Abstract
Intracellular deposition of fibrillar aggregates of alpha synuclein (αSyn) characterizes neurodegenerative diseases such as Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). However, recent evidence indicates that small αSyn oligomeric aggregates that precede fibril formation may be the most neurotoxic species and can be found extracellularly. This new evidence has changed the view of pathological αSyn aggregation from a self-contained cellular phenomenon to an extracellular event and prompted investigation of the putative effects of extracellular αSyn oligomers. Here, we report that extracellular application of αSyn oligomers detrimentally impacts neuronal welfare and memory function. We found that oligomeric αSyn increased intracellular Ca2+ levels, induced calcineurin (CaN) activity, decreased cAMP response element binding protein (CREB) transcriptional activity and resulted in calcineurin-dependent death of human neuroblastoma cells. Similarily, CaN induction and CREB inhibition were observed when αSyn oligomers were applied to organotypic brain slices, which opposed hippocampal long-term potentiation (LTP). Furthermore, αSyn oligomers induced CaN, inhibited CREB and evoked memory impairments in mice that received acute intracerebroventricular injections. Notably, all these events were reversed by pharmacological inhibition of CaN. Moreover, we found decreased active calcineurin (CaN) and reduced levels of phosphorylated CREB in autopsy brain tissue from patients affected by DLB, which is characterized by deposition of αSyn aggregates and progressive cognitive decline. These results indicate that exogenously-applied αSyn oligomers impact neuronal function and produce memory deficits through mechanisms that involve CaN activation.
Keywords: alpha synuclein, oligomers, calcineurin, CREB, LTP, memory, DLB
INTRODUCTION
The signature event in synucleinopathies such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) is the misfolding, aggregation and intracellular accumulation of alpha synuclein (αSyn) (Goedert 2001). αSyn is a 140 amino acid long protein abundant in pre-synaptic terminals of neurons where it may play a role in vesicular trafficking (Burre et al. 2010). Under normal physiological conditions αSyn occurs as a α-helix rich tetramer that is not prone to aggregation (Bartels et al. 2011). However, under disease conditions αSyn misfolds into β-sheet-rich conformations and aggregates into fibrils that are the main components of Lewy bodies (LB) and Lewy neurites (Spillantini et al. 1998, Braak et al. 1999). These deposits are accompanied by progressive neuronal dysfunction and eventually death of affected neuronal populations (Braak & Del Tredici 2008). Behavioral and cognitive deficits are concomitant with these pathological changes (Braak et al. 2005, Caviness et al. 2011).
The discovery of three missense mutations in the SNCA gene (encoding αSyn) that are linked to rare forms of familial PD (Kruger et al. 1998, Polymeropoulos et al. 1997, Zarranz et al. 2004) have highlighted the potential importance of αSyn in the pathogenesis of these diseases, likely due to toxic gain-of-function. Indeed, αSyn knock-out mice are normal, but mice overexpressing αSyn display a synucleopathic phenotype (Kahle 2008). However, αSyn fibrils are thought to be protective because they may sequester more toxic intermediate aggregates such as oligomers (Olanow et al. 2004). αSyn oligomer toxicity is exemplified by a juvenile form of PD in which extensive neurodegeneration occurs in the absence of LB formation (Mori et al. 1998, Olanow et al. 2004, Takahashi et al. 1994). Also, microscopic and biochemical analysis showed that LB-bearing neurons do not exhibit cytotoxic signs (Gertz et al. 1994, Tompkins et al. 1997). More directly, in vitro and in vivo experiments recently illustrated that αSyn oligomers are toxic to neural cells whereas fibrils are not (Kayed et al. 2003, Putcha et al. 2010, Winner et al. 2011).
Classically, the aggregation and deposition of αSyn has been considered an intracellular phenomenon (McNaught & Olanow 2006). However, more recent evidence demonstrates the existence of extracellular αSyn oligomers and suggests that they play key roles in disease progression (Lee 2008, Brown 2010). For example, αSyn oligomers are released from cultured cells and primary neurons (Emmanouilidou et al. 2010, Danzer et al. 2011) and are detectable in the cerbrospinal fluid of PD patients (El-Agnaf et al. 2006) as well as in the soluble protein fraction from brains of DLB patients (Paleologou et al. 2009). In addition, αSyn can be transmitted from neuron to neuron (Desplats et al. 2009) or neuron to astroglia (Lee et al. 2010), fetal tissue grafts in the brain of PD patients acquire LB pathology (Li et al. 2008, Chu & Kordower 2009), and stem cells or fetal tissue transplanted into the CNS of transgenic mice overexpressing human αSyn show intracellular deposits formed by host αSyn (Desplats et al. 2009, Hansen et al. 2011). Collectively, these findings suggest that αSyn oligomers are found extracellularly.
The presence of extracellularly-released αSyn oligomers suggests that they are involved in synucleinopathic human diseases (Lee 2008). It also illustrates the importance of determining the effects of extracellular αSyn on neurons, specifically at synapses where αSyn is thought to be released from the αSyn-enriched pre-synaptic terminals (Schulz-Schaeffer 2010). With this goal in mind, the present study used in vitro, ex vivo and in vivo models to investigate the effects of exogenously-applied αSyn oligomers on Ca2+-related signaling, hippocampal synaptic plasticity and cognitive function. We focused on two proteins important in plasticity, learning and memory: calcineurin (CaN), a CNS-abundant Ca2+/calmodulin-dependent phosphatase (Mansuy et al. 1998, Mansuy 2003), and cAMP response element binding protein (CREB), a transcription factor indirectly regulated by CaN (Bito et al. 1996, Pittenger & Kandel 1998, Silva et al. 1998, Kinney et al. 2009). We further report that similar alterations in active CaN and CREB as elicited in our model systems are also observed in autopsy human brain specimens from patients affected by DLB.
METHODS
Cell culture
Cultured human neuroblastoma (SY5Y) cells from American Type Culture Collection (ATCC, Manassas, VA) were maintained at 37 °C in a humidified cell incubator under a 5% CO2 atmosphere in T-125 culture flasks containing 10 mL of DMEM/F12 supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma, St. Louis, MO). Every two days, half of the medium was replaced; when cultures reached confluence the cells were dislodged by vigorous shaking and divided into two flasks.
SEAP transfection
Transfections were performed using liposome-mediated plasmid introduction in SY5Y cells. The liposome used was DMRIIE-C (Invitrogen, Carlsbad, CA). Cultures at 40% to 50% confluence received 1.2 pmole mL−1 of DNA coupled to DMRIIE-C at a ratio of 1:3 diluted in serum-free OptiMEM medium (Invitrogen). The liposome-DNA mix was replaced with fresh culture medium with serum after 3 h, and the cells were allowed at least 48 h of recovery prior to addition of αSyn with or without 10 µM forskolin (Sigma, St. Louis, MO).
Secreted alkaline phosphatase activity (SEAP; Clontech, Palo Alto, CA) was used to measure the effects of test compounds on transcription factor activity. Plasmids containing the SEAP gene coupled to either a control promoter (pTAL-SEAP) or enhancer sequences specific to binding CRE (CRE-SEAP) were transiently transfected as described above. SEAP activity was assayed directly from the culture medium using the Great EscAPe Chemiluminescent Detection Kit according to manufacturer’s instruction (Clontech).
LDH assay
Release of lactate dehydrogenase (LDH) from cultured cells was used as an indirect measure of cell death. Extracellular LDH levels were determined using a sample of the culture medium and a commercially available kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions.
Phosphatase activity assay
The activity of CaN (PP2B) and combined activity of PP1/PP2A were assayed from lysed cytosolic cells and tissue extracts using a commercially available colorimetric kit (EMD Biosciences, San Diego, CA) according to the manufacturer’s instructions.
Ca2+ imaging
SY5Y cells grown on poly-D-lysine coated coverslips were loaded with Fura-2 acetoxymethyl ester (Invitrogen) according to manufacturer’s instruction. Slides were then mounted in a stage containing 1 mL of serum-free media. Ca2+ imaging was performed on an inverted Nikon Eclipse TE200 microscope with a Nikon S Fluor 20X, 0.75 numerical aperture objective at the Optical Microscopy Core, UTMB-Galveston. The samples were excited with a DG4/DG5 xenon source of illumination at 340nm and 380 nm using MetaFluor software (Universal Imaging, Dowingtown, PA, USA). Emission spectra were collected with a 71000av2 filter cube (Chroma Technology Corp., Bellows Fall, VT) equipped with a Fura-2 emitter D510/80m.
