Abstract
Herpes simplex virus-1 (HSV) infection of the cornea leads to a potentially blinding immuno-inflammatory lesion of the cornea that is termed herpetic stromal keratitis (HSK). It has also been demonstrated that one of the factors that limits inflammation of the cornea is the presence of Fas ligand (FasL) on corneal epithelium and endothelium. In this study the role that FasL expression in the cornea plays following acute infection with HSV-1 was determined. HSV-1 infection of both BALB/c and C57BL/6 (B6) mice were compared to their lpr and gld counterparts. Results indicated that mice bearing mutations in the Fas antigen (lpr) displayed most severe disease while the FasL defective gld mouse displayed an intermediate phenotype. It was further demonstrated that increased disease was due to lack of Fas expression on bone-marrow derived cells. Interestingly, while virus persisted slightly longer in the corneas of mice bearing lpr and gld mutations, the persistence of infectious virus in the trigeminal ganglia was the same for all strains infected. Furthermore, B6 mice bearing lpr and gld mutations were also more resistant to virus-induced mortality than wild-type B6 mice. Thus neither disease nor mortality correlated with viral replication in these mice. Collectively, these findings indicate that the presence of FasL on the cornea restricts the entry of Fas+ bone marrow-derived inflammatory cells and thus reduces the severity of HSK.
INTRODUCTION
Herpetic stromal keratitis (HSK) is a potentially blinding corneal inflammation that accompanies herpes simplex virus (HSV) infection of the eye. The disease course in HSK begins with a primary infection by HSV followed by a period during which the virus enters latency in sensory and autonomic ganglia. Many studies have shown that clinical disease is the result of a cocktail of inflammatory cells, consisting of PMN’s, macrophages and T cells (both CD4+ and CD8+) that are recruited to the corneas of patients with HSK (1–4).
In the face of this potentially blinding inflammatory attack, the cornea has the ability to reduce inflammation. These include the presence of immunosuppressive factors such as TGF-β (5), lack of vascularization (6,7) and the presence of Fas ligand (FasL) (8–14). It is the presence of FasL that is the focus of this project.
Various studies have clearly demonstrated that the presence of FasL in the eye is an important barrier to both inflammatory cells (8–10) and new blood vessels (11–13), both of which are intimately involved in the pathology of HSK. Control of inflammation is also known to be a significant component of the immune privilege of the eye (8,9). FasL expressed on ocular tissues induces apoptosis in Fas+ lymphoid cells that invade the eye in response to viral infection (8) or corneal grafting (10,11). FasL expressed in the retina and the cornea also controls new vessel growth beneath the retina and in the cornea by inducing apoptosis of Fas expressing vascular endothelial cells (15–17). These studies clearly indicate that the presence of FasL in ocular tissues restricts inflammatory responses.
An understanding of the cellular interactions between virus-specific immune cells and cells of the cornea and nervous system are crucial in determining the underlying mechanisms of HSK. In order to more fully examine the role of Fas and FasL during primary HSK, we utilized mice deficient in Fas (lpr) and FasL (gld). To determine if host genetic background influences the role of T cell subsets in recurrent corneal disease, we performed our experiments in HSV susceptible (BALB/c) and HSV resistant (C57BL/6) strains of mice. Our findings indicate that mice that are defective in either Fas or FasL experience increased HSK disease following infection with HSV-1.
MATERIALS AND METHODS
Virus and cells
The viruses used in these studies were the McKrae and KOS strains of HSV-1. A plaque-purified stock was grown and assayed on Vero cells in minimum essential medium with Earle’s balanced salts (MEM-EBS) containing 5% fetal bovine serum, 100U/ml Penicillin and 100 μg/ml Streptomycin. (18). Virus titers in eye swabs were determined by standard plaque assay (18).
