Abstract
Aim
The multifactorial etiology of bacterial vaginosis (BV) impedes development of effective treatment and prevention strategies. Herein, we evaluated the effects of herpes simplex virus type 2 (HSV-2), a suspected BV risk factor, on vaginal flora composition.
Methods
Relationships between HSV-2 infection and BV were prospectively explored among 12 HSV-2 seropositive women with asymptomatic BV that were asked to collect daily vaginal swab specimens for Gram stain analysis of vaginal flora and determination of HSV-2 shedding frequencies during the 1 month before and after metronidazole therapy.
Results
Unlike prior longitudinal studies that reported rapid fluctuations in vaginal flora composition and frequent episodes of spontaneously resolving BV, we found that 99.4% (310/312) of vaginal smears collected before initiation of metronidazole were consistent with a diagnosis of BV. Effectiveness of metronidazole therapy was also much lower than previously reported in studies not restricting enrollment to HSV-2 seropositive women; we observed a BV recurrence rate of 89% in the first month after completion of therapy while the median time to this recurrence occurred only 14 days after treatment.
Conclusions
Our study demonstrates BV recalcitrance among HSV-2 infected women and provides additional evidence for a linkage between this chronic viral infection and abnormal vaginal flora. Additional work will be needed to define mechanisms responsible for this relationship and to determine if vaginal flora health of HSV-2 infected women is improved by medications that suppress HSV-2 shedding.
Keywords: bacterial vaginosis, herpes simplex virus type 2, metronidazole
Introduction
Bacterial vaginosis (BV) is a common genital tract condition dually characterized by reduced numbers of vaginal lactobacilli and overgrowth of facultative and anaerobic flora [1]. Although this re-proportioned microbiota composition can produce vaginal irritation and malodorous vaginal discharge, more frequently BV remains asymptomatic [2]. Reported associations with preterm delivery [3] and HIV acquisition [4, 5] make BV a healthcare issue of considerable interest, but BV pathogenesis remains poorly understood. Also poorly understood are reasons why current antimicrobial therapies are so ineffective for prevention of BV recurrence. As example, while 1 week of oral metronidazole therapy, the most frequently prescribed therapy, achieved 1-month cure rates of ~ 85% [6–9], only a third of women responding to treatment maintained normal vaginal flora composition during the following year [10]. Taken together, the above observations suggest improved delineation of the mechanisms responsible for adverse clinical outcomes will better inform BV pathogenesis, and development of improved BV treatment strategies will benefit from better understanding of the risk factors promoting reduced numbers of vaginal lactobacilli and overgrowth of pathologic vaginal flora [11].
Two recent reports newly suggested that antecedent herpes simplex virus type 2 (HSV-2) infection represents an important risk factor for overgrowth of abnormal vaginal flora or reduced BV treatment efficacy [12, 13]. Definitive evidence for causal connection between genital herpes infections and BV, however, is lacking as neither study determined HSV-2 genital tract shedding frequencies while both studies evaluated vaginal microbiota composition at infrequent 3–4 month intervals. Herein, we prospectively followed HSV-2 infected women with asymptomatic BV during the 1 month before and after completion of oral metronidazole therapy, asking each to collect daily vaginal swab specimens. These specimens were used to assess: 1) the relationship between vaginal flora composition and HSV-2 genital tract shedding; 2) BV persistence prior to antimicrobial therapy; and 3) BV recurrence rates subsequent to completion of this antimicrobial therapy.
