Abstract
Background
A low level of response (i.e., a low LR) to alcohol is a genetically influenced phenotype that predicts later alcoholism. While the low LR reflects, at least in part, a low brain response to alcohol, the physiological underpinnings of the low LR have only recently been addressed.
Methods
Forty-nine drinking but not yet alcoholic matched pairs of 18-25-year-old subjects (N = 98; 53% female) with low and high LRs as established in separate alcohol challenges were evaluated in two event-related functional magnetic resonance imaging (fMRI) sessions (placebo and ~ 0.7 ml/kg of alcohol) while performing a validated stop signal task. The high and low LR groups had identical blood alcohol levels during the alcohol session.
Results
Significant high versus low LR group and LR group × condition effects were observed in blood oxygen level dependent (BOLD) signal during error and inhibitory processing, despite similar LR-group performance on the task. In most clusters with significant (corrected p<.05, clusters >1344 μl) LR group × alcohol/placebo condition interactions, the low LR group demonstrated relatively less, whereas the high LR group demonstrated more, error and inhibition-related activation after alcohol compared to placebo.
Conclusions
This is one of the first fMRI studies to demonstrate significant differences between healthy groups with different risks for a future life threatening disorder. The results may suggest a brain mechanism that contributes to how a low LR might enhance the risk for future heavy drinking and alcohol dependence.
Keywords: alcohol, reaction, risk, fMRI
I. Introduction
The risk for heavy drinking and alcohol use disorders (AUDs) reflects both genes and environment (Goldman et al., 2005; Schuckit, 2009). Each of these domains of influence is heterogeneous, with the genes reflecting at least four separate families of risk factors. These include gene variations related to: alcohol metabolizing enzymes, impulsivity and disinhibition, several additional psychiatric disorders (e.g., schizophrenia and bipolar disorder), and a person's type of response to alcohol (Schuckit, 2009). Additional heterogeneity is observed within each of these families of genetic contributors. Using the level of response (LR) to alcohol as an example, some variance may relate to a person's susceptibility to alcohol's stress dampening effects, and some to possible exaggerated responses to alcohol administered rapidly and/or intravenously (Newlin and Renton, 2010). In addition, some results reflect a low LR that is primarily observed at peak and falling blood alcohol concentrations (BACs) (Schuckit and Gold, 1988).
Perhaps the most thoroughly studied of the LR-related phenomena is the low level of response (low LR) to alcohol that can be documented either through less intense reaction at a given blood alcohol concentration (BAC) or a retrospective report of the need for a larger number of drinks for a range of effects. Each of these measures indicates that low LR values are more often seen in the individuals at higher risk for AUDs (e.g., family histories of alcoholism or members of ethnic groups with higher rates of problematic drinking) (Chiu et al., 2004; Ehlers et al., 1998; Luczak et al., 2002), and are seen in individuals with recent heavy drinking and/or alcohol problems (Daeppen et al., 2000; Chung and Martin, 2009; Kerr et al., 2006). Perhaps most importantly, a lower LR at one point earlier in life predicts heavier drinking and more alcohol problems in the future, even after controlling for the original use and problem patterns (e.g., Chung and Martin, 2009; Heath et al., 1999; Schuckit et al, 2007, 2008, 2009; Volavka et al., 1996). Both alcohol challenge-based and retrospective questionnaire-based measures of LR have heritabilities of 40% to 60% (Heath et al., 1999; Schuckit et al., 2001; Vicken et al., 2003).
There is evidence that the low LR phenotype appears to operate relatively independently of other genetically influenced risk factors (e.g., alcohol metabolizing enzymes and impulsivity/externalizing conditions) (Schuckit, 2009; Vicken et al., 2003; Schuckit et al., 2000). This low LR characteristic also appears to reflect, at least in part, biological differences in brain response to ethanol. Support for this conclusion comes from results of alcohol challenges where, despite matching LR groups on recent drinking histories (i.e., differences in acquired tolerance across groups was unlikely) and evaluating them at identical BACs, those with a low LR demonstrated less alcohol-related increases in the anterior pituitary hormone, prolactin, less alcohol-related increases in adrenocorticotropin hormone (ACTH), as well as the subsequent diminished increase in cortisol after drinking (Schuckit and Gold, 1988; Schuckit et al., 1988b, 1987a, 1987b; King et al., 2006). LR group CNS differences after alcohol were also supported by electrophysiological measures where, despite similar BACs, those with a low LR evidenced less alcohol-related changes in background cortical electroencephalogram (EEG) measures in the alpha power range, and more evanescent prolongations of the latency of the P300 wave in event-related potentials (Gabrielli et al., 1991; Ehlers et al., 2004; Volavka et al., 1996; Schuckit et al., 1988a). Also, regarding differences in brain responses to alcohol across low and high LR groups, several variations in genes active in the brain have been reported to potentially relate to the low LR phenotype, including polymorphisms for the serotonin transporter, potassium channels, gamma-aminobutyric-acid receptor subunits, nicotinic receptors, and glutamate related systems (Joslyn et al., 2008, 2010; Schuckit, 2009).
Additional efforts to understand processes related to a low LR have used brain imaging. One study focused on arterial spin labeling (ASL) to compare alcohol-related changes in cerebral blood flow (CBF) between low and high LR groups matched on demography and substance use patterns (Tolentino et al., in press). Here, while the two LR groups were similar on CBF after placebo and each demonstrated the expected increase in CBF following alcohol, at identical BACs the low LR group evidenced significantly less increase in CBF with alcohol, especially in frontal regions. Related studies have used blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) to examine differences in response contrast between low and high LR subjects during performance of a visual working memory paradigm (Paulus et al., 2006; Tapert et al., 2004; Trim et al., 2010). These studies have consistently revealed that while the low and high LR groups showed similar behavioral task performance, the low LR relative to the high LR group demonstrated higher BOLD response contrasts during placebo or no substance challenge sessions. When imaging sessions were carried out following an alcohol challenge, the alcohol either greatly diminished the LR group differences that had been seen after placebo or reversed the direction of the LR-based imaging results, with relatively less activation following alcohol for the low LR and relatively more activation following alcohol for the high LR group (Paulus et al., 2006; Trim et al., 2010). These fMRI results were most prominent in frontal, parietal, and cingulate regions, but it is important to note that regional distributions of these LR-related findings were likely influenced by the cognitive demands of the visual working memory task.
