Abstract
Rotenone, a widely used pesticide, reproduces Parkinsonism in rodents and associates with increased risk for Parkinson’s disease. We previously reported rotenone increased superoxide production through stimulating microglial phagocyte NADPH oxidase (PHOX). The present study identified a novel mechanism by which rotenone activates PHOX. Ligand-binding assay revealed that rotenone directly bound to membrane gp91phox, the catalytic subunit of PHOX; such binding was inhibited by diphenyleneiodonium, a PHOX inhibitor with a binding site on gp91phox. Functional studies showed both membrane and cytosolic subunits were required for rotenone-induced superoxide production in cell-free systems, intact phagocytes, and COS7 cells transfected with membrane subunits (gp91phox/p22phox) and cytosolic subunits (p67phox and p47phox). Rotenone-elicited extracellular superoxide release in p47phox-deficient macrophages suggested rotenone enabled to activate PHOX through a p47phox-independent mechanism. Increased membrane translocation of p67phox, elevated binding of p67phox to rotenone-treated membrane fractions, and co-immunoprecipitation of p67phox and gp91phox in rotenone-treated wild-type and p47phox-deficient macrophages indicated p67phox played a critical role in rotenone-induced PHOX activation via its direct interaction with gp91phox. Rac1, a Rho-like small GTPase, enhanced p67phox-gp91phox interaction; Rac1 inhibition decreased rotenone-elicited superoxide release. In conclusion, rotenone directly interacted with gp91phox; such an interaction triggered membrane translocation of p67phox, leading to PHOX activation and superoxide production.
Keywords: rotenone, macrophages, NADPH oxidase, gp91phox, superoxide, PHOX, NOX2, Rac1
Introduction
Parkinson’s disease (PD) is a movement disorder characterized by a progressive degeneration of the nigrostriatal dopaminergic pathway [1]. Although its etiology remains unclear, many lines of evidence have indicated a close association of environmental exposure with this disease [2–6]. For example, a recent epidemiological study has reported that PD is positively associated with lifetime use of pesticide rotenone in a population with well-characterized pesticide exposure [7]. Rotenone replicates Parkinsonism features when chronically administrated to rodents [8–11]. Mechanistically, rotenone impairs neuronal mitochondrial complex I [12, 13] and disrupts the microtube-based transport of neurotransmitter vesicles [14, 15]. Recent evidence indicates that microglia, the resident immune cells in the brain, also participate in rotenone-induced neurodegeneration [16, 17]. We previously reported that microglial activation greatly augmented dopaminergic neurodegeneration induced by rotenone, especially at low doses [16]; the enhanced neurodegeneration elicited by rotenone was primarily mediated by microglial NADPH oxidase [18].
Phagocyte NADPH oxidase (PHOX, often referred to as NOX2), an activity-dependent enzyme complex, is widely expressed in various immune cells including microglia, macrophages, and neutrophils. It is the key extracellular superoxide-producing enzyme in phagocytes and plays a critical role in inflammation. Superoxide and its downstream products are highly reactive and they engage in diverse cellular processes. Excessive production of these free radicals increases peroxidation products that damage proteins, lipids, DNA, and RNA, leading to cell dysfunction and eventual death [19]. Oxidative stress has long been recognized as a central pathological event during chronic progression of neurodegenerative diseases including PD [20–22]. Ample evidence strongly suggests that over-activated PHOX plays a pivotal role in inflammation-mediated neurodegeneration [22, 23].
It is well established that the activation of PHOX requires assembling of its multiple subunits into a complex. Upon stimulation, cytosolic subunit p47phox is phosphorylated, which initiates translocation of the cytosolic subunits (p47phox and p67phox) to the plasma membrane where they associate with the membrane component, flavocytochrome b558, to assemble a functional oxidase. Flavocytochrome b558 is a heme-containing heterodimer composed of gp91phox and p22phox. Cytosolic subunits p40phox and two small G-proteins, Rac1 and Rap1A, are also involved in the activation process of PHOX [24–26]. Our previous studies demonstrated that the genetic ablation of gp91phox, the catalytic subunit of PHOX, diminished rotenone-elicited extracellular superoxide release from microglia, thereby mitigating rotenone-induced neurodegeneration [18]. These findings lend strong support to an important role of PHOX activation in rotenone-mediated neurodegeneration. However, how rotenone activates this enzyme remains unknown. Here, we investigate the detailed molecular mechanisms underlying rotenone-induced activation of PHOX.
Materials and methods
Animals and cell cultures
Wild-type (C57BL/6J) and B6.129S6-Cybbtm1Din/J (gp91phox-deficient) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). B6.129S2-Ncf1tm1Shl (p47phox-deficient) mice and Fischer 334 rats were purchased from Taconic (Hudson, NY) and Charles River Laboratories (Wilmington, MA), respectively. Housing and breeding of the animals were performed in strict accordance with National Institutes of Health guidelines.
Wild-type and transfected monkey kidney COS7 cells were gifts from Dr. Mary Dinauer (Indiana University, IN) and cultured as described previously [27, 28]. Briefly, wild-type COS7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 50 U/ml penicillin, and 50 µg/ml streptomycin (Sigma, St. Louis, MO). COS7 cells stably expressed with flavocytochrome b558 (COS-cytob558 cells) were maintained in the same medium supplemented with additional 0.8 mg/ml genectin (Invitrogen) and 10 µg/ml puromycin (InvivoGen). COS7 cells stably expressed with flavocytochrome b558, p47phox, and p67phox (COS-phox cells) were maintained in the above DMEM medium containing 0.8 mg/ml genectin, 2 µg/ml puromycin, and 0.35 mg/ml hygromycin B (Invitrogen).
Peritoneal macrophage and neutrophil preparation
Resting peritoneal macrophages were obtained from the peritoneal cavity of rats or mice by using a modified method described previously [29]. The peritoneal cavities of animals were flushed with 5 ml of ice-cold RPMI medium 1640 (Invitrogen). After washed twice in RMPI, cells were preincubated in no-serum medium for 1 h. The cells were then washed twice to remove nonadherent cells. Adherent macrophages were cultured in DMEM containing 10% FBS, 50 U/ml penicillin, and 50 µg/ml streptomycin (Sigma) at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Peritoneal neutrophils were prepared as described [30]. Briefly, mouse peritoneal neutrophils were harvested 16 h after the injection of 1 ml of phosphate buffered saline (PBS) containing 0.9% casein (Sigma). The peritoneal cells were washed twice with HBSS and cell pellets were stored in −80°C until use.
Measurement of extracellular superoxide and intracellular reactive oxygen species (iROS)
The release of superoxide was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of tetrazolium salt (WST-1, 2-[4-Iodophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium) as described [31]. Primary microglia (1 × 105/well), peritoneal macrophages (1 × 105/well), or various COS7 cells (5 × 104/well) were grown overnight in 96-well plates in DMEM containing 10% FBS and switched to phenol red-free HBSS (100 µl/well). Fifty microliters of HBSS containing vehicle, rotenone, or phorbol 12-myristate 13-acetate (PMA) were then added to each well, followed by 50 µl of WST-1 (1 mM) in HBSS, with and without 600 U/ml SOD. The cells were incubated for 30 min at 37°C, and the absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices, CA).
The production of iROS was measured by a fluorescence probe DCFH-DA as described [32]. Briefly, after cultured overnight in 96-well plates, macrophages were incubated with 10 µM DCFH-DA (Invitrogen) for 30 min at 37°C. After 2 washes with HBSS buffer, cells were switched to HBSS containing 1% FBS. After the cells were incubated with vehicle or rotenone (10 nM) at 37°C for 30 min, the fluorescence was read at 488 nm for excitation and 525 nm for emission using a SpectraMax Gemini XS fluorescence microplate reader (Molecular Devices, CA).
Cell-free NADPH-dependent superoxide production assay
Generation of superoxide was measured by determining the rate of SOD-inhibitable ferricytochrome c reduction as described [33, 34]. Briefly, RAW 264.7 cells (a mouse macrophage-like cell line) were suspended to a concentration of 108 cells/ml in ice-cold disruption buffer containing 8 mM Na,K-phosphate buffer (pH 7.0), 131 mM NaCl, 340 mM sucrose, 2 mM NaN3, 1 mM ethylene glycol-bis (β-aminoetyl ether)-N,N,N',N'-tetraacetic acid (EGTA), and proteinase inhibitor cocktail. Cells suspension was sonicated by 5s bursts followed by a 5s rest; the cycle was repeated six times. Sonicated cell lystes were centrifuged at 6,000g for 10 min at 4°C to remove unbroken cells or organelles (e.g. mitochondria). After ultracentrifugation at 110,000g for 2 h at 4°C, the cytosolic fraction (supernatant) and the membrane fraction (pellet) were collected. The membrane pellet was then washed by 1M KCl and suspended in activation buffer containing 65 mM Na,K-phosphate buffer (pH 6.5), 170 mM sucrose, 2 mM NaN3, 1 mM EGTA, and 10 µM FAD. After treatment of the membrane fraction with rotenone (10 nM) at 37°C for 5 min, the cytosolic fraction supplemented with ferricytochrome c (0.1 mM) was reconstituted with the membrane fraction; SOD (600 unit/ml), GTPγS (10 µM) and DPI (1µM) were added as indicated. The reaction was initiated by the addition of freshly prepared NADPH (final concentration of 0.1 mM); the absorbance at 550 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices, CA). Rates of superoxide production were calculated and expressed as “nmol of O2−/min/mg protein” [33].
