Abstract
Objective
To determine the order of bacterial species succession in re-developing supra and subgingival biofilms.
Methods
Supra and subgingival plaque samples were taken separately from 28 teeth in 38 healthy and 17 periodontitis subjects immediately after professional cleaning. Samples were taken again from 7 teeth in randomly selected quadrants after 1, 2, 4 and 7 days of no oral hygiene and analyzed using checkerboard DNA-DNA hybridization. % DNA probe counts were averaged within subjects at each time point. Ecological succession was determined using a modified moving window analysis.
Results
Succession in supragingival biofilms from periodontitis and health was similar. At 1 day, Streptococcus mitis and Neisseria mucosa showed increased proportions, followed by Capnocytophaga gingivalis, Eikenella corrodens, Veillonella parvula and Streptococcus oralis at 1–4 days. At 4–7 days, Campylobacter rectus, Campylobacter showae, Prevotella melaninogenica and Prevotella nigrescens became elevated. Subgingival plaque redevelopment was slower and very different from supragingival. Increased proportions were first observed for S. mitis, followed by V. parvula and C. gingivalis and, at 7 days by Capnocytophaga sputigena and P. nigrescens. No significant increase in proportions of periodontal pathogens was observed in any of the clinical groups or locations.
Conclusions
There is a defined order in bacterial species succession in early supra and subgingival biofilm re-development after professional cleaning.
Keywords: ecology, succession, oral bacteria, periodontal, periodontitis, biofilms, supragingival, subgingival
INTRODUCTION
When dental plaque is removed by self-performed or professional procedures, there is an immediate and often visible reduction in the total number of organisms, followed, within hours, by a return of detectable plaque. Indeed, the re-establishment of dental biofilms in many people is so rapid that it is generally recommended that individuals brush at least twice daily. When the rate of dental biofilm return after professional plaque removal was measured by indices or quantitative assessment, it was estimated that biofilms returned to their pre-cleaning levels in 1 to 2 days1–5. What was not clear from these estimates was whether all bacterial species present in dental biofilms returned at similar rates in periodontally healthy and periodontitis subjects. Further, it was not clear whether there were differences in patterns of bacterial re-colonization between supra and subgingival biofilms. A few studies have examined the shifts in microbial species that occur during in vivo supra- or subgingival plaque development. Ritz6 used selective media to demonstrate that streptococci and “Neisseria” were prominent at 1 day and that Actinomyces species were initially low in proportion but rose by 9 days. Nyvad & Kilian (1987)7 used cultural techniques to follow the early colonization of pieces of enamel and root surfaces mounted in acrylic appliances in human volunteers. They found that streptococci and gram positive pleiomorphic rods dominated in the first 24 hrs. Streptococcus mitis and Streptococcus oralis were major contributors to the biofilm content comprising from 24% to 42% and 1% to 27% of the microbiota respectively. Nyvad & Kilian (1990)8 compared the streptococcal composition of 4 hr biofilms that formed on pieces of enamel mounted on acrylic appliances in the mouths of adolescents. It was found that that the predominant streptococci were S. oralis and S. mitis and that S. sanguinis was in higher proportion in the mouths of caries inactive than in caries active individuals. Socransky et al3. used predominant cultivable microbiota techniques to show that supragingival counts increased at 1 day and leveled off from 2 – 16 days. S. sanguinis was detected at all time points and at increased proportion at 1 day. Actinomyces species were in low proportions at 1 day but increased by 16 days. Using the same techniques, Zee et al4 examined pooled plaque samples from “rapid” and “slow” plaque-forming subjects. Streptococcus species were dominant at day 1 but by day 14 their mean proportions had declined and proportions of Actinomyces species had increased. Studies of very early biofilm development using molecular techniques demonstrated that S. mitis and S. oralis were in high numbers in supragingival biofilm 6 hours after tooth polishing1. Ramberg et al5 used similar techniques to study early biofilm development in mouths with minimal gingivitis. After an intense 2 week preparatory phase involving repeated professional cleaning, the dominant taxa in 0 time supragingival plaque samples were Actinomyces species. During 4 days of no oral hygiene, Streptococcus, Capnocytophaga, Campylobacter and Fusobacterium species as well as Aggregatibacter actinomycetemcomitans increased in levels.
Quirynen et al9 used molecular and cultural techniques to examine the subgingival colonization of ‘pristine’ periodontal pockets by following the development of subgingival biofilms on recently inserted implants. Species were detected in similar frequencies from the one-week microbiota around implants and the undisturbed subgingival plaque of shallow tooth sites but counts of red and orange complex species were higher for tooth sites. Longer-term studies by the same group demonstrated little change in the already established complex microbiota between 2 and 26 weeks except for an increase in red and orange complex species10.
