Abstract
Group B streptococcus (GBS), a major cause of sepsis, induces inflammatory cytokines in strict dependence of bacterial ssRNA and the host molecules MyD88 und UNC-93B. Here, we show that nitric oxide plays an important role in GBS-induced transcriptional activation of cytokine genes. Phagocytosis induced NO in a MyD88-dependent fashion. In turn, NO propagated the acidification of phagosomes and the processing of phagosomal bacterial nucleic acids and was required for potent transcriptional activation of cytokine genes by streptococci. This NO-dependent amplification loop has important mechanistic implications for the anti-streptococcal macrophage response and sepsis pathogenesis.
Keywords: Bacterial infection, RNA, phagocytosis, signal transduction
INTRODUCTION
Streptococci are constituents of the normal mucosal flora. However, once they become invasive they are a significant life threat. Resident tissue macrophages are the outposts of innate immunity. Their primary role is that of sentinel cells. They orchestrate the inflammatory response to single streptococci that have broken through the mucosal surface and reach the subepithelial space. Accordingly, a balanced macrophage response is pivotal for host-microbe coexistance.
Streptococci are typical extracellular bacteria. Yet, the predominantly extracellular life style does not mean that these bacteria are recognized at the cytoplasmic membrane. Clearly, free streptococcal lipoproteins activate the cell surface receptors TLR2/6 and CD14 (1, 2). In contrast, streptococcal organisms as particulate matter do not essentially engage any of the single surface TLRs known to instruct the inflammatory cytokine response to other bacteria (1, 3). Moreover, streptococcal ssRNA, and not the “visible” bacterial surface molecules, such as capsular polysaccharides, lipoteichoic acid or lipoproteins, is the dominant cytokine-inducing molecule in streptococcal organisms (4).
The observation that uptake and lysosomal processing are prerequisites for the transcriptional activation of inflammatory cytokines (5, 6) suggests that the modification of ligand-receptor interactions by the microenvironment of the maturing phagosome is important for inflammatory gene activation. The sampling process of bacterial particles is not a static event, it is a dynamic process of reciprocal modifications on both sides of the host-microbial interface.
An initiating event of bacteria-macrophage interaction in macrophages is the closure of the phagocytic cup. Then follows the formation of specific endosomal pattern recognition receptors and cytoplasmic multiprotein complexes (7). At the same time, host and bacterial molecules are modified, in particular through the three principal activities comprising pH shift, activation of lysosomal proteases and the oxidative forces, reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI). These activities are closely interdependent. For example, ROI indirectly liberate lysosomal proteases from the endosomal proteoglycan matrix (8) and thereby propagate their activity. These processes are likely to transfer specific functional flavors to receptors expressed on both the endosomal membranes, and ligands being accessible for these receptors. It has been reported that inducible nitric oxide synthetase positively regulates the formation of inflammatory cytokines (9). However, the molecular link between lysosomotropic modification of the host-bacteria interface and regulation of inflammatory cytokine genes is still not completely understood.
We report here that the type I macrophage response to Gram-positive bacteria is dependent on phagolysosomal processing of bacterial RNA by a mechanism involving the MyD88 dependent formation of NO and the subsequent acidification of the phagolysosome. This process is accompanied by processing of streptococcal RNA in the endosomal compartment, which finally results in the transcriptional activation of inflammatory cytokine genes.
MATERIAL AND METHODS
Animals and cell lines
Mice lacking MyD88 and those lacking TLRs were kindly provided by Dr. S. Akira, Department of Host Defense, Osaka University (Osaka, Japan). Mice with an UNC-93B-H412R (3D) mutation were kindly provided by Bruce Beutler (LaJolla, CA). Mice lacking iNOS, gp91Phox and C57BL/6J mice were purchased from The Jackson Laboratory inc. The mice used in the study were backcrossed onto C57BL/6J mice according to (10–15).
Macrophage infection
The GBS type III strain COH1 was originally isolated from a newborn infant with sepsis, was cultured to exponential grown phase in chemically defined medium and heat fixed, as previously described (6). Macrophage differentiation and maintenance of immortalized cell line were done according to (16). Before infection, non-adherent cells were removed by washing with PBS, and the medium was replaced by medium supplemented with 10% FBS, with or without GBS. Samples were analyzed at different time points after infection (up to 48 h).
For live bacterial infection, GBS were grown in chemically defined medium up to exponential growth phase. Followed by extensive washing with PBS bacteria were added to monolayers at various MOI (bacteria/cell). Monolayers were washed extensively after 30min at 37°C and incubated for various time intervals in presence of antibiotics (100 µg/ml gentamicin, 5 units/ml penicillin and 5 µg/ml streptomycin). Bacterial count was determined by colony counting on blood agar according to standard procedures. Cell viability was determined by trypan blue exclusion, and was not affected by any of the pre - treatments used in some of the experiments.
Epifluorescence and confocal fluorescence microscopy
Macrophages were grown overnight on glass coverslips. Cells were stimulated and fixed with 3% paraformaldehyde at room temperature for 15 min. Hoechst (Molecular Probes) staining of nuclei was carried out by adding a 1:10 000 dilution in PBS. Staining of nitrated proteins was carried out by using anti 3NTyr antibody (Enzo). Confocal microscopy images were collected using the LSM 710 Laser Scanning Microscopes, Carl Zeiss (Jena, Germany).
Phagocytosis and FACS assay
The assay was adapted from (17). In short, dilutions of heat-inactivated GBS were made in DMEM and stained by Oregon green. Samples of 2.5 × 106 GBS were added to macrophages and were allowed to incubate for 30 min at 37°C with shaking. After washing with ice-cold DMEM, the cells were transferred to FACS tubes, fixed with paraformaldehyde (2%) in PBS, and analyzed in a flow cytometer (FACScan; Becton Dickinson). The percentage of Oregon green-positive macrophages was used as a measure of the phagocytic activity. Percentages of Oregon green-positive cells were corrected with the negative control. Results are expressed as % phagocytosis, resulting from Oregon green-positive macrophages. In similar setup of experiment for intracellular iNOS staining Anti NOS2 (C-11) PE (Santa Cruz Bioechnology) antibody was employed.