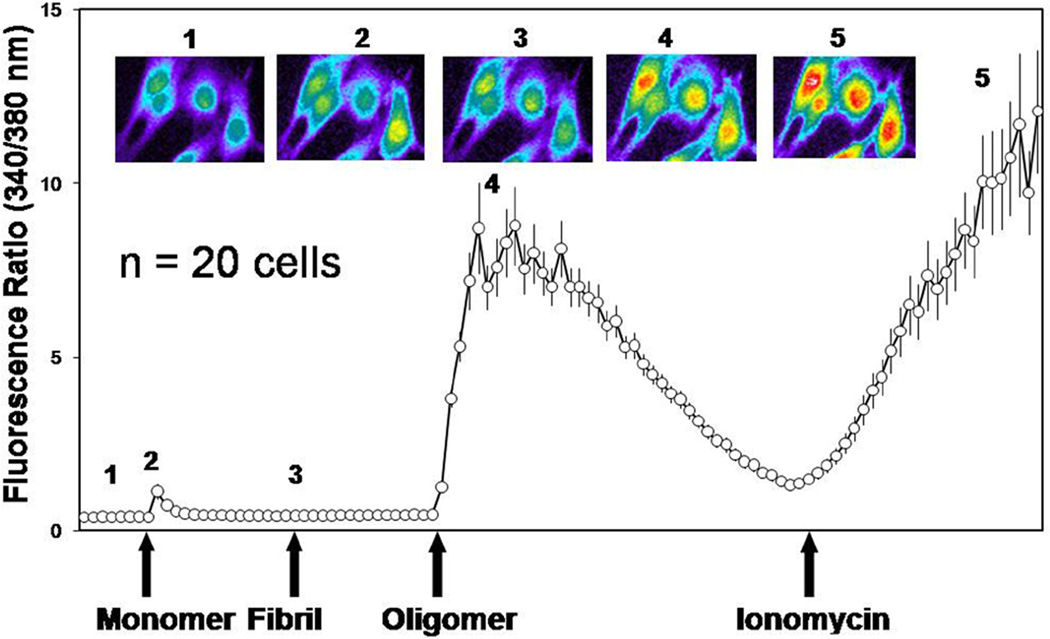
Experiments were performed at 21 °C. The basal Ca2+ ratios were recorded (1). At t = 5 min, 4 µM of monomeric αSyn was added (2). At t = 15 min, 4 µM of fibrillar αSyn was added (3), and at t = 25 min, 4 µM of oligomeric αSyn was added. Finally, at t = 45 min, 2 µM of ionomycin was added as a positive control. Ratiometric measurements and calculations were performed with MetaFluor software. The trace shown is the averaged 340/380 ratio response of 20 cells after background subtraction.
Preparation of αSyn oligomers and fibrils
αSyn oligomer and fibrils were prepared using standardized continuous stirring methods as previously described (Kayed et al. 2003). αSyn aggregation into oligomers and fibrils was initiated by seeding monomeric αSyn with a small amount (1:140 v:v) of amyloid beta (Aβ) oligomers. The final concentration of contaminant Aβ oligomers in the αSyn solution used in these experiments was 250 times lower than the minimum effective Aβ concentration in similar experimental models (Reese et al. 2008). Seed Aβ oligomers were prepared by dissolving 0.3 mg of lyophilized Aβ42 (W.M. Keck Facility, University of Yale) in 200 µL0 of hexafluoro-2-propanol (Sigma-Aldrich) for 10–20 minutes at 21 °C. The resulting Aβ solution was added to distilled, deionized H2O (ddH2O) in a siliconized Eppendorf tube to a final concentration of 66 µM. A magnetic stir bar was placed in the tube and holes were made in the cap to promote HFIP evaporation, and the solution was stirred for 48 h. For preparation of αSyn oligomers or fibrils, 0.1 mg of lyophilized powder of αSyn (rPeptide, Bogart, GA) was dissolved in 230 µL H2O and seeded with the Aβ oligomers (1:140 v:v) and stirred 20 min for oligomers and 5 d for fibrils. Quality and consistency of the resulting preparations were assessed by Western and dot blots using the antibody 4D6, which recognizes aSyn in any aggregated or monomeric state, and A11, which specifically recognizes amyloid oligomers but not monomers or fibrils (Supplementary Fig. 1).
Animals
C57BL/6 mice were used in the intracerebroventricular (ICV) and fear conditioning paradigms. Male Sprague Dawley rats (150–250 g) were employed for electrophysiological experiments. Animals were maintained at the UTMB vivarium under USDA standards (12 h light/dark cycle, food and water ad libitum).
Animals were sacrificed by exposure to halothane vapors followed by decapitation at the end of each experiment. Brains were rapidly removed and either frozen whole (for immunohistochemistry) or dissected into individual areas including hippocampus, cerebellum, frontal cortex, and occipital cortex, then stored at −80 °C until further biochemical analyses.
Ex vivo rat brain slices
According to IACUC-approved protocols, rats were decapitated, and the brain was quickly removed and blocked in cold (4 °C) artificial cerebrospinal fluid (aCSF) containing (in mM): 117 NaCl, 4.7 KCl, 1.2 Na2PO4, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 11 glucose, was oxygenated and equilibrated to pH 7.4 with 95% O2/5% CO2. Coronal brain slices (500 µm) containing the hippocampus were prepared using a Vibroslice (Camden Instruments, London, UK) and equilibrated in aCSF at 21 °C for 30 min prior to the addition of test compounds. At the end of the experiments, slices were rapidly frozen in liquid nitrogen and stored at −80 °C until further analyses were performed.
Patch-clamp recordings
Whole-cell recordings using the blind-patch technique or differential interference contrast (DIC)-enhanced infrared (IR)-videomicroscopy were done as described previously (Li et al. 2011, Fu & Neugebauer 2008). Recording pipettes (3–5 MΩ tip resistance) made from borosilicate glass (1.5 mm o.d., 1.12 mm i.d.; Drummond) were filled with intracellular solution containing (in mM): 122 K-gluconate, 5 NaCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, and 0.4 Na3-GTP; pH was adjusted to 7.2–7.3 with KOH and osmolarity to 280 mOsm/kg with sucrose. A single brain slice was transferred to the recording chamber and submerged in aCSF (31 °C), which superfused the slice at 2 mL/min. A single neuron was recorded in each slice, with a fresh slice used for each new experimental protocol. As the hippocampal regions are easily visible with the aid of light microscopy, recording electrodes were visually positioned in CA1. After tight (>2GΩ) seals were formed, and the whole-cell configuration was obtained, neurons were included if their resting membrane potential was at least −50 mV, and the injection of depolarizing current evoked action potentials overshooting 0 mV. Data acquisition and analysis of voltage and current signals was performed with a dual 4-pole Bessel filter (Warner Instr., Hamden, CT), low-noise Digidata 1322 interface (Axon Instr., Union City, CA), Axoclamp-2B amplifier (Axon Instr.), Pentium PC, and pClamp9 software (Axon Instr.). Headstage voltage was monitored continuously on an oscilloscope to ensure precise performance of the amplifier. Neurons were voltage-clamped at −60 mV. Seal and series resistances were checked throughout the experiment (using pClamp9 membrane test function) to ensure high-quality recordings.
Synaptic transmission and LTP induction
Synaptic transmission was measured at the Schaffer collateral/commissural pathway that provides glutamatergic input to CA1. Using a concentric bipolar stimulating electrode (Kopf Instr., Tujunga, CA) of 22 kΩ resistance, excitatory postsynaptic currents (EPSCs) were evoked in CA1 neurons by focal electrical stimulation (Grass S88 stimulator, Grass Technologies, West Warwick, RI) of the Schaffer collateral/commissural pathway. Test EPSCs were evoked by electrical stimuli (200 µs square-wave pulses) delivered at a frequency of 0.033 Hz. Stimulus intensity was set to evoke a half-maximal response (50 % of EPSC amplitude). Long-term potentiation (LTP) was induced using high-frequency stimulation (HFS) consisting of 3 trains at 100 Hz (1 s duration each; intertrain-interval of 20 s). During HFS stimulus, intensity was raised to evoke an EPSC that was 75% of the maximum amplitude.
ICV injection
Using an IACUC-approved protocol, intracerebroventricular (ICV) injections were performed using a modified free-hand method as previously described (Clark et al. 1968; Dineley et al. 2010). Briefly, mice were deeply anesthetized with an intraperitoneal (IP) injection of ketamine/xylazine mixture (65 and 7.5 mg/kg, respectively), the scalp was shaved and an incision was made through the midline to expose the top of the skull. A 28 gauge needle held with a forceps was lowered 1 mm posterior and 1 mm lateral of the bregma. The needle was connected with 0.28 mm polyethylene tubing to a 25 µL syringe driven by an electronic programmable micro-infuser (Harvard Apparatus, Holliston, MA), which was used to deliver 3 µL/mouse at a rate of 6 µL/min. After injection, the needle was left in place for 1 min and the surgical wound stitched before allowing the mouse to recover on a heated pad under a warm light. Reliability and consistency of injections was routinely tested during actual experiments by injecting India ink in parallel animals and macroscopically observing proper coloration of the ventricles(Dineley et al. 2010). 18 h after ICV, animals were given an IP injection of either 1% DMSO in 0.9% saline or CaN inhibitor FK506 diluted in 0.9% saline at 10 mg/kg (LC Laboratories, Woburn, MA).
Fear Conditioning
All mice were subject to fear conditioning training 6 h after FK506 (or vehicle) injection. In separate experiments, mice were treated with rapamycin (5 mg/kg IP) or vehicle (1% DMSO in 0.9% saline). There were 10 animals per treatment group. The protocol consisted of a training phase when the mice were placed in a particular environment (a chamber with particular lighting, geometry, or odor that constitutes the context conditioned stimulus, CS) and allowed to explore freely for 3 min. An auditory CS (80 dB white noise) was then presented for 30 sec and one footshock (0.8 mA, 2 sec duration; the unconditioned stimulus, US) was delivered during the final 2 sec of the auditory CS. A second presentation of the auditory CS and the US was delivered at the 5 min mark, and the animals remained in the chamber for another 2 min. Twenty-four hours later, the mice were returned to the same training chamber and the context test for fear learning was performed. The amount of freezing the mice exhibited during 5 min in the training chamber was measured. One hour later the cued test was performed in a completely novel context. The animals were placed in the testing chamber and freezing was measured for 3 min before the auditory CS was re-presented and freezing quantified over the next 3 min.
