Abstract
Type-I-interferons are important for direct control of viral infection and for generation of adaptive immune responses. Recently, direct stimulation of CD4+ T cells via type-I-interferon receptor has been shown to be necessary for the formation of function CD4+ T cell responses. In contrast, we find that CD4+ T cells do not require intrinsic type-I-interferon signals in response to combined TLR/anti-CD40 vaccination. Rather, the CD4 response is dependent on the expression of type-I-interferon receptor (IFNαR) on innate cells. Further, we find that DC expression of the TNF ligand, OX40L, was dependent on type I IFN signaling in the DC, resulting in a reduced CD4+ T cell response that could be substantially rescued by an agonistic antibody to the receptor, OX40. Taken together, we show that the IFNαR dependence of the CD4+ T cell response is accounted for exclusively by defects in DC activation.
Introduction
The type-I-interferon cytokine family is vitally important in providing resistance to viral infections and plays a unique role among cytokines by bridging innate and adaptive immunity (1). The marked antiviral effects of type-I-interferon make it clinically useful as an adjuvant to ribavirin as a treatment for hepatitis C virus infection (1, 2). Furthermore, individuals lacking key intermediates both upstream and downstream of type-I-interferon signaling succumb to normally nonlethal viral infection (3, 4). Thus, understanding type-I-interferon biology holds the promise of better understanding human immunity.
In the periphery, type-I-interferon signaling induces infected cells and bystanders to adopt an antiviral state (5) mediated by intracellular factors such as protein kinase R (PKR), Mx1, and others, and characterized by reduced protein synthesis, which limits viral replication (1). Release of type-I-interferon in the context of infection (6), as well as release of byproducts of infection such as double-stranded RNA liberated from lysed cells, leads to the activation of innate cells (7). Activation of peripheral innate cells such as denritic cells (DC) by type-I-interferon leads to the trafficking of these cells to draining lymph nodes (8, 9). There, DC are auto-enhanced by type-I-interferon (10) to present antigens they may have carried from the periphery in the context of costimulation to naïve, primary T cells (10, 11). The culmination of type-I-interferon induction is thus the initiation of an adaptive, antiviral, immune response.
While a picture of the role of type-I-interferon signaling for DC at various points in the in vitro innate immune response has been developed (10, 11), the function of type-I-interferon on DC activation has only recently been explored in vivo (8, 9, 12). IFN-dependent DC activation has been reported as required for vaccine adjuvant efficacy in other model systems (13), and it was recently shown that DC-intrinsic sensing of type-I-interferon was necessary for activation by the Toll-like receptor agonist, polyinosinicpolycytidylic acid (polyI:C) (12). Given the role of DCs in the initiation of adaptive immunity (14–16), it could be expected that defects in DC activation would be a dominant hindrance to the generation of adaptive responses in the absence of type-I-interferon signals. However, there is a body of literature to suggest that type-I-interferons can also have broad, direct effects on responding adaptive immune cells, in particular CD4+ T cells (12, 17–23). Thus, it is presently unknown to what extent failure of adaptive immune responses in the absence of type-I-interferon are attributable to defects in priming DC, and which are due to defects in direct signals to T cells. These parameters are particularly important to establish for the purposes of vaccine development, as many of the adjuvants currently being explored have type I IFN induction as a significant contributor to vaccine efficacy (24).
We have previously shown that combined agonists for Toll-like receptors and CD40 (the anti CD40 antibody, FGK4.5), synergistically promote CD8+ T cell responses (25). Furthermore, CD8+ responses to combined polyI:C and anti-CD40 (polyI:C/CD40) were dependent on the type-I-interferon receptor, IFNαR1 (IFNαR) (25). Our experience with CD4+ T cells revealed that they could also be synergistically activated by polyI:C/CD40 (26), and that the activation of CD4+ T cells by polyI:C/CD40 depended upon signals through the Tumor Necrosis Factor Superfamily (TNFSF) member OX40L. However it remained unknown whether CD4+ T cells activated by polyI:C/CD40 were similarly dependent on signals through IFNαR. Unlike CD4+ T cells, CD8+ T cells require DCs to take up exogenous antigen to be processed and cross-presented on MHC-I, a pathway dependent on type-I-interferon (9). Furthermore, whereas a role for type-I-interferon in CD4+ T cell responses to polyI:C has been established (12), it was unknown whether the addition of a CD40 agonist would abolish the dependence of polyI:C responses on type-I-interferon.
We set out to address the role of type-I-interferon in CD4+ T cell responses initiated by polyI:C/CD40 and confirmed that type-I-interferon is necessary for CD4+ T cell priming. To our surprise, we found that secondary CD4+ T cell responses were, unlike primary responses, relatively intact in IFNαRKO mice. This suggested that antigen-experienced CD4+ T cells were qualitatively different from naïve CD4+ T cells, and that the IFN-dependency was unlikely due to direct IFN stimulation of the T cell. Indeed, the use of mixed bone marrow chimeras revealed that Rag1−/− bone marrow could rescue CD4+ T cell responses in otherwise IFNαRKO mice, demonstrating a role for IFNαR on innate, but not adaptive cells. We further showed that polyI:C/CD40 stimulated IFNαRKO DCs had very low expression of OX40L, and restoration of OX40 signals using an agonistic antibody in IFNαRKO mice enhances CD4+, but not CD8+ T cell priming.
Materials and Methods
MICE
Six- to eight-week old female C57BL/6 mice were obtained through the National Cancer Institute or Harlan Laboratories. Mice deficient in the alpha-1-receptor for type-I-interferon (B6.129PF2/ifnab) were obtained from Laurel Lenz at National Jewish Health and were originally derived by Daniel Portnoy, University of California, Berkeley. These mice were crossed to B6.SJL mice (B6.SJL-PtprcaPep3b/BoyJ) obtained from Jackson Labs. CD40-deficient mice (CD40KO, CD40KO, B6.129P2-Cd40tm1Kik/J), were also obtained from Jackson Labs. Mice deficient for the Rag1 gene (B6.129S7-Rag1tm1Mom/J) were a gift of Dr. Phillippa Marrack, National Jewish Health. Mice were housed at the Biological Resource Center at National Jewish Health. The Institutional Animal Care and Use Committee at National Jewish Health approved all animal procedures. Mice were maintained on Harlan Teklad 2919 chow and water ad libitum for the breeding and the duration of experiments.