Materials and Methods
Study design
A pre- versus post-treatment study design was used to daily evaluate vaginal microbiota composition and HSV-2 shedding frequency among HSV-2 seropositive women with asymptomatic BV in the 1 month before and after oral metronidazole therapy (study design approved by the University of Pittsburgh’s Institutional Review Board). Nonpregnant women aged 18–30 years presenting to 2 Pittsburgh, PA healthcare clinics (Magee-Womens Hospital and the Allegheny County Health Department Sexually Transmitted Disease Clinic) were screened for asymptomatic BV and HSV-2 infection. Women denying vaginal symptoms but who were diagnosed with asymptomatic BV (as defined by Amsel [14] and Nugent [15] criteria) and HSV-2 infection (as identified by point-of-care serologic tests [16]) were eligible. Exclusion criteria included pregnancy, current genital tract infection with Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis; vaginal candidiasis; intrauterine device presence; spermicidal, antiviral, or antimicrobial agent usage in the 2 weeks prior to enrollment; or inability to tolerate systemic metronidazole therapy. Informed consent was obtained prior to enrollment, and a urine pregnancy test was performed prior to specimen collection. Vaginal fluids were collected for determination of pH, amine (whiff) testing, microscopic analyses (wet preparation and Gram stain), and culture-based T. vaginalis identification. A Catch-All™ Sample Collection Swab (EPICENTRE® Biotechnologies; Madison, WI) was applied to external and internal genitalia to detect HSV-2 DNA by real-time-PCR, and a second swab placed endocervically for detection of C. trachomatis and N. gonorrhoeae by strand-displacement amplification. Women with current T. vaginalis, C. trachomatis, or N. gonorrhoeae infection were excluded from study participation, and offered treatment in accordance with Centers for Disease Control and Prevention guidelines [17].
Enrolled participants were provided instructions and supplies for daily self-collection of genital tract swab specimens for HSV-2 detection and Gram stain diagnosis of BV. Each morning before bathing, two separate Catch-All™ Sample Collection Swabs were to be rubbed over external (lower mons pubis, clitoral hood, labia minora, and labia majora) and internal (posterior vagina and paracervix) genitalia. Swabs were placed into cryovials containing 1 ml of HSV digestion buffer and refrigerator-stored until women returned to clinic (once a week during study participation). For Gram stain diagnosis of BV, women were instructed to insert a swab into the vaginal vault, turn it several times to ensure contact with vaginal walls, and roll it across a glass microscopy slide. Women were also asked to maintain daily diaries regarding genital tract symptoms. At each follow-up visit, women were assessed for the presence of Amsel criteria. At the fourth follow-up visit, women were provided fourteen 500 mg metronidazole tablets and instructed to ingest 1 tablet twice a day for 7 days. Women were asked to refrain from self-collection of specimens during antimicrobial treatment. Following completion of metronidazole therapy, women were re-assessed for resolution of BV by Amsel criteria. If BV was unresolved, study participants received additional 1 week courses of oral metronidazole therapy. Women were then asked to resume daily home collection of genital specimens, and to return specimens and diaries to clinic at weekly intervals for an additional 4 visits.
Laboratory methods
Each day’s external and internal genitalia swab specimens were pooled to determine HSV-2 shedding frequencies. DNA was extracted using IT 1-2-3 R.A.P.I.D. DNA Purification Kits (Idaho Technologies; Salt Lake City, UT), and HSV-2 detected using real-time PCR amplification of the HSV-2 DNA polymerase gene as previously described [18]. Vaginal smears were Gram stained for evaluation of vaginal flora using a standardized (Nugent) scoring system whereby normal flora received a score of 0–3 points, intermediate flora 4–6 points, and BV 7–10 points [15].
Statistical considerations
Mean, median, and range values were calculated as indicated. Kaplan-Meier survival curves assessed cumulative incidence of abnormal vaginal flora recurrence after completion of metronidazole therapy. Proportional hazards regression models were constructed using STATA® IC version 10 (College Station, TX) to examine relationships between several time-varying factors (e.g., genital tract shedding of HSV-2; sperm, yeast, erythrocyte, or leukocyte detection in vaginal Gram stain smears) and length of time until BV recurrence (p values ≤ 0.05 were considered statistically significant). All other data analyses were performed using GraphPad Prism® 5 Software (La Jolla, CA).