It is of interest to determine whether similar LR group differences in BOLD response are observed with other cognitive tasks. Several recent fMRI studies have shown that alcohol affects functional brain activation during performance of tasks, such as the stop signal paradigm, that require inhibitory control, and that active alcoholics in treatment demonstrated altered functional brain responses in the prefrontal cortex, amygdala, insula, and putamen during performance of a stop signal task (SST) (Anderson et al., 2011; Li et al., 2009). However, regarding the latter, documenting differences between alcoholics and controls does not clarify whether similar findings would be observed prior to the onset of AUDs and in individuals with relatively higher levels of life functioning. To address this issue, we administered a SST to young nonalcoholic subjects with low and high LR in the fMRI environment (Li et al., 2009). While the low LR subjects have not shown abnormal levels of impulsivity or disinhibition,it is worthwhile to see if the LR groups differ in their BOLD response related to: 1) error processing (i.e., errors relative to correct trials), and 2) inhibitory processing (i.e., correct hard-relative-to-easy trials. This paper describes fMRI results from a large group of individuals who have had experience with alcohol but are not yet alcohol dependent, and who have been carefully characterized regarding their LR to alcohol.
II. Methods
Participants and Initial Evaluations
Following approval from the University of California, San Diego (UCSD) Human Research Protections Program committee regarding the entire protocol, 18-to-25-year-old subjects were identified from respondents to a structured questionnaire mailed to random students at UCSD. The items included a brief medical history and questions regarding the use of alcohol/tobacco/illicit substances and related problems, using items extracted from the validated Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Hesselbrock et al., 1999). These data identified individuals who had experience with alcohol, but who never met criteria for dependence on alcohol or illicit substances using criteria from the two most recent versions of the Diagnostic Statistical Manual (DSM) (American Psychiatric Association, 1987, 2000). To aid in a preliminary screen to identify subjects with higher and lower LRs, the mailing also included the retrospective Self-Report of the Effects of Alcohol (SRE) questionnaires, an instrument for measuring LR that has a Cronbach alpha > .90 and a one-year retest reliability of ~.8, as well as a well established relationship between SRE scores and future heavy drinking and alcohol-related problems (Ray et al., 2007; Schuckit et al., 2009). The SRE asks subjects to report the number of standard drinks (10-12 g ethanol) required to produce up to four effects during the approximate first five times of drinking, including the initial feelings of intoxication, the number of drinks required to slur speech, the drinks needed for unsteady or stumbling gait, and the amount of alcohol needed to produce an unwanted falling asleep – with individuals instructed to only report those effects they actually experienced. The SRE score in units of standard drinks for the first five times of drinking is established by summing the numbers of drinks reported and dividing that sum by the number of effects experienced with mean scores for that value that ranged from 1 to > 10 drinks.
Potential subjects were excluded if they had a history of medical problems or were taking medications likely to affect their LR or present a potential danger during drinking, if they ever had dependence on alcohol or illicit drugs, if they were pregnant, and if they reported any contraindications to the fMRI evaluation (left-handedness, irremovable metal, or claustrophobia). Subjects with a probable low LR per drink (as indicated by the need for a greater number of drinks to experience effects – e.g., a mean SRE score ≥4.0 drinks needed for effects) were selected as possible low LR subjects, and each was matched to a potential high LR individual (e.g., ≤ 3.0 drinks on the first-five SRE) on items that might affect the LR, including gender, age, as well as the recent six-month intake pattern of alcohol, nicotine, and illicit drugs.
Matched subjects provisionally determined on the SRE to have higher and lower LRs were then invited individually to the laboratory where they participated in a full face-to-face SSAGA interview to corroborate the preliminary history. If no exclusionary issues were identified, they were scheduled for an alcohol challenge session to definitively establish their LR status (Eng et al., 2005; Schuckit and Gold, 1988). During the challenge, subjects were first determined to have a zero breath alcohol concentration (BrAC) using an Intoximeter (St. Louis, MO), after which they were given 10 minutes to consume an alcoholic beverage (0.75 ml/kg for males and 0.70 for females to produce approximately equivalent blood alcohol levels) (Breslin et al., 1997). The beverage was mixed as a 20% by volume solution with carbonated, noncaffeinated soda and consumed through a straw extending from a thermos that obscured the actual beverage offered (Mendelson et al., 1984). BrAC and a measure of the subjective LR to alcohol using the Subjective High Assessment Scale (SHAS) were determined at baseline and at 15 minutes, 30 minutes and every subsequent half-hour over the > three hours of the challenge. The SHAS is composed of 13 items regarding potential effects of alcohol, each of which is gauged at each time point using a 39-point scale (e.g., Eng et al., 2005). Approximately three-fourths of the subjects determined on the SRE as likely to have high and low LRs were corroborated on alcohol challenges to be in the upper and lower thirds for LR after alcohol challenges and were invited to participate in two fMRI sessions. If either member of an original provisional pair was no longer in the appropriate ~upper and lower thirds of the LR range, an alternate subject from the relevant LR group was selected and evaluated as described above.
fMRI Sessions
Challenges using the same dose of alcohol and placebo (with the identical straw and container system but placebo had only 1.0 ml of ethanol suspended in the straw), were then conducted in random order just prior to an event-related fMRI session (the same order of beverages was given to members of each LR pair). Because a breathalyzer device could not be used in the scan room, subjects had an intravenous cannula placed in an antecubital vein in the nondominant arm so that BAC levels could be obtained during the scan session, using an analysis kit from Roche Pharmaceuticals that incorporated a photometric enzymatic approach. After the low and high LR individuals consumed the beverage (placebo or alcohol), they were placed into the scanner at the time of rising BACs, 22 minutes after the start of the beverage administration, with subsequent BACs, SHAS evaluations (reported verbally during the scan session), and performance of a stop signal task determined during imaging. In all alcohol sessions, subjects were not allowed to leave the laboratory until their BAC was < .01 g/dl, and they were escorted home by a friend, a member of the staff, or by taxi.
Scans were carried out with a 3 Tesla CXK4 scanner from General Electric (Milwaukee, WI) incorporating an eight-channel head array coil. A sagittal high resolution Spoiled Gradient Recalled anatomical sequence was acquired at the beginning of each session (25 cm field of view; 256 × 256 matrix; 172 1.0 mm thick slices; with 4.8 ms echo time, and 8 ms repetition time). The stop signal task was administered ~40 minutes after entering the scanner (~60 minute(s) since beginning alcohol administration. While performing the stop signal task, T2*-weighted echo planar imaging was carried out using 32 ms echo time, 90° flip angle, 3.43 × 3.43 × 2.6 mm voxels with a 1.4 mm gap, 30 whole brain axial slices, repetition time of 2,000 ms, and 256 repetitions. During the protocol, any possible LR group differences in alcohol-related changes in cerebral blood flow (CBF) were determined using arterial spin labeling (ASL) following the method described Liu and Wong (2005) involving a flow-sensitive alternating inversion recovery (FAIR) sequence. As described in more detail in a recent paper (Tolentino et al., in press), perfusion was determined using a single-subtraction saturation pulse (22 cm field of view, a 64 × 64 matrix, echo time = 3.2 ms, 2500 ms repetition time, inversion time = 600 ms, with an inversion time 2 of 1600 ms) (Wong et al., 1998).