The above investigations provided a starting point for understanding changes in species composition during in vivo biofilm development. However, there was a need for larger scale supragingival biofilm studies and studies that would involve subgingival plaque biofilm samples from periodontally healthy and periodontitis sites. In an earlier publication11, we described microbial shifts in re-developing supra and subgingival dental biofilms over a 7 day period in the absence of oral hygiene in periodontally healthy and periodontitis subjects. It was shown that supragingival biofilm re-development was similar in both groups. Mean total DNA probe counts (i.e., mean number of bacterial cells) reached pre-cleaning levels by 2 days with Veillonella parvula and Neisseria mucosa showing the greatest increase. Re-development of subgingival biofilm was somewhat different. Significant differences between clinical groups occurred in subgingival biofilm samples by 7 days for 17 species, including Actinomyces and Fusobacterium species as well as Aggregatibacter actinomycetemcomitans, Prevotella intermedia and Porphyromonas gingivalis, all of which were higher in the periodontitis group. Species counts were useful for determining increases in total biomass and indicating the species that achieved markedly elevated numbers. However, changes in species proportions are more sensitive for the detection of abrupt shifts in taxa than counts; particularly for species that are present in low numbers in biofilm samples. The observation of such abrupt changes during a specific time period allows the identification of an orderly and predictable pattern of change in a community over time, known as ecological succession12. In the present study, changes in proportions of species were used to seek evidence of bacterial succession and identify increases followed by decreases in proportions, highlighting so-called “microbial blooms”. Thus, the purpose of the present investigation was to examine the order of species succession during supra and subgingival biofilm re-development after professional dental cleaning in periodontally healthy and diseased subjects.
MATERIAL AND METHODS
Details regarding the subject population, clinical monitoring, dental cleaning, microbial sample-taking and enumeration have been described by Uzel et al11 and are briefly presented here.
Subject population
Thirty-eight periodontally healthy and 17 chronic periodontitis subjects were enrolled, according to the following criteria:
Inclusion criteria for healthy subjects: > 20 years of age, > 24 teeth, no pocket depth or attachment level measurements > 3 mm, < 20% of sites with overt gingival redness and/or bleeding on probing and willingness and ability to sign informed consent.
Inclusion criteria for periodontitis subjects: > 20 years of age, > 20 teeth, > 8 teeth with pocket depth and/or attachment level > 4 mm and willingness and ability to sign informed consent.
Exclusion criteria: Pregnancy or nursing, periodontal or antibiotic therapy in the previous 3 months, systemic conditions which might influence the course of periodontal disease or treatment (e.g. diabetes, AIDS), systemic conditions which required antibiotic coverage for routine periodontal procedures (e.g. heart conditions, joint replacements), soft tissue lesions (e.g. leukoplakia, lichen planus) and smoking.
Attempts were made to recruit approximately equal numbers of males and females. In addition, subjects of any racial/ethnic group were accepted for the study. All subjects were recruited at The Forsyth Institute. The study was approved by The Forsyth Institute Institutional Review Board and all subjects signed informed consent prior to entering the study. The baseline clinical characteristics of the subjects in the 2 groups are shown in Table 1.
Table 1.
Mean clinical parameters (± SD) of subject groups at baseline.
| Health | Periodontitis | p (Mann Whitney) | |
|---|---|---|---|
| N subjects | 38 | 17 | |
| Age | 32.3 ± 9.3 | 44.9 ± 11.9 | p<0.001 |
| Number of missing teeth | 0.9 ± 1.6 | 2.2 ± 2.4 | p<0.05 |
| % Males | 39 | 35 | NS |
| % Past smokers | 29 | 29 | NS |
| Pocket Depth (PD) (mm) | 1.9 ± 0.3 | 2.7 ± 0.3 | p<0.001 |
| Attachment Level (AL) (mm) | 1.5 ± 0.6 | 3.0 ± 1.2 | p<0.001 |
| Plaque Index | 1.2 ± 0.7 | 1.6 ± 0.3 | NS |
| % of sites with: | |||
| Redness | 47.7 ± 30.7 | 62.3 ± 34.8 | p<0.001 |
| BOP | 7.3 ± 6.9 | 27.2 ± 12.8 | p<0.001 |
| Suppuration | 0.0 ± 0.0 | 0.2 ± 0.4 | p<0.01 |
| PD > 6 mm | 0.0 ± 0.0 | 0.8 ± 1.0 | p<0.001 |
| PD 4–6 mm | 0.9 ± 2.2 | 15.3 ± 7.2 | p<0.001 |
| PD < 4 mm | 99.1 ± 2.2 | 83.8 ± 7.3 | p<0.001 |
| AL > 4 mm | 0.0 ± 0.0 | 5.3 ± 10.7 | p<0.001 |
| AL 4–6 mm | 1.8 ± 6.6 | 21.6 ± 17.2 | p<0.001 |
| AL < 4 mm | 98.2 ± 6.6 | 73.1 ± 26.1 | p<0.001 |
Clinical monitoring and treatment protocols
All subjects were clinically monitored at entry. Clinical measurements were taken at 6 sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) on all teeth excluding third molars (a maximum of 168 sites per subject) as previously described13. The clinical parameters were measured in the following order: 1) Gingival Index14; 2) Plaque Index15; 3) Pocket Depth (mm); 4) Attachment level (mm); 5) Bleeding on probing (0 or 1); 6) Suppuration (0 or 1).
Pocket depth and attachment level measurements were made to the nearest mm using a North Carolina periodontal probe. They were performed twice and the average of the pair of measurements was used for analysis. The first set of supra and subgingival plaque samples were taken prior to the clinical measurements. Samples were taken by the same calibrated examiner at all sampling visits for a given subject.