Real time PCR quantification of GBS DNA/ RNA processing in phagosomes
The ratio of bacterial DNA to mouse DNA was used to monitor bacterial DNA processing in BMDM. Similarly, the ratio of bacterial c-DNA to mouse c-DNA was used to monitor bacterial RNA processing in BMDM. Purity of RNA was verified with non-reverse transcribed RNA control to exclude contamination with residual DNA. The GBS-specific caf gene was used for determination of GBS DNA or RNA from respective Ct values. Mouse-specific gapdh gene was used for determination of macrophage DNA or RNA from its raw Ct values. For determination of GBS DNA or RNA processing, Ct curves were generated by using the 2−ΔCt method, where ΔCt is the difference between raw Ct values of the bacteria-specific gene and the raw Ct values of the BMDM specific gene. The levels of GBS DNA or RNA are relative to the level of BMDM, which was set at 1 (18).
RNA Preparation and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from samples using the RNAeasy mini kit, according to instruction manual (Qiagen). For quantitative two-step RT-PCR, 2 µg of total RNA were reverse-transcribed to first-strand cDNA with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Aliquots of 20 ng of first-strand cDNA were subsequently used as a template for quantitative PCR with gene-specific primers. The mouse specific gapdh gene served as a control for constitutive gene expression. Gapdh expression was unchanged after bacterial inoculation when compared with the amount of 18S ribosomal RNA. Amplifications were performed in 20µl of SYBR green JumpStart Taq ReadyMix (Sigma–Aldrich) with 350 nM oligonucleotides, using an Eppendorf realplex thermal cycler (Eppendorf). After an initial activation step at 95°C for 7 min, 40 cycles (94°C for 30 s, 60°C for 30 s, 72°C for 30 s, and 82°C for 15 s) were performed, and a single fluorescent reading was obtained after the 82°C step of each cycle. A melting curve was determined at the end of cycling to ensure amplification of only a single PCR product. Ct values were determined with the Realplex V2.0 software. Comparative expression levels (2ΔCt) were calculated according to (18). Expression levels are relative to the level of gapdh expression, which was constant in all RNA samples used and was set to 1. Values are the representative of six samples of two biological experiments assayed by quantitative PCR in triplicates.
Nitrite Determination & Real-Time Live Cell Imaging of NO
The nitrite accumulation in macrophage supernatant was measured as an indicator of NO production based on the Griess reaction. Briefly, 100 µl of cell culture medium was mixed with 100 µl of Griess reagent [equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylethylenediamine–HCl, incubated at room temperature for 10 min. Then the absorbance at 540 nm was measured in a sunrise microplate reader (Tecan). Fresh culture medium was used as the blank in all experiments. The amount of nitrite in the samples was compared to a sodium nitrite serial dilution standard curve and nitrite production was calculated.
Real-time live cell imaging of nitric oxide induction was performed using the DAF-FM DA (Molecular probes) fluorescent stain. BMDM were preloaded with DAF-FM DA according to product manual followed by stimulation with rhodamine labeled GBS at MOI 50. Phagocytosis and NO induction was imaged in real-time up to 300 min using confocal laser scanning microscope Zeiss 710 equipped with live cell imaging station.
Determination of TNF and IL-6 Levels
TNF levels in macrophage culture medium were quantified by ELISA kits according to the manufacturer’s instructions (R&D Systems).
All of the experiments were performed at least three times. Statistical significance was evaluated using the Student's t test for selected experiments.
Oligonucleotides used in this study
Streptococcus agalactie cAMP gene (caf) (Acc No X72754) 5’TAATCAAGCCCAGCAAATGG3’ & 5’GTTGGCACGCAATGAAGTCT3’; Mus musculus glyceraldehyde-3-phosphate dehydrogenase (GAPDHs) (Acc No NM_008084) 5’TCTCCATGGTGGTGAAGACA3’ & 5’ACTCCACTCACGGCAAATTC3’; Mus musculus tumor necrosis factor (Tnf) (Acc No NM_013693) 5’TCGTAGCAAACCACCAAGTG3’ & 5’CCTTGTCCCTTGAAGAGAACC3’; Mus musculus nitric oxide synthase 2 (iNOS) (Acc No NM_010927) 5 ’ GCTTCACTTCCAATGCAACA3’ & 5’GGCTGGACTTTTCACTCTGC3’
RESULTS
MyD88 is not necessary for phagocytosis of GBS, but mediates phagosomal processing of GBS nucleic acids
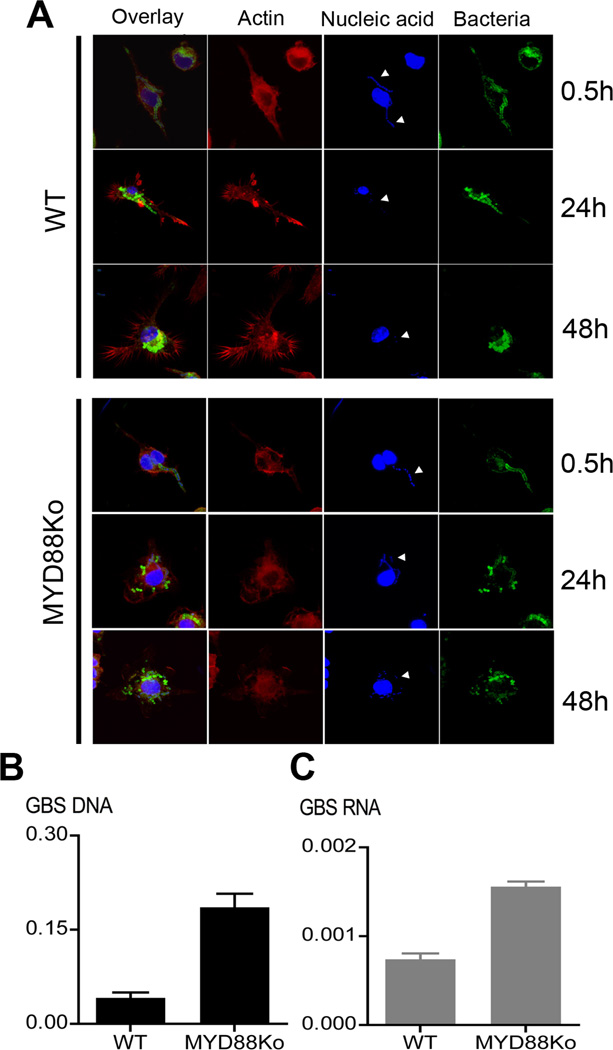
The type I cytokine response to GBS organisms requires recognition of bacterial ssRNA in phagosomes. This is mediated by the TLR adapter protein MyD88 (4). Accordingly we questioned whether MyD88 was involved in the processing of phagosomes containing bacteria. As depicted in supplemental fig. S1, wild type and MyD88-deficient macrophages were indistinguishable in GBS phagocytosis, both with respect to the kinetics of the process and the amount of ingested bacteria. Next we carefully analyzed the kinetics of GBS processing in macrophages by confocal microscopy for up to 48h after exposure to GBS. Thirty minutes after infection, many GBS were found in phagocytic vacuoles (Fig. 1A). Analysis at subsequent intervals from 1 to 4h after inoculation revealed increasing numbers of intracellular bacteria. After 24h, extensive loss of nucleic acids from the GBS particles occurred, as revealed by the loss of Hoechst 33258 staining of the bacteria (Fig. 1A). Expression of MyD88 essentially contributed to the loss of phagosomal GBS nucleic acids (Fig. 1A). Analysis by quantitative PCR revealed that loss of both macrophage associated GBS DNA and RNA was reduced in MYD88Ko macrophages compared to WT macrophages (Fig. 1B&C). These findings strongly suggested that MyD88 was modulating the phagosomal processing of bacterial nucleic acids.