Mice
Investigations with mice conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 (B6) and BALB/c mice were purchased from NCI. The B6Smn.C3-Tnfsf6gld/J and B6.MRL-Tnfsf6lpr/J mice were purchased from Jackson Labs and maintained in our colony. For the purposes of this manuscript we will refer to these mice as B6-gld and B6-lpr respectively. We also bred the B6-gld and B6-lpr mice to BALB/c mice for a minimum of 12 generations. The resultant strains designation will be C.B6- Tnfsf6gld and C.B6- Tnfsf6lpr (19,20) However, we will refer to them as BALB-gld and BALB-lpr respectively. In order to assure that these mice retain their mutations, tail DNA is isolated from individual mice and PCR tested for either the gld or lpr mutation.
Infection of Mice
Eight to twelve week old normal and mutant mice were infected as previously described (21). Briefly, following corneal scarification, 1 × 107 Plaque Forming Units (PFU) of HSV-1 KOS strain when infecting BALB/c mice or 1 × 106 PFU of HSV-1 McKrae strain when infecting C57BL/6. A volume of 5 μl of MEM-EBS containing HSV-1 was placed onto the surface of scarified corneas of BALB/c (HSV sensitive) or C57BL/6 (HSV resistant) mice.
Clinical evaluation
On the designated days after viral infection or UV-B reactivation, a masked observer examined mouse eyes through a binocular-dissecting microscope in order to score clinical disease. Stromal opacification was rated on a scale of 0 to 4, where 0 indicates clear stroma, 1 indicates mild stromal opacification, 2 indicates moderate opacity with discernible iris features, 3 indicates dense opacity with loss of defined iris detail except pupil margins, and 4 indicates total opacity with no posterior view. Corneal neovascularization was evaluated as described (18,21) using a scale of 0–8, where each of four quadrants of the eye is evaluated for the amount of vessels that have grown into them. Periocular disease was measured in a masked fashion on a semiquantitative scale as previously described (23).
Viral tittering from tissues
Eye swab material was collected and assayed for virusby standard plaque assay as previously described (18). Trigeminalganglia and 6-mm biopsy punches of periocular skin were removedand placed in preweighed tubes containing 1-mm glass beads and1 ml of medium. Trigeminal ganglia and periocular skin homogenateswere prepared by freezing and thawing the samples, mechanically disrupting in a Mini-Beadbeater-8 (Biospec Products, Bartlesville,Okla.), and sonicating. Homogenates were assayed for virus bystandard plaque assay, and the amount of virus was expressedas PFU per milliliter of tissue homogenate.
Construction of Bone Marrow Chimeras
Radiation bone marrow chimeras between BALB/c and BALB-lpr mice were prepared as follows. Briefly, mice were irradiated with 700 Rads from an XRAD 320 irradiator (Precision X-Ray, North Branford, CT) and reconstituted with an equal mixture of 2×107 bone marrow and spleen cells.The level of chimerism was determined by PCR genotype analysis of peripheral blood cells 30 days after bone marrow transplantation.
Viral reactivation assay
Trigeminal ganglia were removed from infected mice 30 to 40 days post-infection. To assess reactivation, individual trigeminalganglia were dissociated (19) and plated on collagen-coated 12-wellplates. Supernatants were assayed every 12 h for progeny virusfrom 1 to 5 days post plating.
Assays of antibody titers
Serum was collected from mice at weekly intervals following infection and examined for HSV-specific antibody content as previously described (24). Briefly, for enzyme linked immunosorbant assays (ELISA), serial four fold dilutions of mouse serum were incubated for 2 hours in duplicate wells of a 96 well plate coated with purified HSV-1 glycoprotein. Biotinylated goat anti-mouse IgG was subsequently used in a colorimetric assay to determine specific IgG amounts based on comparison to a standard curve generated as previously described (24).
Flow Cytometric Analysis
Cells were isolated from corneas as previously described (25). Briefly, corneas were excised at 18 and 23 dpi and incubated in PBS-EDTA at 37°C for 15 minutes at 37°C. Stromas were separated from overlying epithelium and digested in 84 U collagenase type 1 (Sigma-Aldrich, St. Louis, MO) per cornea for 2 hours at 37°C and then were triturated to form a single-cell suspension. Suspensions were filtered through a 40-μm cell strainer cap (BD Labware, Bedford, MA) and washed and then stained. Suspensions were stained with: PerCP-conjugated anti-CD45 (30-F11) and Alexa Fluor700-Gr-1 (RB6-8C5) (from BioLegend, San Diego, CA); FITC conjugated anti-CD4 (RM4–5), PE-conjugated anti-CD8α (53–6.7), PE-Cy7-conjugated anti-CD11c (HL3), (all BD PharMingen); eFluor450-conjugated CD11b (M1/70) (from eBiosciences, San Diego, CA). Cells were then analyzed on a flow cytometer (FACSAria with FACSDIVA data analysis software; BD Biosciences).