IMMUNIZATIONS, DEPLETIONS, AND ANTIBODY BLOCKADE
Unless indicated otherwise, mice were immunized intraperitoneally with 500μg of whole chicken ovalbumin protein (Sigma-Aldrich, St. Louis, MO), 100μg of the Eα-derived 2W1S peptide (EAWGALANWAVDSA, custom synthesized by Pi Proteomics, Huntsville, AL) (27), 50μg of CD40 agonist antibody (clone FGK4.5, Bio × Cell, West Lebanon, NH), and 50μg poly-Inosine:Cytosine (polyI:C, Amersham/GE Healthcare, Piscataway, NJ). All vaccinations were prepared by mixing each component together in PBS, and injected in 200μL. PolyI:C was stored in frozen aliquots in PBS at −20°C and was reconstituted prior to injection by melting at 56°C for 10 minutes and then allowing the solution to cool to room temperature in order to limit concatamerization. All reagents were found to contain minimal LPS content by Limulus Amoebocyte Assay (Lonza, Walkersville, MD). Blocking antibody for OX40L (RM134L) and agonistic anti-OX40 (OX86) were obtained from BioXcell. OX40L blockade was facilitated by intraperitoneal injection of 250μg of RM134L in PBS on days −1 and 0 relative to immunization. On day 0, blocking antibodies were mixed with prepared vaccines and both were delivered in a single injection. For OX40 agonist administration, 250μg of OX86 in PBS were mixed with prepared vaccine on day 0 and given together as a single injection. For experiments involving footpad immunization, mice were anesthetized with isofluorane and injected with 50μL of PBS containing 40μg of polyI:C, 40μg of FGK4.5, and 50μg of OVA protein.
FLOW CYTOMETRY
For T cell assays, mice were sacrificed 7 days post primary immunization or 5 days post boost immunization, peripheral blood was harvested from the abdominal aorta, and spleens were harvested and minced with forceps in HBSS containing 5mM EDTA. Single cell suspensions were made by passing minced spleens through nylon mesh strainers. Red blood cells were lysed in peripheral blood samples using ACK lysis solution (BioSource, Rockville, MD). All samples were resuspended in RPMI 1640 medium containing 2.5% heat-inactivated fetal calf serum, 2-mercaptoethanol, L-glutamine, non-essential amino acids, HEPES, sodium pyruvate, penicillin, and streptomycin. Cells were stained with phycoerytherin-labeled, tetramerized MHC molecules bearing antigens of interest. Drosophila S2 cells transfected with 2W1S-IAb monomers were a gift of Marc Jenkins at the University of Minnesota (28). Secreted 2W1S-IAb was harvested from supernatants as described (29), but did not require additional biotinylation due to co-transfection of the BirA enzyme (James Moon and Marc Jenkins). Tetramerized 3K-IAb was prepared as described (29). Kb reagents were purified as described (30) and were loaded with OVA (SIINFEKL) antigen prior to staining. Cells were coincubated with tetramer for 1 hour at 37°C prior to staining with surface antibodies. Surface antibodies were purchased from BioLegend (San Diego, CA), or eBioscience (San Diego, CA). For cytokine assays, cells were incubated for 3.5 hours at 37°C with 6μg/mL brefeldin A in complete media as above. Cells were restimulated with 10μg/mL 2W1S and SIINFEKL peptides, and following the incubation period cells were fixed, permeablized, and stained for intracellular cytokines as described (31). Dendritic cells were isolated from spleens in EHAA media (Invitrogen) containing DNAse (Worthington, Lakewood, NJ) and Collagenase D (Roche Diagnostics, Indianapolis, IN) as described (30). Crude preparations were made by passing digested spleens through nylon mesh strainers, and were purified over Nycodenz (Nycoprep Universal, Accurate Chemical & Scientific Corp., Westbury, NY) according to manufacturer's instructions. Both dendritic cells and T cells were washed and stained in FACS buffer containing 10% 2.4G2 supernatant (B cell hybridoma blocking Fcγ receptors). Cells were gated for forward scatter, side scatter, pulse width, and, in the case of T cells, were MHCII−, DX5−, and CD3+ prior to gating on CD4+ and CD8+ events. DC were gated for forward scatter, side scatter, pulse width, and were CD19−, CD3−, and CD11c+.
MIXED BONE-MARROW CHIMERAS
Recipient mice were lethally irradiated with 900 rads in the morning and were grafted via the tail vein in the afternoon with 4×106 total T cell-depleted donor bone marrow cells suspended in 200μL of PBS. Bone marrow was depleted of T cells using magnetic removal of CD3+ cells (Miltenyi Biotec, Auburn, CA). Most mixed bone marrow chimeras were grafted at a ratio of 1:1, representing 2×106 cells from each of two donors. However Rag-deficient bone marrow was enriched relative to competitor bone marrow 3:1 to enhance engraftment. Screening of recipients of B6.SJL and RagKO bone marrow revealed that the 3:1 ratio was optimal for the generation of equal numbers of NK cells derived from each donor. Chimeric mice were rested a minimum of 12 weeks before being immunized for experiments. Chimeric mice were fed tirmethoprim-sulfamethoxazole-containing chow (Harlan Teklad 6596) for 6 weeks following reconstitution to reduce the risk of bacterial infection, but were switched to standard chow (Harlan Teklad 2919) well before immunization.
IN VITRO CULTURE
Spleen cells were harvested as described above using DNAse and collagenase and cultured unfractionated at 1×106 cells/mL in 24-well, flat-bottom plates. Cells were stimulated with 0.5 μg/mL anti-CD3ε (clone 2C11) in complete RPMI medium supplemented as above and with 10% FCS. In some cases, cells were incubated with 105 U/mL recombinant IFNα prepared as described (32). At indicated timepoints, cells were harvested by washing plates with FACS buffer and stained as above.