The stop signal task used here was based on the approach of Matthews et al. (2005, 2009) in which subjects viewed an “X” or an “O” as “go” stimuli projected on a small computer screen with black background while in the scanner. Participants were instructed to press the left computer mouse button whenever they saw an “X,” to press the right button when they saw an “O,” and to inhibit pressing either mouse button when they heard a tone (the “stop” stimulus). In 25% of the trials, the go signal was followed by an auditory tone (i.e., the stop signal). Stop trials were pseudo-randomized throughout the task to avoid order bias. Each trial lasted ~1300 ms or until the subject issued a response, with a 200 ms interstimulus interval.
Prior to scanning, subjects performed an abbreviated SST outside the scanner. As reported in prior papers (Matthews et al., 2005, 2009), the timing of the subsequent scanner trials were then based on each individual's previously determined personal mean reaction time (MRT), which was calculated from successfully inhibited non-stop trials from the pre-scan session. During one 8.5 minute portion during the fMRI protocol, at about the time of peak BAC, each subject was given both individualized hard to inhibit stop signals (where the tone was delivered at or close to the person's own MRT (i.e., MRT, 100 ms less than MRT, or 200 ms less than MRT), and individualized easy trials (where the tone was delivered at 300 ms less than MRT, up to 400 ms less, or up to 500 ms less than MRT). While the reaction time was established for each individual, all subjects received the same number of hard and easy trials. Subjects performed 288 total trials, including 72 stop trials which were pseudo-randomized through the task, and counterbalanced. Six blocks were performed, each containing 48 trials (12 stop and 36 non-stop) with task instructions presented for 12s between blocks. The two major scores for this task include the percentage of correct responses during hard to inhibit and easy to inhibit conditions (reflecting an individual's ability to successfully inhibit during the two types of trials), as well as the mean reaction time during the sessions overall.
Data Processing and Analyses
The focus of the analyses was on whole brain BOLD contrasts related to two performance measures: errors (i.e., unsuccessfully inhibited) relative to correct trials, and performance during harder vs. easier to inhibit trials. In these contrasts, all functional image processing incorporated Analysis of Functional NeuroImages software (AFNI; http://afni.nimh.nih.gov; Cox, 1996). The effects of head motion were corrected through co-registering each repetition to the maximally stable base image using a 6-parameter algorithm (3dvolreg). Analysis of time series data utilized multiple regression (3dDeconvolve) controlling for baseline signal, linear drift, and motion corrections applied in three rotational orientations. Task regressors were multiplied, or convolved, with a modified gamma variate function (Boynton et al., 1996) that modeled anticipated hemodynamic response. Data were resampled to 4 × 4 × 4 mm voxels, a Gaussian filter (FWHM 4 mm) was used to account for anatomical variations for individuals, and the data for each subject were transformed to standard space (Talairach and Tournoux, 1988). Where needed, the percent signal change was generated through dividing signal in during the task by the baseline signal in each voxel.
In these whole brain analyses, for regions demonstrating significant LR group differences on the key measures, group by condition interaction effects were determined through follow-up 3dANOVA3s carried out in AFNI, which used thresholds that considered the intensity of the statistical effect of each voxel, as well as the number of contiguous voxels with the same effect. Monte Carlo simulations were used to guard against Type I error, as employed within AFNI using AlphaSim to determine the number of contiguous voxels each with an effect of p < 0.01, yielding an overall volume-wise < 5% probability of a false-positive finding. For regions with significant contrast, the average percent signal change was evaluated for each participant from each condition using ANCOVA to control for the effects of any background characteristic that might have been significantly between LR groups. Confirmation of labels for brain activation used Talairach Daemon software (Lancaster et al., 2000). CBF for any brain regions with significant BOLD response effects were determined using the method of Liu and Wong (2005), as described above.
III. Results
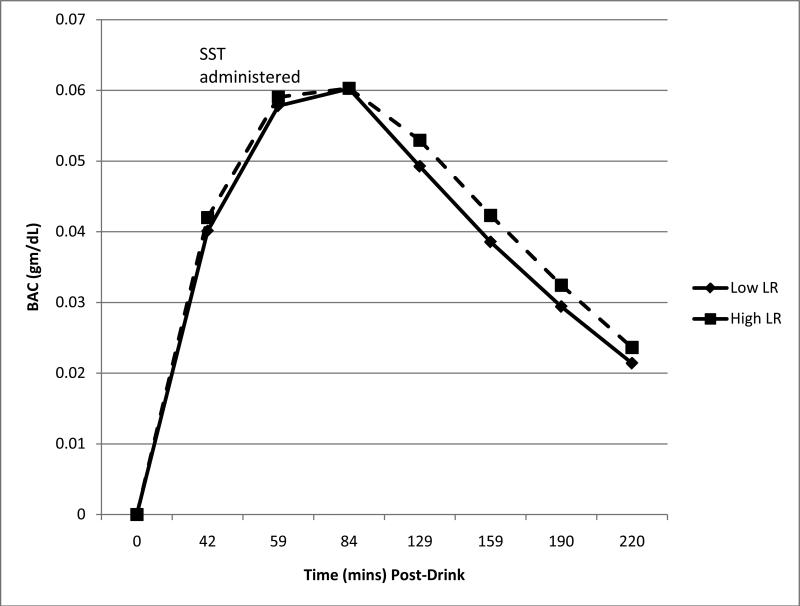
The 98 subjects evaluated here were composed of 49 matched pairs of low and high responders to alcohol. As shown in Table 1, the groups had an average age of about 20 years, 53% were female, and most were in their second year of college. None met criteria for alcohol or drug dependence, and all had tried alcohol. Low LR and high LR groups were similar on demography, the past six month tobacco and cannabis use, and on lifetime conduct-related problems as reported on the SSAGA interview. As shown in Table 1 and Figure 1, the BACs at 60 minutes during the alcohol challenge session were identical between the two groups, and the patterns of rising, peak and falling BACs over the 220 minutes of the test session were very similar. Consistent with the hypothesis that a low LR to alcohol contributes to the quantity of alcohol intake, the low responders consumed more drinks per occasion, although they did not drink more frequently than the high responders. Reflecting these data, the major analyses reported below control for differences in the usual drinking quantity.
Table 1.