Fig. 1. MyD88 is involved in phagosomal processing of GBS nucleic acids.
Confocal microscopic evaluation of phagocytic processing of GBS nucleic acid (A). BMDM from wild type and MYD88-deficient mice were incubated with 106/ml labelled GBS for 0.5h; 24h or 48h. White arrow heads indicate GBS nucleic acid in phagosome. B&C. GBS were incubated with BMDM of the indicated genotypes for 24h, the cell lysates were analyzed by quantitative PCR for GBS DNA (black) and GBS RNA (gray) as outlined in the methods section; Y axis represents the amount of amplified GBS DNA or RNA relative to amount of mouse DNA. Data is mean ± SD.
Phagocytosis, phagosomal maturation and expression of MyD88 are required for GBS-induced formation of NO
GBS and other Gram-positive bacteria such as staphylococci and streptococci induce inflammatory cytokines in macrophages in strict dependence of phagocytosis and MyD88. However, the signaling events underlying regulation of phagosomal properties upon processing of bacteria by macrophages are less well established. Here we assessed how the inducible lysosomotropic NO was regulated, both with respect to uptake and requirement of individual TLRs and TLR adapters.
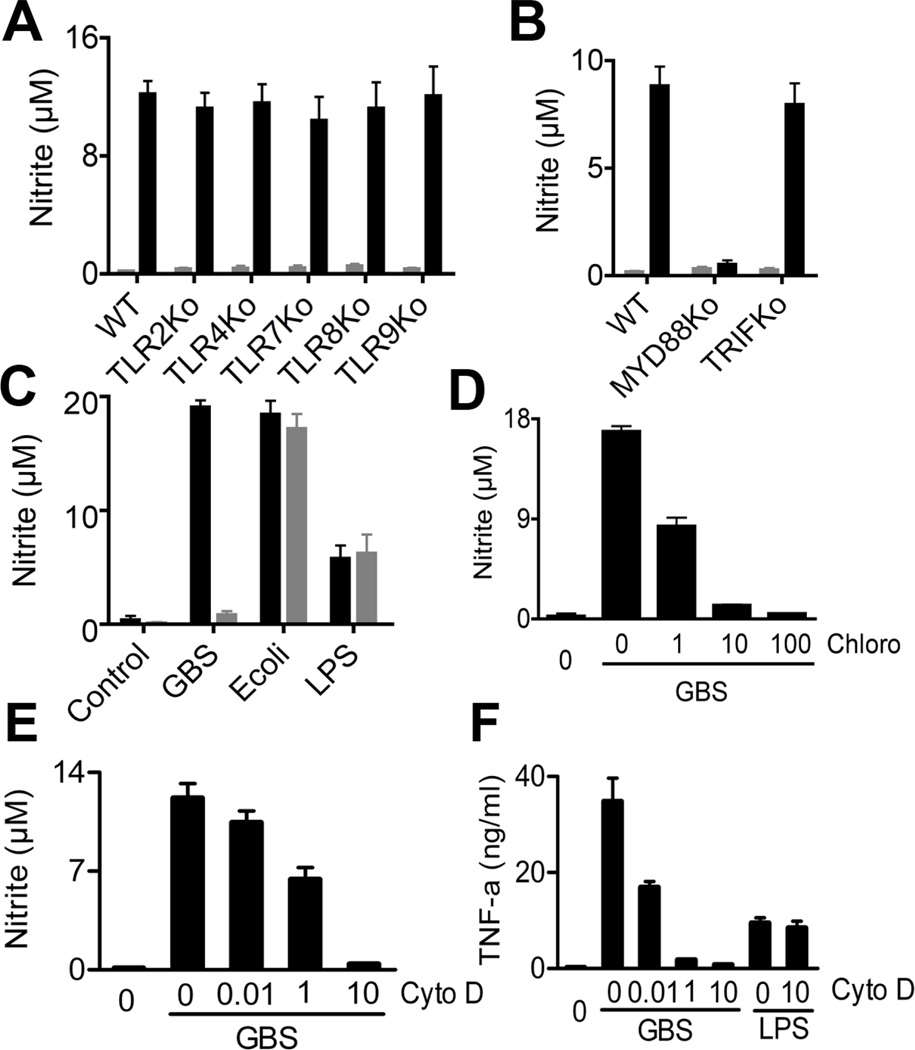
MyD88 was absolutely required for NO formation, whereas the individual TLRs 2, 4, 7, 8 and 9 were dispensable (Fig. 2A & B). Furthermore, TLR3 signaling was not involved, since deletion of the essential TLR3 adapter TRIF did not impair the NO response (Fig. 2B). UNC-93B, which is used primarily by endosomal nucleic acid recognizing TLRs, has recently been demonstrated to play an important role in cytokine production in response to GBS (4). In accordance with these data, we found that bone-marrow-derived macrophages (BMDM) from UNC-93B mutant mice were unable to mount a NO response to GBS, whereas the response to the Gram-negative rod E. coli and to its TLR4 ligand LPS were independent of UNC-93B (Fig. 2C).