Results
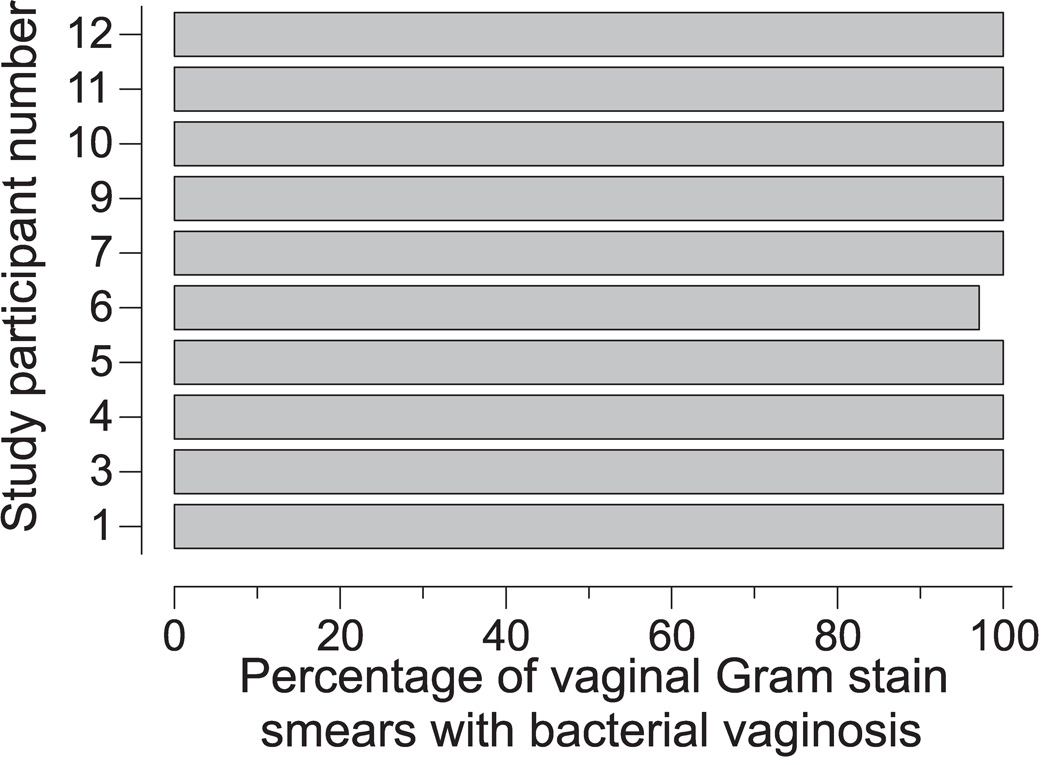
12 HSV-2 seropositive women with asymptomatic BV were enrolled. Two of these women were lost to follow-up within 2 weeks of enrollment, and excluded from analyses. A third woman lost to follow-up after initiating metronidazole therapy at study midpoint was excluded from BV incidence analyses. 8 of 10 long-term study participants described themselves as black and 2 as white. Mean age of study participants was 25.3 years (range 20–29). 5 of 10 women were sexually active with a single male partner in the month prior to enrollment and for duration of the study, while the other 5 denied sexual activity. The 5 sexually active study participants reported consistent condom usage. The average number of daily specimens collected per study participant was 63.9 (range 25–83). Among the 9 women who returned to clinic after receiving metronidazole therapy, an average number of 37.1 specimens (range 33–43) were collected per study participant after completion of this therapy. All study participants demonstrated a remarkable persistence of BV (defined by a vaginal Gram stain score ≥ 7) between the time of their enrollment and their initiation of metronidazole therapy; 99.4% (310/312) of the vaginal smears collected during this time were consistent with a diagnosis of BV (Figure 1). These results were surprising because of their marked difference from prior longitudinal evaluations in which numerous, 2–3 day long episodes of spontaneously resolving BV were observed among study participants [19, 20].
Figure 1. Pathologic flora is highly persistent among HSV-2 seropositive women diagnosed with asymptomatic BV.
Each bar represents the percentage of vaginal Gram stain smears from individual study participants prior to initiating a 7-day course of oral metronidazole at study mid-point that possessed a Nugent score consistent with BV. Average number of daily specimens collected prior to antimicrobial therapy was 33.4 (range 32–39) (n=10).