Immunofluorescence
Immunofluorescence was performed as described previously (Reese et al. 2011). Briefly, mouse or human brain sections (10 µm) were cut using a cryostat and affixed to Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) for storage at −80 °C until use. Slides were equilibrating to 21 °C, rinsed in 0.1 M PBS and fixed in ice-cold 4% paraformaldehyde for 15 min. After two brief washes in 0.1 M PBS, the sections were blocked and permeabilized for 1 h in 0.1 M PBS containing 10% goat serum, 0.03% Triton-X (ICN Radiochemicals, Irvine, CA), and 0.1% phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA). Incubation with primary antibodies in 0.1 M PBS containing 10% serum and 0.1% phosphatase inhibitor was carried out overnight at 4°C. Antibodies used were CREB (mouse, 1:100; Cell Signaling, Danvers, MA) and phospo-CREB (Ser133, rabbit, 1:100; Millipore, Billerica, MA). Slides were washed and incubated for 1 h with Alexa Fluor 488 and 594 secondaries (1:600; Invitrogen, Carlsbad, CA) in the same solution as primaries. Slides were again rinsed twice in PBS and once in ddH2O before Vectashield containing 4',6-diamidino-2-phenylindole (DAPI) was applied (Vector Laboratories, Burlingame, CA), and coverslips were mounted. Nail polish was applied to seal the edges and preserve fluorescence.
Western blotting
Cells or frozen tissue were lysed in sodium dodecyl sulphate (SDS) lysis buffer containing 5 mM ethylenediaminetetraacetic acid (EDTA), 50 mM Tris, 2% SDS, 1 mM DTT, 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1% protease cocktail inhibitors (Sigma, St. Louis, MO). Following lysis, the cells were sonicated for 15 s,centrifuged at 20,000 × g for 5 min, and the supernatants were collected. Protein samples (30 µg as determined by the BCA assay, Pierce, Rockford, IL), were subjected to SDS-polyacrylamide gel electrophoresis using 12% gels, followed by electrophoretic transfer to nitrocellulose membranes (Biorad, Hercules, CA). Membranes were then incubated with appropriate dilutions (usually 1:1000 v:v) of the primary antibody, washed in TBS-T, then incubated with a horseradish peroxidase (HRP) conjugated secondary antibody (Biorad, Hercules, CA) against rabbit IgG (for polyclonal primaries) or mouse IgG (for monoclonal primary antibodies). Signals were detected with Enhanced Chemiluminescence (ECL) substrate (Pierce, Rockford, IL).
Human Tissue
Frozen frontal lobe tissue was obtained through a Materials Transfer Agreement with the Oregon Brain Bank at Oregon Health and Science University in Portland. Table 1 shows the diagnosis, age, sex, post mortem interval (PMI), Braak staging, plaque load and presence of αSyn pathology (macroscopic aggregates and Lewy bodies) in cortex, amygdala and midbrain of the cohort in this study. Collectively, these cases have post-mortem intervals (PMI) ranging from 3–48 hours (control: 10.55 ± 7.60; DLB: 24.43 ± 14.73; p=0.0846, Student’s t-test) and a mean age of 77 years (control: 81.8 ± 7.66; DLB: 73.8 ± 6.57; p=0.0823, Student’s t-test).
Table 1. Demographic characteristics of the cohort of subjects donating the brain sample specimens used in this study.
PMI: Post Mortem Interval; Braak stage 1–6; Plaque load 0–3; DLB: Dementia with Lewy bodies; PD: Parkinson Disease; αSyn pathology: presence of αSyn aggregates/LBs as detected by immunohistochemistry in cortex, amygdala and mid brain.
| αSyn pathology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case# | State | Age | Sex | PMI (h) | Braak | Plaque | Cortex | Amyg | MdBrain | Clinical Dx |
| 1720 | Control | 73 | M | 5.25 | 2 | 1 | No | No | No | |
| 1731 | Control | 74 | F | 7.5 | 2 | 1 | No | No | No | |
| 1761 | Control | 86 | M | <24 | 1 | 0 | No | No | No | |
| 1052 | Control | 87 | M | 8 | 2 | 1 | No | No | No | |
| 1728 | Control | >89 | F | 8 | 4 | 0 | No | No | No | |
| 198 | DLB | 72 | M | <24 | 4 | 3 | Yes | Yes | Yes | Dementia |
| 727 | DLB | 76 | F | 19 | 6 | 3 | Yes | Yes | Yes | Dementia/PD |
| 887 | DLB | 67 | F | 12 | 5 | 3 | Yes | Yes | Yes | Dementia/PD |
| 1764 | DLB/PD | 73 | M | 33 | 2 | 1 | Yes | Yes | Yes | Dementia/PD |
| 1783 | DLB/HS | 78 | M | 48 | 2 | 1 | Yes | Yes | Yes | Dementia/PD |
| 1850 | DLB/PD | 85 | M | 31.5 | 1 | 1 | Yes | Yes | Yes | Dementia/PD |
| 1859 | DLB/PD | 66 | M | 3.5 | 2 | 0 | Yes | Yes | Yes | Dementia/PD |
Statistical Analysis
Where appropriate (two group comparisons), statistical differences were assessed by Student’s t test. Analyses with more than two groups were subject to one-way analysis of variance (ANOVA) and, if overall p < 0.05, was followed by Fisher least significant difference (LSD). Columns and error bars represent mean ± S.D., in all figures.
RESULTS
CaN is a key negative modulator of synaptic plasticity and memory function (Mansuy 2003, Lee & Ahnn 2004). We have previously reported that CaN hyperactivation mediates some of the detrimental effects that Aβ oligomers exert on synapses and cognition in Alzheimer’s disease models (Dineley et al. 2007, Dineley et al. 2010, Reese et al. 2008). These neurotoxic aggregate species share structural and functional similarities with αSyn oligomers (Glabe & Kayed 2006). As such, we chose to examine whether specific aggregates of αSyn also induce CaN activity and subsequent detrimental effects on synaptic and cognitive function.
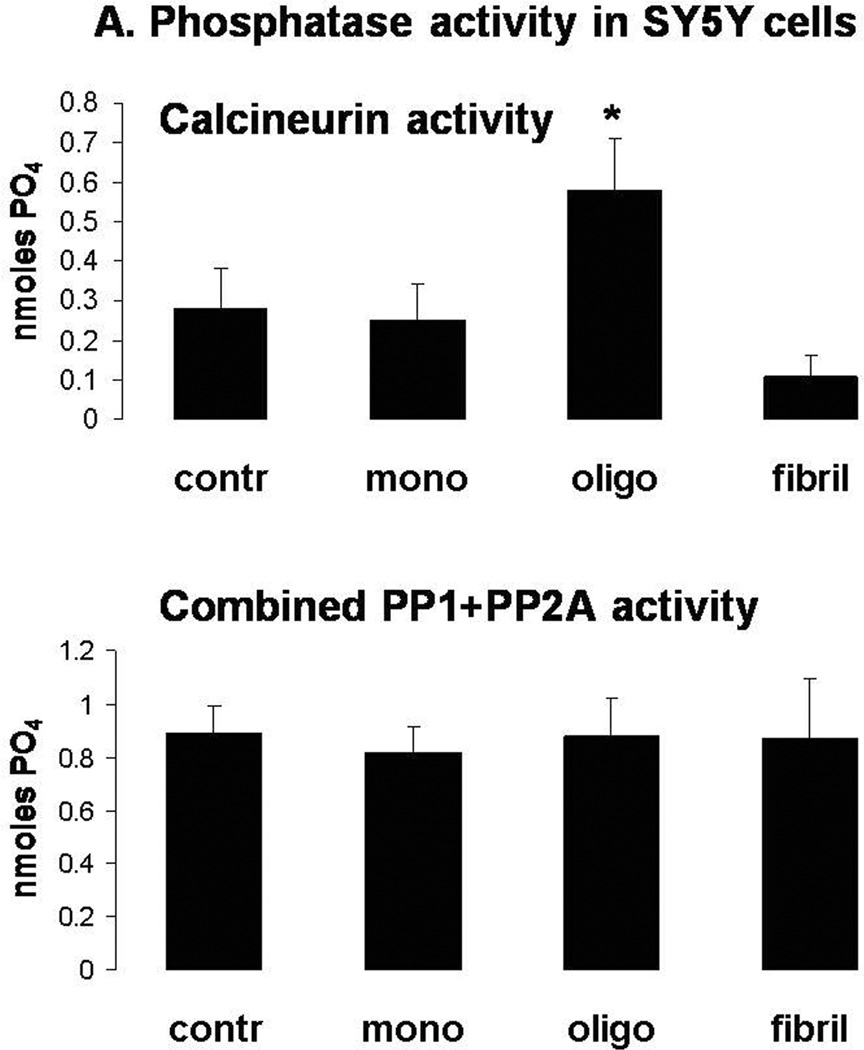
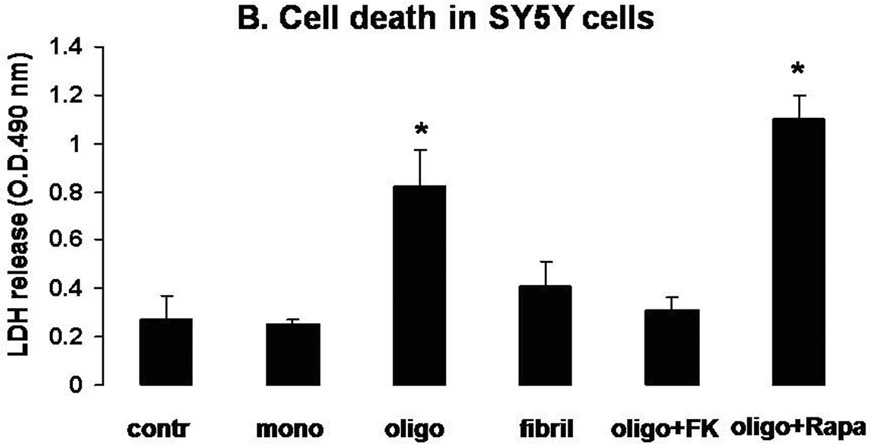
Consistent with previous reports on other oligomeric amyloid proteins (Demuro et al. 2005), treatment of SY5Y cells with oligomeric αSyn augmented intracellular Ca2+ levels (Fig. 1). Monomeric and fibrillar αSyn were ineffetive, even though they were added to the very same cells that were responsive to oligomeric αSyn. Increased intracellular Ca2+ may result in activation of the Ca2+/calmodulin-dependent phosphatase CaN, which directly dephosphorylates the pro-apoptotic protein BAD (Wang et al. 1999). Therefore, we determined phosphatase activity and cell survival in SY5Y cells treated with monomeric, oligomeric, and fibrillar αSyn (Figs. 2a and 2b). Consistent with previous results (Kayed et al. 2003), we observed that only oligomeric αSyn increased the activity of CaN and induced cell death as measured by the amount of LDH in the culture medium. Conversely, the combined activity of the phosphatases PP1 and PP2A, which are related to CaN but are not activated by Ca2+/calmodulin, did not increase, suggesting that the observed cell death is coincident with increased CaN activity. Indeed, we found that cytotoxicity was attenuated by treatment with the CaN inhibitor FK506, but not by its analog Rapamycin, which has no effect on CaN activity although both act as immunosuppresants (Abraham & Wiederrecht 1996). Incubation with monomeric and fibrillar αSyn preparations did not cause an appreciable increase of cytotoxicity.