Fig. 2. MyD88 and UNC-93B mediate GBS- induced NO formation.
A&B; BMDM lacking the indicated TLRs or TLR adaptors were stimulated (black) or not (gray) with GBS (107/ml). After 24 h, NO in the supernatant was determined. C. BMDM with a H417R mutation in UNC-93B were stimulated with GBS (107/ml), E. coli (107/ml) and LPS 100 ng/ml. After 24 h, NO in the supernatant was determined. D&E. BMDM were pre-treated with the indicated concentrations of cytochalasin D (µg/ml) or chloroquine (µM) for 1h before stimulation with GBS (106/ml). After 24h, NO in the supernatant was determined. F. BMDM were pre-treated with the indicated concentrations of cytochalasin D (µg/ml) for 1h before stimulation with GBS (106/ml) or LPS (100 ng/ml). After 24h, TNF and NO in the supernatant was determined. All the data is mean ± SD.
In addition to MyD88 and UNC-93B, phagocytosis was essential for GBS-induced NO formation, as indicated by the dose-dependent inhibitory effect of cytochalasin D, which interferes with F-actin polymerisation (Fig. 2E). Ablation of phagocytosis by cytochalasin D treatment was verified by microscopy and FACS analysis (data not shown). These data were in line with previous observations (19). In addition, acidification of the GBS containing phagosome was necessary for NO induction, as clearly indicated by the inhibitory effect of chloroquine on this process (Fig. 2D).
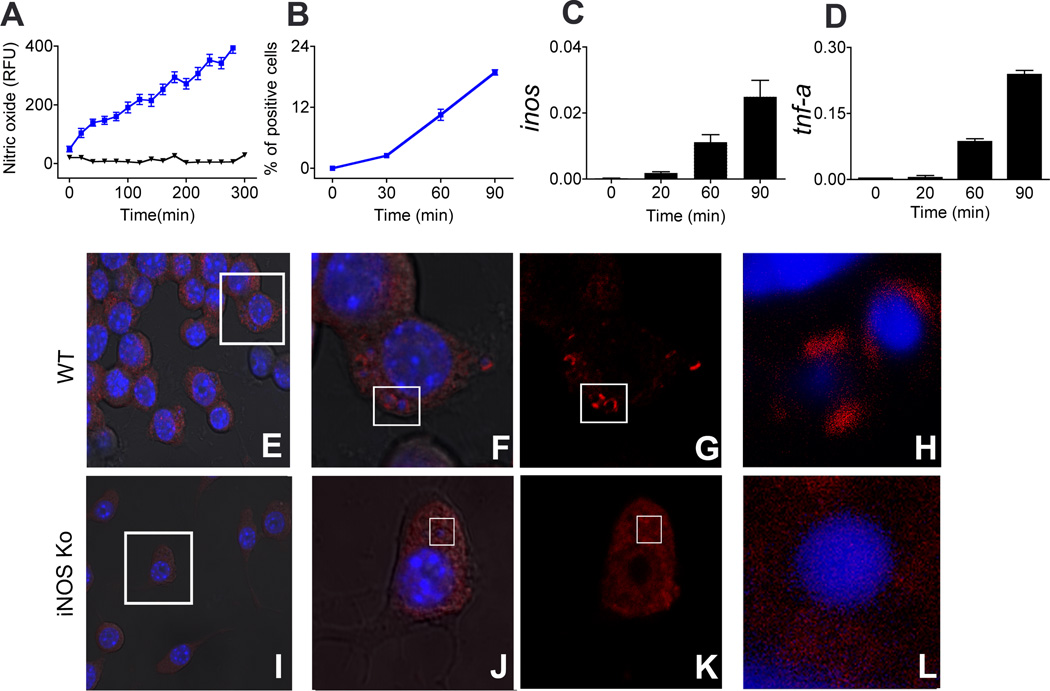
Next, we determined the kinetics of iNOS and NO formation and its effects on host structures. We found that inos transcription was induced as early as 20 to 30 min after initial contact with GBS. This was accompanied by induction of NO at the GBS containing phagosomes, as determined by time-resolved live cell microscopy using the sensitive NO indicator 4-amino-5-methylamino-2',7'-difluororescein diacetate (DAF-FM DA) and rhodamine labeled bacteria. In addition, formation of iNOS protein was measured 1h after stimulation by immunostaining and FACS analysis (Fig. 3 A–D, supplementary video 1&2;). NO potentially modulates protein function through post translational nitration of amino acids (7). In macrophages processing GBS, we found nitration of tyrosin residues in vicinity of the GBS containing phagosomes of BMDM from WT, but not iNOSKo or MYD88Ko mice (Fig. 3 E–L and data not shown). Accordingly, GBS rapidly induced NO which in turn modified the protein scaffold of the bacteria bearing phagosome.
Fig. 3. GBS induces nitric oxide early during phagocytosis that in turn nitrate proteins at phagosome, iNOS dependently.
A; BMDM were followed for nitric oxide production with (blue) and without (black) GBS phagocytosis in real time live cell imaging for indicated time using fluorescent nitric oxide indicator DAF-FM DA. Fluorescence intensities were calculated using Image J for 20 cells per experiment. Data demonstrated is a representative cell out of three independent repetitions each. B; BMDM were stimulated with GBS and fixed at different time intervals, iNOS protein formation was measured by FACS. C&D; Kinetics of transcriptional activation of inos (C) and tnf-α (D) upon stimulation with GBS. Data is mean ± SD. E–L; WT (E–H) and iNOSKo (I–L) BMDM were stimulated with GBS for 30 min, and stained intracellularly for nitrated proteins using anti-nitrotyrosin antibody (Red). FG and JK is magnification from inset in E and I respectively. H&L are magnification of inset in FG and JK respectively. Bacterial and cell nucleic acid was stained with Hoechst (blue); H is turned 90° and magnified from inset in G.