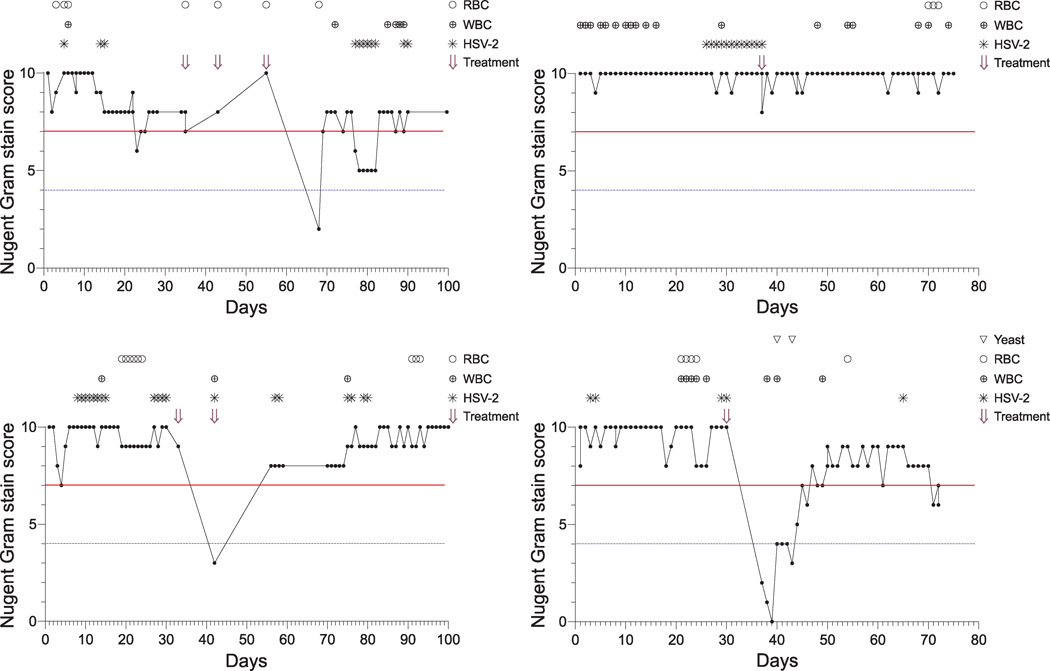
As acquisition of abnormal vaginal flora has been associated with early menstrual cycle stages, sexual activity, and antecedent vaginal candidiasis [10, 21–24], we next analyzed our data for the presence of time-varying factors associated with compositional shifts of the vaginal microbiota. Variables examined included menses (as suggested by the presence of erythrocytes on vaginal Gram stain smears), HSV-2 genital tract shedding, and vaginal sperm, leukocyte, or yeast detection on vaginal Gram stain smears (representative results displayed in Figure 2). Proportional hazards models, with inclusion of single predictor variables in each model, were unable to delineate statistically significant associations between BV recurrence and any time-varying factors examined. However, compared to an absence of vaginal leukocytes, leukocyte detection on vaginal Gram stain smear was associated with a nearly 4 times greater risk of incident BV (hazard ratio = 3.82; p = 0.11) (data not shown). While this elevated hazard ratio did not achieve statistical significance, it may be worth reporting given the small sample size present in our investigation.
Figure 2. BV is recalcitrant to systemic metronidazole therapy among HSV-2 seropositive women.
Longitudinal results from representative HSV-2 seropositive study participants diagnosed with asymptomatic BV at enrollment. Red continuous horizontal line: BV (Nugent score ≥ 7); blue discontinuous horizontal line: abnormal vaginal flora (Nugent score ≥ 4). Explanation of figure legend: (○) RBC, vaginal red blood cells; (⊕) WBC, vaginal white blood cells; (✳) HSV-2, genital tract detection of HSV-2 DNA; and (⇓) Treatment, initiation of 7 day course of oral metronidazole therapy.