Figure 1. αSyn oligomers, but not monomers or fibrils, increases intracellular Ca2+ levels in SY5Y cells.
Graph showing a 35 min time course of Ca2+-dependent fluorescence recorded and averaged from 20 fluo-3 loaded SY5Y cells in response to consecutive application of αSyn monomers, fibrils, and oligomers (2 µM for 10 min each). Cells were challenged with ionomycin (2 µM) at the conclusion of the experiment. Each point represents the average of the 340/380 nm readings from 20 individual cells. Representative images (top) depict the response of 4 individual cells at the time points indicated by the corresponding number on graph. Warmer colors correspond to a higher level of fluorescence.
Figure 2. Selective induction of CaN activity and CaN-dependent cell death by oligomeric αSyn in SY5Y cells.
A. CaN (top) and combined PP1+PP2A (bottom) activity in SY5Y cells treated for 24 h with 2 µM αSyn monomers, oligomers or fibrils. Graph is representative of 2 independent experiments returning similar results. n=3 independent measurements per condition; *: p<0.05 vs. control group (ANOVA).
B. LDH release in the culture medium of SY5Y cells treated for 24 h with 2 µM αSyn monomers, oligomers or fibrils. Separate dishes of oligomer-treated cells were additionally treated with FK506 (10 µM) or rapamycin (10 µM). Graph is representative of 2 independent experiments returning similar results. n=4–6 independent measurements per condition; *: p<0.01 vs. control group (ANOVA).
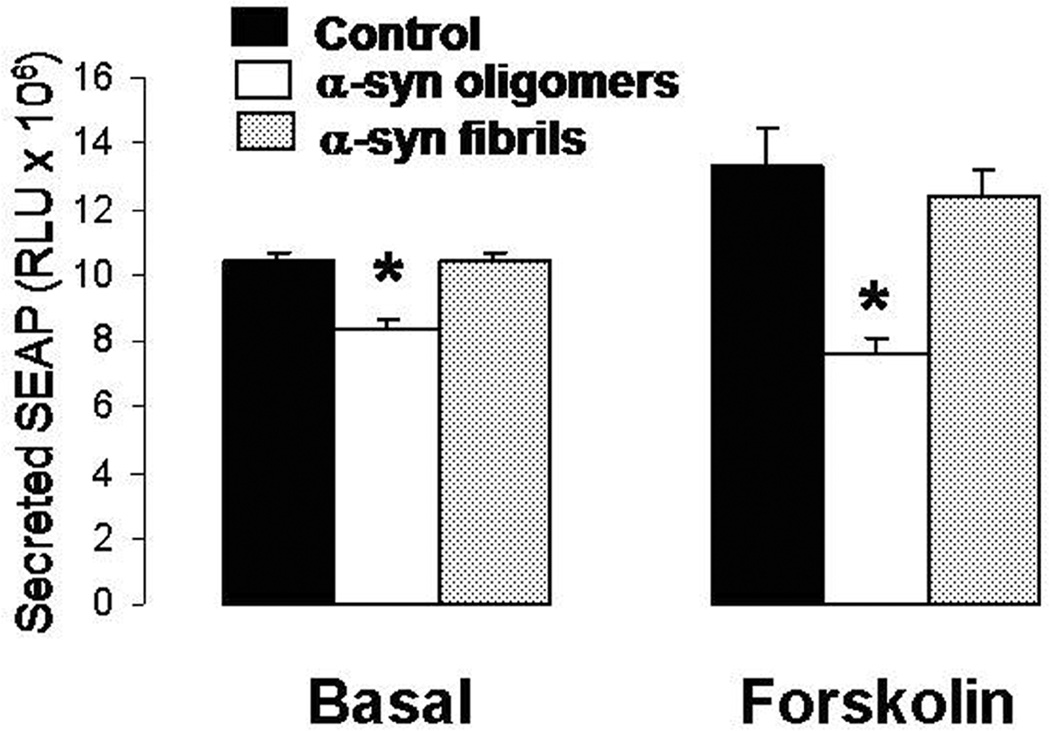
Through either direct or indirect dephosphorylation of the transcription factor CREB, CaN exerts negative effects on synaptic plasticity and LTP (Hotte et al. 2007, Josselyn et al. 2004). In order to ascertain if these events might be triggered by αSyn oligomers, we first sought to determine if the oligomeric and fibrillar forms of αSyn had any effect on CREB transcriptional activity (Fig 3). SY5Y cells were transiently transfected with a cDNA construct encoding a secreted alkaline phosphatase (SEAP) reporter gene driven by a CREB-sensitive promoter (pCRE-SEAP). All cells were additionally transfected with a vector encoding renilla luciferase to ensure equal transfection efficiency among samples (data not shown). Twenty-four hours after transfection, cells were treated with 2 µM of either oligomeric or fibrillar αSyn, in the presence or absence of the adenylate cyclase (AC) activator forskolin (10 µM). The amount of SEAP in the culture medium, assayed 3 h after treatment, (well before the observance of any cell death) is indicative of CREB-driven transcription. Under basal conditions, release of SEAP was significantly diminished by treatment with oligomeric αSyn whereas cells treated with fibrillar αSyn were unaffected. Because the level of CREB-driven transcription in SY5Y cells is quite low under basal conditions, we performed a second experiment using cells treated with forskolin, which greatly induced PKA-promoted CREB transcription activity, evidenced by the increased SEAP release (Fig. 3, right). Under these conditions, treatment of the stimulated cells with oligomeric αSyn significantly opposed the release of SEAP; those treated with fibrillar αSyn were not affected.
Figure 3. CREB-promoted transcriptional activity is inhibited by oligomeric αSyn in both basal and AC/PKA-stimulated conditions in SY5Y cells.
Release of the reported gene product SEAP in SY5Y cells transiently transfected with the CREB-sensitive CRE-SEAP construct and treated for 3 h with oligomeric or fibrillary αSyn (2 µM) in the presence or absence of the AC/PKA activator forskolin (10 µM). n=6 replicates per group; *: p<0.01 vs. control cells (ANOVA).
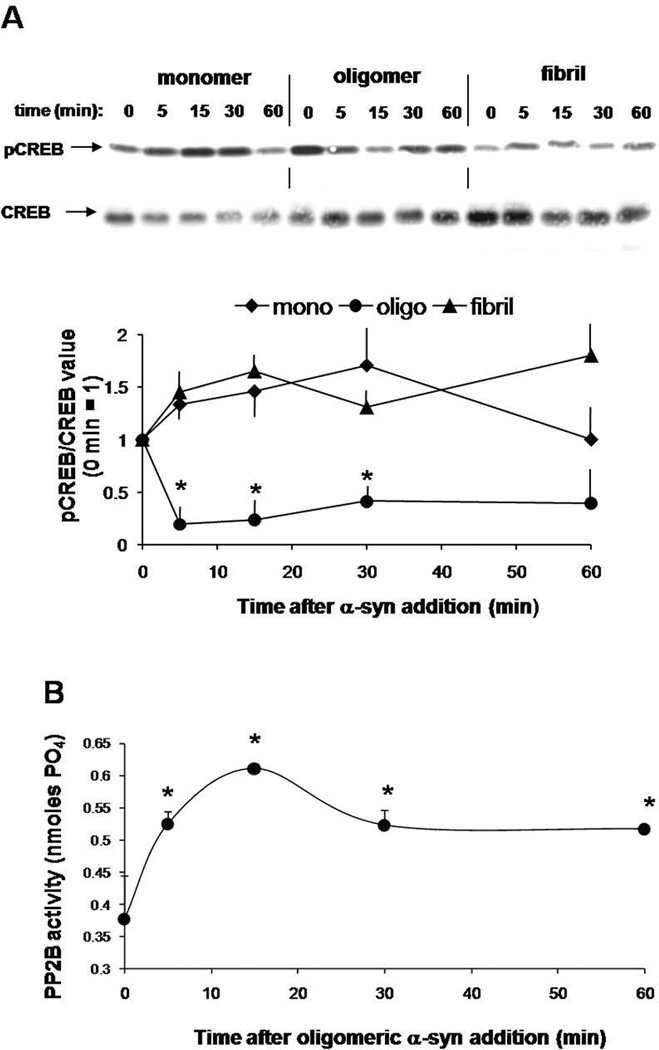
Next, we expanded on the initial findings from SY5Y cells in ex vivo rat brain slices. Figure 4a shows a time course experiment in acutely cultured rat brain slices to assess pCREB by Western blot. pCREB was maximally decreased in total protein extracts from brain slices treated with oligomeric αSYn for 15 minutes but recovered by the 30 to 60 minute time points. Conversely, monomeric and fibrillar αSyn did not affect the phosphorylation of CREB. In fact, treatment with monomer appeared increase pCREB (although not statistical significant). The effect of oligomeric αSyn on pCREB was closely mirrored by a dramatic rise in CaN activity that also peaked at 15 minutes and remained significantly increased up to 60 minutes (Fig. 4b).
