL, Rossini PM. Sources of cortical rhythms in adults during physiological aging: a multi-centric EEG study. Human Brain Mapp. 2006;27:162–172. doi: 10.1002/hbm.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Binetti G, Tombini M, Del Percio C, Ferreri F, Ferri R, Frisoni G, Lanuzza B, Nobili F, Parisi L, Rodriguez G, Frigerio L, Gurzì M, Prestia A, Vernieri F, Eusebi F, Rossini PM. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer’s disease. Eur J Neurosci. 2007;25:3742–3757. doi: 10.1111/j.1460-9568.2007.05601.x. [DOI] [PubMed] [Google Scholar]
- Başar E. EEG–brain dynamics. Relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. p. 412. [Google Scholar]
- Başar E. Brain oscillations I: principles and approaches. Heidelberg: Springer; 1998. [Google Scholar]
- Başar E. Brain function and oscillations: II. Integrative brain function. Neurophysiology and cognitive processes. Heidelberg: Springer; 1999. [Google Scholar]
- Başar E. Memory and brain dynamics: oscillations integrating attention, perception, learning and memory. Florida: CRC Press; 2004. [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Güntekin B, Tülay E, Yener G. Evoked and event related coherence of alzheimer patients manifest differentiation of sensory-cognitive networks. Brain Res. 2010;1357:79–90. doi: 10.1016/j.brainres.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Başar E, Hari R, Lopes da Silva FH, Schürmann M (eds) (1997) Brain alpha activity—new aspects and functional correlates. Int J Psychophysiol, special issue, pp 5–29
- Başar-Eroğlu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Schmiedt-Fehr C, Marbach S, Brand A, Mathes B. Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 2008;1235:143–152. doi: 10.1016/j.brainres.2008.06.114. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Schmiedt-Fehr C, Mathes B, Zimmermann J, Brand A. Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol. 2009;71:75–83. doi: 10.1016/j.ijpsycho.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen I. Bericht. Archiv Fuer Psychiatrie und Nervenkrankheiten. 1929;87:527–570. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O’Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Towey JP, Fredman D, Tekne CE, Voglmaier MM, Leite P, Cohen P, Quitkin FM. Abnormal cerebral laterality in bipolar depression: convergence of behavioral and brain event-related potential findings. Biol Psychiatry. 1992;32:3–47. doi: 10.1016/0006-3223(92)90140-U. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients and their first degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, Hodes RL, Lang PJ. Preparedness and phobia: effects of stimulus content on human visceral conditioning. J Abnorm Psychol. 1986;95:195–207. doi: 10.1037/0021-843X.95.3.195. [DOI] [PubMed] [Google Scholar]
- Degabriele R, Lagopoulos J. A review of EEG and ERP studies in bipolar disorder. Acta Neuropsychiatrica. 2009;21:58–66. doi: 10.1111/j.1601-5215.2009.00359.x. [DOI] [Google Scholar]
- Dewan MJ, Haldipur CV, Boucher MF, Ramachandran T, Major LF. Bipolar affective disorder II: EEG, neuropsychological, and clinical correlates of CT abnormality. Acta Psychiatry. 1988;77:677–682. doi: 10.1111/j.1600-0447.1988.tb05187.x. [DOI] [PubMed] [Google Scholar]
- El-Badri SM, Ashton CH, Moore PB, Mursh VR, Ferrier IN. Electrophysiological and cognitive function in young euthymic patients with bipolar affective disorder. Bipolar Disord. 2001;3:79–87. doi: 10.1034/j.1399-5618.2001.030206.x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the structured interview for DSM-IV axis I disorders—research version (SCID-I, version 2.0, February 1996 final version) New York: Biometrics Research; 1996. [Google Scholar]
- Ford JM, Roach B, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerez M, Tello A. Clinical significance of focal topographic changes in the electroencephalogram (EEG) and evoked potentials (EP) of psychiatric patients. Brain Topogr. 1992;5:3–10. doi: 10.1007/BF01129964. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Saatçi E, Yener G. Decrease of evoked delta, theta and alpha coherence in Alzheimer patients during a visual oddball paradigm. Brain Res. 2008;1235:109–116. doi: 10.1016/j.brainres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, Alloy LB, Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Friedman L, Schulz SC. Meta-analysis of brain size in bipolar disorder. Schizophr Res. 1999;37:177–181. doi: 10.1016/S0920-9964(98)00149-2. [DOI] [PubMed] [Google Scholar]
- Huang C, Wahlund LO, Dierks T, Julin P, Winblad B, Jelic V. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol. 2000;11:1961–1967. doi: 10.1016/S1388-2457(00)00454-5. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Bilateral electrodemal habituation-dishabituation and resting EEG in remitted schizophrenics. J New Menr Dis. 1982;170:91–101. doi: 10.1097/00005053-198202000-00005. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Kato N, Kato T. Possible relationship between electroencephalogram finding and lithium response in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:903–907. doi: 10.1016/S0278-5846(02)00203-8. [DOI] [PubMed] [Google Scholar]
- Itil TM, Saletu B, Davis S. EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol Psychiatry. 1972;5:1–13. [PubMed] [Google Scholar]
- Itil TM, Saletu B, Davis S, Allen M. Stability studies in schizophrenics and normals using computer-analyzed EEG. Biol Psychiatry. 1974;8:321–335. [PubMed] [Google Scholar]
- Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Kano K, Nakamura M, Matsuoka T, Iida H, Nakajima T. The topographical features of EEGs in patients with affective disorders. Electroencephalogr Clin Neurophysiol. 1992;83:124–129. doi: 10.1016/0013-4694(92)90025-D. [DOI] [PubMed] [Google Scholar]
- Karadağ F, Oral ET, Yalçın FA, Eten E. Validity and reliability of young mania rating scale in Turkey. Turk J Psychiatry. 2002;13:107–114. [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, Jelic V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005;26:165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Koles ZJ, Lind JC, Flor-Henry P. Spatial patterns in the background EEG underlying mental disease in man. Electroencephal Clin Neurophysiol. 1994;91:319–328. doi: 10.1016/0013-4694(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116(3):614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, et al. Gamma frequency range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D. From mapping to the analysis and interpretation of EEG/EP maps. In: Maurer K, editor. Topographic brain mapping of EEG and evoked potentials. Berlin: Springer; 1989. pp. 53–75. [Google Scholar]
- Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A. Cortical gray matter deficit in patients with bipolar disorder. Schizophrenia Res. 1999;40:219–227. doi: 10.1016/S0920-9964(99)00063-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugué E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Tanaka K, Hagimoto H, Miura T, Kishimoto H, Matsushita M. Computerised EEG in schizophrenic patients. Biol Psychiatry. 1990;28:488–494. doi: 10.1016/0006-3223(90)90482-H. [DOI] [PubMed] [Google Scholar]
- Muir WJ, St. Clair DM, Blackwood DHR. Long latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21:867–879. doi: 10.1017/S003329170002986X. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Tunca Z, Başar E. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Kocaaslan S, Tunca Z, Başar E. Event related oscillations in euthymic patients with bipolar disorder. Neurosci Lett. 2008;444:5–10. doi: 10.1016/j.neulet.2008.07.081. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Atagün İ, Turp B, Oral ET, Başar E. Decrease of long distance event related gamma coherence in bipolar patients. Int J Psychophysiol. 2010;77:313. doi: 10.1016/j.ijpsycho.2010.06.222. [DOI] [Google Scholar]
- Özerdem A, Güntekin B, Saatçi E, Tunca Z, Başar E. Disturbance in long distance gamma coherence in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:861–865. doi: 10.1016/j.pnpbp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Roy PD, Zipursky RB, Saint-Cyr JA, Bury A, Langevin R, Seeman MV. Temporal horn enlargement is present in schizophrenia and bipolar disorder. Biol Psychiatry. 1998;44:418–422. doi: 10.1016/S0006-3223(98)00105-X. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic depressive psychosis. Biol Psychiatry. 1999;45:98–106. doi: 10.1016/S0006-3223(98)00208-X. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Başar-Eroğlu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schulz C, Mavrogiorgou P, Schroter A, Hegerl U, Juckel G. Lithium-induced EEG changes in patients with affective disorders. Neuropsychobiology. 2000;42:33–37. doi: 10.1159/000054850. [DOI] [PubMed] [Google Scholar]
- Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry. 2004;161:1564. doi: 10.1176/appi.ajp.161.9.1564. [DOI] [PubMed] [Google Scholar]
- Small JG, Milstein V, Kellams JJ, Miller MJ, Boyko OB, Small IF. EEG topography in psychiatric diagnosis and drug treatment. Ann Clin Psychiatry. 1989;1:7–17. doi: 10.3109/10401238909149858. [DOI] [Google Scholar]
- Small JG, Milstein V, Malloy FW, Klapper MH, Golay SJ, Medlock CE. Topographic EEG studies of mania. Clin Electroencephalogr. 1998;29:59–66. doi: 10.1177/155005949802900203. [DOI] [PubMed] [Google Scholar]
- Souza VB, Muir WJ, Walker MT, Glabus MF, Roxborough HM, Sharp CW, Dunan JR, Blackwood DHR. Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry. 1995;37:300–310. doi: 10.1016/0006-3223(94)00131-L. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiserm M. Resting EEG in first-episode and chronic-schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitans of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/S0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- Wilder-Willis KE, Sax KW, Rosenberg HL, Fleck DE, Shear PK, Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. 2001;3:58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yener G, Güntekin B, Öniz A, Başar E. Increased frontal phase-locking of event-related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int J Psychophysiol. 2007;64:46–52. doi: 10.1016/j.ijpsycho.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Yener G, Güntekin B, Başar E. Event related delta oscillatory responses of Alzheimer patients. Eur J Neurol. 2008;15:540–547. doi: 10.1111/j.1468-1331.2008.02100.x. [DOI] [PubMed] [Google Scholar]
- Zappoli R, Versari A, Paganini M, Arnetoli G, Muscas GC, Gangemi PF, Arneodo MG, Poggiolini D, Zappoli F, Battaglia A. Brain electrical activity (quantitative EEG and bit-mapping neurocognitive CNV components), psychometrics and clinical findings in presenile subjects with initial mild cognitive decline or probable Alzheimer-type dementia. Ital J Neurol Sci. 1995;16:341–376. doi: 10.1007/BF02229172. [DOI] [PubMed] [Google Scholar]