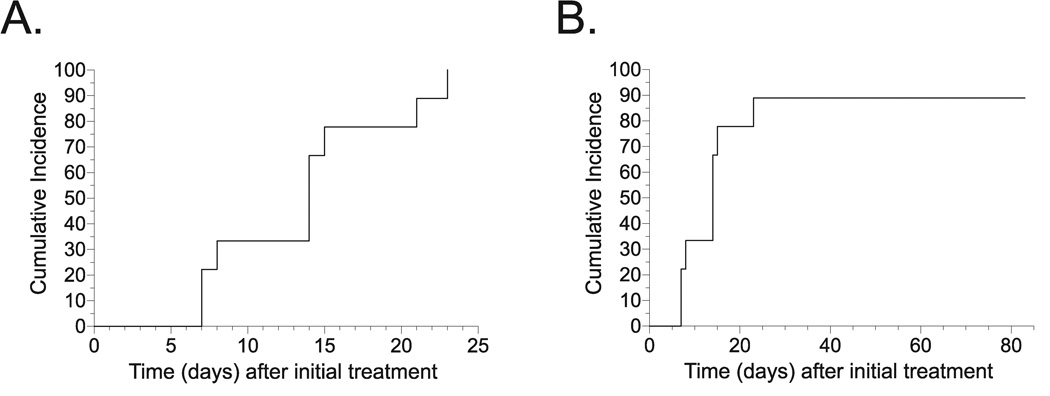
In addition to the highly durable persistence of BV seen prior to metronidazole therapy, a large majority of women in our investigation either failed to acquire or rapidly lost normal vaginal flora subsequent to completion of antimicrobial therapy (representative results displayed in Figure 2). In fact, Kaplan-Meier survival curves showed all 9 study participants with available follow-up results exhibited recurrence of abnormal vaginal flora (vaginal Gram stain score ≥ 4) within 24 days of completing metronidazole therapy (Figure 3A), while 8/9 (89%) of these women also demonstrated rapid BV recurrence (Figure 3B). Among women demonstrating recurrence, the median time to diagnosis of either abnormal vaginal flora or BV was 14 days (data not shown). Taken together, our findings argue for BV recalcitrance among HSV-2 seropositive women.
Figure 3. BV recurs rapidly among HSV-2 seropositive women.
Kaplan-Meier survival curves demonstrating: A) recurrence of abnormal vaginal flora (Nugent score ≥ 4), and B) recurrence of BV (Nugent score ≥ 7) after 7-day course of oral metronidazole (n = 9).
Discussion
The synergy between HSV-2 infection and abnormal vaginal flora has been incrementally exposed. Cross-sectional studies revealed higher prevalences of BV and HSV-2 infection were mutually associated with one another [25, 26], while longitudinal studies suggested linkage between antecedent HSV-2 infection and incident BV [12, 13]. Our investigation provides further insight into both phenomena. First, the remarkable persistence of BV in our cohort prior to metronidazole therapy corroborates prior cross-sectional data demonstrating positive reciprocal association between prevalences of BV and HSV-2 infection. In addition, nearly 90% of our study participants had recurrence of BV within a month of completing antimicrobial therapy, a finding consistent with earlier studies that reported increased susceptibility for BV acquisition among HSV-2 infected women. Although based on a small sample size, our study contains findings requisite for better acceptance of a synergism between HSV-2, a persistent viral genital tract infection, and acquisition of pathologic vaginal flora.