Figure 4. Oligomeric αSyn reduces pCREB levels and coincidentally increases CaN activity in organotypic rat brain slices.
A. Representative Western blot (top) detecting pCREB in total protein extracts from organotypic brain slices treated with 0.75 µM of monomeric, oligomeric and fibrillar αSyn for the time length shown. The blot was stripped and re-probed for total CREB to control for sample gel loading. Densitometry band quantification (bottom) confirmed that pCREB levels were significantly reduced by aSyn oligomers but not monomers or fibrils. The graph shows the average from 3 independent experiments. *: p<0.05 vs. time 0 control (ANOVA).
B. CaN activity assay in homogenates from rat brain slices treated with 0.75 µM oligomeric αSyn for the times indicated. n=3 replicates per time point. *: p<0.05 vs. time 0 control (ANOVA).
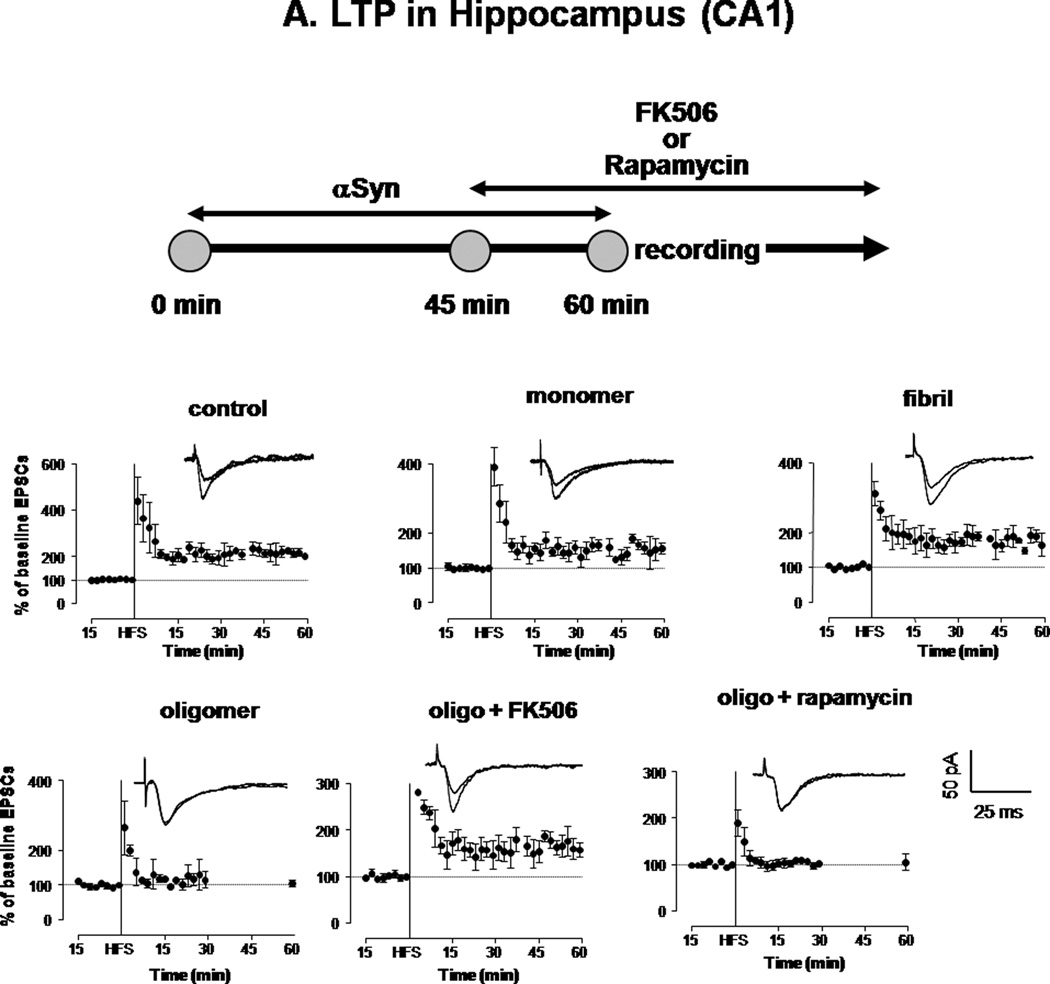
Previous studies have shown that aberrant CaN activity is sufficient to oppose expression of LTP (Winder et al. 1998, Belmeguenai & Hansel 2005). To determine if oligomeric αSyn could affect LTP through inducing CaN activity, we performed electrophysiological recordings in hippocampal (CA1) brain slices from wild-type rats exposed to αSyn. In control slices, as well as those pretreated for 1 hour with 0.5µM monomeric and fibrillar αSyn, Schaffer collateral high frequency stimulation resulted in enhanced CA1 EPSCs (Fig. 5a). However, when slices were incubated with 0.5 µM oligomeric αSyn, LTP expression was clearly opposed. This deficit in LTP expression could be rescued by adding the CaN inhibitor FK506 to the superfusion buffer (10 µM) 15 min before recording, whereas rapamycin had no effect.
Figure 5. Oligomeric αSyn decreases LTP expression in hippocampal neurons in a CaN-dependent fashion.
A. HFS-induced expression of LTP in the hippocampus (CA1) of rat brain slices treated for 1 h with vehicle (control) or with 0.5 µM monomeric, oligomeric or fibrillar αSyn. A parallel set of oligomer-treated slices were additionally treated (45 min after addition of αSyn) with FK506 (10 µM) or rapamycin (10 µM), which remained in the perfusion buffer throughout the recording. Each symbol is the average of 10 monosynaptic EPSCs. Peak amplitudes were measured and expressed as percent of baseline values before HFS. Individual traces in inserts show averaged EPSCs recorded before and 30 min after HFS. Monosynaptic EPSCs were evoked by electrical stimulation (200 µs square-wave pulses at 0.033 Hz) of the Schaffer collateral/commissural pathway. LTP was evoked by 3 high-frequency trains (100 Hz, 1 s duration each; intertrain-interval 20 s). For illustration purposes, all scales were set to a maximum of 400 %.
B. CaN (top) and PP1+PP2A combined activity (bottom) assayed in the same brain slices shown in A at the end of the LTP recording. Columns represent mean ± S.D.; n=3 per group; *: p<0.05 vs. control (ANOVA).
Immediately following electrophysiological recordings under these various conditions, slices were assayed for CaN activity (Fig. 5b). Consistent with results from cultured cells and hippocampal slices, only αSyn oligomers significantly increased CaN activity. This was prevented in slices that were co-treated with FK506, but not in those co-treated with rapamycin.
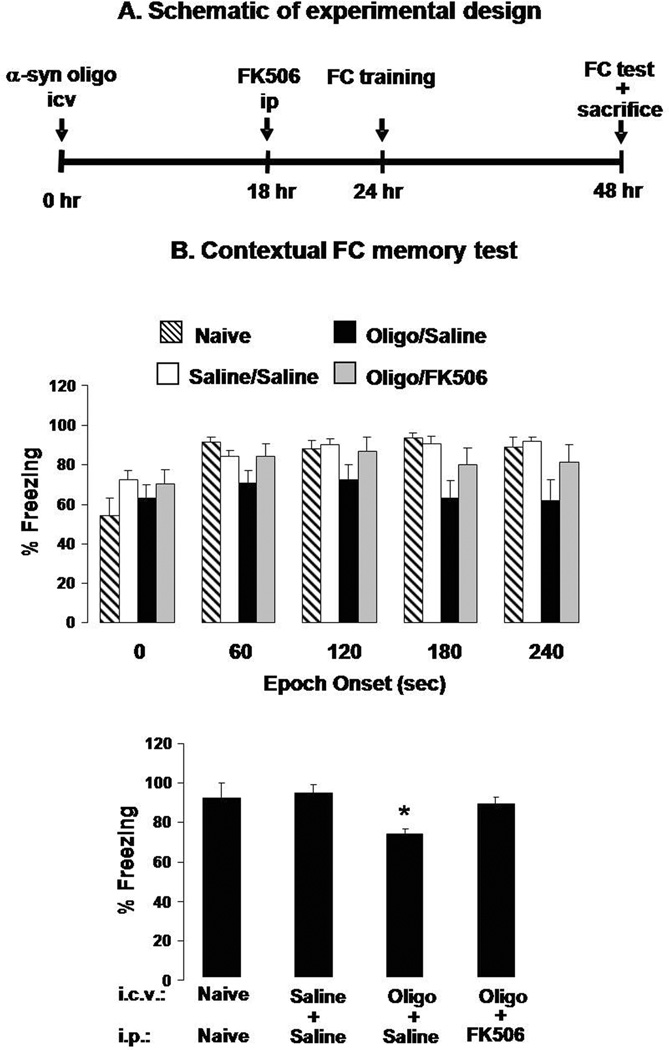
Given that hippocampal slice LTP is a cellular model for learning and memory (Howland & Wang 2008, Goosens & Maren 2002, Barnes 1995), we tested the hypothesis that αSyn oligomers could induce CaN-dependent memory deficits in vivo. Our behavioral paradigm employed a 2-pair rodent fear conditioning paradigm in which a white noise cue (CS) was paired with a foot shock (US) (Dineley et al. 2007). Twenty-four hours after training the mice were tested for both contextual and cued fear learning. In the contextual test, there are no stimuli, and the animal instead relies on visuospatial and olfactory cues acquired during the training session and consolidated during the previous 24 h. Freezing behavior during a 5 min test phase is a reliable and robust measure of hippocampus-dependent associative learning and memory and therefore especially appropriate for studying putative adverse effects of oligomeric αSyn on memory function (Freichel et al. 2007). Indeed, we found that mice treated with oligomeric αSyn have a marked reduction in freezing behavior in each epoch of the contextual test (Fig. 6b). However, these mice performed as well as control animals if they received FK506 6 h prior to training. FK506 treatment was designed to optimize the CNS drug concentration, which remains relatively stable 5–24 h following injection (Butcher et al. 1997, Fukudo et al. 2005) and does not affect freezing behavior per se (Dineley et al. 2007). Mice injected ICV with αSyn oligomers exhibited no significant differences in the amygdala-dependent cued fear conditioning (Supplementary Fig. 2), indicating that the hippocampus may be more sensitive to αSyn-induced alterations of CaN activity.