Comparison of Participant Characteristics as Percents and Mean (and Standard Deviations) Across Low and High Level of Response to Alcohol (LR) Matched-Pair Groups
| Low LR (n=49) M (SD) or % | High LR (n=49) M (SD) or % | t-statistic or χ2 (p value) | |
|---|---|---|---|
| Age (years) | 19.8 (1.47) | 20.2 (1.56) | -1.40 (.17) |
| % Female | 53.1 | 53.1 | -- |
| Years of education completed | 13.6 (1.12) | 13.7 (1.21) | -0.44 (.67) |
| Height (inches) | 68.5 (4.05) | 68.1 (3.92) | 0.41 (.69) |
| Weight (pounds) | 155.7 (25.66) | 153.6 (25.69) | 0.40 (.69) |
| Days/month used alcohola | 4.6 (4.48) | 3.8 (3.92) | 0.91 (.36) |
| Usual drinks per occasion*a | 4.0 (1.79) | 3.2 (1.85) | 2.17 (.03) |
| Days/month of tobacco usea | 0.5 (1.08) | 1.2 (3.32) | -1.35 (.18) |
| Tobacco units/occasiona | 0.3 (0.55) | 0.2 (0.47) | 0.79 (.43) |
| % Ever used cannabis | 59.2 | 46.9 | 1.48 (.23) |
| Lifetime cannabis use occasions | 28.1 (78.97) | 20.1 (73.86) | 0.52 (.60) |
| Number of conduct problems | 0.41 (0.64) | 0.31 (0.59) | 0.82 (.41) |
| BAC at 60 minutes (mg/dL) | 0.06 (0.02) | 0.06 (0.02) | -0.32 (.75) |
p < .05
data for prior 6 months
Figure 1.
Blood alcohol concentration (BAC) measured at baseline through 84 minutes, and breath alcohol concentration (BrAC) for 129 to 220 minutes for low and high LR groups (N=98). No significant group differences at any time point (p's>.05).
Table 2 presents the comparison of low and high responders on the two key performance measures of the stop signal task during placebo and alcohol conditions. The groups did not differ on mean reaction time, or percent of stop signal trials with successfully inhibited responses. A mixed design ANOVA for reaction time indicated no LR effect (F = 0.08, p = .78) nor a LR by condition (F = 0.08, p = .37) interaction. A similar analysis for correctly inhibited responses revealed no LR group effect (F = 0.00, p = .99), and no LR group by placebo/alcohol condition interaction (F = 0.01, p = .93). Thus, the imaging data can be contrasted between low LR and high LR subjects without concern for group differences in task performance.
Table 2.
Comparisons of Behavioral Performance on Stop Signal Task for Low and High Level of Response to Alcohol (LR) Matched-Pair Groups
| Mean Reaction Time (msec) | |||
|---|---|---|---|
| Low LR (n=49) M (SD) | High LR (n=49) M (SD) | t (p value) | |
| Placebo | 649.92 (155.94) | 645.98 (144.90) | 0.13 (.90) |
| Alcohol | 648.78 (153.27) | 667.90 (140.31) | -0.64 (.52) |
| % Responses Successfully Inhibited | ||||
|---|---|---|---|---|
| Low LR (n=49) M (SD) | High LR (n=49) M (SD) | t (p value) | ||
| Placebo | Easy | 90.60 (9.17) | 89.74 (8.08) | 0.49 (.62) |
| Hard | 55.01 (19.45) | 56.12 (17.35) | -0.30 (.77) | |
| Alcohol | Easy | 88.52 (12.23) | 87.95 (9.63) | 0.26 (.80) |
| Hard | 53.73 (18.43) | 54.07 (16.82) | -0.10 (.92) | |
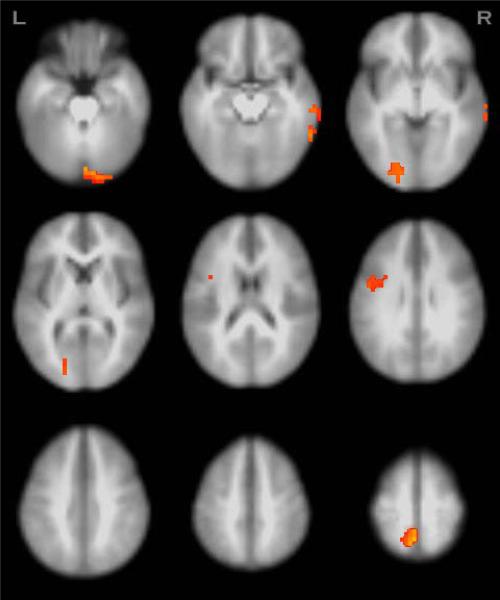
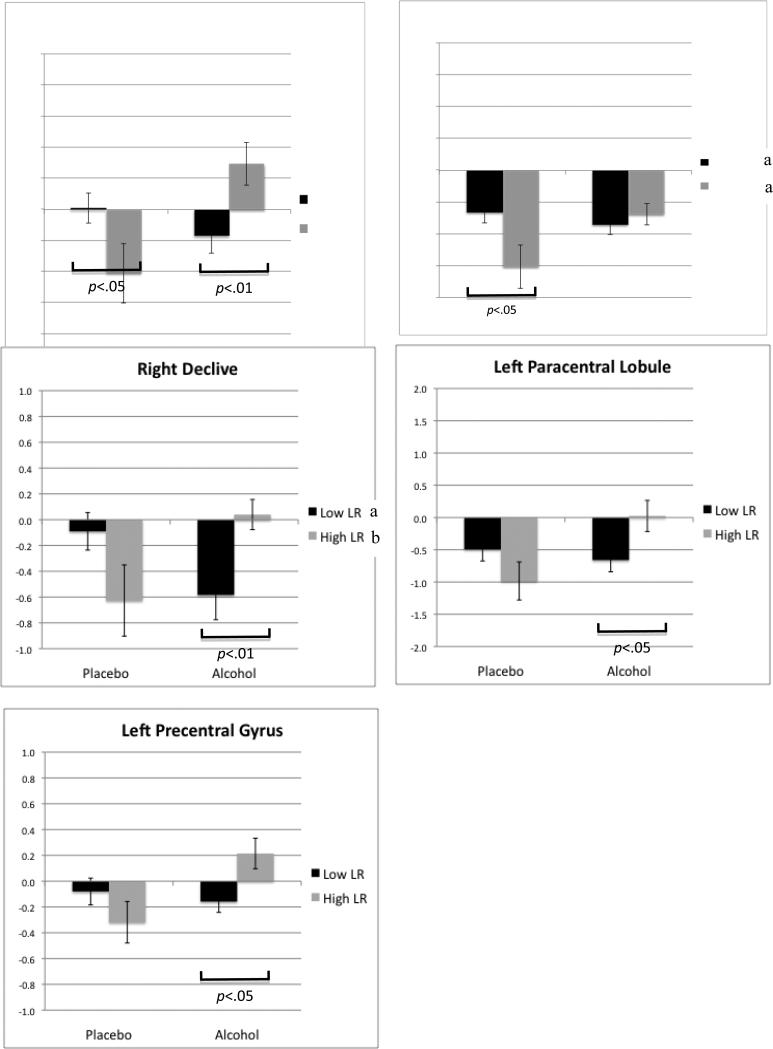
Tables 3 and 4, and Figures 2 to 5 present data regarding clusters from whole brain analyses showing significant LR group main effects or group by condition interaction (alcohol/placebo) effects on task-related functional brain activity. Beginning with Table 3, the columns indicate first the brain regions showing a group by condition effect for error trials relative to correct response trials, and then the corresponding cluster volume, Talairach coordinates, and percent signal change in BOLD response for errors relative to correct trials for each group during each condition. In general, the low LR group demonstrated less error-related contrast after consuming a moderate dose of alcohol as compared to placebo, with the trend for the high LR group in the opposite direction (relatively more error-related contrast with alcohol than placebo). While the statistical analyses revealed no significant LR group differences overall, there were consistent LR group by alcohol/placebo condition interactions for all five regions (see Figure 4).