Plasma membrane preparation
Plasma membranes of macrophages or neutrophils were isolated followed a published protocol [35]. Briefly, cells were suspended in isolation buffer (10 mM Tris-Cl, pH 8.0, 0.25 M sucrose, 1 mM EDTA and protease inhibitor cocktails), and cell membranes were broken by Dounce homogenization. The cell lysates were centrifuged at 6,000 X g for 10 min at 4°C to remove unbroken cells, cell debris, and mitochondria; afterwards, pellets of membranes were obtained by ultracentrifugation at 100,000g for 1 h at 4°C. After washed by 1M KCl, membrane pellets were either freshly used or stored at −80°C. Mitochondrial contamination of isolated plasma membranes were detected by Western blot analysis using antibody specific for VDAC (voltage-dependent anion channel, a mitochondrial membrane marker); crude mitochondrial fractions (pellets harvested by low-speed centrifugation of homogenized cell lystes) were used as a positive control of mitochondria.
[3H] labeled-rotenone binding assay
Rotenone’s binding was mainly performed using plasma membranes.. Briefly, membrane pellets were suspended in binding buffer (50mM Tris-Cl, pH 8.0, 100 mM NaCl, 1% BSA and 1 mM PMSF) and divided into aliquots of 0.25 mg protein per tube. Protein concentrations were determined by BCA Protein Kit (Pierce, Rockford, IL). For binding assay, [3H]-labeled rotenone ([3H] Dihydrorotenone, 50 Ci/mmol, American Radiolabeled Chemicals Inc, St Louis, MO) was added into membrane aliquots at a final concentration of 10 nM. For saturation studies, the concentration of [3H]-labeled rotenone ranged from 1 to 80 nM, and nonspecific binding was determined in the presence of additional 100 µM rotenone. Binding assay was terminated by filtration through glass microfibre filters (GF/C, Whatman) after binding samples were incubated on a rotator for 2 h at 4°C. Filters were immediately washed six times using binding buffer and transferred to the scintillation vials. After the filters were solublized in 1 M NaOH, radioactivity was counted with a liquid scintillation counter (Perkin Elmer, MA).
Since rotenone is a highly lipophilic molecule and easily penetrates and is incorporated into biological membranes, rotenone shows high non-specific binding in various binding assays. In order to encounter these intrinsic obstacles in binding assay of rotenone, we combined immunoprecipitation using anti-gp91 antibody and radiolabeled binding assay (IP-binding). Specifically, we prepared plasma membranes from wild-type and gp91phox-deficient macrophages, COS7WT cells lacking endogenous PHOX, and COS7 cells stably expressed with gp91phox and p22phox. Membrane pellets were suspended in 0.5 ml IP buffer (10 mM HEPES [pH 7.4], 1% Triton X-100, 5 mM Mg2Cl, 10 mM KCl, 1 mM PMSF, 1 mM dithiothreitol [DTT], 10% glycerin and protease inhibitor cocktails). After pre-incubation with [3H]-labeled rotenone (10 nM) for 30 min, membrane aliquots (0.25 mg protein) were incubated overnight with the addition of control IgG or anti-gp91phox polyclonal antibody (Santa Cruz Biotech, Santa Cruz, CA) at 4°C. Immunoprecipitation was performed by protein A/G beads (Santa Cruz Biotech, Santa Cruz, CA) saturated in 100 µM rotenone. After 4-hour incubation at 4°C, protein beads were washed 8 times by IP buffer. Then the beads were eluted twice by 0.5 ml glycine buffer (0.1 M at pH 2.5), and radioactivity was counted with a liquid scintillation counter. The binding capacity was determined by subtracting the binding of control IgG from that of gp91-antibody.
Cell-free membrane binding assay
Isolated membrane pellets (100 µg) from resting peritoneal macrophages were suspended in 100 µl binding buffer containing protease inhibitors and were incubated with vehicle or rotenone (50 nM) for 30 min at 37 °C. Samples were then incubated with corresponding cell cytosol extracts at 37°C for another 30 min. G-protein stimulator GTPγS (10 µM) was added in some groups. Since SDS has been reported to induce p47phox binding to cell membranes [36] and to stimulate PHOX-mediated superoxide production in cell-free systems [37], we used SDS (0.1 mM) as a positive control. The pellets of treated membranes were re-collected by centrifugation at 100,000 g for 1 h and then analyzed by western blotting. Membrane binding capacities of p67phox and p47phox were determined, and gp91phox was used as a membrane marker.
Immunoprecipitation for determining interactions between gp91phox and p67phox
Immunoprecipitation studies were performed using a method described previously [38]. Peritoneal macrophages were homogenized in extraction buffer (10 mM Hepes, pH 7.4, 0.25 M sucrose, 1 mM EDTA and protease inhibitor cocktails). Differential centrifugation of cell lysates removed debris and mitochondria; membrane pellets and cytosol were obtained by ultracentrifugation at 100,000g for 1 h. Isolated membrane pellets (250 µg) were suspended in 0.5 ml lysis buffer (10 mM HEPES [pH 7.4], 2% Triton X-100, 5 mM Mg2Cl, 10 mM KCl, 1 mM PMSF, 1 mM dithiothreitol [DTT] and protease inhibitor cocktails). After pre-incubation with vehicle or rotenone (50 nM) at 37°C for 30 min, solubilized membrane proteins were incubated with 0.5 ml cytosol extract. Immunoprecipitation was performed by the addition of anti-gp91phox polyclonal antibody and protein A/G beads (Santa Cruz Biotech, Santa Cruz, CA) saturated in BSA. After 4-hour incubation at 4°C, protein beads were washed 5 times; bound proteins were denatured in Laemmli sample buffer by boiling at 100°C for 5 min.
Gel electrophoresis and Western blotting analysis
Membranes of peritoneal macrophages or various cultured cells were homogenized in SDS lysis buffer (2% SDS and 50 mM Tris-HCl, pH 7.5) or RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 1:100 protease inhibitor cocktails), sonicated and heated to 100°C for 10 min. Extracted protein samples were resolved on SDS polyacrylamide gels, and immunoblot analyses were performed using antibodies against gp91phox, p67phox, or p47phox. Monoclonal anti-GAPDH antibody was included as an internal standard to monitor loading errors.
Statistics analysis
Data were expressed as mean ± S.E.M. Statistical significance was assessed by ANOVA followed by Bonferroni’s t test using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). A value of p < 0.05 was considered statistically significant.