Scaling and root planning or dental prophylaxis
At the entry visit, after the initial monitoring and sampling, all periodontitis subjects received full mouth scaling and root planning (SRP) at a single visit, using manual curettes and ultrasonic devices, followed by polishing and flossing. Periodontally healthy subjects received a dental prophylaxis using a rubber cup and paste, followed by dental flossing. After the initial prophylaxis or SRP, subjects refrained from oral hygiene procedures for 7 days.
Microbiological sample taking and enumeration of organisms
Individual supra and subgingival plaque samples were taken separately from the mesio-buccal aspect of up to 28 teeth in each subject at entry and immediately after tooth cleaning. Thus, up to 28 samples per subject were taken at 2 visits (baseline and immediately after professional cleaning) from 2 locations (supragingival and subgingival) for a total of up to 6160 samples (55 subjects × 28 teeth × 2 visits × 2 locations). Each quadrant in each subject was randomly assigned to be sampled at the 1, 2, 4 and 7 day time points (i.e., up to 7 teeth from the same quadrant were sampled at a given time point). Seven supra and separately 7 subgingival samples were taken at those time points providing up to an additional 3080 samples (55 subjects × 7 teeth × 4 visits × 2 locations).
Supragingival plaque samples were taken separately from each tooth using individual sterile Gracey curettes. After removal of any remaining supragingival plaque, subgingival plaque samples were taken separately from each tooth and evaluated as described above. Each sample was placed in individual tubes containing 0.15 ml TE (10 mM Tris-HCL, 0.1 mM EDTA, pH 7.6). 0.15 ml of freshly-prepared 0.5 M NaOH was added. The samples were boiled for 5 min and neutralized using 0.8 ml 5 M ammonium acetate and placed into the extended slots of a Minislot (Immunetics, Cambridge MA) and then concentrated onto a positively charged nylon membrane (Roche, Indianapolis, IN) by vacuum and fixed to the membrane by exposure to ultraviolet light followed by baking at 120°C for 20 min. The counts of the 41 species in each sample were determined using checkerboard DNA-DNA hybridization16, 17.
Data Evaluation
The percent of the total DNA probe count comprised by each of the 41 test species in the individual supragingival and subgingival biofilm samples was computed. The percent for each species was averaged within each subject at each time point and then averaged across subjects at that time point in the 2 clinical groups separately. Up to 28 supra and 28 subgingival samples were averaged per subject immediately after tooth-cleaning (day 0), and 7 samples at days 1, 2, 4 and 7.
Major significant increases or decreases over time in the proportions of species in each location (supragingival or subgingival) in each clinical group (periodontally healthy or periodontitis) were sought using a modification of a “moving window” approach18. The “moving-window” approach is commonly used in “macro-ecology” to identify changes in communities, patterns of community assembly and factors associated with the development of community structure in forests, lakes and soil19–21. It has also been used for the study of environmental microbial ecology22, 23. It allows the analysis of multivariate data across a gradient, which, in the present study was time (7 days) and is particularly useful for the detection of sharp transitions in species composition in one ecosystem24.
The mean proportions of each species at each time point in a location/clinical group were compared from the first time point with mean proportions of the same species in samples from the same subjects at each of the later time points and a t statistic >1.96 between tested time points was considered to be different. Thus, the t statistic was used as a “measuring stick” to discriminate meaningful differences in mean proportions of a species at 2 time points. These differences were considered to be “significant”.
The technique of visual inspection of the data18 revealed that in some instances (see for example, Capnocytophaga gingivalis in periodontally healthy subjects in Fig. 1), increases occurred not in the samples immediately after cleaning (day 0), but at later visits such as from 2 days to 4 and 7 days in the cited example. Thus, the moving window sought differences not just from day 0 to days 1, 2, 4, and 7; but also from day 1 to days 2, 4, and 7, from day 2 to days 4 and 7, and from day 4 to day 7.
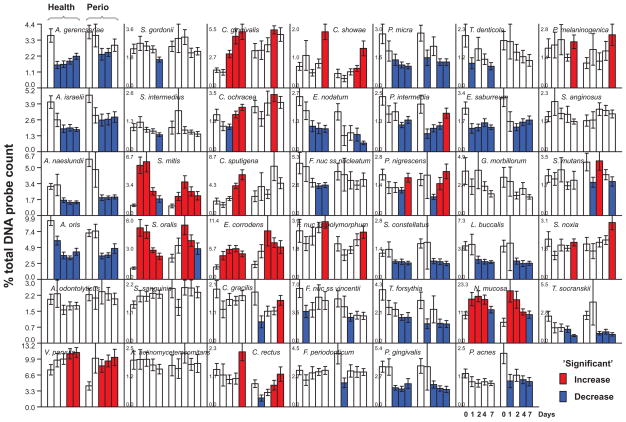
Fig. 1.
Bar charts of the mean % of the total DNA probe count of 41 bacterial species in samples of supragingival biofilm taken immediately after dental cleaning (day 0) and after 1, 2, 4 and 7 days of biofilm accumulation in the absence of oral hygiene procedures from periodontally healthy subjects (left set of bars in each panel) and subjects with periodontitis (right set of bars in each panel). The % of the total DNA probe count was computed for each species in each sample, averaged within the subject at that time point, and then averaged across subjects for the individual time points separately for subjects who did or did not have periodontitis. The bars indicate the mean values and the whiskers indicate the SEM. Note that y axis values differ for each species and are indicated in each panel. The red bars indicate a significant increase in the mean proportion from a mean value for an earlier time point for that species in that clinical group (see data analysis). In a similar fashion, the blue bars indicate a significant reduction in mean proportions of species from an earlier time point. Species are ordered according to subgingival microbial complexes (Socransky et al 1998).