In conclusion, these experiments suggested that the rapid NO response to GBS required phagocytosis of the bacteria and MyD88 expression of the macrophages.
NO is involved in the acidification of bacteria-containing phagosomes and in the processing of streptococcal nucleic acids
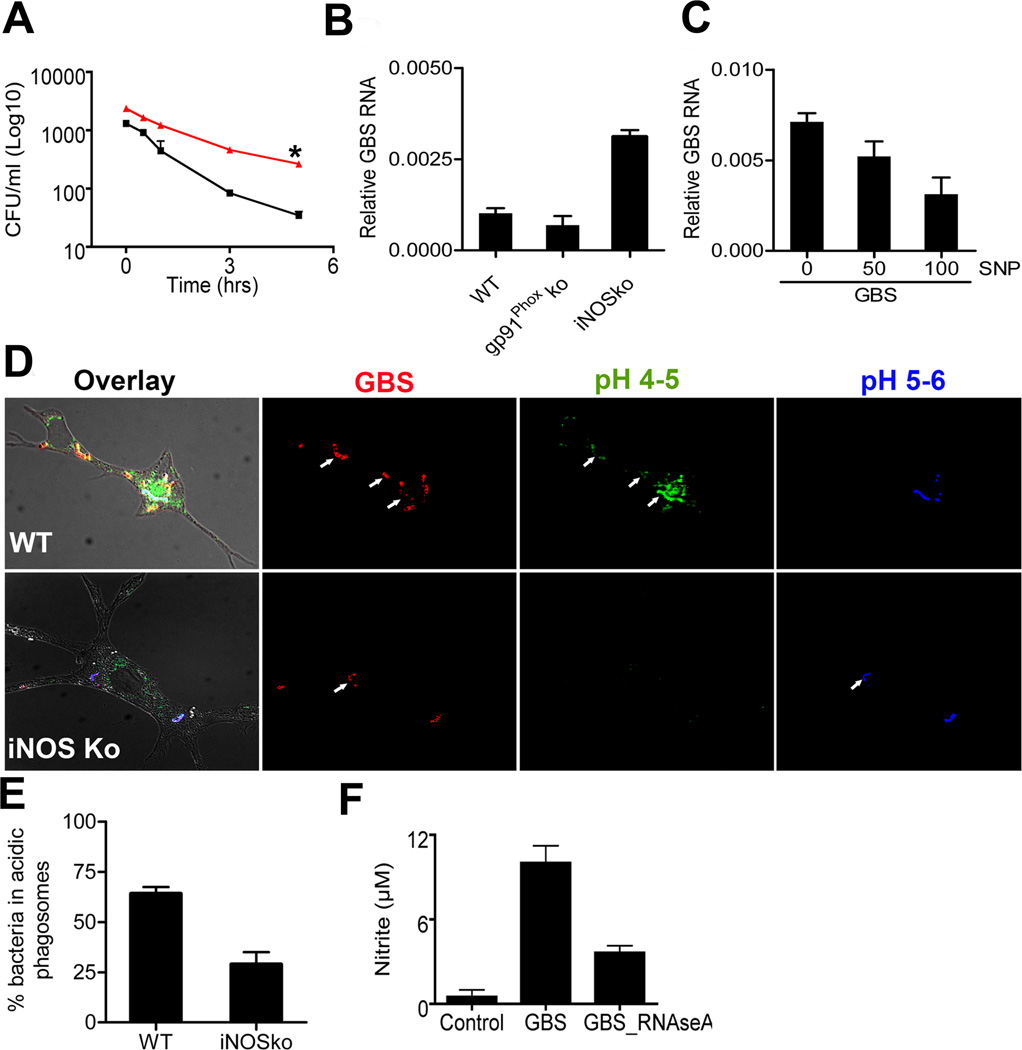
Since MyD88 was involved in both NO formation and lysosomal processing of nucleic acids from GBS, we addressed whether both processes were directly interlinked, i.e. whether MyD88 exerted its lysosomotropic, cytokine modulating properties at least partially via NO. First, we assessed whether NO was involved in the intracellular killing of GBS in macrophages from iNOSKo mice. There we found that NO was an important component of the killing machinery of GBS, since the average GBS half live was 11±1min in iNOSKo macrophages as opposed to 7.5±2min in WT macrophages (Fig. 4A and data not shown). Notably, as early as one hour after macrophage infection, bacterial loads were higher in iNOSKo compared to WT macrophages. This is in line with the observation presented above that formation of GBS-induced NO is a rapid process, which can be detected as early as 20 minutes after initial stimulation. It remains to be established whether differences in basal iNOS between iNOSKo and WT mice additionally contribute to differences in killing. We further determined, if NO plays a role in the processing of GBS nucleic acids. Interestingly, iNOSko macrophages were substantially retarded in phagosomal GBS RNA processing, when compared to WT macrophages (Fig. 4B). In contrast, macrophages from gp91Ko mice, which do not form reactive oxygen species in response to GBS, were normal in their ability to process RNA (Fig. 4B). In order to independently substantiate, whether NO played a direct role in RNA processing, we preincubated GBS with the potent nitrosating agent sodium nitroprusside (SNP) (20) for 30 min at room temperature in the dark, before adding the bacteria to the macrophages. Addition of SNP propagated processing of GBS RNA in macrophages in a dose dependent manner (Fig. 4C). This finding suggested a role of NO in the processing of GBS nucleic acids in the phagosome, whereas inducible ROS appeared to be less important. Next we asked, whether the propagation of nucleic acid processing was part of a broader role of NO in phagosomal maturation. Accordingly, we measured the acidification of bacteria-containing phagosomes in iNOS-deficient and -sufficient macrophages. Specifically, we assessed the number of GBS-containing vesicles with either pH of 4–5 or pH 5–6 as compared with pH standards (Fig. 4D). Vesicles in at least 50 cells per condition in each experiment were counted and the data from three independent experiments were combined. We found that after 24h of incubation 70% of GBS were localized in acidic phagososmes in wild type macrophages, whereas the same was true for only about 30% of GBS in iNOS-deficient macrophages (Fig. 4E).