Results
Rotenone-induced superoxide release from phagocytes required the presence of PHOX
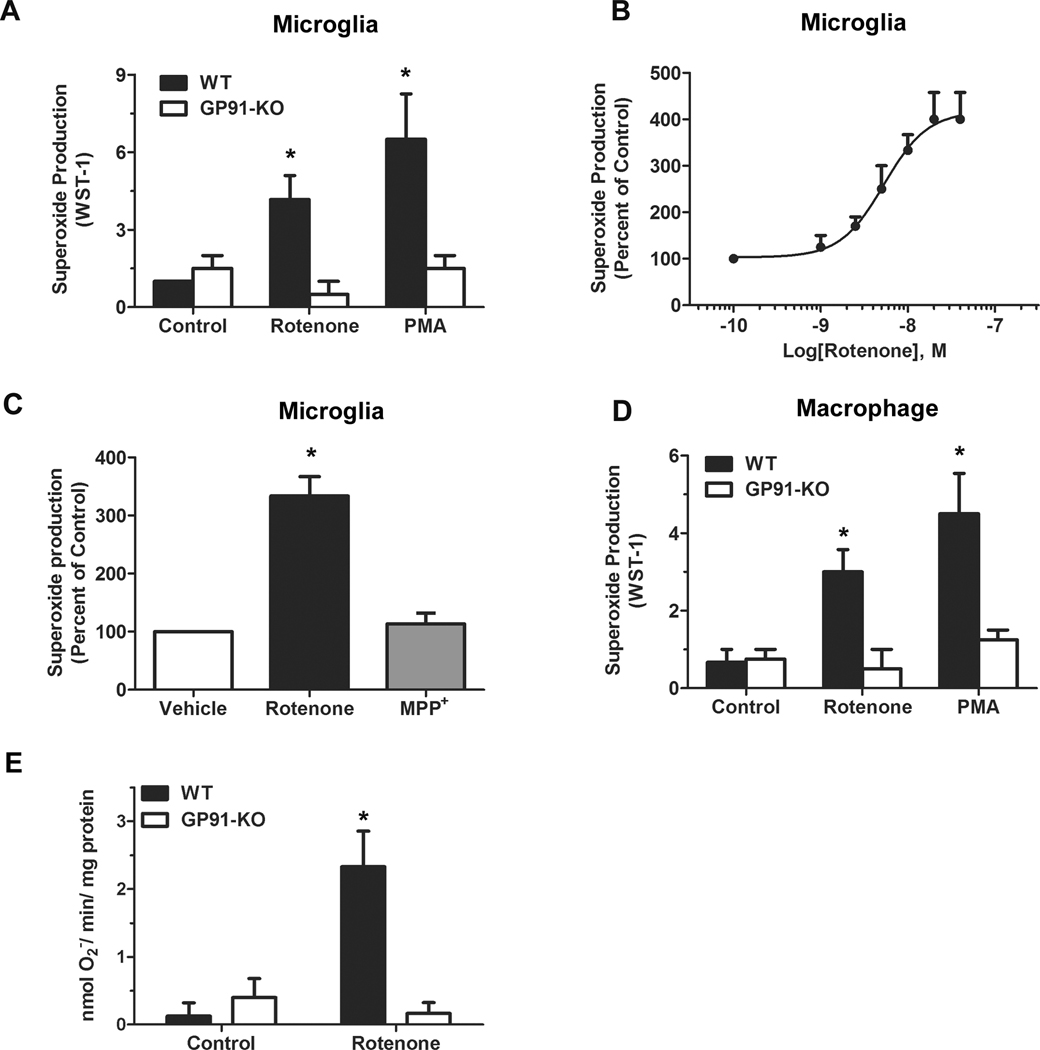
Like PMA (an activator of PHOX; a positive control), rotenone (10 nM) induced superoxide release in both primary microglia (Fig.1A) and peritoneal macrophages (Fig.1D) from wild-type mice, but failed to do so in cells from gp91phox-deficient mice. These data indicated that PHOX was indispensable for rotenone-induced extracellular superoxide release from microglia and macrophages. Similar dose-response curve of superoxide production elicited by rotenone was observed in primary microglia (Fig.1B) and macrophage (data not shown). The EC50 value of rotenone was 5.34 × 10−9 M. Ten nanomolar rotenone was used in all following studies, since at this concentration rotenone reached the 83% of the plateau of superoxide production. In contrast to rotenone, MPP+ (1-methyl-4-phenylpyridinium ion, 5 µM) did not produce superoxide in microglia (Fig. 1C) or macrophages (data not shown), although both toxins are well-known inhibitors of mitochondrial complex I [39]. The dose of rotenone (10 nM) used in the present study is much lower than the doses used for mitochondrial inhibition and mitochondrial ROS production (100–2000 nM) [40–42], which further implied that mitochondria did not participate in the PHOX activation and consequent extracellular superoxide release elicited by a low dose of rotenone. In order to definitely confirm that rotenone-induced PHOX activation is independent of mitochondria, we measured NADPH-dependent superoxide production in a cell-free system. The results showed that rotenone (10 nM) induced superoxide production in the cell-free system reconstituted from wild-type microglial membrane and cytosolic fractions but not from gp91phox-deficient microglial membrane and cytosolic fractions.
Fig. 1. Rotenone induced extracellular superoxide release in wild-type, but not gp91phox-deficient phagocytes.
Extracellular superoxide release from primary microglia (A) and peritoneal macrophages (D) of wild-type and gp91phox-deficient mice was measured by the SOD-inhibitable reduction of WST-1 after treatment with vehicle, rotenone (10 nM) or PMA (40 nM). (B) Dose-response curve of superoxide production in primary microglia induced by rotenone. Curve-fitting analyses were done with GraphPad Prism. (C) Unlike rotenone (10 nM), another mitochondrial complex I inhibitor MPP+ (5 µM) did not produce superoxide in microglia. (D) Rotenone (10 nM) induced superoxide production in the cell-free system reconstituted from wild-type microglial membrane and cytosolic fractions but not from gp91phox-deficient microglial membrane and cytosolic fractions. Data were shown as mean ± SEM from three independent experiments in triplicate. *, P < 0.05 compared with corresponding vehicle-treated control.
To further elucidate the mechanism underlying the activation of PHOX by rotenone, we used peritoneal macrophages and neutrophils instead of microglia in the present study for the following reasons: 1) microglia are considered as the macrophages in the brain, and both cells are derived from bone marrow monocytes; 2) in response to rotenone stimulation, the production of extracellular superoxide is qualitatively similar in peripheral macrophages and in microglia (Fig. 1); 3) macrophages and neutrophils have high levels of PHOX activity; 4) it is difficult to generate a great amount of primary microglia required for this study.
Rotenone directly interacted with flavocytochrome b558
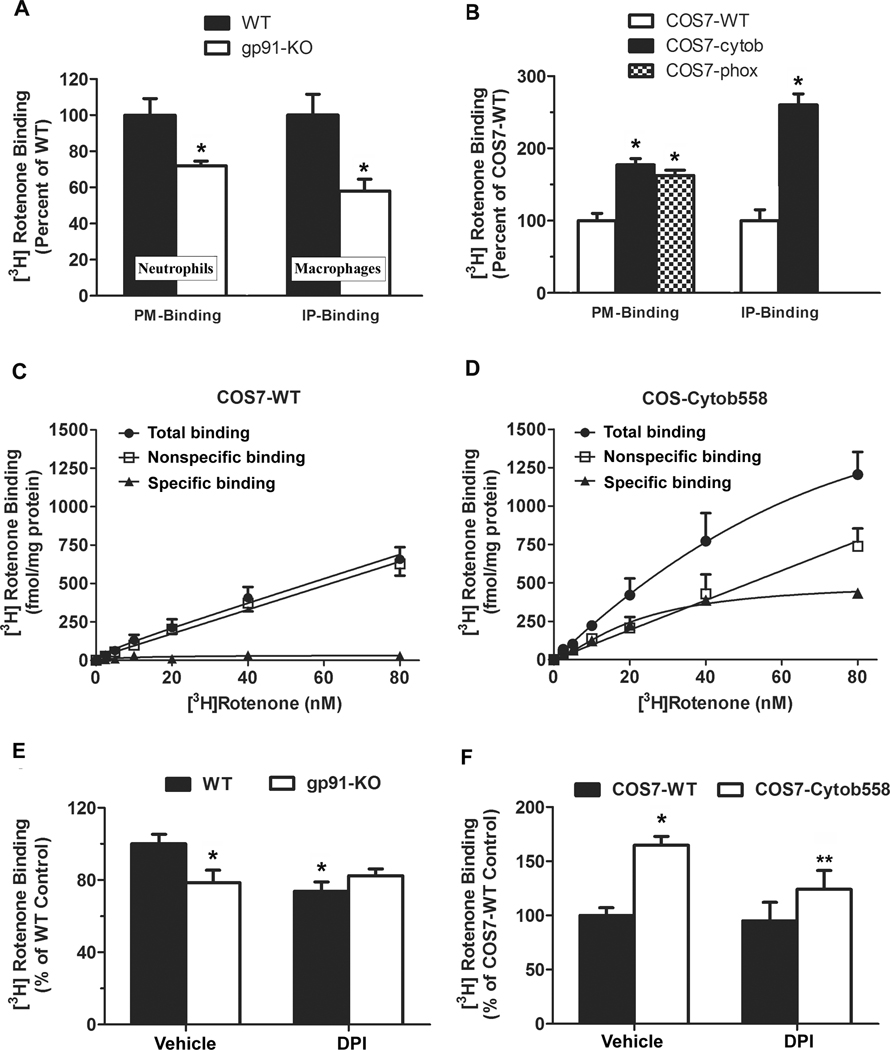
We isolated plasma membranes of peritoneal neutrophils from wild-type and gp91phox-deficient mice for binding assay, since PHOX was much abundant in neutrophils. The results revealed that more rotenone bound to wild-type neutrophil membranes than bound to gp91phox-deficient neutrophil membranes (Fig. 2A). After enrichment of gp91phox protein from wild-type primary macrophage membranes by immunoprecipitation using anti-gp91 antibody, the radiolabeled rotenone binding showed greater differences in the presence and absence of gp91phox (Fig. 2A). We also compared the binding capacity of rotenone to plasma membranes prepared from COS7 cells that lack endogenous expression of p22phox and gp91phox [27] or COS7 cells stably transfected with flavocytochrome b558 alone (COS-cytob558) or in combination with p47phox and p67phox (COS-phox). The reason for the co-transfection of gp91phox and p22phox is that p22phox facilitates stable expression of gp91phox in plasma membrane of COS7 cells [43]. Western blotting assay using VDAC (voltage-dependent anion channel, a mitochondrial membrane marker) antibody did not show detectable mitochondrial contamination in isolated membranes (Fig. S1A). [3H]-labeled rotenone binding assay showed that plasma membranes from COS-cytob558 and COS-phox cells had 50~60% higher binding capacity to rotenone than membranes from wild-type COS7 cells (Fig. 2B). After immunoprecipitation using anti-gp91 antibody, about 2.5 folds increase in the binding of [3H]-labeled rotenone to immunoprecipitated COS7-cytob membrane proteins compared with immunoprecipitated COS7WT membrane proteins (Fig. 2B). These binding results together implied that rotenone could directly interact with flavocytochrome b558, and specially, subunit gp91phox. The saturation study was performed by using wild-type COS7 and COS-cytob558 cells. In wild-type cells, no significant specific binding was observed since the total binding and the non-specific binding displayed very similar linear curves (Fig. 2C). On the contrary, the specific binding was clearly observed in COS-cytob558 transfected cells (Fig. 2D). [3H]-labeled rotenone binding was saturable with an affinity in the low nanomolar range, and the calculated Kd value was 21.18 ± 3.47 nM.