RESULTS
Bacterial succession in supragingival dental biofilms in periodontally healthy individuals
Fig. 1 presents mean proportions of 41 bacterial taxa in supragingival biofilms obtained from periodontally healthy subjects (as well as periodontitis patients) immediately after prophylaxis (day 0) and after 1, 2, 4 and 7 days of biofilm accumulation in the absence of self-performed oral hygiene. The first species to significantly increase in mean proportions, at 1 day, were Streptococcus mitis, Streptococcus oralis, Eikenella corrodens and N. mucosa. S. mitis and S. oralis maintained their high proportions at day 2 but began to decrease in proportions thereafter. E. corrodens increased from immediate post-cleaning (day 0) mean values to significantly higher values at 1, 2, 4 and 7 days, while N. mucosa increased at 1, 2 and 4 days and declined slowly thereafter. C. gingivalis began to increase significantly at 2 days and continued to increase in mean proportions at 4 and 7 days. V. parvula, Capnocytophaga ochracea and Capnocytophaga sputigena increased significantly in mean proportions at 4 days. At 7 days, significant increases were observed for Campylobacter rectus and Campylobacter showae. Major significant decreases in mean proportion were observed for Actinomyces species, Fusobacterium nucleatum subspecies, P. intermedia, P. gingivalis, Treponema denticola, Eubacterium nodatum, Parvimonas micra and Streptococcus constellatus after cleaning.
Bacterial succession in supragingival dental biofilms in subjects with periodontitis
At day 1, only S. mitis and N. mucosa increased significantly in mean proportions in the supragingival samples from subjects with periodontitis (Fig. 1). By day 2, significant mean increases were observed for S. oralis, E. corrodens and V. parvula. C. gingivalis, C. ochracea, C. rectus, Prevotella nigrescens and C. showae increased in mean proportions by 4 days and Fusobacterium nucleatum ss polymorphum, P. intermedia, C. gracilis, Prevotella melaninogenica and Selenomonas noxia by 7 days. Decline in mean proportions in supragingival biofilm samples obtained from periodontitis subjects was observed for Actinomyces species, P. gingivalis, T. forsythia, E. nodatum, Fusobacterium nucleatum ss vincentii, S. constellatus and Treponema socranskii.
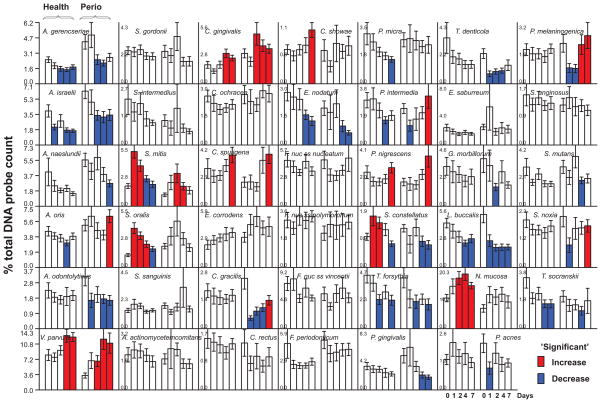
Bacterial succession in subgingival dental biofilms in periodontally healthy individuals
At 1 day, the only species that increased significantly in mean proportions in the subgingival biofilms of periodontally healthy subjects were S. mitis, S. oralis and S. constellatus (Fig. 2). At 2 days, N. mucosa; at 4 days, V. parvula and C. gingivalis, and at 7 days C. sputigena, C. showae and P. nigrescens also showed significant increases in mean proportions in the periodontally healthy subject group. Actinomyces israelii, Actinomyces gerencseriae, T. forsythia, E. nodatum, P. intermedia, L. buccalis, P. micra, and T. socranskii decreased in mean proportions during biofilm re-development in this group.
Fig. 2.
Bar charts of the mean % of the total DNA probe count of 41 bacterial species in samples of subgingival biofilm taken immediately after dental cleaning and after 1, 2, 4 and 7 days of biofilm accumulation in the absence of oral hygiene procedures. The subject population, computation of mean species proportions and determination of significant increases or decreases in proportions were performed as described for Fig. 1.
Bacterial succession in subgingival dental biofilms in subjects with periodontitis
The earliest significant increase in mean proportions occurred at day 2 for V. parvula, S. mitis and C. gingivalis in subgingival biofilm samples from the subjects with periodontitis (Fig. 2). From days 4 to 7, mean proportions of Actinomyces oris, C. sputigena, C. gracilis, S. noxia, P. intermedia, P. nigrescens and P. melaninogenica also increased significantly. Notable decreases in mean proportions occurred for Actinomyces species, S. constellatus, E. nodatum, T. forsythia, P. gingivalis and T. denticola.