Figure 6. An acute ICV injection of αSyn oligomers coincidentally induces CaN activity, decreases pCREB, and impairs FC memory in mice.
A. A schematic illustrating the experimental design used in these experiments. αSyn oligomers (100 pmole/3 µl/mice) were injected ICV 24 h prior to FC training (48 h prior to FC test and sacrifice). Prior to FC training half of the mice were treated with FK506 (10 mg/kg IP) and half received saline.
B. Contextual FC test scores in naïve mice and mice treated with saline ICV followed by saline IP (Saline/Saline) or αSyn ICV followed by saline ip (Oligo/Saline) or FK506 ip (oligo/FK506). Percent freezing (reflecting memory recollection) for each group is shown for each 60 sec block of a 240 sec test period (upper panel) or as cumulative data for the entire test period (lower panel). n=15 mice per group. **: p<0.01 vs. Saline/Saline or oligo/FK506 (ANOVA).
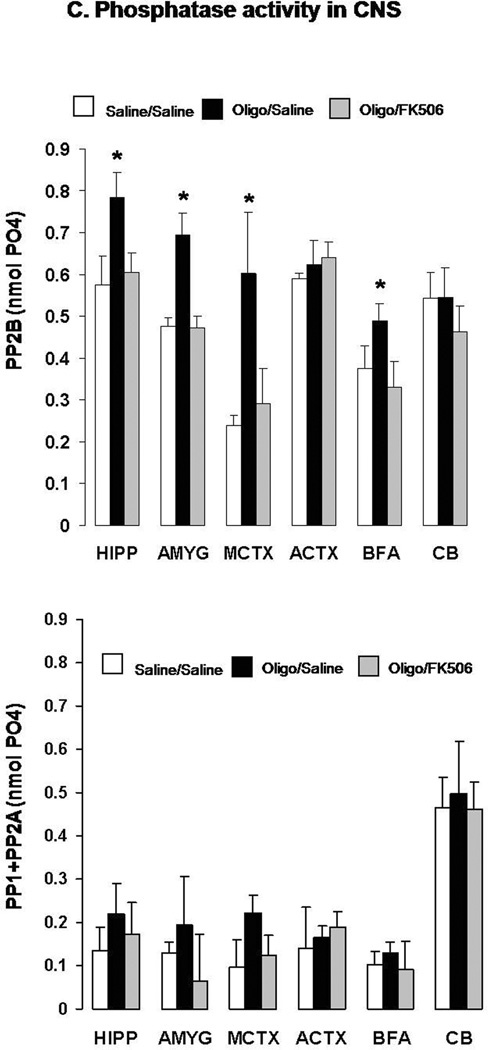
C. CaN (top) and PP1+PP2A combined activity (bottom) in the hippocampus (HIPP), amygdala (AMYG), medial cortex (MCTX), anterior cortex (ACTX), basal forebrain area (BFA) and cerebellum (CB) of the same mice sacrificed at the end of the FC memory tests.
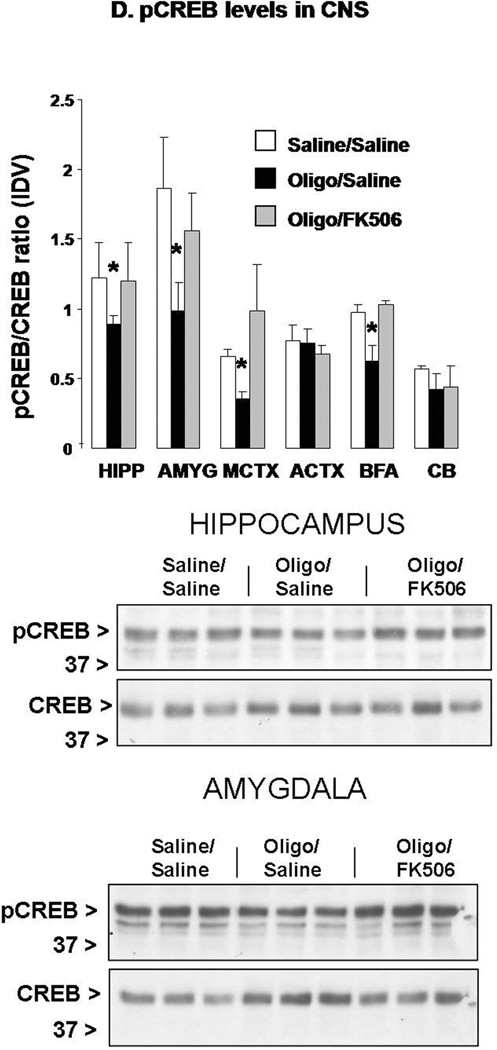
D. Phosphorylated (active) CREB levels assayed by Western blot in the same brain areas from the same mice as shown in (D). Membranes were stripped and re-probed for total CREB and final values were expressed as ratio of pCREB/CREB calculated for each sample. Representative Western blots from this experiment detecting pCREB and CREB in the hippocampus and amygdala are shown at the bottom. n=5 mice per group (randomly chosen from the 15 per group used in behavioral studies). *: p<0.05 vs. Saline/Saline or oligo/FK506 (ANOVA).
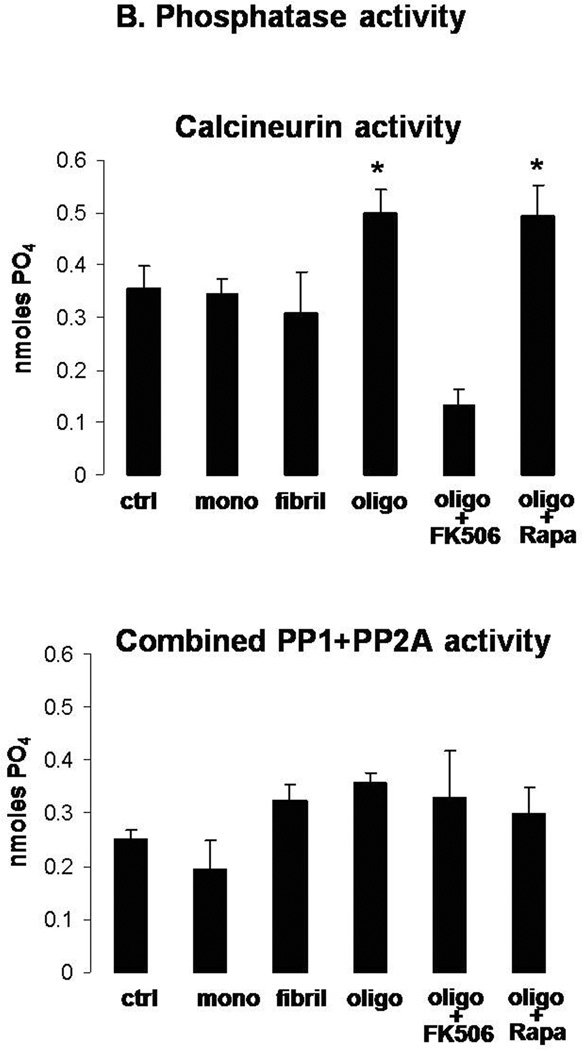
After testing, these mice were sacrificed and brain regions rapidly dissected. The hippocampus (HIPP), amygdala (AMYG), medial cortex (MCTX), anterior cortex (ACTX), basal forebrain/septum (BFA), and cerebellum (CB) were assessed for CaN activity and combined PP-1/PP-2A activity on freshly homogenized tissue (Fig. 6d). We found that CaN activity was significantly elevated in HIPP, AMYG, MCTX, and BFA of mice subjected to icv injection of αSyn compared to saline-treated mice. While ineffective when administered alone (Dineley et al. 2007), FK506 restored CaN activity to control levels in these brain areas of αSyn-treated mice. Conversely, combined PP-1/PP-2A enzymatic activity was not significantly affected by oligomeric αSyn or FK506 treatment in any of the CNS areas assayed, although there was a general trend toward increased values following αSyn oligomer treatment.