Fig. 4. iNOS is essential for the processing of nucleic acids from group B streptococcus.
A. WT BMDM (black) and BMDM lacking iNOS (red) were incubated with GBS (MOI 5), bacterial killing was measured and expressed as the bacteria surviving (CFU/ml) after different time intervals up to 5h. Statistical significance: P-value *<0. 05 B. Quantitative PCR of amplifiable GBS RNA was measured 24 h after stimulated with GBS (106/ml) in BMDM lacking gp91Phox or iNOS. C. Quantitative PCR of amplifiable GBS RNA measured 24 h after stimulation with the indicated concentrations of SNP (µM) in iNOSKo BMDM. D. Phagosomes of WT and iNOSko BMDM were loaded with the pH sensitive stain LysoSensor and stimulated with WGA-TRITC-labeled GBS (106/ml). Blue fluorescence indicates pH 5 - pH 6, whereas green fluorescence indicates more acidic environments. % GBS in acidic phagosome were measured by confocal microscopic analysis in BMDM lacking iNOS gene and WT. E. Quantitative analysis of GBS in phagosomes < pH5. F. BMDM from WT mice were stimulated with GBS (107/ml), which had been depleted or not for ssRNA by RNAseA enzymatic treatment. After 24 h, NO in the supernatant was determined. All the data is mean ± SD.
The impact of both MyD88 and NO on RNA processing was intriguing, since streptococcal ssRNA is essential for the activation of type I inflammatory cytokines by whole streptococci in macrophages (4). In contrast, depletion of DNA with DNAse remained without effect on cytokine formation by GBS (4). Furthermore the NO response was dependent on streptococcal ssRNA (Fig. 4F). Hence it was important to address whether NO modulated cytokine formation through its effects on streptococcal RNA.
NO, but not ROS and lysosomal proteases, modulates GBS-induced cytokine formation
We compared the TNF response of wild type, gp91PhoxKo, iNOSKo and MYD88Ko macrophages upon stimulation with various concentrations of GBS. Cathepsin B, which has previously been suggested to be involved in phagosomal processes leading to the activation of the inflammasome (21), was not critical for cytoplasmic recognition of GBS ssRNA. Inhibition of cathepsin B remained without effect on GBS-induced cytokine formation (supplemental Fig. S3).
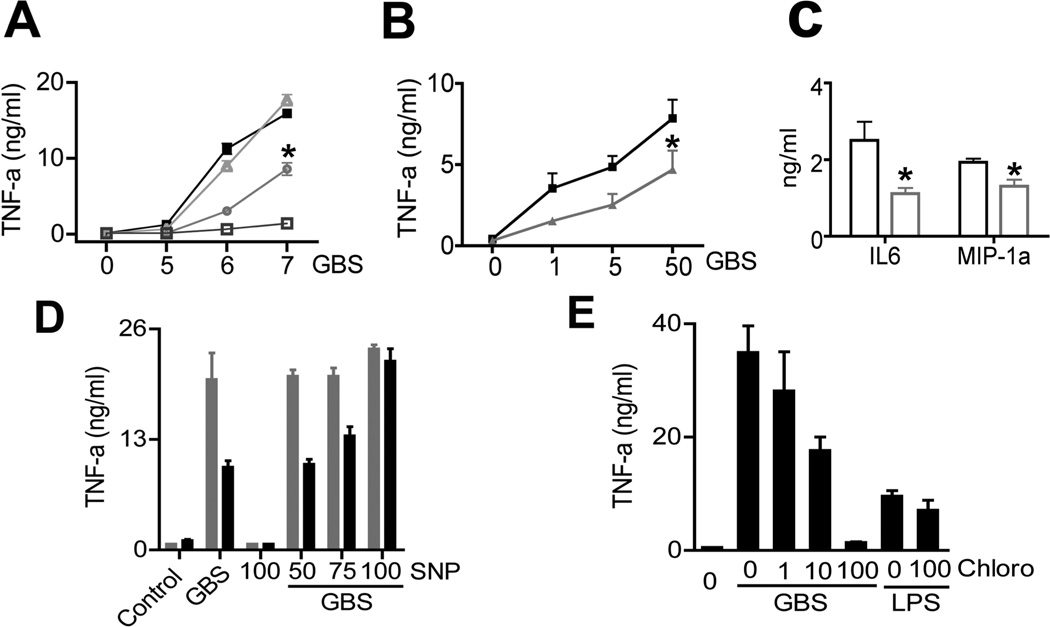
Whereas gp91Phoxko macrophages exhibited a normal TNF response to GBS, iNOSko macrophages had an approximately 50% reduced TNF response compared to WT macrophages (Fig. 5A). This effect of iNOS was not limited to TNF, but was also found with respect to formation of IL-6 and MIP-1 alpha (Fig. 5C). The contribution of iNOS to cytokine formation was not only evident in the context of fixed GBS, which corresponds to a predominantly particle-driven activation mode, but was also observed upon infection of primary macrophages with live GBS (Fig. 5B). This seemed important, since GBS survived longer in iNOSKo macrophages than in WT macrophages. Therefore the phagosomal presence of metabolic GBS products in iNOSKo macrophages likely exceeds that in WT macrophages. Based on this observation, we analyzed whether the enzyme iNOS or its product NO contributed to the TNF response. We found that NO was an essential intermediate in the GBS-induced cytokine formation, since the NO donor SNP fully reconstituted the TNF response in iNOS-deficient cells. Importantly, SNP alone did not affect TNF formation (Fig. 5D). Therefore, the iNOS product NO in the phagosome appeared to propagate cytokine formation. In order to determine whether the compensatory effect of SNP occurred at the transcriptional level, we analyzed TNF gene expression in SNP-treated samples. Similar to protein formation, activation of tnf by GBS was iNOS dependent and was compensated for by SNP in iNOSKo macrophages (supplemental fig. S2). It appears that the effect of iNOS on phagosomal acidification was the putative link to its effect on TNF formation, since the alkalanizing agent chloroquine inhibited GBS-induced TNF formation in a dose-dependent manner (Fig. 5E). Importantly, SNP did not reconstitute the cytokine response to GBS in MyD88ko macrophages (data not shown). Therefore, NO is an important, however not a sufficient downstream intermediate of MyD88 in the cytokine response to GBS.