EXPERIMENTAL AND STATISTICAL ANALYSIS
Spleen cells were quantified on a Vi-Cell cell viability analyzer (Beckman-Coulter). Cytometry samples were acquired on a CyAn ADP (Dako Cytomation) using Summit acquisition software. Samples were analyzed using FloJo software (Tree Star, Inc. Ashland, OR). In most cases, results from FloJo analysis were imported into Prism (GraphPad, La Jolla, CA) and pairwise statistical analyses were made between samples using the Students' t-Test. In vivo experiments used in this manuscript were completed independently at least twice with a minimum three individuals per group. In vitro experiments were completed independently at least three times.
Results
IFNαRKO MICE HAVE DEFECTIVE PRIMARY RESPONSES TO COMBINED POLYI:C AND CD40 STIMULUS
We have previously found that combined adjuvants poly-Inosine:Cytosine (polyI:C) and an agonistic CD40 antibody (FGK4.5) synergisitically promote CD4+ T cell responses (25, 26, 32), whereas responses to either single agonist alone were as much as 10-fold lower than combined stimulus (26). We also showed that polyI:C/CD40 stimulus promoted CD8+ T cell responses that were type-I-interferon dependent (25). Whereas CD4+ T cell responses to immunizations containing polyI:C alone as an adjuvant are known to be dependent on type-I-interferon (12), we were interested in whether type-I-interferon was also required for promotion of CD4+ T cell responses in our polyI:C/CD40 adjuvant system.
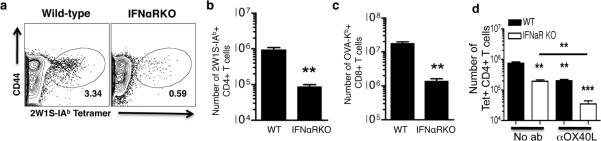
We began by immunizing wild-type B6 mice and interferon-α-receptor-deficient (IFNαRKO) B6 mice intraperitoneally with both a CD4+ T cell antigen (the 2W1S antigen, EAWGALANWAVDSA), and a CD8+ T cell antigen (whole ovalbumin), in the presence of polyI:C and CD40 agonists (polyI:C/CD40). Immunization with polyI:C/CD40 promoted robust CD4+ T cell proliferative responses in wild-type mice (Figure 1, a and b), as measured by the percents (Figure 1a) and numbers (Figure 1b) of 2W1S-IAb-tetramer-specific cells in the spleen. Similar to previously published results for CD8+ T cell responses (Figure 1c and (26), absence of type-I-interferon signaling in IFNαRKO mice reduced CD4+ T cell proliferative responses to polyI:C/CD40 up to 10-fold in the spleen (Figure 1, a and b) and peripheral blood (not shown and see Figure 2). Thus, type-I-interferon signaling is critical for the synergistic effects of polyI:C/CD40 for both CD4+ and CD8+ T cells, and are not unique to any particular cell type.
Figure 1. CD4+ and CD8+ T cell responses to combined polyI:C/CD40 stimulus are IFN-dependent.
Wild-type B6 or B6 IFNαRKO mice were immunized with antigen, polyI:C, and FGK4.5 anti-CD40 antibody as described in materials and methods. Seven days later mice were sacrificed and spleens harvested and stained for antigen specific CD4+ and CD8+ T cells. (a) Representative FACS plot depicting antigen specific CD4+ T cells stained with 2W1S-IAb tetramers and the activation marker CD44 from wild-type (left) and IFNαRKO mice (right). (b and c) Spleen cells from mice seven days after immunization as in (a) were quantified and stained with 2W1S-IAb (b) or OVA-Kb (c) tetramers. (d) IFNαRKO mice demonstrate a defect in eliciting cytokine-producing cells following immunization. Cells from mice in (b) were stained with 2W1S-IAb tetramer as described in materials and methods. Shown are total numbers of tetramer+ CD4+ T cells per spleen. Data are representative of at least two independent experiments with at least three mice per group. Error bars represent standard error of the mean. Statistics were calculated using Students' t Test and represent p<0.05 (*), p<0.01 (**), and p<0.005 (***).
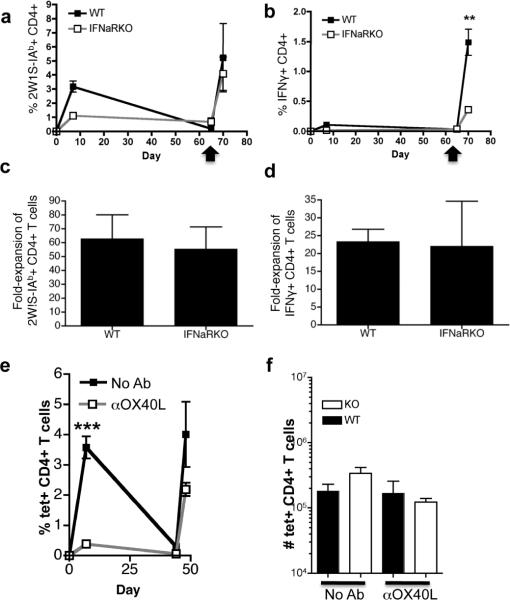
Figure 2. Primary CD4+ T cell responses are IFN- and OX40L-depednent while secondary CD4+ T cell responses are IFN- and OX40L-indepednent.
(a) Plot of percent 2W1S-IAb tetramer-positive CD4+ T cells in the peripheral blood of mice showing frequencies of antigen-specific CD4+ T cells as determined by 2W1S-IAb tetramer at different timepoints relative to immunization with 2W1S and polyI:C/CD40 (set to Day 0 on x axis) as described in Figure 1. Mice were re-stimulated with a second dose of 2W1S peptide and polyI:C/CD40 as indicated by the arrow (day 65) and were harvested 5 days later. (b) Plot showing the frequency of 2W1S antigen-specific IFNγ+ CD4+ T cells restimulated with 2W1S pepetide over time relative to immunization on day 0 as in (a). (c) Fold expansion of CD4+ T cells calculated as the ratio of antigen-specific cells in peripheral blood pre- and post-boost of a separate experiment performed as in (a). (d) Fold expansion of 2W1S-specific IFNγ+ CD4+ T cells of a separate experiment performed as in (b). (e) Mice were immunized with 2W1S peptide and polyI:C/CD40 and OX40-OX40L interactions were blocked by injecting 250μg of RM134L intraperitoneally on days −1 and 0 relative to priming, as described in materials and methods. 67 days later mice were rechallenged with antigen and polyI:C/CD40, and after 5 days spleens were harvested and stained with 2W1S-IAb tetramer. Shown are percentages of antigen-specific CD4+ T cells in peripheral blood over time. (f) Mice were primed with antigen and polyI:C/CD40 and were re-immunized 70 days after priming with antigen and polyI:C/CD40. OX40L was blocked only during the secondary immunization by administering 250μg of RM134L intraperitoneally on days −1 and 0 relative to rechallenge. Shown are numbers of antigen-specific splenic CD4+ T cells 5 days following rechallenge. Data are representative of at least two independent experiments containing at least three mice per group. Statistics were calculated using Student's t Test and are pairwise comparisons to control unless otherwise indicated. P values are p<0.05 (*), p<0.01 (**), and p<0.005 (***).