Statistical analysis
All statistical analyses were performed with the aid of Sigma Stat for Windows, version 2.0 (Jandel, Corte Madera, CA). The Student’s unpaired t-test was used to compare corneal disease scores, virus titer, and antibody titer data. Fisher’s exact Χ2 tests were used to compare limiting dilution assay data.
RESULTS
Herpetic stromal keratitis (HSK) is a disease that results from the infection of corneal epithelium by herpes simplex virus type I (HSV-1) (1–4). At the heart of this disease is an immune-mediated inflammatory attack on the cornea. As previously described, corneal inflammation is controlled by a number of cell free and surface proteins including the presence of FasL (5–14). Previous studies attempting to determine the role of FasL in controlling HSK were not able to distinguish differences in corneal disease when the corneas of B6 and their lpr and gld counterparts were infected with the KOS strain of HSV-1 (26). However, since B6 mice are highly resistant to developing HSK when infected with the KOS strain of HSV-1 following corneal infection, we thought that this was not a fair means of testing the role that these apoptotic molecules might play during HSK. Consequently, we decided to test the more susceptible BALB/c strain of mouse with the KOS strain of HSV-1. When BALB/c, BALB-gld, and BALB-lpr mice were infected with the KOS strain of HSV-1, we observed that both BALB-lpr and to a lesser extent, BALB-gld mice displayed significantly greater disease scores (opacity, neovascularization (Fig. 1), blepharitis (Fig. 2)) than wild-type BALB/c mice. In addition to displaying the highest ocular disease scores, BALB-lpr mice also displayed significantly more disease symptoms, including weight loss, ruffled fur, hunched posture, and temporary limb weakness (data not shown). We had anticipated that mice expressing a mutation in FasL would have a similar disease profile as those mice that carry a mutation in Fas. Unexpectedly, BALB-gld mice were intermediate between wild-type BALB/c and BALB-lpr mice in their disease profile, providing further evidence that mice carrying the gld mutation are not equivalent to those lacking FasL (27).
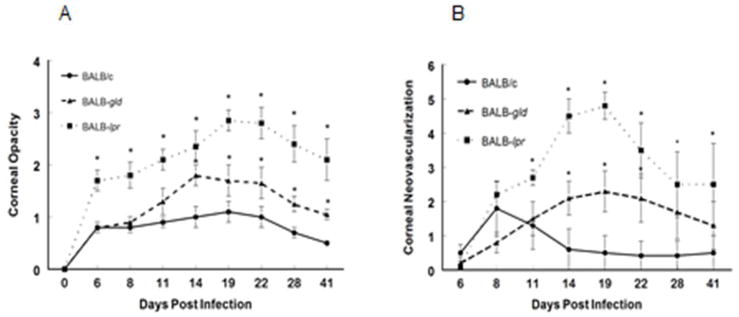
Figure 1.
Defective expression of both Fas and to a lesser extent FasL, results in increased HSK following infection with HSV-1, KOS strain. Eyes of BALB/c wild-type (n=30), BALB-lpr (n=25) and BALB-gld (n=25) mice were infected with 107 pfu of HSV-1, KOS strain. Corneal opacity (A) and corneal neovascularization (B) were measured and compared between these strains of mice. Significant virus-induced corneal opacity was observed for BALB-lpr (P<0.001) at all time points when compared to BALB/c controls. BALB-gld mice displayed significantly more opacity than did BALB/c controls at days 14–35 (P<0.05–0.01). BALB-lpr displayed significantly greater neovascularization at days 11–41 (P<0.05–0.001) and BALB-gld mice had greater neovascularization at days 14–22 (P<0.05–0.01) than did BALB/c controls.