Fig. 5. iNOS is essential for the cytokine induction.
A. BMDM from mice lacking gp91Phox (triangle), iNOS (circle), MYD88 (open square) and WT mice (filled square) were stimulated with GBS (105, 106, 107/ml). B. BMDM from WT mice (black) and iNOSKo mice (gray) were stimulated with live GBS for 2 hrs at MOI 10. Then, macrophages were washed extensively, and incubated for 8 hrs; Finally, TNF in the supernatants was measured by ELISA. Statistical significance: P-value *<0. 05 C. BMDM from WT mice (black) and iNOSKo mice (gray) were stimulated with GBS (106/ml), IL-6 & MIP-1 alpha in the supernatants was measured by ELISA. D. BMDM from WT mice were stimulated with GBS (106/ml) plus the indicated concentrations of sodium nitroprusside (SNP, µM) or SNP alone. E. WT-BMDM were pre-treated with chloroquine (µM) for 1h before stimulation with GBS (106/ml) or LPS (100ng/ml). After 24 h, TNF in the supernatants was measured by ELISA. Data is mean ± SD.
DISCUSSION
In this study, we introduce a complex novel model of reciprocal interactions between GBS and macrophages during phagosomal processing of the bacteria, which results in the recognition of GBS ssRNA. During this process, the highly reactive and diffusible NO is formed by iNOS, which is tied to a signaling cascade downstream of MyD88 (22). NO propagates nucleic acid processing and acidification of the bacteria-containing phagosome, and mediates inflammatory activation by streptococci.
Streptococci are well adapted to a coexistence with the host and mucosal sites constitute typical interfaces. GBS colonizes up to 20% of all adults (genital and intestinal tract) und 10% of newborn infants (23). Compared to this physiological situation, invasive infections are relatively rare (for GBS only in 1% of colonized infants). In view of this, it is tempting to visualize a model whereby the colonizing bacterial community exploits the innate immune system as a shepherd that punishes individual bacteria with deviating invasive behavior in order to guarantee persistence of the colonizing community.
The role of macrophages in the regulation of colonization has been demonstrated by the increase of invasive streptococcal disease in a mouse colonization model where local macrophages are eliminated by intra-nasal chlodronate (24). The potential costs of a highly alert monocytic defense system have been demonstrated with respect to so-called inflammatory monocytes, since overexpression of the chemokine receptor CCR2 in the lung induces chronic inflammatory changes (25). A powerful and timely cellular reaction requires a potent response by cells that have seen low number of bacteria (since otherwise the system may be overwhelmed by the bacterial load). Hence it makes inherent sense that processing of bacterial particles is intercalated into the inflammatory activation of the macrophage.
NO is clearly part of the cellular killing machinery for bacteria, since gp91phox/iNOS dKO mice are much more prone to invasive bacterial disease than conventional gp91Phox knock-out mice (26). We show here that NO contributes to the bactericidal activity in macrophages. Moreover, we find NO exerts more global lysosomotropic effects beyond its direct antibacterial function. NO propagates acidification of the bacteria containing phagolysosome. Modulation of the intraluminal pH has several important direct and indirect implications for phagolysosomal processes such as modification of microbial substructures. As an example, interaction of hypomethylated CpG DNA with TLR heavily depends on an acidic intraluminal environment (27). Furthermore, lysosomal proteases exert their function in a pH-dependent fashion (28).
The short lived NO seems to be an ideal intermediate for regulating the activation level of the single macrophage and the intercellular network in the macrophage vicinity. The rapid induction of NO and accumulation of nitrated proteins at the vicinity of GBS bearing phagosome, and the role it plays in bacterial processing, puts NO very upstream in the signaling propagation cascade, well before secondary products such as TNF will reciprocally modify cellular activity (19). This interdependance of particle processing and inflammatory activation of macrophages may be especially important under non-opsonizing conditions, e.g. in children lacking protective antibodies and in the serum-starved submucosal tissue where ITAM-dependent activation through FC gamma receptors is poorly developed.
The data presented here may provide the molecular basis for the previous observation of an unresolved proinflammatory role of NO in GBS sepsis mortality (9). However, it remains to be established, whether NO exerts phagosomal acidification through nitration of phagosomal proteins, via chemical properties of the NO product peroxinitrite or any other mechanism. Importantly, ROS are fully dispensable in this context.
In summary, this study identifies NO as a macrophage autonomous signaling modifier in the ssRNA-induced response to streptococci.
Supplementary Material
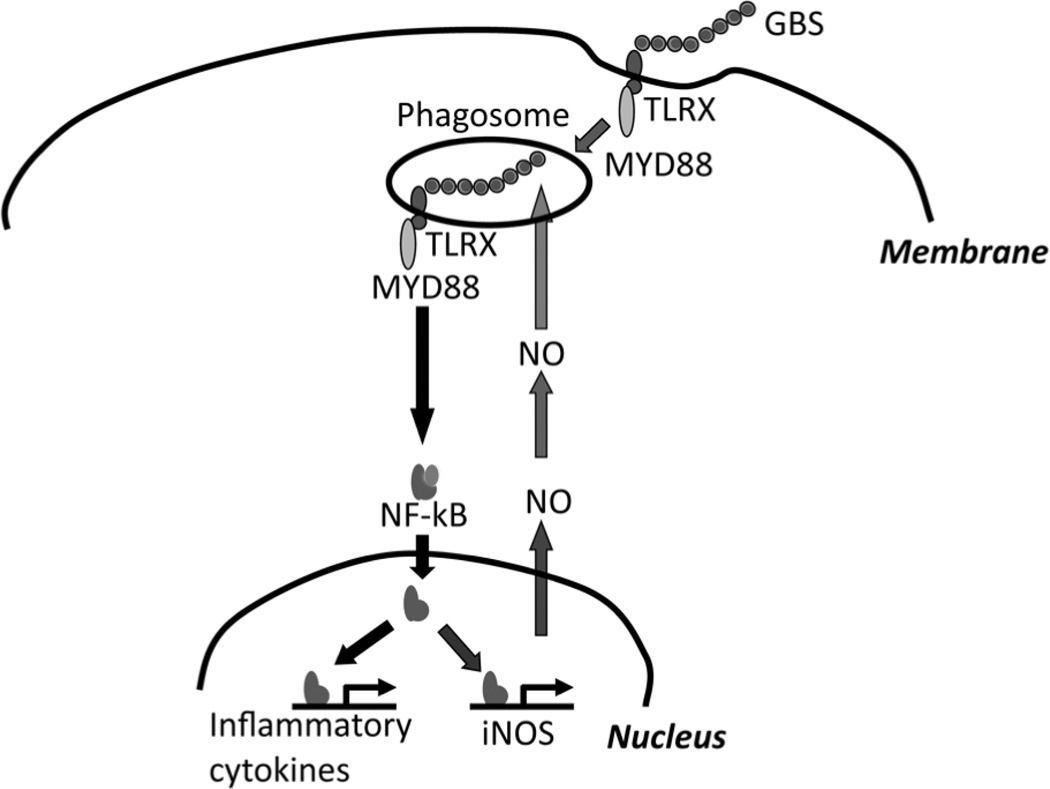
Fig. 6. Model on the role of NO in the induction of inflammatory cytokines.