We previously found that OX40L is required for optimal CD4+ T cell proliferation in wild-type mice (26). We verified the role of OX40L in CD4+ T cell responses in both wild-type and IFNαRKOs by using the blocking antibody, RM134L. Mice were injected intraperitoneally with 250μg of blocking antibodies on days −1 and 0 relative to immunization with 2W1S and OVA antigens and polyI:C/CD40. Consistent with previous data (26), we found that CD4+ T cell responses in wild-type mice were reduced in the presence of RM134L blocking antibody (Figure 1d) while CD8+ T cell responses were unimpaired following OX40L blockade ((26, 33) and data not shown). Somewhat to our surprise, the CD4+ T cell response in unblocked IFNαRKO mice were brought down even further in the presence of RM134L. Again, CD8+ T cell responses in IFNαRKO mice were unaffected by RM134L (data not shown). Thus, even the remnant CD4+ T cell response in the IFNαRKO mice was largely dependent on OX40/OX40L interactions. Collectively the data demonstrate the central importance of both type I IFN and OX40 in the generation of CD4+ T cell immunity following combined polyI:C/CD40 immunization.
THE DEFECT IN IFNαRKO MICE IS LIMITED TO PRIMARY, BUT NOT SECONDARY RESPONSES
We were interested to know whether the defect in primary CD4+ T cell responses in IFNαRKO mice led to impaired secondary responses. To assess the role of type-I-interferon in CD4+ T cell memory, we immunized B6 or IFNαRKO mice with 2W1S antigen and polyI:C/CD40 as described above. Mice were followed by staining peripheral blood for the presence of antigen-specific CD4+ T cells at different time points. After allowing a recovery period of 40–70 days, the peripheral blood was monitored for the numbers of antigen specific memory cells as determined by tetramer staining (pre boost). The mice were the rechallenged with a boosting dose of 2W1S and polyI:C/CD40, and secondary responses were measured 5 days later (post boost).
We reasoned that poor primary responses would predict poor secondary responses in IFNαRKO mice. To our surprise, we found that secondary proliferative responses in IFNαRKO mice were largely intact and almost equivalent to secondary responses in B6 mice (Figure 2a). In particular, the fold-expansion of 2W1S-specific CD4+ T cells from pre-boost levels to post-boost levels was equivalent in IFNαRKO mice and B6 mice (Figure 2c). Other authors have noted that defects in type-I-interferon signaling during priming can lead to secondary responses with intact proliferation, but defective cytokine production (34). In line with these previous observations (34), we found that secondary cytokine responses by CD4+ T cells in peripheral blood were generally reduced in IFNαRKO mice relative to wild type mice (Figure 2b). However, in the spleen, the total number of cells producing IFNγ were more similar between WT mice and IFNαRKOs (not shown), and in all cases, the fold expansion of cytokine producing cells from pre- to post-boost was similar between wild-type and IFNαRKOs (Figure 2d). Thus, our data indicate that secondary proliferation and cytokine-production by CD4+ T cells are minimally dependent on type-I-interferon.
Curiously, we also found that wild-type and IFNαRKO mice responded equally well to secondary immunization, regardless of whether OX40L was blocked during the primary (Figure 2e) or secondary (Figure 2f) challenge. Blockade of OX40L during the primary response, while reducing the primary expansion of antigen specific CD4+ T cells, does not prevent the formation or response of memory CD4+ T cells (Figure 2e). Further, blockade of OX40L only after boosting vaccination has minimal impact on CD4+ T cell recall responses (Figure 2f). Broadly, we conclude from these data that OX40L plays an important role in determining the magnitude of the primary CD4+ T cell responses, but not the generation of memory nor the response of the memory cells following rechallenge.
THE IFNαR DEPENDENCY OF PRIMARY CD4+ T CELL IMMUNE RESPONSES IS T CELL EXTRINSIC
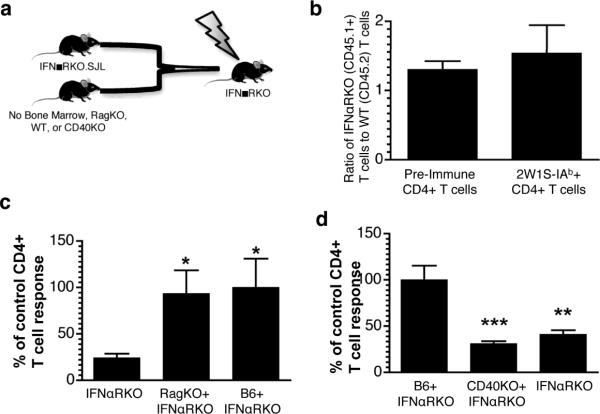
The fact that IFNαRKO CD4+ T cell could mount an effective secondary response suggested that the IFN-dependency of the primary CD4+ T cell response was not due to a requirement for IFNR expression on T cells. To more specifically address whether the effects of IFNαR deficiency are T cell intrinsic or extrinsic, we generated mixed bone marrow chimeras in lethally-irradiated IFNαRKO recipients to isolate defects in type-I-interferon signaling to bone-marrow-derived cells (Figure 3, a). We used congenically marked CD45.1+ IFNαRKO bone marrow and mixed it 1:1 with wild-type B6 bone marrow (Figure 3, a). We reasoned that, if IFNαR were required on responding T cells, we would observe a disproportionate response of the WT bone marrow derived T cells as compared to the congenic, IFNαRKO bone marrow-derived T cells. Instead, we found that T cells derived from IFNαRKO bone marrow competed very well with wild-type bone marrow, if anything, responding better than the wild-type T cells (Figure 3, b). This indicated that IFNαR was dispensable on responding CD4+ T cells to promote immune responses. Similar to previous reports of a CpG-based adjuvant system (13), our data suggested that the dependency of the polyIC/CD40 elicited response on type I IFN must be due to a requirement for IFNR expression on the APCs (12). We therefore reconstituted irradiated IFNαRKO hosts with Rag-recombinase deficient (RagKO) bone marrow mixed 3:1 (to enhance engraftment of RagKO marrow) with IFNαRKO bone marrow. The resulting host has T cells that are exclusively IFNαR-deficient but both WT and IFNαR-deficient APCs. As anticipated, the CD4+ T cell responses were rescued in these RagKO × IFNαRKO chimeras (Figure 3, c). These data demonstrate that IFNαR is dispensable on responding CD4+ T cells, and that cells derived from RagKO bone marrow are sufficient to restore IFNαR-dependent pathways in IFNαRKO mice.