Comparison of bacterial succession in periodontal health and disease
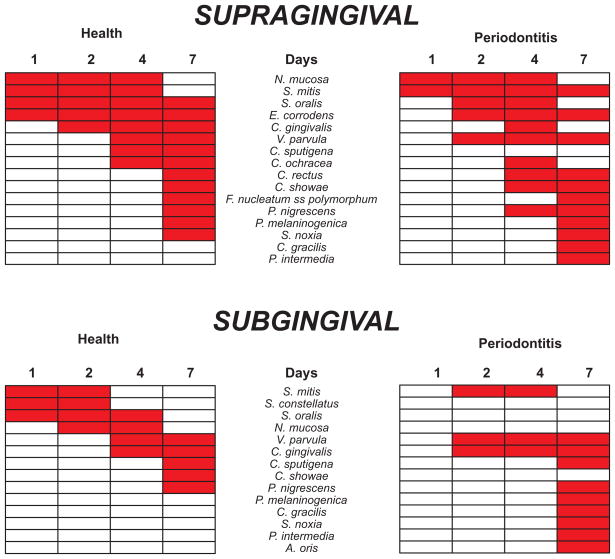
Fig. 3 summarizes the significant increases in mean proportions that took place in supragingival and subgingival biofilm samples from subjects who were periodontally healthy or had periodontitis. In supragingival samples from both periodontal health and periodontitis, early increases in mean proportions at 1 and 2 days were seen for S. mitis, S. oralis, E. corrodens and N. mucosa. V. parvula increased significantly in proportion at 2 days in periodontitis subjects and at 4 days in healthy individuals. C. gingivalis increased significantly at 2 or 4 days in both clinical groups. At 4 –7 days, increases were observed for both clinical groups in proportions of C. rectus, C. showae, C. ochracea and P. nigrescens. Species that increased significantly in periodontitis subjects but not in health included C. gracilis and P. intermedia. C. sputigena increased in health but not in periodontitis.
Fig. 3.
Taxa that showed “significant” increases in mean proportions during the 7 days of biofilm re-development in the absence of oral hygiene procedures. The top pair of panels indicate supragingival samples, the bottom pair of panels indicate subgingival samples. Panels to the left represent samples from periodontally healthy subjects, while the panels to the right present data from subjects with periodontitis. The red rectangles indicate time points at which the mean proportions were significantly higher than the mean proportions in samples from an earlier time point (see Materials and Methods). The taxa have been ordered according to the earliest “significant” increases in periodontally healthy subjects.
In subgingival samples, increases in specific species proportions started at 1 day in samples from health and 2 days in the samples from periodontitis. At 1 to 2 days only S. mitis increased significantly in proportions in subgingival biofilm samples in both health and disease. V. parvula and C. gingivalis increased in proportions from days 2 to 4 while C. sputigena and P. nigrescens increased in mean proportions in both clinical groups at 7 days. In subgingival samples from periodontal health, S. oralis and S. constellatus increased significantly at day 1, N. mucosa at 2 days and C. showae at 7 days. In subgingival samples from periodontitis subjects, P. melaninogenica increased significantly at 4 days, C. gracilis, A. oris, P. intermedia and S. noxia at 7 days.
DISCUSSION
The purpose of the present investigation was to define the early ecological succession of bacterial species during 7 days of no oral hygiene after professional removal of supra and subgingival plaque from periodontally healthy and periodontitis subjects.
Information about ecological succession in periodontal biofilms is limited to a few studies of the development of supragingival plaque in healthy individuals 1–5, 7, 8, 25 and subgingival biofilm formation around implants9, 10. Hence, there is a gap in knowledge of microbial succession in supragingival and subgingival plaque, in both periodontal health and disease. This information is important because dental biofilms have a direct impact on periodontal stability, disease initiation and progression26–30. The identification of critical periods for pathogen colonization and proliferation would be helpful in the prevention and management of periodontal diseases.
Studies to date using in vitro and in situ models have provided guidance in understanding possible growth requirements, spatial organization as well as synergistic and antagonistic relationships among different species and perhaps can provide clues to possible ecological succession31–37. However, only in vivo studies can demonstrate actual ecological succession and provide the opportunity to test conceptual models or assumptions.
In the present manuscript, we demonstrated that supragingival plaque re-development was similar in periodontal health and disease, but the re-development of subgingival plaque was quite different in the two clinical groups. In supragingival plaque, specific taxa increased or decreased at similar time points in both groups. Proportions of S. mitis and N. mucosa were significantly elevated at day 1, corroborating their proposed role as early colonizers1–4, 6. This role might be due to their ability to attach to hydroxyapatite or the salivary glycoproteins that cover hard surfaces, an ability to proliferate in the presence of oxygen and to metabolize dietary or salivary sources of carbohydrate. Proportions of C. gingivalis and E. corrodens increased significantly in both groups on days 2–4. This time frame coincides with the period when plaque biomass typically surpasses pre-cleaning levels in studies of plaque development in the absence of oral hygiene4, 5, 11. Interestingly, those species have been shown to significantly contribute to the increase of biomass in supragingival biofilms38.
At 4 days, V. parvula showed increases in mean proportions and increased from 5% of the post-cleaning plaque to almost 10% by day 7. This increase was observed days after the increase in S. mitis and S. oralis, supporting a likely metabolic food chain in dental biofilms39. We also observed that a more complex bacterial community ensued in both clinical groups following the increase in proportion of V. parvula. This finding is in accord with a recent publication that regarded Veillonella as a critical genus that guides the development of multispecies communities when saliva is the main nutritional source40. Conceivably, Streptococcus species set the stage for the growth of Veillonella, which, in turn, set the stage for other taxa.