CREB phosphorylation at Ser133 was measured in the same brain areas described above and we found that mice treated ICV with αSyn oligomers exhibited significantly reduced pCREB in the same brain regions that showed increased CaN activity (Fig. 6e). FK506 abolished αSyn-induced reduction in CREB phosphorylation, suggesting that it was indeed CaN-dependent. It is worth noting that total CREB levels remained unchanged, illustrating that reduction of pCREB by αSyn oligomers could not be ascribed to an overall decrease of CREB, but rather reflected a decrease in protein phosphorylation.
Immunofluorescent examination of the hippocampi of mice injected ICV with either saline or αSyn oligomers (Supplementary Fig. 3) confirmed that neuronal pCREB was significantly decreased by αSyn oligomers. As well, expression of total CREB remained unchanged after ICV injection of αSyn oligomers, although a trend toward reduced expression was noticeable. On the other hand, ICV injection of a parallel set of mice with αSyn fibrils did not elicit any change in pCREB or CREB expression as determined by immunohistochemistry (Supplementary Fig. 3), further illustrating that, consistent with the in vitro and ex vivo experiments described above, these effects were selectively induced only by oligomeric αSyn.
Collectively, the results described above illustrate that CaN activation and the subsequent decrease in pCREB consistently characterize the impact of extracellular-applied αSyn oligomers on neurons, regardless of the model system. In order to extend these experimental observations to the actual human disease, we measured active CaN and pCREB levels in the brains of patients with clinically diagnosed DLB and age-matched controls. Immunofluorescence analysis of frontal cortex demonstrates the presence of highly immunoreactive macroscopic αSyn aggregates (Lewy bodies and Lewy neurites) in DLB brains (Supplementary Fig. 4).
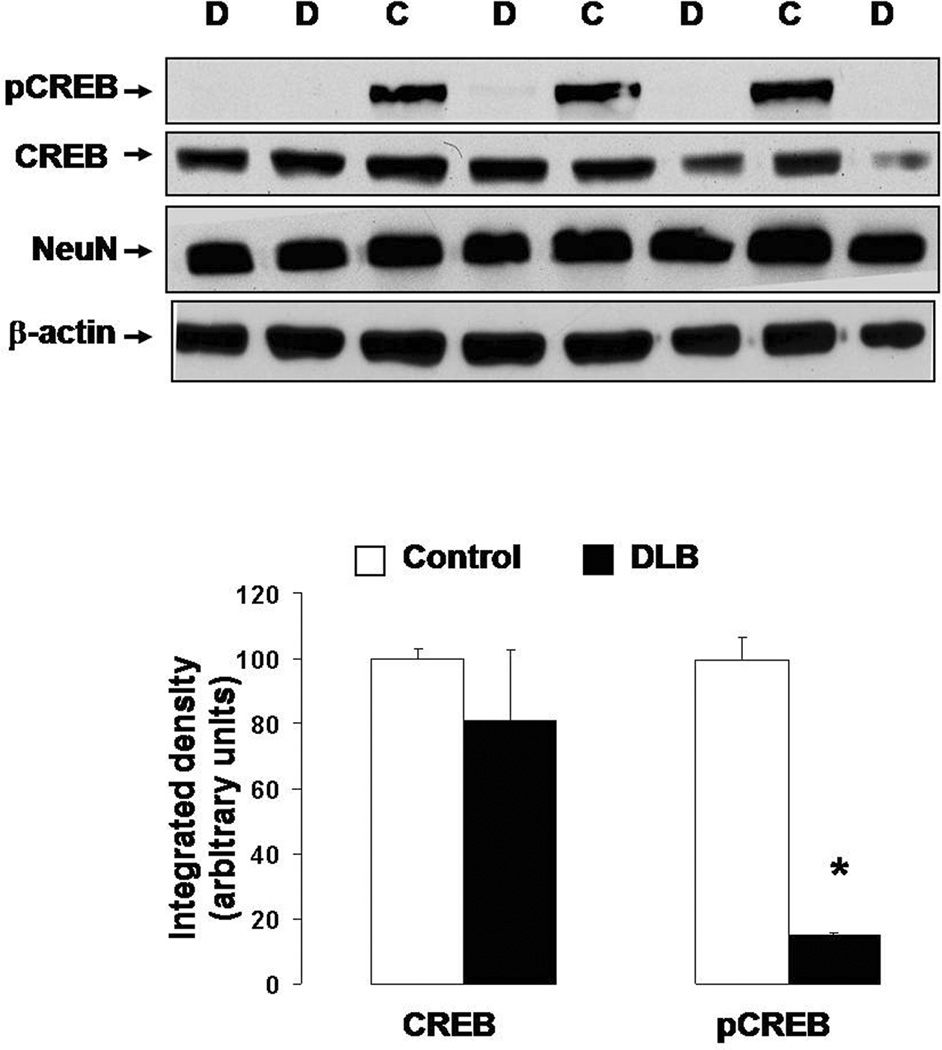
In order to determine CaN activation we measured levels of the truncated 57 kDa form of subunit A of CaN (CaN-A). Upon activation, subunit A is cleaved to reveal the active site (Liu et al. 2005). Therefore, levels of truncated CaN-A are an indirect measure of CaN activity. This approach is necessary when using human autopsy specimens because CaN is rapidly deactivated by post-mortem oxidation, making direct measurements of CaN enzymatic activity highly unreliable (Wang et al. 1996). Western blot analysis revealed that truncated CaN was increased in the DLB samples relative to age-matched control (Fig. 7a), suggesting increased CaN activity in the DLB brains. Along with increased truncation of CaN, levels of phosphorylated CREB were dramatically reduced in the nuclear fraction from the DLB samples as compared to controls, whereas levels of total CREB were relatively unchanged between the two groups (Fig. 7b). Confocal imaging confirmed the marked reduction of pCREB in the samples from DLB patients (Supplementary Fig. 5). Notably, double staining with NeuN, a neuronal marker, showed that pCREB was reduced in both neuronal (NeuN+) and non-neuronal (NeuN−) cells. It is unlikely that the observed changes were due to differential protein degradation in samples collected at different post-mortem intervals (PMI). Indeed, CaN and pCREB levels did not correlate with PMI (CaN: r2=0.158, p=0.852; pCREB: r2=−0.390, p=0.387).
Figure 7. Increased truncated (active) CaN and decreased pCREB in frontal cortex of DLB brains.
(A) (Top) Representative Western blot detecting CaN-A in protein extracts from frontal cortex autopsy specimens from DLB patients (D) and age-matched controls (C). Membrane was stripped and re-probed for β-actin to ensure equal loading. (Bottom) Densitometric analysis of immunoreactive bands comparing the levels of the truncated CaN-A (corrected to β-actin) in frontal cortices from DLB patients (n=7) and age-matched controls (n=5). The average density value in control cases was arbitrarily set at 100. *: p<0.05 vs. control (ANOVA).
(B) (Top) Representative Western blot detecting pCREB in protein extracts from the frontal cortex of DLB patients (D) and age-matched controls (C). Membranes were stripped and re-probed for total CREB, NeuN (neuronal marker), and β-actin (loading control). (Bottom) Densitometric analysis of immunoreactive bands comparing CREB and pCREB levels (both corrected to β-actin) in frontal cortices from DLB (n=7) and age-matched controls (n=5). The average density value in control cases was arbitrarily set at 100. *: p<0.001 vs. control (ANOVA).
DISCUSSION
Taken together, our results indicate that the extracellular application of αSyn oligomers to in vitro, ex vivo and in vivo experimental models has profound effects on neuronal signaling that result in loss of neuronal viability, altered functional markers, decreased synaptic plasticity and memory deficits. In every model system these effects were specifically attributable to oligomeric αSyn, rather than monomeric or fibrillar concoctions. A model emerges whereby extracellularly applied oligomeric αSyn increases intracellular Ca2+, which activates CaN, resulting in the net dephosphorylation of key signal transduction molecules that are required for synaptic plasticity, learning and memory. We further observe that higher levels of truncated, active CaN and lower levels of pCREB co-exist in the DLB human brain. It is therefore prudent to propose that αSyn oligomers are capable of disrupting signaling pathways that underlie synaptic function and may play a role in cognitive decline that accompanies certain synucleopathies such as DLB and possibly PD (Schulz-Schaeffer 2010).
As previously noted, the crucial roles of CaN and pCREB in LTP expression have been well established, and there is ample consensus that LTP constitutes the cellular basis of hippocampus-dependent memory formation. As such, and in light of the LTP and behavioral experiments described here, we propose that at least one functional outcome of extracellular-applied αSyn oligomers on neuronal networks is CaN-dependent deficits in synaptic plasticity and cognitive function. It is important to note that in both LTP and behavior experiments, the CaN inhibitor FK506 was applied minutes to hours after αSyn oligomers, respectively, indicating that the impairments in LTP or memory elicited by αSyn oligomers under these experimental conditions are reversible and further suggesting that these effects of αSyn oligomers are not the result of αSyn-induced cell death. Indeed, we detected no change in the level of phosphorylation of the pro-apoptotic BAD assayed in brain tissue samples from the same animals receiving ICV injection of αSyn oligomers and behavioral analysis (Supplementary Fig. 6), and CaN-mediated dephosphorylation/activation of the pro-apoptotic protein BAD has been described as a central event in neuronal apoptosis (Wang et al. 1999, Springer et al. 2000, Shou et al. 2004, Yang et al. 2004).