Figure 3. IFN-dependency of CD4+ T cell responses is T cell extrinsic.
(a) Model for mixed bone-marrow chimeras. Bone marrow was harvested from congenically marked, IFNαRKO mice (IFNαRKO.SJL), Rag recombinase-deficient mice (RagKO), CD40-deficient mice (CD40KO) or wild-type B6 mice, mixed as indicated, and transplanted via the tail vein into lethally-irradiated IFNαRKO recipients. All mice were rested 12 weeks or more prior to immunization with polyI:C/CD40 and antigen. (b) 2×106 T cell-depleted bone marrow cells derived from each IFNαRKO.SJL (CD45.1) and wild-type (WT, CD45.2) mice was mixed 1:1 (4×106 total cells) and injected into lethally irradiated CD4 IFNαRKO CD45.2 recipients. The ratio of IFNαRKO (CD45.1+) to WT (CD45.1−) CD4+ T cells prior to immunization, and the ratio of 2W1S-specific IFNαRKO (CD45.1+) to WT (CD45.1−) following polyI:C/CD40 and 2W1S immunization, is shown. Data represent consolidated samples from two independent experiments (n=7). (c) IFNαRKO bone marrow was harvested (IFNαRKO), mixed 1:3 with RagKO bone marrow (IFNαRKO+Rag), or 1:1 with wild-type bone marrow (IFNαRKO+B6) and transplanted into lethally-irradiated IFNαRKO recipients. Peripheral blood from chimeric mice was stained with 2W1S-IAb tetramers seven days following immunization with 2W1S and polyI:C/CD40. Results were normalized to the percent of tetramer+ CD4+ T cells in the IFNαRKO+B6 control (mean=1.3%). Data are representative of two independent experiments pooled with at least three mice per group. (d) Lethally-irradiated IFNαRKO mice were reconsitituted with 4×106 T cell-depleted bone marrow cells from B6 mixed 1:1 with IFNαRKO, CD40KO mixed 1:1 with IFNαRKO, or IFNαRKO alone. Results represent two independent experiments containing 3 or more mice per group normalized to B6+IFNαRKO controls (mean=3.5×105 cells). Statistics were calculated using Students' t Test and represent p<0.05 (*), p<0.01 (**), and p<0.005 (***).
We have previously shown that CD40 expression on innate cells was sufficient for the synergistic effects of agonistic anti-CD40 combined with polyI:C (26). Our finding that IFNαR expression was also sufficient on innate cells raised the question of whether CD40 and IFNαR were required on the same cells, or whether CD40 stimulus and polyI:C stimulus could work in trans for promotion of optimal CD4+ T cell responses to polyI:C/CD40. To determine whether CD40 and IFNαR were required on the same cells, we reconstituted lethally-irradiated IFNαRKO mice with bone marrow derived from IFNαRKO.SJL mice alone, IFNαRKO.SJL and CD40KO bone marrow mixed 1:1, and IFNαRKO.SJL and B6 bone marrow mixed 1:1. We found that IFNαRKO.SJL and CD40KO bone marrow were not able to reconstitute responses in IFNαRKO recipients (figure 3d), suggesting that both CD40 and IFNαR need to be expressed by the same APC in order for polyI:C/CD40 to promote robust CD4+ T cell immune responses. By extension, trans signals elaborated by polyI:C/CD40 are not sufficient for the combined effects of both agonists.
IFNαRKO DENDRITIC CELLS DEMONSTRATE DEFECTIVE ACTIVATION IN RESPONSE TO POLYI:C AND CD40 STIMULUS
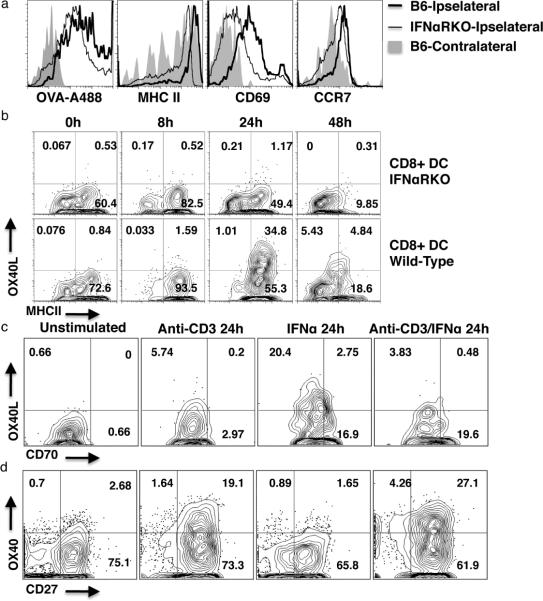
The data thus far indicate that the dependency of the CD4+ T cell response elicited by combined polyI:C/CD40 immunization must be due to the action of IFN on the antigen presenting cell. Other authors have also shown that DCs can be regulated in an intrinsic IFNαR-dependent manner (8, 12). To understand how IFNαR-deficiency might affect DC activation, we immunized B6 and IFNαRKO mice in one footpad with fluorescent OVA antigen and polyI:C/CD40 stimulus and isolated DCs from the ipsilateral and contralateral popliteal lymph nodes 18–24 hours later. FACS histograms revealed that IFNαRKO DCs (Figure 4, a) had relative defects in antigen uptake, MHC II expression, CD69 expression, and CCR7 expression compared with wild-type mice (Figure 4, a). Importantly, DCs from IFNαRKO mice did upregulate these molecules relative to cells from contralateral nodes in wild-type mice (Figure 4a – shaded area), suggesting that DC activation was not completely absent in IFNαRKO mice, but was impaired.