Phagocytosis of GBS activates NFκB and induces transcriptional activation of iNOS in a MYD88 dependent fashion. NO is critical for the acidification of the phagosome and thereby modifies nucleic acids of internalized GBS. NO-modified single stranded GBS RNA eventually results in further transcriptional activation of cytokine genes.
ACKNOWLEDGMENTS
The authors thank S. Akira, S. Bauer and M. Freudenberg for the provision of knockout mice, B. Kremer, K. Bruckner, S-H. Seibel, L. Fuchs, and A. Imm for outstanding technical assistance and A-M Eades-Perner for help in preparing the manuscript.
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF 01 EO 0803), the Deutsche Forschungsgemeinschaft (He 3127/2-3 and 3-1), and from the National Institutes of Health (ROI AI052455-06A1, to DTG).
ABBREVIATIONS
- GBS
Group B streptococci
- S. aureus
Staphylococcus aureus;
- BMDM
Bone marrow-derived macrophages
- TNF
Tumor necrosis factor
- PAMP
Pathogen-associated molecular pattern
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Henneke P, Dramsi S, Mancuso G, Chraibi K, Pellegrini E, Theilacker C, Hubner J, Santos-Sierra S, Teti G, Golenbock DT, Poyart C, Trieu-Cuot P. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J Immunol. 2008;180:6149–6158. doi: 10.4049/jimmunol.180.9.6149. [DOI] [PubMed] [Google Scholar]
- 2.Henneke P, Golenbock DT. TIRAP: how Toll receptors fraternize. Nature immunology. 2001;2:828–830. doi: 10.1038/ni0901-828. [DOI] [PubMed] [Google Scholar]
- 3.Gratz N, Siller M, Schaljo B, Pirzada ZA, Gattermeier I, Vojtek I, Kirschning CJ, Wagner H, Akira S, Charpentier E, Kovarik P. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. The Journal of biological chemistry. 2008;283:19879–19887. doi: 10.1074/jbc.M802848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshmukh SD, Kremer B, Freudenberg M, Bauer S, Golenbock DT, Henneke P. Macrophages recognize streptococci through bacterial single-stranded RNA. Embo Rep. 2011;12:71–76. doi: 10.1038/embor.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip WK, Sokolovska A, Charriere GM, Boyer L, Dejardin S, Cappillino MP, Yantosca LM, Takahashi K, Moore KJ, Lacy-Hulbert A, Stuart LM. Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to Staphylococcus aureus. J Immunol. 2010;184:7071–7081. doi: 10.4049/jimmunol.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenzel S, Santos-Sierra S, Deshmukh SD, Moeller I, Ergin B, Fitzgerald KA, Lien E, Akira S, Golenbock DT, Henneke P. Role of p38 and early growth response factor 1 in the macrophage response to group B streptococcus. Infection and immunity. 2009;77:2474–2481. doi: 10.1128/IAI.01343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart LM, Ezekowitz RAB. Phagocytosis: Elegant Complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 9.Timmer AM, Nizet V. IKKbeta/NF-kappaB and the miscreant macrophage. The Journal of experimental medicine. 2008;205:1255–1259. doi: 10.1084/jem.20081056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-Like Receptor 4 (TLR4)-Deficient Mice Are Hyporesponsive to Lipopolysaccharide: Evidence for TLR4 as the Lps Gene Product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature immunology. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science (New York, N.Y. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 16.Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-Independent Type I Interferon Induction in Response to an Extracellular Bacterial Pathogen via Intracellular Recognition of Its DNA. Cell host & microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velasco A, Alonso S, Garcia JL, Perera J, Diaz E. Genetic and functional analysis of the styrene catabolic cluster of Pseudomonas sp. strain Y2. Journal of bacteriology. 1998;180:1063–1071. doi: 10.1128/jb.180.5.1063-1071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Goodrum KJ, McCormick LL, Schneider B. Group B streptococcus-induced nitric oxide production in murine macrophages is CR3 (CD11b/CD18) dependent. Infection and immunity. 1994;62:3102–3107. doi: 10.1128/iai.62.8.3102-3107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aga RG, Hughes MN, Robert KP. Methods in Enzymology. Academic Press; 2008. The Preparation and Purification of NO Gas and the Use of NO Releasers: The Application of NO Donors and Other Agents of Nitrosative Stress in Biological Systems; pp. 35–48. [DOI] [PubMed] [Google Scholar]
- 21.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. Journal of cell science. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 23.Henneke P, Berner R. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infection and immunity. 2006;74:3085–3095. doi: 10.1128/IAI.01551-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of Clinical Investigation. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Moser C, Louboutin J-P, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM. Toll-Like Receptor 4 Mediates Innate Immune Responses to Haemophilus influenzae Infection in Mouse Lung. The Journal of Immunology. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 26.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of Mice and Macrophages Deficient in Both Phagocyte Oxidase and Inducible Nitric Oxide Synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 27.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nature immunology. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 28.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. The EMBO journal. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.