Fig. 2. Rotenone directly interacted with gp91phox and this interaction was attenuated by DPI.
Plasma membranes isolated from wild-type and gp91phox-deficient neutrophils and macrophages (A), wild-type COS7 cells (COS7-WT), COS7 cells stably expressed with flavocytochrome b558 alone (COS7-cytob558) or in combination with p47phox and p67phox (COS-phox) (B) were used to perform the radiolabeled binding directly (PM-binding) or to conduct immunoprecipitation using anti-gp91 antibody and the radiolabeled binding assay (IP-binding assay). Data were shown as a percentage of the wild-type control. Results were presented as mean ± SEM from three independent experiments in duplicate. *, P < 0.01 compared with wild-type. (C, D) Saturation curves of [3H]-rotenone binding were performed by using wild-type COS7 (C) and COS-cytob558 cells (D). Total binding (●) was measured by the inclusion of [3H]-labeled rotenone at concentrations ranging from 1 to 80 nM, while nonspecific binding (□) was determined in the presence of additional 100 µM rotenone. Saturation isotherms were analyzed in Graphpad prism 5. Specific binding (▲) was calculated by the difference of total binding and nonspecific binding. This experiment was performed three times with similar results. (E, F) DPI inhibited the binding of [3H]-rotenone only in gp91phox-expessed membranes. Plasma membranes prepared from wild-type and gp91phox-deficient neutrophils (E), COS7-WT cells or COS7-cytob558 cells (F) were incubated with [3H]-rotenone (10 nM) containing DPI (10 µM) or vehicle for 2 h at 4 °C. Data were shown as a percentage of wild-type control after normalized to vehicle-treated wild-type membranes. Results were presented as mean ± SEM from three independent experiments in duplicate. *, P < 0.05 compared with vehicle-treated wild-type membrane. **, P < 0.05 compared with vehicle-treated COS7-cytob558 membrane.
It is known that diphenyleneiodonium (DPI) can suppress gp91phox activity through interacting with C-terminal flavin-binding domain of gp91phox [44]. Although it blocks multiple flavin-containing enzymes, DPI has been widely used as a NADPH oxidase inhibitor. It is because that DPI shows more potent inhibition on NADPH oxidase than on other flavin-containing targets, such as flavin components of mitochondrial electron transport chain [45] and nitric oxide synthases [46]. Our results revealed that pre-incubation of membranes with DPI (10 µM) for 30 min at 4°C decreased the binding capacity of [3H]-rotenone to gp91phox-expressed membranes isolated from wild-type macrophages and COS7-cytob558 cells. DPI failed to attenuate [3H]-rotenone binding to membranes that lack gp91phox (gp91phox-deficient macrophage membrane or COS7-WT cell membrane) (Fig. 2E, F). Western blotting assay using VDAC (voltage-dependent anion channel, a mitochondrial membrane marker) antibody did not show detectable mitochondrial contamination. No mitochondrial contamination in isolated membranes (Fig. S1A), combined with the finding that DPI could not affect the binding of [3H]-rotenone at 10 nM concentration to mitochondrial fractions (Fig. S1B), further excludes the involvement of mitochondria in DPI-induced reduction of [3H]-rotenone binding to gp91phox-expressed membranes. These data together demonstrated an interaction between rotenone and membrane gp91phox. Here, the binding inhibition by DPI is not necessary to imply that rotenone and DPI compete for the same binding site. It is possible that rotenone and DPI have different binding sites on gp91phox. Conformational changes of gp91phox after DPI binding may reduce rotenone’s binding to gp91phox. It remains to be determined whether rotenone binds to heme, the C-terminal flavin domain, or any specific domain of gp91phox.
Rotenone failed to induce superoxide production in COS7 cells transfected with flavocytochrome b558
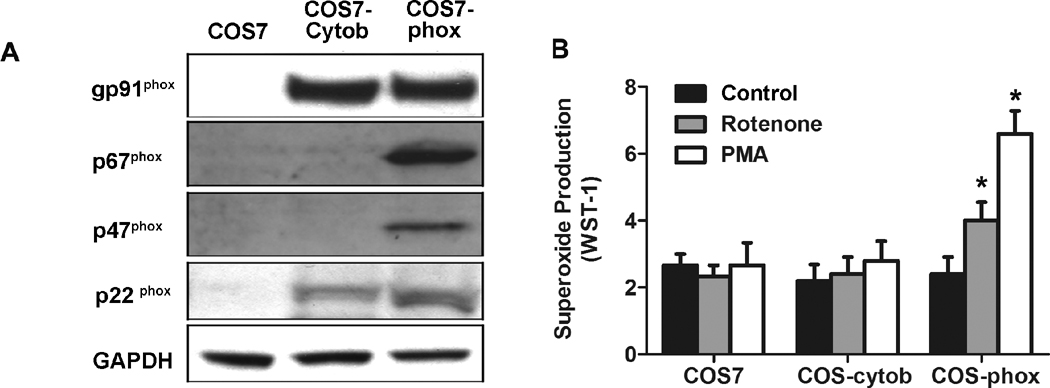
To investigate the possibility that rotenone directly activates gp91phox to initiate the production of superoxide, we measured extracellular superoxide release in wild-type COS7 cells, COS7 cells transfected with flavocytochrome b558 (COS-cytob558), and COS7 cells transfected with flavincytochrome b558 and p47phox/p67phox (COS-phox). The expression levels of various subunits in different cells were shown in Fig. 3A. As expected, PMA (40 nM) stimulated extracellular superoxide release only in COS-phox cells (Fig.3B), since it is well known that PMA activated PHOX through a p47phox-dependent pathway in which the phosphorylation of p47phox is an essential step. Rotenone (10 nM) also induced superoxide release only in COS7-phox cells, which indicated that rotenone-induced activation of PHOX required the attendance of cytosolic subunit(s): p47phox, p67phox, or both.
Fig. 3. Both membrane and cytosolic subunits were required for rotenone-induced extracellular superoxide release.
(A) Western blotting shows expression of PHOX subunits in COS7, COS-cytob558 (COS7 cells stably transfected with flavocytochrome b558), and COS-phox cells (COS7 cells stably transfected with both flavocytochrome b558 and cytosolic subunits p47phox and p67phox). (B) Extracellular superoxide release was measured by the SOD-inhibitable reduction of WST-1 after treatment with vehicle, rotenone (10 nM), or PMA (40 nM). Data were shown as mean ± SEM from three independent experiments in triplicate. *, P < 0.01 compared with corresponding vehicle-treated controls.