Figure 4. DC OX40L expression is type I IFN dependent.
(a) Representative FACS histograms of popliteal CD11c+ cells harvested 24 hours following footpad immunization with polyI:C/CD40. Shown from left to right are fluorescent antigen (OVA) uptake, MHCII expression, CD69 expression, and CCR7 expression. Shown are (relative to injection site) ipselateral nodes from wild-type mice (thick black line), ipselateral nodes from IFNαRKO mice (thin black line), and contralateral nodes from wild-type mice (gray shading). Data are typical of three independent experiments containing three or more mice per group. (b) Representative FACS plot of splenic CD8+ CD11c+ cells, harvested at the given timepoints following i.p. imunization with polyI:C/CD40, and stained for OX40L and MHCII. Data are typical of two independent experiments containing at least three mice per group. (c and d) Representative FACS plots of ex vivo (left panels, c and d) and in vitro cultured splenocytes stimulated with 0.5μg/mL anti-CD3 (2C11), 105U/mL recombinant IFNα, or combined anti-CD3 and IFNα for the indicated period. Shown are CD11c+ cells (c) and CD4+ T cells (d). Data are typical of four independent experiments.
Given the established importance of OX40/OX40L signaling in mediating the CD4+ T cell response to combined polyIC/CD40 immunization (Figure 1 and (26)), we were interested to know whether expression of OX40L on activated DCs was also influenced by the presence or absence of IFN. We chose to focus on CD8+ DCs, as OX40L expression is more pronounced on this subset, and we have found that CD8+ DC OX40L expression correlates to CD4+ T cell responses (26). We found that both OX40L expression and MHC II expression on CD8+ DCs were reduced at multiple timepoints in IFNαRKOs relative to wild-type mice (Figure 4, b).
While the loss of DC OX40L expression was not absolute in the IFNabRKOs (as evidenced by the fact that OX40L blockade can still reduce the residual T cell response in these hosts -Figure 1d), the majority of OX40L expression appeared to be IFN-dependent (Figure 4b). These data suggested that the introduction recombinant IFNα might be sufficient to promote DC OX40L expression. We harvested naïve splenocytes and stained them directly (Figure 4, c), or cultured them in the presence of anti-CD3 (2C11), recombinant IFNα, or anti-CD3 and recombinant IFNα. Intriguingly, anti-CD3 alone and anti-CD3 with IFNα did not lead to appreciable expression of OX40L in these cultures (Figure 4c). However, administration of recombinant IFNα alone was sufficient to massively upregulate OX40L expression on cultured DCs (Figure 4, c). Furthermore, DCs increased expression of CD70 in the presence of recombinant IFNα (Figure 4, c), regardless of the presence of anti-CD3.
It was interesting that IFNα alone, but not IFNα with anti-CD3, led to upregulation of OX40L on cultured DCs (Figure 4, c). The antibody used to detect OX40L expression, RM134L, is also useful for blockade of OX40L-OX40 interactions (26). We hypothesized that activation of responding T cells in the presence of anti-CD3 and recombinant IFNα may have led to upregulation of OX40 which, in turn, might prevent detection of OX40L on the DCs, either by down-modulating OX40L expression or by directly interfering with antibody binding to OX40L. We therefore assessed the expression of OX40 on responding CD4+ T cells. We found that OX40 expression was high in the presence of anti-CD3 alone +/− recombinant IFNα, but not on naïve cells or on cells stimulated with IFNα alone (Figure 4d). This result is consistent with the finding that, in vivo, priming of CD4+ T cells does not require IFNαR on the cells themselves. Similarly, expression of another TNF receptor, CD27, was high on CD4+ T cells under all conditions, but appeared to increase in the presence of anti-CD3 (Figure 4d). This suggests that regulation of DC TNF ligand expression could be a direct consequence of type-I-interferon, but regulation of the receptors on responding CD4+ T cells is likely to be a consequence of CD3-mediated T cell receptor stimulus.
OX40 RECEPTOR STIMULATION RESCUES CD4+ T CELL RESPONSES IN IFNαR KO MICE
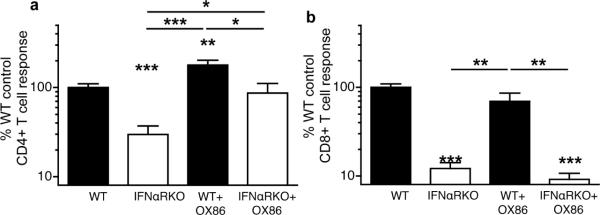
As OX40L signals were required for optimal CD4+ T cell priming, but were defective in IFNαRKO mice, we hypothesized that restoration of signaling through OX40 might restore CD4+ T cell responses in IFNαRKOs. We therefore administered single doses of the OX40-agonistic antibody, OX86 (35, 36), to wild-type and IFNαRKO mice coincident with immunization by antigen and polyI:C/CD40. We found that agonistic OX40 antibody largely enhanced CD4+ T cell responses (Figure 5a), but not CD8+ T cell responses (Figure 5b), in wild-type and IFNαRKO mice. This data confirmed a unique role for OX40 in CD4+ T cell, but not CD8+ T cell priming, which is reflected by the literature (37). In all cases, cytokine production by CD4+ and CD8+ T cells reflected the tetramer responses shown here (not shown). Thus, augmentation of OX40-OX40L signals in IFNαRKO mice can substantially enhance CD4+ T cell, but not CD8+ T cell primary responses.
Figure 5. OX40 stimulation rescues primary CD4+ T cell responses in IFNαRKO mice.