On days 4 and 7, significant increases in both groups occurred mostly among orange complex species, including C. showae, C. rectus P. nigrescens and F. nucleatum ss polymorphum. Additional orange complex taxa increased in proportions in the periodontitis group, including P. intermedia and C. gracilis. Orange complex species have been associated with gingivitis and periodontitis41, 42. In addition, local inflammation has been shown to influence the composition of the supragingival microbiota43, 44. Hence, it is possible that inflammatory responses elicited by dental cleaning procedures, incomplete healing after mechanical therapy or early plaque re-development might have favored their growth and, thus, might have influenced microbial succession. Pathogenic species such as E. nodatum, T. forsythia, P. gingivalis and T. denticola decreased in both clinical groups. This finding is in accord with other studies1, 5 and suggests that the habitats that were once conducive to the growth of these fastidious strict anaerobes were disrupted by cleaning procedures and these habitats might take much longer than 7 days to re-establish.
Overall, the subgingival environment exhibited fewer significant changes in proportions of taxa (Fig. 2), suggesting that this ecosystem may take longer than supragingival biofilms to redevelop. This may be due in part to the physical confinement of this location, which is surrounded by hard and soft surfaces and thus has limited access to certain dietary nutrients. This seclusion might also have shielded the site from potential colonizing bacterial cells that can be disseminated after supra and subgingival debridement45. This suggests that the likely source of re-colonizing species in subgingival biofilm is the bacterial cells left behind after cleaning. Finally, professional cleaning might have altered the surfaces for attachment, the reservoir sources for re-colonization and the tissue source of nutrients, all factors that can affect biofilm development44, 46–49.
Significant changes in subgingival biofilm development began somewhat later in periodontitis patients in comparison with periodontally healthy subjects, although, by day 7 more significant increases were observed in the former. At that time point, three orange complex species, C. gracilis, P. intermedia and P. nigrescens were significantly elevated in the periodontitis group. That might suggest a possible shift towards a pathogenic microbiota, even though no “classic” periodontal pathogens showed significant increase in proportions in either group. In fact, E. nodatum, P. gingivalis, T. forsythia and T. denticola decreased in proportions in periodontitis subjects. These findings are in accord with those of Quirynen et al9. Although biofilm development may be somewhat different around implants, it is worth reporting that the authors found that subgingival colonization of shallow and moderate pockets around implants were more similar to the undisturbed microbiota present in shallow pockets around teeth than moderate pockets associated with teeth. These observations describe the level of taxa one week after abutment connection and remained virtually unchanged until 4 weeks. Among all shallow and moderate sites, the implant-associated pockets sites had the lowest levels of orange complex species among all shallow and moderate sites and also extremely low levels of “classic” periodontal pathogens. The authors suggested that these complexes might take longer to establish, in part because they might require the presence of appropriate conditions, provided by earlier colonizers. In a follow-up paper, the observation period was extended to 26 weeks10. After week 2, a clear increase in levels of all taxa was observed. Increases in levels of orange and red complex species began by week 4, were clear at week 13 and continued to week 26.
While biomass was restored within days after careful dental cleaning in the subjects in this study11, the climax community typical of the supra and subgingival tooth surfaces was not fully re-established50. Actinomyces species were in lowered proportions in supragingival biofilms at 7 days, but data in the literature suggest that their return would be more robust by 14 to 21 days51. The lowered proportions of the red complex species, T. forsythia, P. gingivalis and T. denticola, might take even longer (months to years) to return to their pre-instrumentation levels28, 52. Thus, time is a critical factor affecting biofilm formation. Clearly, internal “remodeling” of tooth-associated biofilms takes place over time, enhancing the prominence of some species and decline of others. The time constraints of this study could not follow these changes further.
The present paper focused on bacterial succession; i.e., it used the proportions of 41 bacterial species in samples to define the sequence of species “blooms” that occurred as biofilms redeveloped following a “catastrophic” event (tooth-cleaning). One limitation of the study was that the oral cavity harbors many more species than the 41 taxa examined53–56. However, the species selected for study represent about 60% of dental biofilm isolates recovered by culture57. Further, 8 of 11 of the most common taxa detected by clonal analysis of biofilm samples by Dewhirst et al55 were among the 41 taxa examined in the present study. In addition, prominent taxa in this study, V. parvula and S. mitis, accounted for the largest number of clones described in the Dewhirst et al survey.
The design of the study precluded following changes in biofilm re-development over time on individual tooth surfaces. While this would have been an ideal goal, it would have necessitated one of two approaches. The first would have been to clean each surface, then take a sample immediately post-cleaning, another sample from that surface at 1 day, then again at 2 days, 4 days and 7 days. Unfortunately, when you take, for example, the 1 day sample, you have inadvertently altered the validity of the upcoming 2 day sample by removing a major portion of the developing biofilm. For this reason, this design was rejected. The alternative approach would be to start again from a re-cleaned tooth surface, each time creating a new time 0. The second design was precluded for two reasons. First, the repeated subgingival re-cleaning of each tooth surface (4 times for each surface) might have affected the adjacent periodontal tissues and altered the typical pattern of re-colonization. Further, the subjects would have had to refrain from home care for 2 weeks (0 to 1, 0 to 2, 0 to 4, 0 to 7 days). Two weeks without oral hygiene was not acceptable to the IRB, of concern to the clinical staff, particularly for the subjects with periodontitis and would have severely compromised subject recruitment. Thus, the second design was also rejected and a compromise design involving sampling randomly assigned quadrants only once was utilized. This design lost the ability to follow the microbial changes on the same individual surfaces over time but it was better in that unknown effects of repeated sampling without cleaning a single tooth surface or repeat cleaning of each surface were eliminated. Variability in initial bacterial re-colonization of oral surfaces has been demonstrated58 and confirmed in this study. The use of randomly assigned quadrants did not allow us to follow changes in individual surfaces and, therefore, fully assess such variability. However, it did permit us to follow the general pattern of species succession in the supra and subgingival biofilms present in periodontally healthy and diseased subjects.