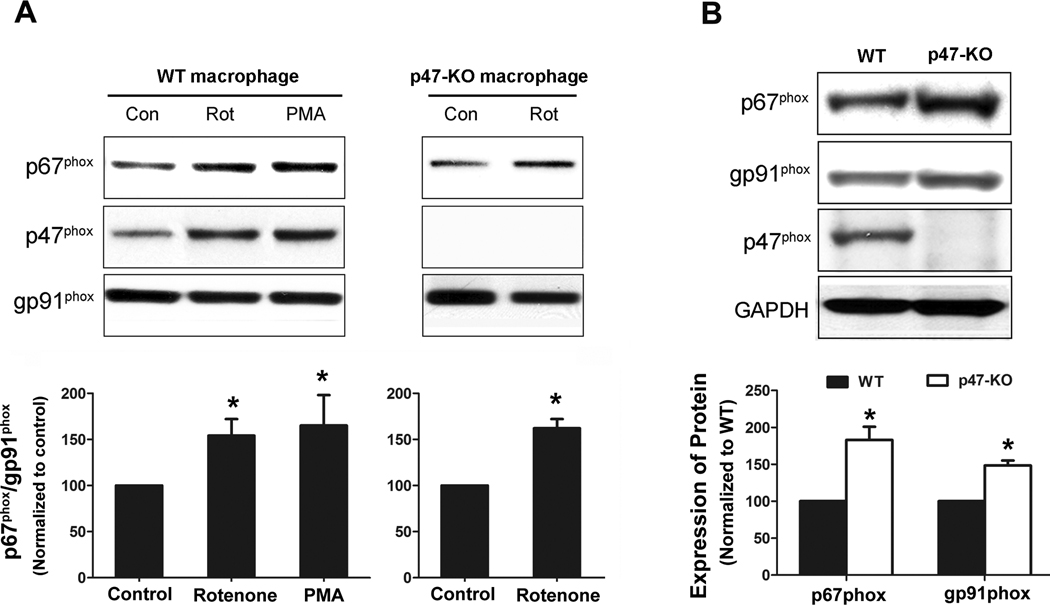
Rotenone induced superoxide production in p47phox-deficient macrophages
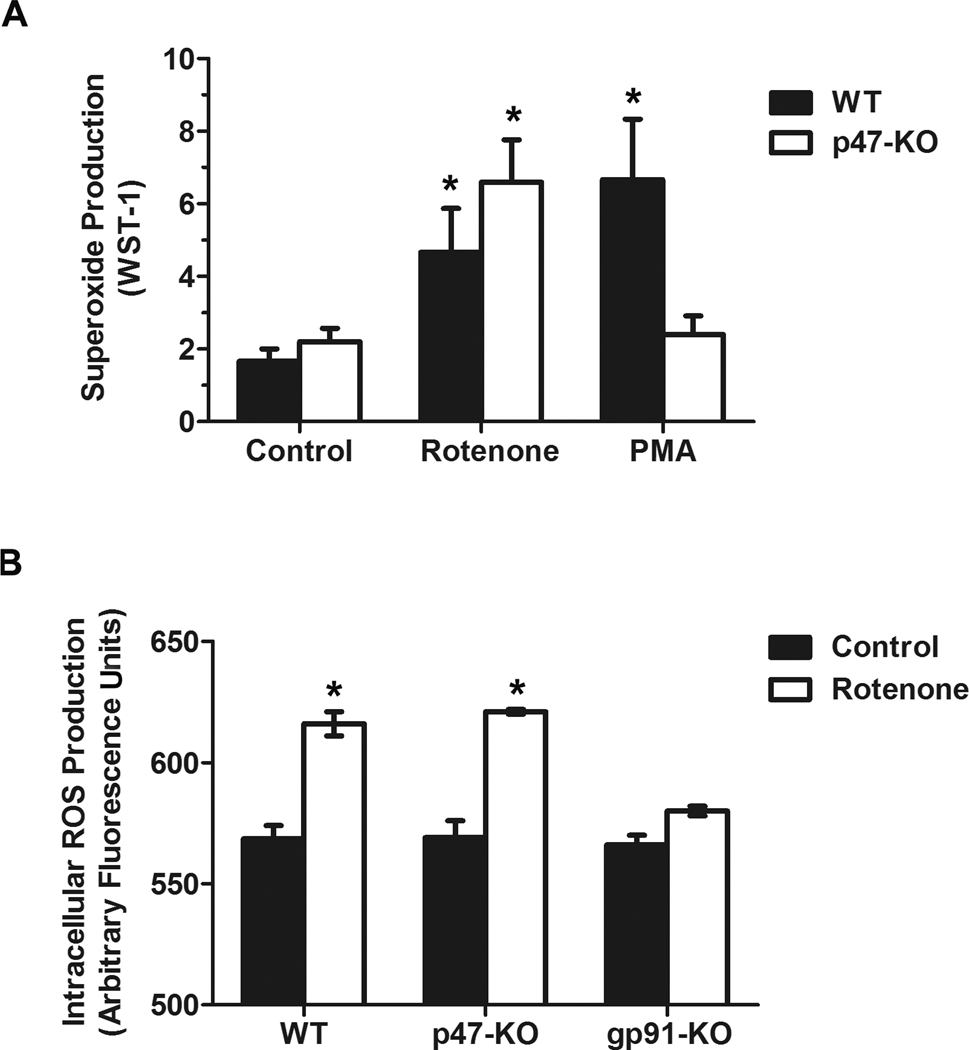
We next used peritoneal p47phox-deficient macrophages generated from p47phox-knockout mice to determine the role of cytosolic subunit p47phox in rotenone-induced PHOX activation. As expected, both rotenone and PMA induced superoxide production in wild-type macrophages. In contrast, differential effects of PMA and rotenone were observed in p47phox-deficient macrophages. Specifically, rotenone, but not PMA, was still capable of increasing the production of superoxide even in the absence of p47phox (Fig. 4A). Rotenone treatment led to significantly less production of iROS in gp91phox-deficient macrophages than in wild-type and p47phox-deficient macrophages (Fig. 4B). The dose of rotenone (10 nM) used in this study is much lower than the doses used for mitochondrial inhibition and ROS production (100–2000 nM) [40–42]. Thus, iROS production induced by a low dose of rotenone was mainly from PHOX. Collectively, these data indicated that p47phox was not indispensable for PHOX activation induced by rotenone, suggesting a p47phox-independent pathway.
Fig. 4. Rotenone induced superoxide production in p47phox-deficient macrophages.
(A) Extracellular superoxide release was measured by the SOD-inhibitable reduction of WST-1 after peritoneal macrophages from wild-type and p47phox-deficient mice were treated with vehicle, rotenone (10 nM), or PMA (40 nM). Data were expressed as mean ± SEM from three independent experiments in triplicate. *, P < 0.05 compared with corresponding vehicle-treated control. (B) Intracellular ROS production was measured by fluorescence probe DCFH-DA as described in Methods. Data were collected at 30 min after the addition of vehicle or rotenone into cells and shown as arbitrary fluorescence units. Results were presented as mean ± SEM from three independent experiments in triplicate. *, P < 0.05 compared with corresponding vehicle-treated control.
Rotenone induced translocation of cytosolic subunits to cell membranes
As described above, both membrane and cytosolic subunits were required for rotenone-elicited PHOX activation. Therefore, we explored how these subunits interacted after rotenone treatment. While it is well established that translocation of cytosolic subunits to the membrane is essential for PHOX activation, the involvement of individual cytosolic subunit in this process remains unclear. Here, we used PMA as positive control for the translocation study. PMA is known to activate protein kinase C (PKC), leading to phosphorylation of p47phox. Phosphorylated p47phox forms a complex with p67phox and the complex translocates to cell membrane. The distribution of p67phox in cell membranes isolated from rotenone-treated wild-type and p47phox-deficient macrophages indicated that rotenone could trigger p67phox translocation to membranes (Fig. 5A). Interestingly, although p47phox was not required for rotenone-induced activation of PHOX (Fig. 4), rotenone treatment caused translocation of p47phox to membrane in wild-type macrophages, as shown by western blotting (Fig. 5A and quantified intensity in Fig. S2A) and immunofluorescence staining (Fig. S2B). Considering the existence of p47phox-independent pathway, we hypothesized that the membrane translocation of p67phox may play a key role in rotenone-induced PHOX activation. Worth noting is elevated levels of gp91phox and p67phox in p47phox-deficient macrophages (Fig 5B) This result may suggest a compensatory role of p67phox and gp91phox in the absence of p47phox.
Fig. 5. Rotenone induced membrane translocation of cytosolic subunits in wild-type and p47phox-deficient macrophages.
Peritoneal macrophages from wild-type or p47phox-deficient mice were treated with vehicle or rotenone (10 nM) for 15 min. PMA-treated wild-type macrophages were used as positive controls. Distribution of p47phox and p67phox in membrane fractions was detected by western blot assays (A). The ratios of densitometry values of p67phox to values of membrane gp91phox (loading control) were normalized to corresponding controls. *, P < 0.01 compared with vehicle-treated control. (B) The expression levels of p67phox and gp91phox in wild-type and p47phox-deficient macrophages were determined by western blotting. The ratios of densitometry values of p67phox or gp91phox relative to loading control GAPDH were normalized to wild-type. All experiments shown in this figure have been performed at least three times. *, P < 0.05 compared with corresponding wild-type.