Wild-type (WT) or IFNαRKO mice were immunized with antigen and polyI:C/CD40 and were administered 250μg of an agonistic antibody for OX40 (OX86) i.p. on day 0 relative to primary immunization. Seven days following immunization spleens were harvested and stained with 2W1S-IAb tetramer (a) or OVA-Kb (b). Data represent combined results from three independent experiments containing three or more mice per group normalized to control (WT), with mean responses of 7.2×105 CD4+ T cells in (a) and 1.8×106 CD8+ T cells in (b). Statistics represent pairwise comparisons to wild-type controls unless otherwise indicated. P values were calculated using Students' t Test and represent p<0.05 (*), p<0.01 (**), and p<0.005 (***). Error bars represent standard error of the mean.
Discussion
Collectively, our data contribute to the growing literature of the influence of type-I-interferon on adaptive immunity by adding a critical role for type-I-interferon in DC OX40L expression and subsequent stimulation CD4+ T cells through OX40. In addition, our data challenge the paradigm that direct stimulation of T cells by IFN is required for their capacity to respond to primary challenge. Several authors have written on the role of direct type-I-interferon signals for T cell immunity (12, 20, 38–40), and in particular the role of type-I-interferon in Th1-type immune responses is controversial (39). However, we could find no evidence that the Th1 phenotype of responding CD4+ T cells was different between wild-type and IFNαRKO mice. Rather, we noted reduced responses across the board in IFNαRKOs relative to B6 controls in both proliferation and cytokine production, suggesting a defect in CD4+ T cell proliferation, but not necessarily differentiation. Furthermore, using a mixed bone marrow chimeric system, we were able to show that absence of IFNαR on responding CD4+ T cells does not disfavor their participation in primary immune responses with competing wild-type cells. In fact, we show isolated IFNαRKO CD4+ T cells, in a system without any cells able to sense type-I-interferon save for bone-marrow-derived, Rag-independent cells, have the capacity to mount effective primary immune responses to interferon-dependent stimuli. Granted, the outcomes we measure, such as CD4+ T cell proliferation, are different from the outcomes others have measured, such as antibody production (38), or CD8+ T cell differentiation (20). Nevertheless, our data support a minimal role for type-I-interferon signaling to CD4+ T cells during primary responses.
We note also that, in our dual-adjuvant system, it is unlikely that CD40 stimulus is rescuing interferon-dependent responses. We have shown that CD40 is dispensable on responding CD4+ T cells in the setting of polyI:C/CD40 stimulus (26), and we show here that IFNαR and CD40 must be on the same cells to promote synergistic immune responses. This is consistent with our previous data as well, where we have shown that CD40 is sufficient on innate cells for polyI:C/CD40-induced priming (26). Although CD40 agonist by itself is a sufficient adjuvant to initiate adaptive immune responses (41), and is not known to be dependent on type-I-interferons, it is interesting that the phenotype of IFNαRKO mice dominates to limit expansion of CD4+ T cells dramatically compared to wild-type mice. It is possible, however, that CD40 agonism may reverse some of the defects in CD4+ T cell responses that other authors have observed in IFNαRKO mice (17, 21). If true, taken with our previous data that CD40 is necessary on innate cells (26) our data would suggest that CD40 agonism acts indirectly, through antigen-presenting cells, to rescue CD4+ T cell memory in IFNαRKO mice.
We have previously shown that recombinant IFNα will directly synergize with agonistic anti-CD40 antibody to promote CD8+ T cell responses when combined together (32). However, we have not been able to show that recombinant IFNα would synergize with anti-CD40 to promote CD4+ T cell responses (data not shown). This implies that while IFNαR is indispensable for the effects of polyI:C/CD40 immunization, IFNα itself is not sufficient to synergize with CD40 agonists for CD4+ T cells. We account for this by noting that CD4+ and CD8+ T cells in this setting require slightly different priming conditions (26), and that these could be affected by the dose of recombinant IFNα used. It is possible that CD4+ and CD8+ T cell priming is initiated by different antigen presenting cell subsets (42, 43), and these might have different sensitivities to recombinant IFNα. Alternatively, IFNα is one of many types of type-I-interferon, all of which require the IFNαR to function (44), and any of these may differentially impact antigen presentation to CD4+ and CD8+ T cells, either by having variable effects on different APC subsets, or by directly impacting presentation through MHC-II or MHC-I in an paralogue-dependent manner.
A larger question raised by these data concern our findings that both interferon and OX40 are dispensable for secondary CD4+ T cell responses but required for primary responses. This disagrees with previous observation (17, 21, 23), and is particularly interesting given the finding that, in the case of lymphocytic choriomeningitis (LCMV) viral infection, survival of CD4+ T cells is dependent on cell-intrinsic type-I-interferon signaling (21). In contrast, our bone marrow chimera suggests that intrinsic roles for type-I-interferon are dispensable and that the primary role for interferon in CD4+ T cell responses is T cell extrinsic. Consistent with this, cell-intrinsic type-I-interferon signaling was not required for CD4+ T cell responses to bacterial infection (21) while studies conflict with regard to the necessity for direct IFN signaling to T cells in response to vaccinia virus challenge (40, 45). There are a number of non-mutally exclusive ways to reconcile our data with these findings. One is that our dual-agonist immunization system exploits a pathway that permits CD4+ T cell survival similar to the result found for bacterial infection (discussed above), (21). Another possibility is that, during lymphotrophic viral infection such as LCMV, the inability to respond to interferon signals leads to killing of CD4+ T cells by direct infection. Third, as has been previous suggested (40), the inflammatory environment present during the initial stimulation of the T cells may dictate the degree of dependence or independence of direct IFN signaling into the T cells. A final possibility is that aberrant interferon responses by CD4+ T cells in infected tissue leads to lysis by NK cells or CTL.
Our data show that the causes of poor primary responses that are attributable to IFNαRKO mice are not as relevant for secondary responses as they are for primary responses. To some extent this is not surprising as primary and memory CD4+ T cells are qualitatively different from each other (46, 47) and our work adds to this body of literature. However, our data showing memory responses either in the absence of type-I-interferon signaling or OX40L blockade or begs the question of how WT-levels of secondary T cell expansion can occur after such a compromised primary burst size. One explanation is that larger clonal burst sizes lead to shorter half-lives of daughter progeny, which is an extrapolation of recent data showing that population size of CD4+ T cells is a determinant of survival (48). An alternative, but not mutually exclusive explanation for these data is that a population of T cells with high avidity for antigen, which is thought to be important for CD4+ T cell memory formation (47), is able to become activated in wild-type and IFNαRKO mice regardless of costimulation. Thus, addition of extra costimulation and antigen presentation in wild-type mice in this model might provide activation and recruitment of more lower avidity cells, increasing the primary burst size but not the memory pool. Similarly it is possible that memory formation is facilitated by a minimal threshold of activation beyond which extra proliferation yields extra effector cells, but not necessarily more memory. Thus, type-I-interferon and OX40L costimulation may promote effector cell generation beyond what is required for memory formation, and the blockade or loss of these signaling pathways reduces clonal burst size by reducing effectors. All possibilities outlined here are validated for OX40 by recent data (49, 50).