One final limitation of the present study was our inability to describe the spatial relationships that occur among bacterial species during biofilm re-development. For example, Dige et al58 have demonstrated that streptococci are early biofilm colonizers on clean surfaces and that these organisms form “chimney-like” structures in association with other taxa, often A. naeslundii, in the central portion of the chimney. Such findings suggest that combinations of approaches will be needed to fully appreciate biofilm development. Studies such as the present one provide a broad quantitative assessment of microbial changes during biofilm development. However, finer details of inter-microbial association, particularly spatial relationships, are better measured by other techniques, including FISH and confocal microscopy
In summary, there was clear evidence of bacterial succession in both supra and subgingival biofilm samples from periodontally healthy and diseased subjects. Species showing early “blooms” included S. mitis, S. oralis, N. mucosa, V. parvula, E. corrodens and C. gingivalis. Species thought to play a role in periodontal pathogenesis such as P. gingivalis, T. forsythia, T. denticola and E. nodatum showed a decline in proportions during the early supra and subgingival re-colonization period. Succession in supragingival plaque re-development was found to be similar in periodontal health and disease, but the re-development of subgingival plaque was different in the two clinical groups. This suggests that the bacterial species, the surfaces for colonization and the bulk fluid comprising the supragingival ecosystem are generally more similar in health and disease than they are in the subgingival ecosystem. Understanding the sequence of bacterial succession that leads to blooms in pathogenic taxa could lead to new approaches to controlling their levels before they achieve sufficient numbers to elicit or contribute to damage to the periodontal tissues.
Acknowledgments
This work was supported in part by NIH/NIDCR R01-DE-14368 (S.S.), R03 DE 021742-01 (F.T.) and the Eleanor and Miles Shore Fellowship Program for Scholars in Medicine (The Forsyth Institute/Harvard Medical School) (F.T.).
References
- 1.Li J, Helmerhorst EJ, Leone CW, et al. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. 2004;97:1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharawy AM, Sabharwal K, Socransky SS, Lobene RR. A quantitative study of plaque and calculus formation in normal and periodontally involved mouths. J Periodontol. 1966;37:495–501. doi: 10.1902/jop.1966.37.6.495. [DOI] [PubMed] [Google Scholar]
- 3.Socransky SS, Manganiello AD, Propas D, Oram V, van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977;12:90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 4.Zee KY, Samaranayake LP, Attstrom R. Predominant cultivable supragingival plaque in Chinese “rapid” and “slow” plaque formers. J Clin Periodontol. 1996;23:1025–1031. doi: 10.1111/j.1600-051x.1996.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramberg P, Sekino S, Uzel NG, Socransky S, Lindhe J. Bacterial colonization during de novo plaque formation. J Clin Periodontol. 2003;30:990–995. doi: 10.1034/j.1600-051x.2003.00419.x. [DOI] [PubMed] [Google Scholar]
- 6.Ritz HL. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967;12:1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- 7.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 8.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 9.Quirynen M, Vogels R, Pauwels M, et al. Initial subgingival colonization of ‘pristine’ pockets. J Dent Res. 2005;84:340–344. doi: 10.1177/154405910508400409. [DOI] [PubMed] [Google Scholar]
- 10.Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res. 2006;17:25–37. doi: 10.1111/j.1600-0501.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 11.Uzel NG, Teles FR, Teles RP, et al. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 38:612–620. doi: 10.1111/j.1600-051X.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fierer N, Nemergut D, Knight R, Craine JM. Changes through time: integrating microorganisms into the study of succession. Res Microbiol. 161:635–642. doi: 10.1016/j.resmic.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Haffajee AD, Socransky SS, Goodson JM. Comparison of different data analyses for detecting changes in attachment level. J Clin Periodontol. 1983;10:298–310. doi: 10.1111/j.1600-051x.1983.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 16.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 17.Socransky SS, Haffajee AD, Smith C, et al. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 18.Legendre PLL. Numerical Ecology. Amsterdam: Elsevier; 2003. [Google Scholar]
- 19.Dale MDD, Fortin M, Legendre P, Myears D, Rosenberg M. Conceptual and mathematical relationships among methods for spatial analysis. Ecography. 2002;25:558–577. [Google Scholar]
- 20.Carlson MFL, Gillet F, Mitchell E. Community development along a proglacial chronosequence: are above-ground and below-ground community structure controlled more by biotic than abiotic factors? J Ecol. 2010;98:1084–1095. [Google Scholar]
- 21.Parn JRK, Mander U. Correspondence of vegetation boundaries to redox barriers in a Northern European moraine plain. Basic and Applied Ecology. 2010;11:54–64. [Google Scholar]
- 22.Redford AFN. Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb Ecol. 2009;58:189–198. doi: 10.1007/s00248-009-9495-y. [DOI] [PubMed] [Google Scholar]