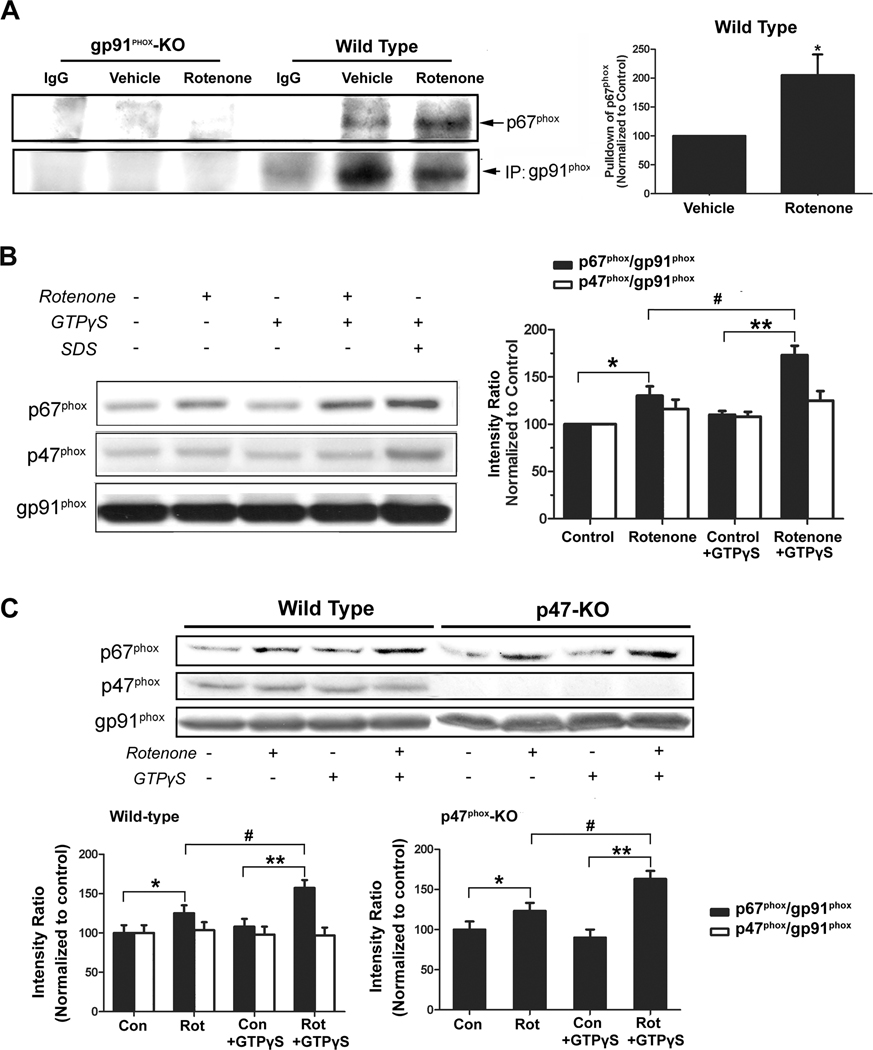
Rotenone induced cytosolic p67phox binding to gp91phox
Immunoprecipitation assay was employed to examine the interaction between p67phox and gp91phox after rotenone treatment. As shown in Fig.6A, p67phox was co-immunoprecipitated with gp91phox by anti-gp91phox antibody. No immunoprecipitation by control IgG and minimal nonspecific immunoprecipitation by anti-gp91phox antibody in gp91phox-deficient cell lysates confirmed the observed binding between gp91phox and p67phox was specific. Cell-free binding assay was used to further validate rotenone-elicited p67phox-gp91phox interaction. As shown by Figure 6B, rotenone-treated macrophage membranes, prepared from rats and wild-type mice, exhibited higher binding capacity for p67phox than vehicle-treated membranes. The binding capacity was further increased by the addition of GTPγS, a non-hydrolyzable G-protein-activating GTP analog. These findings implied that p67phox interacted with rotenone-bound gp91phox and G-proteins facilitated and stabilized such an interaction (Fig. 6B). Similar results were observed in membranes from p47phox-deficient macrophages (Fig. 6C). The binding of p47phox to macrophage membranes was not significantly changed after the addition of vehicle, rotenone, or GTPγS (Fig. 6B,C). Collectively, the binding of rotenone to gp91phox could facilitate p67phox-gp91phox interaction in a p47phox-independent manner, and such p67phox-gp91phox interaction was a critical step in PHOX activation by rotenone. The finding that GTPγS enhanced p67phox-gp91phox interaction in a cell-free binding assay suggests that Rac1 or Rap1A (two small G-proteins that have been implicated in NADPH oxidase activation) may play some role in rotenone-induced PHOX activation.
Fig. 6. Rotenone treatment recruited p67phox from cytosol to the membrane where it bound to gp91phox.
(A) Immunoprecipitation for NADPH oxidase subunits gp91phox and p67phox was performed by using membrane fractions of macrophages from wild-type and gp91-deficent mice (the latter as a negative control). P67phox was co-immunoprecipitated by the anti-gp91phox antibody. Experiments were performed three times with similar results. (B, C) Cell membrane fractions of peritoneal macrophages prepared from rats, C57BL/6J mice, or p47-knockout mice were incubation with vehicle or rotenone (10 nM) for 30 min at 37 °C; afterwards, membrane fractions were reconstituted with the corresponding cytosol extracts and were incubated at 37°C for another 30 min. SDS (0.01%, a positive control) and GTPγS (10 µM) were added as indicated. Occurrence of p67phox and p47phox in membrane fractions was detected by western blot assays. The ratios of densitometry values of p67phox or p47phox relative to gp91phox (used as a membrane marker) were normalized to vehicle-treated control. Experiments have been performed at least four times. *, #, P < 0.05 and **, P < 0.01 were considered statistically significant.
Rac1 participates in rotenone-induced PHOX activation
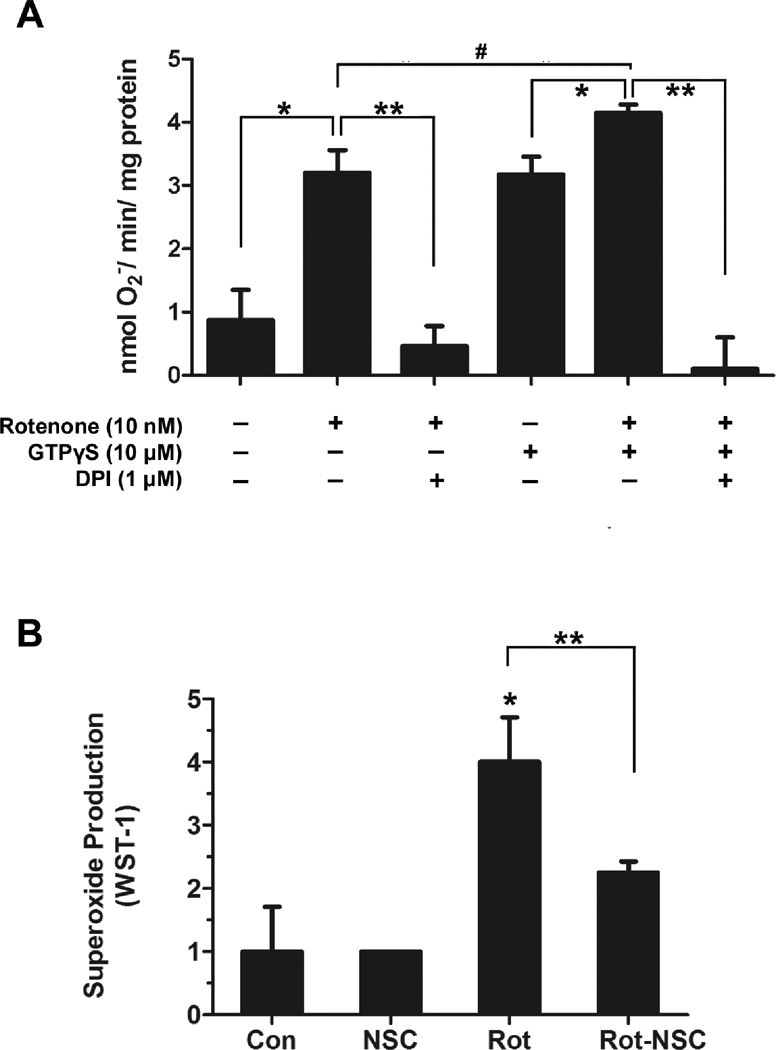
We next discern the role of Rac1, a Rho-like small GTPase, in rotenone-elicited PHOX activation. As seen in Fig. 7A, GTPγS (10 µM) increased rotenone-induced NADPH-dependent superoxide production in a cell-free system, and such superoxide production was blocked by DPI. Inhibition of Rac activity by NSC 23766, a Rac1 inhibitor, significantly attenuated rotenone-induced extracellular superoxide release from primary microglia (Fig. 7B). These results together revealed an important role of Rac1 in rotenone-induced PHOX activation through enhancing p67phox-gp91phox interaction.