While we observed what appears to be a causal association between CD4+ T cell priming, IFN and DC OX40L expression, there are likely other defects APC function that contribute to the IFN-dependency of the T cell response. DC activation (10, 12, 13), trafficking (8), and antigen presentation (9) are all impaired in the absence of type-I-interferon. We have confirmed many of these defects in our system by observing reduced antigen uptake, expression of MHC-II, CD69, CCR7, CD80 and CD86 (Figure 4 and data not shown), all of which contribute to poor CD4+ T cell priming in the absence of IFN. In particular, we show that stimulation through the T cell receptor is important in vitro for OX40 upregulation on CD4+ T cells. We extrapolate that impaired TCR stimulus might impair CD4+ T cell competence for OX40 stimulus. This explains why IFNαRKO mice, where MHCII expression is impaired, are not completely rescued by OX86 to the level of wild-type mice treated with OX86. Nevertheless, the connection we make to OX40L demonstrates that defects in IFNαR signaling can have very concrete molecular consequences for the ability of DCs to prime CD4+ T cells, and that these defects may be partially reversed by agonist treatment of key pathways absent in IFNαRKOs.
Collectively, our data contribute to the growing body of evidence that the induction of type I IFN in a vaccine setting can have a powerful influence on the generation of cellular responses largely through an influence on APC function. These data imply that the application of IFN or IFN-inducing modalities to the generation of T cell immunity will need to take into consideration the fact that the primary function of IFN would be early in the generation of the response and that continued application of IFN beyond the window of necessary APC function will likely only be self limiting. Thus, while the primary clinical regimen of chronic IFN dosing is appropriate to capitalize on the direct antiviral and cytostatic properties of IFN signaling, the use of IFN as a therapeutic intervention to augment the adaptive response will likely need to consider a shorter course of IFN treatment. Our data indicate that OX40-OX40L interactions will be a critical component by which type-I-interferon elicits CD4+ T cell responses with hopeful protective and/or therapeutic potential.
Acknowledgements
The authors thank Hideo Yagita, Juntendo University School of Medicine, Tokyo, Japan, for providing the OX40L and CD70 antibodies.
This work was supported by grants from the NIH (AI06877, AI066121) and the Department of Defense (W81XWH-07-1-0550). DoD support was associated with funding for the Center for Respiratory Biodefense at National Jewish Health.
References
- 1.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feld JJ, Lutchman GA, Heller T, Hara K, Pfeiffer JK, Leff RD, Meek C, Rivera M, Ko M, Koh C, Rotman Y, Ghany MG, Haynes-Williams V, Neumann AU, Liang TJ, Hoofnagle JH. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology. 2010;139:154–162. e154. doi: 10.1053/j.gastro.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jouanguy E, Zhang SY, Chapgier A, Sancho-Shimizu V, Puel A, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Human primary immunodeficiencies of type I interferons. Biochimie. 2007;89:878–883. doi: 10.1016/j.biochi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 6.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 7.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 8.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 9.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 10.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 11.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 12.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilz A, Kratky W, Stockinger S, Simma O, Kalinke U, Lingnau K, von Gabain A, Stoiber D, Sexl V, Kolbe T, Rulicke T, Muller M, Decker T. Dendritic cells require STAT-1 phosphorylated at its transactivating domain for the induction of peptide-specific CTL. J Immunol. 2009;183:2286–2293. doi: 10.4049/jimmunol.0901383. [DOI] [PubMed] [Google Scholar]
- 14.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166:182–194. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guery JC, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II-restricted T cells after administration of protein in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos HJ, Davis AM, George TC, Farrar JD. IFN-alpha is not sufficient to drive Th1 development due to lack of stable T-bet expression. J Immunol. 2007;179:3792–3803. doi: 10.4049/jimmunol.179.6.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenner CA, Guler ML, Macatonia SE, O'Garra A, Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- 20.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 21.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ. Type I interferon (IFN alpha) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol. 2009;183:2915–2920. doi: 10.4049/jimmunol.0801607. [DOI] [PubMed] [Google Scholar]
- 23.Davis AM, Ramos HJ, Davis LS, Farrar JD. Cutting edge: a T-bet-independent role for IFN-alpha/beta in regulating IL-2 secretion in human CD4+ central memory T cells. J Immunol. 2008;181:8204–8208. doi: 10.4049/jimmunol.181.12.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Comparison of OX40 ligand and CD70 in the promotion of CD4+ T cell responses. J Immunol. 2010;185:2106–2115. doi: 10.4049/jimmunol.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T Cell Frequency Varies for Different Epitopes and Predicts Repertoire Diversity and Response Magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 30.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T Cells Compete for Access to Antigen-bearing Antigen-presenting Cells. J. Exp. Med. 2000;192:1105–1114. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009 doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28:1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 34.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.al-Shamkhani A, Birkeland ML, Puklavec M, Brown MH, James W, Barclay AN. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 37.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 38.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 39.Berenson LS, Gavrieli M, Farrar JD, Murphy TL, Murphy KM. Distinct characteristics of murine STAT4 activation in response to IL-12 and IFN-alpha. J Immunol. 2006;177:5195–5203. doi: 10.4049/jimmunol.177.8.5195. [DOI] [PubMed] [Google Scholar]
- 40.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 41.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 42.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 43.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 44.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 46.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 47.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 49.Soroosh P, Ine S, Sugamura K, Ishii N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol. 2007;179:5014–5023. doi: 10.4049/jimmunol.179.8.5014. [DOI] [PubMed] [Google Scholar]
- 50.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]