- 23.Wittlebolle MVW, Boon N. The inoculum effect on the amonia-oxidizing bacterial communities in parallel sequential batch reactors. Water Research. 2009;43:4149–4158. doi: 10.1016/j.watres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Kent MGW, Weaver R, Armitage R. Landscape and plant community boundaries in biogeography. Progress in Physical Geography. 1997;21:315–353. [Google Scholar]
- 25.Diaz PI, Chalmers NI, Rickard AH, et al. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamma JJ, Nakou M, Baehni PC. Clinical and microbiological characteristics of smokers with early onset periodontitis. J Periodontal Res. 1999;34:25–33. doi: 10.1111/j.1600-0765.1999.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 27.Haffajee AD, Uzel NG, Arguello EI, Torresyap G, Guerrero DM, Socransky SS. Clinical and microbiological changes associated with the use of combined antimicrobial therapies to treat “refractory” periodontitis. J Clin Periodontol. 2004;31:869–877. doi: 10.1111/j.1600-051X.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 28.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–36. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 29.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 30.Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000. 2006;42:219–258. doi: 10.1111/j.1600-0757.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 31.Gmur R, Wyss C, Xue Y, Thurnheer T, Guggenheim B. Gingival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur J Oral Sci. 2004;112:33–41. doi: 10.1111/j.0909-8836.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 32.Gmur R, Thurnheer T. Direct quantitative differentiation between Prevotella intermedia and Prevotella nigrescens in clinical specimens. Microbiology. 2002;148:1379–1387. doi: 10.1099/00221287-148-5-1379. [DOI] [PubMed] [Google Scholar]
- 33.Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 34.Dalwai F, Spratt DA, Pratten J. Modeling shifts in microbial populations associated with health or disease. Appl Environ Microbiol. 2006;72:3678–3684. doi: 10.1128/AEM.72.5.3678-3684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer RJ, Jr, Diaz PI, Kolenbrander PE. Rapid succession within the Veillonella population of a developing human oral biofilm in situ. J Bacteriol. 2006;188:4117–4124. doi: 10.1128/JB.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Periasamy S, Kolenbrander PE. Aggregatibacter actinomycetemcomitans builds mutualistic biofilm communities with Fusobacterium nucleatum and Veillonella species in saliva. Infect Immun. 2009;77:3542–3551. doi: 10.1128/IAI.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009;191:6804–6811. doi: 10.1128/JB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haffajee AD, Teles RP, Patel MR, Song X, Veiga N, Socransky SS. Factors affecting human supragingival biofilm composition. I. Plaque mass. J Periodontal Res. 2009;44:511–519. doi: 10.1111/j.1600-0765.2008.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Periasamy S, Kolenbrander PE. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J Bacteriol. 192:2965–2972. doi: 10.1128/JB.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolenbrander PE. Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. Int J Oral Sci. 3:49–54. doi: 10.4248/IJOS11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvi GE, Franco LM, Braun TM, et al. Pro-inflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: a proof-of-concept study. J Clin Periodontol. 37:9–16. doi: 10.1111/j.1600-051X.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- 43.Rudiger SG, Carlen A, Meurman JH, Kari K, Olsson J. Dental biofilms at healthy and inflamed gingival margins. J Clin Periodontol. 2002;29:524–530. doi: 10.1034/j.1600-051x.2002.290609.x. [DOI] [PubMed] [Google Scholar]
- 44.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 45.Beikler T, Abdeen G, Schnitzer S, et al. Microbiological shifts in intra- and extraoral habitats following mechanical periodontal therapy. J Clin Periodontol. 2004;31:777–783. doi: 10.1111/j.1600-051X.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 46.Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32 (Suppl 6):57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 47.Salvi GE, Ramseier CA, Kandylaki M, Sigrist L, Awedowa E, Lang NP. Experimental gingivitis in cigarette smokers: a clinical and microbiological study. J Clin Periodontol. 2005;32:441–447. doi: 10.1111/j.1600-051X.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 48.Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 49.Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol. 38(Suppl 11):28–35. doi: 10.1111/j.1600-051X.2010.01673.x. [DOI] [PubMed] [Google Scholar]
- 50.Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol. 2000;27:722–732. doi: 10.1034/j.1600-051x.2000.027010722.x. [DOI] [PubMed] [Google Scholar]
- 51.Lie MA, van der Weijden GA, Timmerman MF, Loos BG, van Steenbergen TJ, van der Velden U. Oral microbiota in smokers and non-smokers in natural and experimentally-induced gingivitis. J Clin Periodontol. 1998;25:677–686. doi: 10.1111/j.1600-051x.1998.tb02505.x. [DOI] [PubMed] [Google Scholar]
- 52.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–334. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 53.Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keijser BJ, Zaura E, Huse SM, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 57.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 58.Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. Actinomyces naeslundii in initial dental biofilm formation. Microbiology. 2009;155:2116–2126. doi: 10.1099/mic.0.027706-0. [DOI] [PubMed] [Google Scholar]