Fig. 7. Rac1 participates in rotenone-induced PHOX activation.
(A) In the cell-free system reconstituted from membrane and cytosolic fractions of RAW 264.7 cells, rotenone-induced superoxide production was blocked by DPI. GTPγS significantly increased rotenone-elicited superoxide production; such superoxide production was also prevented by DPI. (B) Rac1 inhibitor NSC 23766 (100 µM) significantly attenuated rotenone-induced extracellular superoxide release in primary microglia.
Discussion
In the present study, we elucidated a unique mechanism by which rotenone activates PHOX. We found that rotenone directly interacted with gp91phox (the catalytic subunit of PHOX), which facilitated the interaction between gp91phox and cytosolic subunit p67phox, thus triggering the formation of activated enzyme complex in phagocytes. While Rac1 enhanced rotenone-elicited superoxide production through stabilizing p67phox-gp91phox interaction, the genetic deletion of p47phox did not affect rotenone-induced superoxide production. The later finding indicates that p47phox was not required for functional enzyme assembly in the presence of rotenone. Thus, rotenone could activate PHOX through a p47phox-independent mechanism.
Recent case-control and epidemiological studies suggests that exposure to pesticide rotenone is associated with an increased risk for PD [5, 7]. However, the mechanism of rotenone-induced neurotoxicity is still unclear. Although potential relevance of mitochondrial complex I impairment to PD pathogenesis has been demonstrated in several in vivo models created by rotenone [8, 11], the doses of rotenone used were not sufficient to alter mitochondrial respiration in isolated brain mitochondria [11]. Doses of rotenone for PHOX activation range from 1 to 20 nM (Fig. 1B), which is consistent with our previous studies [16, 18]. The doses used for mitochondrial inhibition and mitochondrial ROS production are much higher (100–2000 nM) [40–42]. Thus, rotenone neurotoxicity must not result solely from mitochondrial complex I inhibition and subsequent bioenergetic defect. In rotenone-infusion rodents, relatively selective oxidative stress in the midbrain and olfactory bulb was observed [10, 47]. Antioxidants rescue the behavioral deficits and attenuate dopamine neuronal loss in drosophila and rats exposed to rotenone [48, 49].
Reactive oxygen species (ROS) generated by leakage from mitochondria was considered as major source of oxidative stress in rotenone-induced PD animal models [47], since impaired complex I activity enhances ROS formation [50, 51]. A recent study has reported that cultured dopaminergic neurons from wild-type and mitochondrial complex I-deficient (ndufs4−/−) mice exhibited similar vulnerability to rotenone neurotoxicity [52], which implies that rotenone may damage neurons through alternative targets other than mitochondrial complex I. It is noticeable that in the rat rotenone PD model, selective microglial activation has been observed in the striatum and the substantia nigra, the main brain regions damaged in PD patients [17]. In addition, our previous in vitro studies using cells from PHOX-deficient (gp91phox−/−) mice suggested that microglial PHOX-derived superoxide markedly enhanced rotenone-induced degeneration of dopaminergic neurons [18]. Superoxide and its downstream free radical products caused oxidative damages to neurons, eventually leading to neuronal death. Thus, PHOX is perhaps an alternative target of rotenone. In the present study, we explored this possibility and elucidated how rotenone activated PHOX. The results showed that rotenone directly interacted with gp91phox subunit (Fig. 2, 6). Rotenone (≤ 10 nM) elicited superoxide production in both intact wild-type phagocytes and the cell-free system reconstituted from phagocyte membrane and cytosolic fractions from wild-type mice; in the absence of gp91phox, rotenone did not induce superoxide production in these systems (Fig. 1 & 7). However, the finding that rotenone failed to stimulate superoxide production in COS7 cells stably transfected with flavincytochrome b558 containing gp91phox and p22 phox (Fig. 3) indicated that rotenone did not directly activate gp91phox and cytosolic subunits or other cofactors were required for rotenone-induced PHOX activation (Fig. 1–3).
In the resting state, p47phox separates from p67phox and is functionally inactive [53]. Upon stimulation, p47phox is phosphorylated, and the auto-inhibition region (AIR) of p47phox was released, allowing its interaction with p22phox and p67phox. The complex of p47phox and p67phox translocates to cell membrane, where p47phox binds to membrane p22phox and p67phox binds to gp91phox, leading to PHOX activation [24]. In fact, p47phox serves as an organizer, while p67phox is considered as an activator [24, 54]. Traditionally, p47phox was considered essential for PHOX activation. Mutants in p47phox gene have been linked to some patients with chronic granulomatous disease (CGD), a diverse group of hereditary diseases with deficiency in PHOX activity [55]. Consistent with previous publications [56], we found that p47phox was required for PMA-induced free radical production (Fig. 4). However, it has also been reported that p47phox is not indispensable for the activation of PHOX in some cell-free studies [57–59]. For instance, the pioneer study conducted by Freeman and Lambeth has demonstrated that in the absence of p47phox, the PHOX activity is still preserved, but this requires the presence of p67phox and Rac1; Rac1 is consider to stabilize the interaction between gp91phox and p67phox. Our findings that rotenone was able to activate PHOX and led to production of free radicals in p47phox-deficient macrophages (Fig. 4–6) indicated the existence of a p47phox-independent pathway in rotenone-induced activation of PHOX in intact phagocytes.
We then pinpointed a crucial role of cytosolic subunit p67phox in rotenone-induced PHOX activation. Firstly, rotenone activated PHOX in COS7-phox cells that contain cytosolic subunits p47phox and p67phox (Fig. 3), while p47phox was not indispensable (Fig. 4). Secondly, rotenone caused membrane translocation of p67phox in both wild-type and p47phox-deficient macrophages (Fig. 5). Thirdly, membrane binding assay and immunoprecipitation study showed that rotenone-bound gp91phox exhibited higher binding affinity to p67phox than vehicle-treated gp91phox (Fig. 6). Therefore, rotenone-induced interaction between gp91phox and p67phox was crucial for assembling the active enzyme complex and for producing superoxide. It is thought that the direct protein-protein interaction between gp91phox and p67phox may require a conformational change of gp91phox [60] ; this change can be induced by p47phox binding to p22phox or activated-Rac1 binding to gp91phox [59]. Rotenone-bound gp91phox may undergo a similar conformational change. Such a conformational change might be provided by rotenone’s interaction with C-terminal of gp91phox, since rotenone’s binding to isolated plasma membrane was inhibited by DPI that binds to flavin-binding domain in C-terminal of gp91phox. In addition, C-terminal phosphorylation of gp91phox has been reported to regulate the assembly of the enzyme complex possibly through some conformational changes [38]. However, at present, it remains to be determined whether rotenone binds to heme, the C-terminal flavin domain, or any specific domain of gp91phox. Taken together, our findings suggest that rotenone’s binding may induce a conformational change of gp91phox, which facilitated the interaction between gp91phox and p67phox leading to PHOX activation. Thus, rotenone provided an opportunity to bypass the regulatory role of p47phox and activated PHOX through a novel mechanism. These findings imply that the interaction between p67phox and gp91phox is the major event in oxidase activity, while p47phox may not be indispensable for certain stimulators.
Although we identified a p47phox-independent pathway in rotenone-induced PHOX activation, we also observed the membrane translocation of p47phox in wild-type macrophages after rotenone treatment (Fig 5A, Fig S2). Additionally, in cell-free system, maximal activation of PHOX still requires the attendance of p47phox [58]. As described previously, activation of PHOX has the feature of propagation [59] and superoxide-mediated superoxide production may keep sustained activation of PHOX [61]. Thus, it is possible that superoxide produced by rotenone-elicited macrophages through a p47phox-independent mechanism may stimulate multiple downstream oxidative signaling pathways, which could induce p47phox membrane translocation to participate in PHOX activation. However, the precise mechanism of p47phox-dependent activation of PHOX by rotenone warrants further investigation.
Certainly, in addition to p67phox and p47phox, other cytosolic subunits might be also involved in rotenone-induced activation of PHOX. We noticed that GTPγS significantly increased the binding of p67phox to isolated macrophage membranes after rotenone treatment (Fig. 6B, C). GTPγS is a non-hydrolyzable G-protein-activating GTP analog. Rac-1 and Rap1A, two small GTPases, are considered as the regulatory subunits of PHOX. Upon stimulation, Rac-1 most likely moves to membrane from cytosol, and perhaps interacts with gp91phox [25, 62]. GTP-bound Rac-1 also interacts with tetratricopeptide (TPR) domain of p67phox, which is proposed to stabilize the interaction between p67phox and gp91phox [59]. The following findings from the present study indicate that Rac1 play an important role in rotenone-induced PHOX activation through enhancing p67phox-gp91phox interaction: 1) GTPγS enhanced the binding between p67phox and gp91phox after rotenone treatment in a cell-free binding assay (Fig. 6B,C); 2) GTPγS increased rotenone-induced NADPH-dependent and DPI-inhibitable superoxide production in a cell-free system (Fig. 7A); 3) Rac1 inhibition decreased rotenone-elicited extracellular superoxide release from microglia (Fig. 7B).