Table 3.
Regions Showing Significant Group by Condition Interaction Effects (p <.05, clusters ≥1344 μl) for BOLD Signal for Errors Relative to Correct Responses
| Anatomic Regionx | Brodmann Areas | Volume (μl) | Talairachx | Low LR | High LR | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Placebo Mean | Alcohol Mean | Placebo Mean | Alcohol Mean | |||
| R middle temporal gyrus, extending to the R inferior temporal gyrus | 37,21,20 | 3072 | -58 | 49 | -8 | 0.01a | -0.17b | -0.41 | 0.30 |
| L lingual gyrus, extending to the L cuneus | 18,17 | 1792 | 22 | 77 | -4 | -0.67aaa | -0.86 | -1.51aa | -0.69 |
| R declive, extending to the R uvula | -- | 1600 | -6 | 85 | -16 | -0.09aa | -0.58b | -0.63bb | 0.04 |
| L paracentral lobule, extending to the L precuneus | 5,7 | 1600 | 2 | 45 | 64 | -0.49 | -0.66a | -0.98 | 0.02 |
| L precentral gyrus, extending to the L inferior frontal gyrus | 6,9 | 1472 | 46 | 1 | 28 | -0.08 | -0.16a | -0.32 | 0.21 |
Talairach coordinates refer to peak effect group difference within the cluster.
Abbreviations: R right; L left; LR level of response to alcohol.
Follow-up t-test showing significant group differences for the condition, p<.05
Follow-up t-test showing significant group differences for the condition, p<.01
Follow-up t-test showing significant condition effects within the group, p<.05
Follow-up t-test showing significant condition effects within the group, p<.01
Table 4.
Regions Showing Significant Group or Group by Condition Effect (p <.05, clusters ≥1344 μl) in BOLD Response Contrast for Hard Relative to Easy Inhibition Trials
| Anatomic Regionx | Brodmann Areas | Volume (μl) | Talairachx | Low LR | High LR | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Placebo Mean | Alcohol Mean | Placebo Mean | Alcohol Mean | |||
| L superior frontal gyrus, extending to the L medial frontal gyrus, L precentral gyrus, L middle frontal gyrus, L cingulate gyrus, and L paracentral lobuley | 6,31,5 | 7360 | 10 | 1 | 68 | 0.05a | 0.12b | -0.12 | -0.14 |
| L anterior cingulate, extending to L cingulate gyrus, L medial frontal gyrus, L middle frontal gyrusy | 32,9,24 | 3968 | 2 | -27 | 24 | -0.04 | 0.10b | -0.20 | -0.18 |
| L lingual gyrus, extending to L middle occipital gyrus, L cuneusy | 18,19,17 | 5120 | 18 | 89 | -8 | -0.29b | -0.25b | 0.06 | 0.13 |
| R cuneus, extending to the R middle occipital gyrusy | 18,19 | 6016 | -10 | 89 | 12 | -0.32a | -0.30b | 0.01 | 0.09 |
| L angular gyrus, extending to L inferior parietal lobule and L precuneusz | 39,19 | 2560 | 50 | 61 | 36 | 0.01aa | -0.18a | -0.21 | 0.13 |
| R fusiform gyrus, extending to R lingual gyrus and R inferior occipital gyrusz | 18,17 | 4480 | -18 | 89 | -12 | 0.07 | -0.34a | -0.16aa | 0.29 |
Talairach coordinates refer to peak effect group difference within the cluster.
Abbreviations: R right; L left; LR level of response to alcohol.
Significant group (Low vs. High LR) main effect.
Significant group by condition interaction effect.
Follow-up t-test showing significant group differences for the condition, p<.05
Follow-up t-test showing significant group differences for the condition, p<.01
Follow-up t-test showing significant condition effects within the group, p<.05
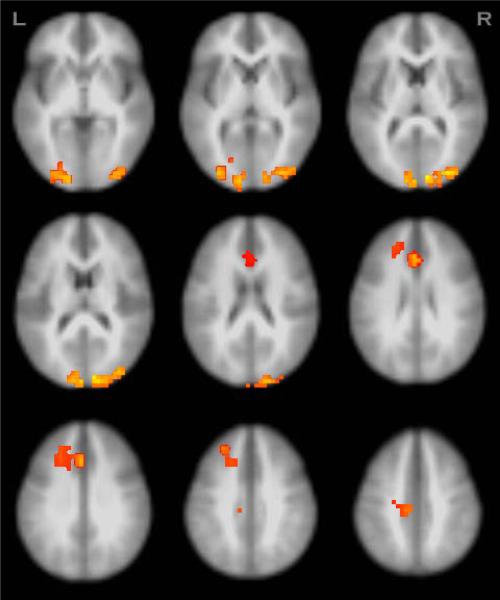
Figure 2.
Regions showing significant group by condition interaction effects in BOLD signal from erroneous relative to correct responses (N=98, p<.05, clusters≥1344μl).
Figure 5.
Graphs with standard error bars for each significant group main effect or group by condition interaction effect in BOLD response contrast for hard relative to easy inhibition trials (p<.05, clusters≥1344 μl). Significant differences across high and low LR groups with placebo and with alcohol conditions are indicated with a bracket under the bars. Significant differences within each LR group across alcohol and placebo conditions are noted in each graph's legend (a p<.05, b p<.01).
Figure 4.
Graphs with standard error bars for each significant group by condition interaction in BOLD signal contrast for erroneous relative to correct response trials (p<.05, clusters≥1344 μl). Significant differences across high and low LR groups with placebo and with alcohol conditions are indicated with a bracket under the bars. Significant differences within each LR group across alcohol and placebo conditions are noted in each graph's legend (a p<.05, b p<.01).