Additional studies will be needed to elucidate a biological basis for these resolute shifts to abnormal vaginal flora and high rates of BV recurrence among HSV-2 infected women. Hydrogen peroxide-producing vaginal lactobacilli, thought to provide protection against BV acquisition [27, 28], are less prevalent among HSV-2 seropositive women [29], while antecedent HSV-2 infection increases the chance these bacteria are lost from the vagina [13]. As biologic activity of HSV-2 during latency is limited to reactivation and mucosal shedding, we posit that connections between chronic HSV-2 infection, increased loss of hydrogen peroxide-producing lactobacilli, and increased susceptibility for BV acquisition are sequelae to HSV-2 genital tract shedding. Because this shedding is controlled by genital tract infiltration of HSV-specific CD8+ lymphocytes [30], we further hypothesize that increased vaginal mucosal inflammation elicited in response to HSV-2 shedding provides linkage between HSV-2 infection and BV recalcitrance. Our findings support these hypotheses as the detection of vaginal leukocytes was associated with greater risk of BV recurrence. It is plausible, therefore, that medications that suppress HSV-2 reactivation will decrease abnormal vaginal flora recurrences or improve efficacies of antimicrobial BV therapies among HSV-2 infected women. In addition to providing the construct for a testable BV prevention strategy, rapid recurrence of BV among HSV-2 seropositive women suggests that similar cohorts will be ideally suited for focused, cost-effective investigation of BV pathogenesis.
Unlike prior studies that reported rapid fluctuation of vaginal microbiota composition and numerous episodes of spontaneously resolving BV [20–23, 31], our cohort of HSV-2 seropositive women exhibited a remarkable persistence of BV. Similar to the study design we utilized, these prior investigations asked women to serially self-collect vaginal smears for Gram stain diagnosis of BV. However, our study appears to be the first to identify the HSV-2 serostatus of its participants. This distinction allows us to speculate that rapidly changing vaginal flora compositions and episodes of spontaneously resolving BV may be more frequently seen among investigational cohorts that contain both HSV-2 seronegative and seropositive women. In a prospective study with eligibility criteria similar to our own (inclusion criteria included a vaginal Gram stain score ≥ 7 or a Gram stain score of 4–6 and ≥ 3 Amsel criteria), a 1-month cure rate after metronidazole therapy (400 mg p.o. for 7 days) of 77% and a median time of 176 days until BV recurrence was reported [10]. Conversely, we detected a 1-month BV cure rate of 11% and a median time of 14 days after treatment until BV recurrence. It is interesting to speculate that our study’s exclusion of HSV-2 seronegative women may have, at least in part, contributed to such highly discrepant results.
Although our study suggests correlation between antecedent HSV-2 infection and vaginal microbiota, it is unable to delineate strength of this association or the mechanisms responsible for results so disparate from prior investigations. We hypothesize increased mucosal tissue inflammation in response to HSV-2 shedding adversely impacts vaginal ecosystems, but are also unable to identify direct connections between HSV-2 shedding and BV acquisition. It seems likely that our inability to form such conclusions is limited by the small number women we enrolled. Enrollment into future investigations will benefit from inclusion of HSV-2 seropositive women with symptomatic or asymptomatic BV as well as a BV positive/HSV-2 seronegative control group. Our conclusions may be further limited by the fact we asked study participants to collect daily vaginal specimens while other BV treatment trials collected specimens at more infrequent (1-month or 3-month) intervals. Thus increased sampling frequency, rather than HSV-2 infection, may be responsible for higher rates of BV recurrence. Our conclusions may also be limited by selection bias and resultant identification of spurious relationships between HSV-2 and BV - the possibility we preferentially enrolled women with factors other than HSV-2 infection that promoted BV recalcitrance. Despite these limitations we provide important new epidemiologic data in support of the synergistic relationships emerging between genital herpes and abnormal vaginal microbiota. Additional work is needed to define mechanisms responsible for this relationship and to determine if vaginal flora health of HSV-2 infected women is improved by medications suppressing genital tract shedding of the virus.
Acknowledgments
Authors wish to express their appreciation to the women who participated in our study. Authors also acknowledge Ingrid Macio, Jamie Haggerty, Lorna Rabe, and Michele Austin for help with study execution; W. Allen Hogge, James Roberts, Yoel Sadovsky, and David Perlmutter for departmental support; Clare Brennan and Toni Darville for manuscript review, and Robert Hendricks, Sharon Hillier, Gerald Nau, and Anna Wald for mentorship.
This work was supported by the National Institutes of Health grants K23AI064396 and U19A1084024.
Footnotes
Authors declare there are no sources of financial support or relationships that may pose a conflict of interest and confirm results of this manuscript are not distorted by research funding or any conflicts of interest.