Our autopsy specimen results are highly suggestive that similar mechanisms linking αSyn aggregates and synaptic plasticity signaling elements may occur in brains from humans affected by clinically diagnosed DLB. Specifically, we found significantly higher levels of truncated, active CaN and lower levels of pCREB. Given the growing evidence for a central role of αSyn oligomers in the clinical manifestation and progression of synucleopathic disorders (Brown 2010, Gadad et al. 2011, Winner et al. 2011, Brundin et al. 2010), and in light of our current results suggesting CaN-dependent synaptic and memory effects of αSyn oligomers in experimental models, it is tempting to hypothesize that αSyn oligomers, through affecting levels of synaptic plasticity and memory proteins such as CaN and pCREB (Josselyn & Nguyen 2005, Mansuy 2003, Silva et al. 1998), may play a role in cognitive decline accompanying DLB progression (Schulz-Schaeffer 2010). As cognitive impairments often occur in several synucleopathies including PD (Ferrer 2011, Braak et al. 2005, Caviness et al. 2011), it is plausible that the present observations may extend beyond the specific mechanisms of DLB pathology. Indeed, we observed a dramatic decrease in CREB phosphorylation in samples from patients affected by DLB. This decrease was tightly associated with increased levels of truncated, active CaN, suggesting that a similar mechanism driven by αSyn oligomers (possibly acting extracellularly) linking increased CaN activity and decreased pCREB may also occur in diseased human brain. To that end, it is interesting that the decrease of pCREB in DLB brains was observed in both neuronal (NeuN+) and non-neuronal (NeuN−) cells, suggesting a global, rather than neuron-specific, phenomenon.
Indeed, extracellular misfolded αSyn oligomers have recently gained much attention because of their potential role in disease progression (Lee 2008, Schulz-Schaeffer 2010). Recent investigations have suggested that αSyn oligomers are released via an atypical secretory pathway. αSyn oligomers are released in the culture medium of cells overexpressing human αSyn (Emmanouilidou et al. 2010), and αSyn oligomers have been found in plasma of PD patients (El-Agnaf et al. 2006). Like all neurodegenerative diseases, PD has a typical neuroanatomical progression that affects the brainstem forward to the frontal cortex (Braak et al. 2003), suggesting prion-like intercellular transmission of misfolded αSyn species (Angot et al. 2010, Brundin et al. 2010). Indeed, neuron-to-neuron and neuron-to-astroglia transmission of misfolded αSyn species has been demonstrated in vitro and in vivo (Desplats et al. 2009, Lee et al. 2010). Furthermore, αSyn pathology deriving from the host αSyn pool has been found in fetal tissue grafted into the CNS of PD patients (Li et al. 2008, Chu & Kordower 2009), as well as in dopaminergic neurons grafted in the CNS of αSyn transgenic mice (Hansen et al. 2011). This collectively implies that aggregated αSyn species may gain access to the extracellular space where they are taken up by neighboring neurons. This mechanism may be of particular significance at the synapse, where αSyn oligomers can be released presynaptically and may impact post-synaptic elements. Indeed, abundant αSyn aggregates are found at presynaptic terminals in PD and DLB (Schulz-Schaeffer 2010) and their pathological presence is associated with loss of pre- and post-synaptic markers (Kramer & Schulz-Schaeffer 2007) and dendritic spine retraction (Revuelta et al. 2008, Zaja-Milatovic et al. 2006, Zaja-Milatovic et al. 2005). This neuro-anatomical evidence suggests that significant synapse loss occurs in the brains of PD and DLB patients and may underlie the cognitive symptoms of these diseases; it also introduces a loss-of-function component to the pathology of diseases like PD. where overall clinical manifestations have classically been ascribed to overt neuronal death in specific brain regions.
Therefore, our current results reveal a previously undocumented mechanism whereby small oligomeric αSyn species possibly present within the extracellular space may disturb synaptic plasticity, thus detrimentally affecting memory function and cognitive ability. Our results further suggest that such pathological mechanisms may be effectively targeted by pharmacological inhibition of CaN and possibly by future immunotherapies aimed at scavenging extracellular oligomeric αSyn.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NINDS grant R01NS059901 (G.T.), Alzheimer’s Association grant IIRG-90755 (G.T.), and a Mitchell Center Neurodegenerative Center Collaborative Grant (G.T). The authors wish to thank Dr. Randall Woltjer at the Department of Pathology, Oregon Health & Science University, Portland, Oregon, for generously providing us the human autopsy brain specimens and for critical feedback.
REFERENCES
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Involvement of LTP in memory: are we “searching under the street light”? Neuron. 1995;15:751–754. doi: 10.1016/0896-6273(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–69. doi: 10.1016/s0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- Brown DR. Oligomeric alpha-synuclein and its role in neuronal death. IUBMB Life. 2010;62:334–339. doi: 10.1002/iub.316. [DOI] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Henshall DC, Teramura Y, Iwasaki K, Sharkey J. Neuroprotective actions of FK506 in experimental stroke: in vivo evidence against an antiexcitotoxic mechanism. J Neurosci. 1997;17:6939–6946. doi: 10.1523/JNEUROSCI.17-18-06939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness JN, Lue L, Adler CH, Walker DG. Parkinson’s disease dementia and potential therapeutic strategies. CNS Neurosci Ther. 2011;17:32–44. doi: 10.1111/j.1755-5949.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2009;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. Faseb J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007 doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res. 2010;88:2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. Faseb J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson’s disease. Parkinsons Dis. 2011:708404. doi: 10.4061/2011/708404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel C, Neumann M, Ballard T, et al. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–1435. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudo M, Yano I, Masuda S, Okuda M, Inui K. Distinct inhibitory effects of tacrolimus and cyclosporin a on calcineurin phosphatase activity. J Pharmacol Exp Ther. 2005;312:816–825. doi: 10.1124/jpet.104.074930. [DOI] [PubMed] [Google Scholar]
- Gadad BS, Britton GB, Rao KS. Targeting Oligomers in Neurodegenerative Disorders: Lessons from alpha-Synuclein, Tau, and Amyloid-beta Peptide. J Alzheimers Dis. 2011;24:223–232. doi: 10.3233/JAD-2011-110182. [DOI] [PubMed] [Google Scholar]
- Gertz HJ, Siegers A, Kuchinke J. Stability of cell size and nucleolar size in Lewy body containing neurons of substantia nigra in Parkinson’s disease. Brain Res. 1994;637:339–341. doi: 10.1016/0006-8993(94)91257-2. [DOI] [PubMed] [Google Scholar]
- Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte M, Thuault S, Dineley KT, Hemmings HC, Jr, Nairn AC, Jay TM. Phosphorylation of CREB and DARPP-32 during late LTP at hippocampal to prefrontal cortex synapses in vivo. Synapse. 2007;61:24–28. doi: 10.1002/syn.20339. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol Learn Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Kahle PJ. alpha-Synucleinopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:87–95. doi: 10.1007/s00401-007-0302-x. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Sanchez-Alavez M, Barr AM, Criado JR, Crawley JN, Behrens MM, Henriksen SJ, Bartfai T. Impairment of memory consolidation by galanin correlates with in vivo inhibition of both LTP and CREB phosphorylation. Neurobiol Learn Mem. 2009;92:429–438. doi: 10.1016/j.nlm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Ahnn J. Calcineurin in animal behavior. Mol Cells. 2004;17:390–396. [PubMed] [Google Scholar]
- Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson’s disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- Mansuy IM. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Olanow CW. Protein aggregation in the pathogenesis of familial and sporadic Parkinson’s disease. Neurobiol Aging. 2006;27:530–545. doi: 10.1016/j.neurobiolaging.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mori H, Kondo T, Yokochi M, Matsumine H, Nakagawa-Hattori Y, Miyake T, Suda K, Mizuno Y. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51:890–892. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Perl DP, DeMartino GN, McNaught KS. Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol. 2004;3:496–503. doi: 10.1016/S1474-4422(04)00827-0. [DOI] [PubMed] [Google Scholar]
- Paleologou KE, Kragh CL, Mann DM, Salem SA, Al-Shami R, Allsop D, Hassan AH, Jensen PH, El-Agnaf OM. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Kandel E. A genetic switch for long-term memory. C R Acad Sci III. 1998;321:91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Putcha P, Danzer KM, Kranich LR, et al. Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J Pharmacol Exp Ther. 2010;332:849–857. doi: 10.1124/jpet.109.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese LC, Laezza F, Woltjer R, Taglialatela G. Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta GJ, Rosso A, Lippa CF. Neuritic pathology as a correlate of synaptic loss in dementia with lewy bodies. Am J Alzheimers Dis Other Demen. 2008;23:97–102. doi: 10.1177/1533317507310565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathologica. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis. Biochem J. 2004;379:805–813. doi: 10.1042/BJ20031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, Tsuji S, Ikuta F. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology. 1994;44:437–441. doi: 10.1212/wnl.44.3_part_1.437. [DOI] [PubMed] [Google Scholar]
- Tompkins MM, Basgall EJ, Zamrini E, Hill WD. Apoptotic-like changes in Lewy-body-associated disorders and normal aging in substantia nigral neurons. Am J Pathol. 1997;150:119–131. [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Omori K, Suzukawa J, Inagaki C. Calcineurin-mediated BAD Ser155 dephosphorylation in ammonia-induced apoptosis of cultured rat hippocampal neurons. Neurosci Lett. 2004;357:73–75. doi: 10.1016/j.neulet.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Keene CD, Montine KS, Leverenz JB, Tsuang D, Montine TJ. Selective dendritic degeneration of medium spiny neurons in dementia with Lewy bodies. Neurology. 2006;66:1591–1593. doi: 10.1212/01.wnl.0000216137.09685.c1. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.