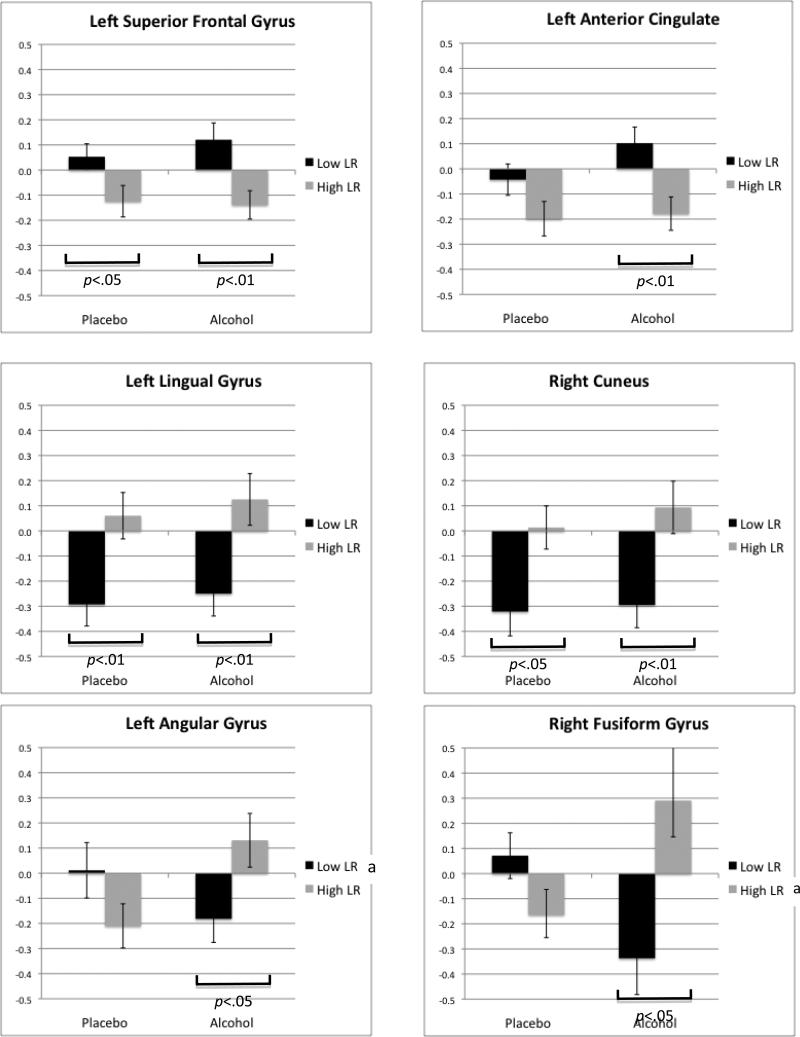
Table 4 describes results for the six clusters showing significant group effects or group by condition interactions on BOLD response for hard to inhibit (i.e., where the stop signal was delivered at or near the subjects’ mean reaction time in the paradigm) versus the easier inhibition trials (i.e., where the stop signal was delivered earlier in time relative to the subjects’ mean reaction time). In four of the six regions there were significant BOLD response differences in inhibition-related functional activity between low and high LR groups (see Figure 5). In two left anterior regions (left superior frontal gyrus and left anterior cingulate cortex), low LR relative to high LR groups showed greater BOLD response contrast for both placebo and alcohol conditions. In two visual cortex regions (left lingual gyrus and right cuneus), low LR subjects showed less BOLD response contrast than high LR subjects. In two posterior temporal regions (left angular gyrus and right fusiform gyrus), interactive effects similar to those in Table 3 and Figure 4 were observed; in each the low LR group showed decreasing BOLD response contrast to hard versus easier to inhibit trials after alcohol as compared to placebo, while the high LR group showed increasing activation after alcohol.
The next step in the analyses was carried out in recognition of the differences in usual drinks per occasion over the prior six months as reported for low and high LR groups in Table 1, as well as the LR group differences in alcohol-related increases in CBF reported in a prior publication (Tolentino et al., in press). Reflecting these data, the statistical analyses reported for Tables 3 and 4 were repeated after covarying for CBF and the usual drinks per occasion. Results indicated all significant effects shared in Tables 3 and 4 remained significant after controlling for usual drinks per occasion and after controlling for CBF.
In summary, Tables 3 and 4 identified seven clusters with LR group by alcohol/placebo condition interactions. In each, the low LR group showed decreasing BOLD response contrast from placebo to alcohol sessions, while the high LR group demonstrated the opposite (increasing BOLD response contrast from placebo to alcohol conditions). These results were seen for both the errors relative to correct responses and the hard relative to easy inhibition trials. LR group differences were observed in four clusters but only for hard relative to easy inhibition trials, with low LR participants showing more activation in frontal regions and less response in visual cortex across conditions as compared to high LR subjects.
IV. Discussion
These results support three major conclusions. First, consistent with the documentation of physiologic differences across LR groups in prior evaluations (e.g., Schuckit et al., 1987a, 1987b, 1988a, 1988b; Tolentino et al., in press), subjects with low relative to high LR values showed altered functional brain responses during error and inhibition processing. Second, also consistent with prior studies (Paulus et al., 2006; Tapert et al., 2004; Tolentino et al., in press; Trim et al., 2010), low and high LR subjects were different during both placebo and alcohol sessions. Third, there were significant interactions between LR status and alcohol versus placebo conditions, with low LR individuals typically showing more activity to erroneous or difficult trials under placebo conditions than matched high LR individuals, but less activity after a moderate dose of alcohol. These results were observed at identical BACs for the two LR groups, and after careful matching on demography, smoking and illicit substance use patterns, as well as most aspects of drinking histories. It is important to note that our findings are among the first reports indicating that a phenotype relatively closely associated with the future risk for a major psychiatric disorder (alcoholism) can be identified using fMRI. At the time of study, the subjects had no major psychiatric disorders, and while they had experience with alcohol, did not meet the criteria for alcohol dependence.
Using our stop signal task, the low and high LR groups did not exhibit behavioral differences (e.g., more errors), suggesting that the observed group fMRI differences were not confounded by behavioral differences between groups. In fact, prior studies have not demonstrated a significant relationship between LR and impulsivity or disinhibition (Schuckit et al., 2000; Schuckit and Smith, 2006). In the current stop signal task and similar to prior studies using a visual working memory task (Tapert et al., 2004; Trim et al., 2010), the high and low LR groups differed on measures of brain activity, but not on task performance. The only two dependent variables available for the major cognitive measures evaluated in the current paradigm involved commission of stop signal errors and reaction time, and, therefore, our findings should not be interpreted to indicate that the LR groups were behaviorally different. The similarities in performance facilitate a direct comparison between groups on BOLD response contrast between task conditions.