References
- 1.Fredricks DN, Fiedler TN, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 2.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 3.Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, et al. Risk factors for prematurity and premature rupture of membranes: A prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150:965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 4.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell AL, Fox AR, Phillips I, Barlow D. Anaerobic vaginitis (non-specific vaginitis): clinical, microbiological, and therapeutic findings. Lancet. 1983;2:1379–1382. doi: 10.1016/s0140-6736(83)90920-0. [DOI] [PubMed] [Google Scholar]
- 7.Hagström B, Lindstedt J. Comparison of two different regimens of metronidazole in the treatment of non-specific vaginitis. Scand J Infect Suppl. 1983;40:95–96. [PubMed] [Google Scholar]
- 8.Andres FJ, Parker R, Hosein I, Benrubi GI. Clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis: a prospective double-blind clinical trial. South Med J. 1992;85:1077–1080. doi: 10.1097/00007611-199211000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Fischbach F, Petersen EE, Weissenbacher ER, Martius J, Hosman, Mayer H. Efficacy of clindamycin versus oral metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol. 1993;82:405–410. [PubMed] [Google Scholar]
- 10.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 11.Koumans EH, Markowitz LE. Hogan V for the CDC BV Working Group. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: a synthesis of the data. Clin Infect Dis. 2002;35 Suppl 2:S152–S172. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 12.Nagot N, Ouedraogo A, Defer M, Vallo R, Mayaud P, Van de Perre P. Association between bacterial vaginosis and herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;83:365–368. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 14.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiological associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 15.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;103:1105–1108. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyland B, Kennedy MR, Wimberly YH, Levine BJ, Cherpes TL. Serologic detection of herpes simplex virus type 2 antibodies among pregnant women using a point-of-care test from Focus Diagnostics. J Clin Virol. 2009;44:125–128. doi: 10.1016/j.jcv.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2002 Guidelines for treatment of sexually transmitted diseases. MMWR Morb Wkly Report. 2002;51:1–132. [Google Scholar]
- 18.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Inf Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 19.Morison L, Ekpo G, West B, Demba E, Mayaud P, Coleman R, et al. Bacterial vaginosis in relation to menstrual cycle, menstrual protection method, and sexual intercourse in rural Gambian women. Sex Transm Infect. 2005;81:242–247. doi: 10.1136/sti.2004.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwebke JR, Morgan SC, Weiss HL. The use of sequential self-obtained vaginal smears for detecting changes in the vaginal flora. Sex Transm Dis. 1997;24:236–239. doi: 10.1097/00007435-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Keane FEA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS. 1997;8:489–494. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 23.Hay PE, Ugwumadu A, Chowns J. Sex, thrush and bacterial vaginosis. Int J STD AIDS. 1997;8:603–608. doi: 10.1258/0956462971918850. [DOI] [PubMed] [Google Scholar]
- 24.Schwebke JR, Richey CM, Weiss HL. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis. 1999;180:1632–1636. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- 25.Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–410. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kaul R, Nagelkerke NJ, Kimani J, Ngugi E, Bwayo JJ, Macdonald KS, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Inf Dis. 2007;196:1692–1697. doi: 10.1086/522006. [DOI] [PubMed] [Google Scholar]
- 27.Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wölner-Hanssen P, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 28.Mijac VD, Dukić SV, Opavski NZ, Dukić MK, Ranin LT. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur J Obstet Gynecol Reprod Biol. 2006;129:69–76. doi: 10.1016/j.ejogrb.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Baeten JM, Hassan WM, Chohan V, Richardson BA, Mandaliya K, Ndinya-Achola JO, et al. Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women. Sex Transm Dis. 2009;85:348–353. doi: 10.1136/sti.2008.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiffer JT, Abu-Radded L, Mark KE, Zhu J, Selke S, Koelle DM, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. PNAS. 2010;107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priestly CJF, Jones BM, Dhar J, Goodwin L. What is normal vaginal flora? Genitourin Med. 1997;73:23–28. doi: 10.1136/sti.73.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]