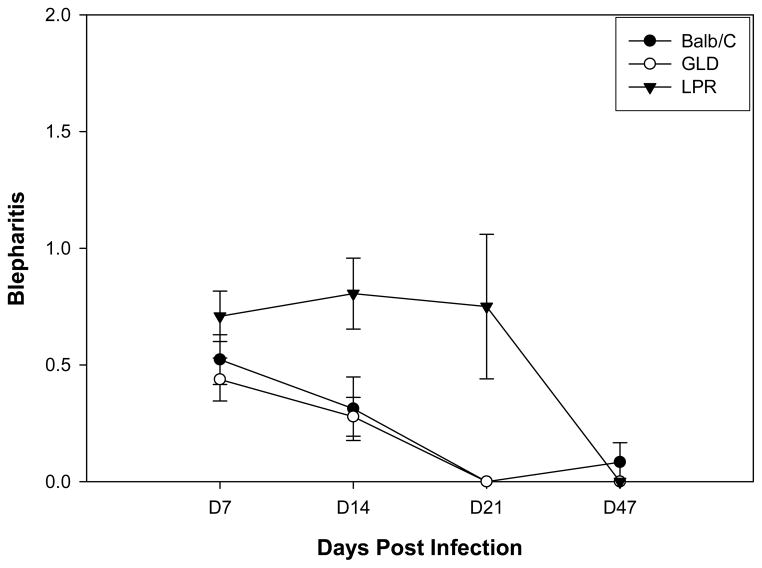
Figure 2.
Defective expression of Fas but not FasL, results in increased blepharitis following infection with HSV-1, KOS strain. Eyes of BALB/c wild-type (n=20), BALB-lpr (n=20) and BALB-gld (n=20) mice were infected with 107 pfu of HSV-1, KOS strain and blepharitis measured. Significant virus-induced blepharitis was observed for BALB-lpr (P<0.01) at days 14 and 21 when compared to both BALB/c controls and BALB-gld mice.
To confirm what we observed with BALB/c mice we also tested whether similar results would be seen when B6, B6-lpr, and B6-gld mice were infected with HSV-1. However, we decided to use the McKrae strain of HSV-1, due to previous publications demonstrating that infection with this strain leads to significant disease in B6 mice (21). Results from infecting B6, B6-lpr, and B6-gld mice displayed a similar disease profile as that observed when BALB/c mice were infected with KOS, namely that B6 mice containing mutations in either Fas or FasL displayed increased HSK disease than did wild-type B6 mice (Fig. 3).
Figure 3.
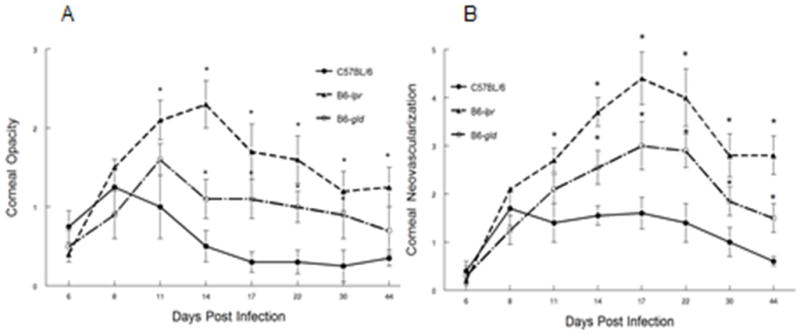
Defective expression of both Fas and to a lesser extent FasL, results in increased HSK following infection of C57BL/6 (B6) mice with HSV-1, McKrae strain. Eyes of B6 wild-type (n=25), B6-lpr (n=22) and B6-gld (n=23) mice were infected with 2×106 pfu of HSV-1, McKrae strain. Corneal opacity (A) and corneal neovascularization (B) were measured and compared between these strains of mice. Significant virus-induced corneal opacity was observed for B6-lpr (P<0.01–0.001) at Days 11–30 when compared to B6 controls. B6-gld mice displayed significantly more opacity than did B6 controls at days 14–30 (P<0.05–0.01). B6-lpr displayed significantly greater neovascularization at days 11–44 (P<0.05–0.001) and B6-gld mice had greater neovascularization at days 14–44 (P<0.05–0.01) than did B6 controls.