The LR group differences were not likely to reflect acquired or intersession tolerance for several reasons. The relevant analyses covaried for recent drinking quantities, and the rates of disappearance of alcohol in Figure 1 were very similar across the two LR groups, indicating no evidence of group differences on pharmacokinetic tolerance. Past studies have documented a correlation between a low LR and heavier drinking in 12-year-olds who have only consumed alcohol on few occasions, with an average intake of three drinks per instance, and the low LR predicted heavy drinking outcomes in individuals who were very light drinkers when first evaluated and unlikely to have developed tolerance. Earlier studies have demonstrated that the low LR and tolerance perform independently in predicting future drinking, and the relationships of a low LR to future heavier drinking and alcohol problems have remained robust even after controlling for prior drinking patterns at the time LR was measured (Schuckit et al., 2007, 2008, 2010).
For the five regions that were differentially affected during error versus correct trials for the low and high LR groups (i.e., in Figures 2 and 4), subjects with low LR demonstrated relatively less BOLD response contrast (i.e., less difference between error and correct trials), whereas those with high LR showed higher error-related BOLD response contrast values (i.e., relative more error response) after alcohol compared to placebo. A similar pattern of BOLD response contrast was observed in two of the six areas showing a significant LR group by alcohol/placebo interaction for hard versus easy inhibition conditions.
Taken together, these findings underscore the existence of biological correlates of the low LR and suggest consistent and potentially important differences in functional brain activity between low and high LR groups after both placebo and alcohol. The results of this and prior studies indicate that some LR group differences reliably exist with no challenge (e.g., Tapert et al., 2004) and with placebo (Paulus et al., 2006; Trim et al., 2010; and this paper). That occurs despite no impaired performance on the cognitive task. The data also document that LR groups differ after alcohol, and that the directions of change between placebo and alcohol are opposite for low- and high-LR groups. We speculate below that the results may help explain why low LR subjects perceive less difference from placebo to alcohol (i.e., feel less alcohol effect). That is interesting, and also potentially important.
However, the underlying brain mechanisms central to the LR phenomenon are likely to be complex and heterogeneous. The mechanisms might relate to the wide range of genetic polymorphisms that have been highlighted in our prior research, as these could impact on CNS functioning in subtle ways. Or, perhaps, some additional phenomena that haven't yet been identified might contribute to both the low LR and CNS differences between LR groups during cognitive tasks. The current results underscore that the differences between LR groups are real and deserve further study.
It is important to note that, in the whole brain analyses reported here, most of the areas that were differentially activated or deactivated across LR groups were in more posterior brain regions known to be relevant to visual and auditory perception. For example, the fusiform gyrus has been found to activate in concert with increasing local detail in visual stimuli, while lingual gyrus activation has been found to increase with added global visual processing complexity demands (Mechelli et al., 2000). Under placebo conditions, the low LR group appeared to rely more on this local visual processing center to approach the hard trials, while the high LR group relied more on the global-oriented area. After a moderate dose of alcohol, both groups remained similar in use of global processing resources, but local processing-related activation increased only for high LR individuals. The general absence of significant LR-group differences in more frontal regions often associated with inhibition tasks is consistent with both the similar performance for low and high LR subjects, and the selection of a non-clinical sample of students not likely to show high levels of impulsivity.
The results may indicate that in the brains of high LR subjects recognition of the level of performance on a task was relatively clear following placebo, but less clear after alcohol. The direction of the difference from placebo to alcohol might contribute to a relatively distinct appraisal of the effects of intoxication. In contrast, at the fixed and modest dose of alcohol used in this experiment, those with a low LR may have felt less affected by the drink and more clearly perceived how they were performing after alcohol compared to placebo. To those with low LR, despite BACs and drinking patterns similar to those of their high LR controls, the alcohol may not have been interpreted as associated with impairment or the appraisal of being in a perturbed state of functioning. This appraisal among those with a low LR of less effort (and possibly less stress) associated with performing a task after drinking might also have contributed to prior findings of less alcohol-related increases in stress hormones of CNS origin such as ACTH (Schuckit et al., 1987a, 1988b). The appraisal of an ability to more accurately recognize errors in performance after drinking might also relate to less change in the alcohol-related alterations in background cortical EEG power in the alpha range often associated with feelings of relaxation (Ehlers et al., 2004) and less effect on event-related potential P3 latency when asked to recognize a rare but difficult to identify stimulus (Schuckit et al., 1988a) for low LR subjects.
In addition to the interaction effects discussed above, four main effects of the LR group were observed for hard- relative to easy-to-inhibit trials on the stop signal task. Consistent with prior findings that low LR subjects utilized more neural resources to complete a task under placebo conditions, here we saw low LR subjects showing more BOLD response contrast than high LR subjects in the superior frontal gyrus and anterior cingulate; yet, this elevated activity persisted after the moderate alcohol dose. In contrast, low LR subjects showed less visual cortex activation (BA 18 and 19, left and right) during placebo and alcohol conditions, for the difficult inhibition trials. This may suggest a tendency for the low LR individuals to rely more on frontal circuitry and less on visual system input when responding to particularly challenging inhibitory demands, and this frontal hyperactive/visual hypoactive difference persisted despite the moderate does of alcohol.
An additional comment is required regarding the absence of a significant alcohol-related change in behavioral performance on the stop signal task. It is important to note that the dose of alcohol used in this experiment was modest, and a bit lower than those incorporated in some prior work in the literature that reported a main effect for alcohol (e.g., Fillmore and Vogel-Sprott, 1999). The use of an fMRI protocol required that we select an alcohol load not likely to produce the nausea and vomiting that might be seen after much higher doses, as those would have had adverse effects on both subjects and fMRI equipment. We also chose a dose similar to the alcohol challenges incorporated in our prior work. Nonetheless, several prior studies reported relatively little general effect of alcohol on the overall performance measures reported here (Fillmore and Blackburn, 2002; Weafer and Fillmore, 2008), and significant BOLD contrast differences between the LR groups were documented in the current study.
A note regarding the selection of the stop signal task is also warranted. One advantage of this paradigm is that hard and easy trials were individualized based on each subject's MRT. This cognitive challenge was chosen in light of the relatively extensive work with the stop signal task that had been carried out in prior fMRI paradigms (e.g., Anderson et al., 2011; Matthews et al., 2009; Li et al, 2009). These included significant differences between alcoholics and controls, as well as fMRI studies of impulsivity and disinhibition, factors generally associated with an increased risk for a wide range of substance use disorders. The current subjects, however, were not alcohol dependent, the low and high LR groups were similar on the number of conduct problems, and prior work with similar LR groups has not supported impressive elevations in externalizing characteristics for subjects selected using inclusion and exclusion criteria similar to the current study (Schuckit and Smith, 2006). In addition, most evaluations of individuals with a low LR to alcohol have found little evidence of a significant connection between the low LR and elevated scores for externalizing measures (e.g., Schuckit et al., 2000). Thus, in the current sample, it is unlikely that LR group differences reflected externalizing characteristics, a finding underscored by the visual working memory task data and the cerebral blood flow data, characteristics not likely to relate to externalizing conditions (Tolentino et al., in press; Trim et al., 2010).