In summary, this study demonstrates that PHOX is a novel target of rotenone. Through direct interaction with gp91phox (the catalytic moiety of PHOX), rotenone may induce conformational changes of gp91phox in phagocytes. These changes facilitated and stabilized the binding of cytosolic subunit p67phox to membrane gp91phox, which led to assembling of a functional oxidase and consequent superoxide generation. Thus, this study identified a distinct p47phox-independent mechanism in rotenone-induced activation of PHOX. Findings from this study advance our understanding of rotenone-induced oxidative stress and neurotoxicity, providing a potential mechanism of environmental toxin-associated neurodegeneration.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences. We thank Jeff Tucker for his assistance in collecting confocal imaging data and Anthony Lockhart for his assistance with animal colony management and maintenance of the timed pregnant mice.
Glossary
List of Abbreviations
- MPP+
1-Methyl-4-phenylpyridinium ion
- WST-1
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
- DPI
diphenyleneiodonium
- PD
Parkinson’s disease
- PHOX
phagocyte NADPH oxidase
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 3.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 4.Ritz B, Yu F. Parkinson's disease mortality and pesticide exposure in California 1984–1994. Int J Epidemiol. 2000;29:323–329. doi: 10.1093/ije/29.2.323. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, Shepherd S. Pesticide/environmental exposures and Parkinson's disease in East Texas. J Agromedicine. 2008;13:37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, Paraquat and Parkinson's Disease. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 9.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong N, Huang J, Zhang Z, Zhang Z, Xiong J, Liu X, Jia M, Wang F, Chen C, Cao X, Liang Z, Sun S, Lin Z, Wang T. Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson's disease. PLoS One. 2009;4:e7878. doi: 10.1371/journal.pone.0007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 12.Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson's disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- 13.Jenner P. Parkinson's disease, pesticides and mitochondrial dysfunction. Trends Neurosci. 2001;24:245–247. doi: 10.1016/s0166-2236(00)01789-6. [DOI] [PubMed] [Google Scholar]
- 14.Marshall LE, Himes RH. Rotenone inhibition of tubulin self-assembly. Biochim Biophys Acta. 1978;543:590–594. doi: 10.1016/0304-4165(78)90315-x. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Feng J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J Neurochem. 2007;103:303–311. doi: 10.1111/j.1471-4159.2007.04741.x. [DOI] [PubMed] [Google Scholar]
- 16.Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett. 2003;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 18.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/a:1020685903186. [DOI] [PubMed] [Google Scholar]
- 20.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 21.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 22.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 25.Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 26.Bokoch GM, Quilliam LA, Bohl BP, Jesaitis AJ, Quinn MT. Inhibition of Rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of Rap1A. Science. 1991;254:1794–1796. doi: 10.1126/science.1763330. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price MO, McPhail LC, Lambeth JD, Han CH, Knaus UG, Dinauer MC. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 29.Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, Arm JP. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J Biol Chem. 2006;281:6691–6698. doi: 10.1074/jbc.M508314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto Y, Fukada Y, Mori D, Tanaka S, Yamane H, Okuno Y, Deai K, Tsuchiya S, Tsujimoto G, Ichikawa A. Prostaglandin E2 stimulates granulocyte colony-stimulating factor production via the prostanoid EP2 receptor in mouse peritoneal neutrophils. J Immunol. 2005;175:2606–2612. doi: 10.4049/jimmunol.175.4.2606. [DOI] [PubMed] [Google Scholar]
- 31.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Zhao J, Zhang X. Inhibition of uncoupling protein 2 by genipin reduces insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2009;486:88–93. doi: 10.1016/j.abb.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Molshanski-Mor S, Mizrahi A, Ugolev Y, Dahan I, Berdichevsky Y, Pick E. Cell-free assays: the reductionist approach to the study of NADPH oxidase assembly, or "all you wanted to know about cell-free assays but did not dare to ask". Methods Mol Biol. 2007;412:385–428. doi: 10.1007/978-1-59745-467-4_25. [DOI] [PubMed] [Google Scholar]
- 34.Bromberg Y, Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J Biol Chem. 1985;260:13539–13545. [PubMed] [Google Scholar]
- 35.Draye JP, Quintart J, Courtoy PJ, Baudhuin P. Relations between plasma membrane and lysosomal membrane. 1. Fate of covalently labelled plasma membrane protein. Eur J Biochem. 1987;170:395–403. doi: 10.1111/j.1432-1033.1987.tb13713.x. [DOI] [PubMed] [Google Scholar]
- 36.DeLeo FR, Nauseef WM, Jesaitis AJ, Burritt JB, Clark RA, Quinn MT. A domain of p47phox that interacts with human neutrophil flavocytochrome b558. J Biol Chem. 1995;270:26246–26251. doi: 10.1074/jbc.270.44.26246. [DOI] [PubMed] [Google Scholar]
- 37.Shpungin S, Dotan I, Abo A, Pick E. Activation of the superoxide forming NADPH oxidase in a cell-free system by sodium dodecyl sulfate. Absolute lipid dependence of the solubilized enzyme. J Biol Chem. 1989;264:9195–9203. [PubMed] [Google Scholar]
- 38.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. Faseb J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krueger MJ, Sablin SO, Ramsay R, Singer TP. Reactivation of NADH dehydrogenase (complex I) inhibited by 1-methyl-4-(4'-alkylphenyl)pyridinium analogues: a clue to the nature of the inhibition site. J Neurochem. 1993;61:1546–1548. doi: 10.1111/j.1471-4159.1993.tb13653.x. [DOI] [PubMed] [Google Scholar]
- 40.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 41.Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang D, Narabayashi H, Sata T, Takeshige K. Kinetics of superoxide formation by respiratory chain NADH- dehydrogenase of bovine heart mitochondria. J Biochem. 1983;94:1301–1306. doi: 10.1093/oxfordjournals.jbchem.a134475. [DOI] [PubMed] [Google Scholar]
- 43.Yu L, Zhen L, Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290(Pt 1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. Faseb J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 47.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nehru B, Verma R, Khanna P, Sharma SK. Behavioral alterations in rotenone model of Parkinson's disease: attenuation by co-treatment of centrophenoxine. Brain Res. 2008;1201:122–127. doi: 10.1016/j.brainres.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 50.Hensley K, Pye QN, Maidt ML, Stewart CA, Robinson KA, Jaffrey F, Floyd RA. Interaction of alpha-phenyl-N-tert-butyl nitrone and alternative electron acceptors with complex I indicates a substrate reduction site upstream from the rotenone binding site. J Neurochem. 1998;71:2549–2557. doi: 10.1046/j.1471-4159.1998.71062549.x. [DOI] [PubMed] [Google Scholar]
- 51.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dagher MC, Pick E. Opening the black box: lessons from cell-free systems on the phagocyte NADPH-oxidase. Biochimie. 2007;89:1123–1132. doi: 10.1016/j.biochi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 54.de Mendez I, Garrett MC, Adams AG, Leto TL. Role of p67-phox SH3 domains in assembly of the NADPH oxidase system. J Biol Chem. 1994;269:16326–16332. [PubMed] [Google Scholar]
- 55.Clark RA, Malech HL, Gallin JI, Nunoi H, Volpp BD, Pearson DW, Nauseef WM, Curnutte JT. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med. 1989;321:647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- 56.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 58.Koshkin V, Lotan O, Pick E. The cytosolic component p47(phox) is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal superoxide production. J Biol Chem. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 59.Mizrahi A, Berdichevsky Y, Ugolev Y, Molshanski-Mor S, Nakash Y, Dahan I, Alloul N, Gorzalczany Y, Sarfstein R, Hirshberg M, Pick E. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. J Leukoc Biol. 2006;79:881–895. doi: 10.1189/jlb.1005553. [DOI] [PubMed] [Google Scholar]
- 60.Paclet MH, Coleman AW, Vergnaud S, Morel F. P67-phox-mediated NADPH oxidase assembly: imaging of cytochrome b558 liposomes by atomic force microscopy. Biochemistry. 2000;39:9302–9310. doi: 10.1021/bi000483j. [DOI] [PubMed] [Google Scholar]
- 61.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem. 2006;281:36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 62.Sarfstein R, Gorzalczany Y, Mizrahi A, Berdichevsky Y, Molshanski-Mor S, Weinbaum C, Hirshberg M, Dagher MC, Pick E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. J Biol Chem. 2004;279:16007–16016. doi: 10.1074/jbc.M312394200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.