The current study and results must be viewed from the standpoint of the methods used. The analyses focused on a large group of individuals and required 196 fMRI sessions (alcohol and placebo evaluations for 49 high LR/low LR pairs), along with 98 alcohol challenge sessions prior to the fMRI analyses (to corroborate the preliminary LR group assignment) and 98 sessions for carrying out with the validated SSAGA interview to establish baseline functioning. The results remained robust after controlling for the modest differences in the usual quantity of drinking and for any possible differences between LR groups on changes in cerebral blood flow. However, the population studied here was Caucasian or white Hispanic, and relatively highly educated. Therefore, the generalizability of these results to other ethnic groups and those from different socioeconomic strata will need to be determined. Also each subject performance only one run on the task which may have affected statistical power for some measures (e.g., errors). This may have contributed to the relatively small effect size of some of the findings. However, in prior studies using the same paradigm (Matthews et al., 2005, 2009), there was sufficient power for detecting significant task and group effects. Finally, as indicated earlier in this discussion, while it is not likely that acquired tolerance explains our results, this possibility cannot be definitely discarded.
Figure 3.
Regions showing significant group main effects or group by condition interaction effects in BOLD response contrast for hard relative to easy inhibition (N=98, p<.05, clusters≥1344μl).
Acknowledgments
This work was supported by NIAAA grant 5R01 AA15760
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IIIR) American Psychiatric Association; Washington DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:1–10. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin FC, Kapur BM, Sobell MB, Cappell H. Gender and alcohol dosing: a procedure for producing comparable breath alcohol curves for men and women. Alcohol Clin Exp Res. 1997;21:928–930. doi: 10.1111/j.1530-0277.1997.tb03860.x. [DOI] [PubMed] [Google Scholar]
- Chiu TM, Mendelson JH, Sholar MB, Mutschler NH, Wines JD, Hesselbrock VM, Mello NK. Brain alcohol detectability in human subjects with and without a paternal history of alcoholism. J Stud Alcohol. 2004;65:16–21. doi: 10.15288/jsa.2004.65.16. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. J Stud Alcohol Drugs. 2009;70:660–667. doi: 10.15288/jsad.2009.70.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Landry U, Pecoud A, Decrey H, Yersin B. A measure of the intensity of response to alcohol to screen for alcohol use disorders in primary care. Alcohol Alcohol. 2000;35:625–627. doi: 10.1093/alcalc/35.6.625. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Sobel DF, Phillips E. Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology. 1998;18:282–292. doi: 10.1016/S0893-133X(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wall TL, Wilhelmsen K, Schuckit MA. EEG alpha and level of response to alcohol in Hispanic- and non-Hispanic-American young adults with a family history of alcoholism. J Stud Alcohol. 2004;65:301–308. doi: 10.15288/jsa.2004.65.301. [DOI] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Gabrielli WF, Jr, Nagoshi CT, Rhea SA, Wilson JR. Anticipated and subjective sensitivities to alcohol. J Stud Alcohol. 1991;52:205–214. doi: 10.15288/jsa.1991.52.205. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden AF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh W, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit MA, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit MA, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcohol Clin Exp Res. 2010;34:800–812. doi: 10.1111/j.1530-0277.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Midanik LT. How many drinks does it take you to feel drunk? Trends and predictors for subjective drunkenness. Addiction. 2006;101:1428–1437. doi: 10.1111/j.1360-0443.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport. 2005;16:755–760. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, McGuire M, Mello NK. A new device for administering placebo alcohol. Alcohol. 1984;1:417–419. doi: 10.1016/0741-8329(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Michelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 2000;267:1909–2013. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. author reply 203-5. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Audette A, DiCristoforo S, Odell K, Kaiser A, Hutchison KE. Behavioral, laboratory, and genetic correlates of low level of response to alcohol. Alcohol Clin Exp Res. 2007;31:131A. [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich L, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;35:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987a;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Serum prolactin levels in sons of alcoholics and control subjects. Am J Psychiatry. 1987b;144:854–859. doi: 10.1176/ajp.144.7.854. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO, Croot K, Finn P, Polich J. P300 latency after ethanol ingestion in sons of alcoholics and in controls. Biol Psychiatry. 1988a;24:310–315. doi: 10.1016/0006-3223(88)90199-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO. Alcohol consumption, ACTH level, and family history of alcoholism. Am J Psychiatry. 1988b;145:1391–1395. doi: 10.1176/ajp.145.11.1391. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs. 2007;68:371–378. doi: 10.15288/jsad.2007.68.371. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Trim R, Bucholz KK, Edenberg HJ, Hesselbrock V, Kramer JJ, Dick DM. An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol Drugs. 2009;70:436–445. doi: 10.15288/jsad.2009.70.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith T, Kalmijn J, Raimo E. The relationship of a low response to alcohol to ERP and personality measures. Alcohol Clin Exp Res. 2000;24:27A. [Google Scholar]
- Schuckit MA, Smith TL. The relationship of behavioural undercontrol to alcoholism in higher-functioning adults. Drug Alcohol Rev. 2006;25:393–402. doi: 10.1080/09595230600876697. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Fukukura T, Allen R. The overlap in predicting alcohol outcome for two measures of the level of response to alcohol. Alcohol Clin Exp Res. 2009;33:563–569. doi: 10.1111/j.1530-0277.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Heron J, Horwood J, Davis J, Hibbeln J, the ALSPAC Study Team The self-rating of the effects of alcohol questionnaire as a predictor of alcohol-related outcomes in 12-year-old subjects. Alcohol Alcohol. 2008;43:641–646. doi: 10.1093/alcalc/agn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01435.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim RS, Simmons AN, Tolentino NJ, Hall SA, Matthews SC, Robinson SK, Smith TL, Padula CB, Paulus MP, Tapert SF, Schuckit MA. Acute ethanol effects on brain activation in low- and high-level responders to alcohol. Alcohol Clin Exp Res. 2010;34:1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Goodwin DW, Gabrielli WF, Jr, Penick EC, Mednick SA, Jensen P, Knop J, Schulsinger F. The electroencephalogram after alcohol administration in high-risk men and the development of alcohol use disorders 10 years later. Arch Gen Psychiatry. 1996;53:258–263. doi: 10.1001/archpsyc.1996.01830030080012. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology (Berl) 2008;201:315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]