In order to better understand the mechanism underlying these observations, we hypothesized that mice expressing either defective Fas (lpr) or FasL (gld) were not able to effectively control inflammatory cell entry into corneas infected with HSV-1. To test whether lack of control of inflammatory cells entering the cornea was the underlying mechanism responsible for increased disease, we constructed bone marrow chimeras between wild-type BALB/c and BALB-lpr mice. BALB-lpr mice were chosen because they displayed the highest HSK disease scores of the strains tested. Thus we predicted that wild-type BALB/c mice reconstituted with BALB-lpr bone marrow would experience greater disease because their inflammatory cells, which do not express functional Fas, would not be controlled by the FasL expressed on corneal endothelium and epithelium (8,10). In contrast, if it were a non-lymphoid cell that was responsible for this disease, BALB-lpr mice reconstituted with wild type BALB/c cells would experience greater disease. As displayed in Figure 4, increased disease phenotype was associated with the genotype of the bone marrow derived lymphoid cells as irradiated BALB/c mice reconstituted with BALB-lpr bone marrow had significantly worse corneal disease than did irradiated BALB-lpr mice reconstituted with BALB/c bone marrow. Thus we concluded that it was lack of control of the inflammatory infiltrate that leads to increased HSK.
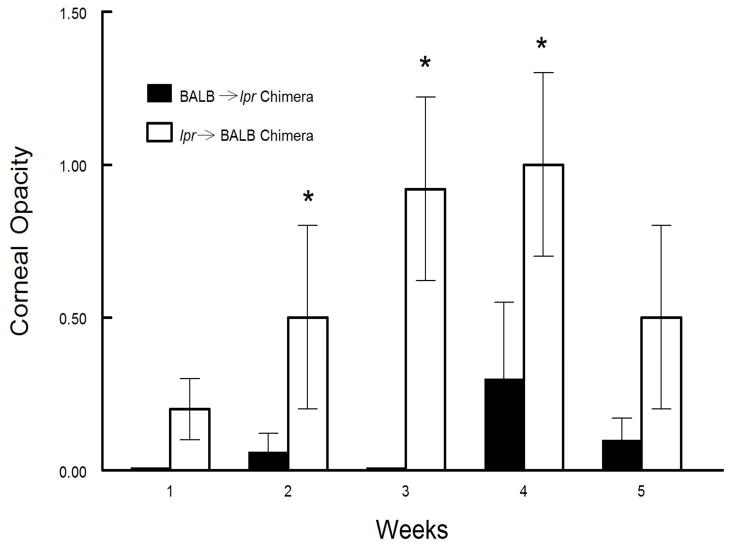
Figure 4.
Increased disease seen in mice carrying the lpr mutation is associated with the genotype of the animals bone marrow derived cells. Chimeric mice were constructed by irradiating either BALB/c or BALB-lpr mice and reconstituting them with bone marrow cells (2×107) from either BALB-lpr and BALB/c respectively. Eyes of chimeric mice were then infected with 107 pfu of KOS strain of HSV-1 with 20 mice in each group. BALB/c mice reconstituted with BALB-lpr bone marrow cells displayed increased disease at weeks 2–4 (P<0.05–0.005).
We next isolated cells from the corneas of mice with both severe disease (opacity scores >2) and with little disease (opacity scores <1) to determine if there were any qualitative differences in the types of cells infiltrating the corneas of BALB/c, BALB-lpr and BALB-gld mice. Surprisingly the nature of the inflammatory infiltrate was remarkably similar between these mice. All mice with severe disease consistently displayed very high percentages of Gr-1+, CD11b+ neutrophils (BALB/c, 73.2% ± 6.9%; BALB-lpr, 83% ± 8.2%; BALB-gld, 77.7% ± 4.7%; see Fig. 5). The percent of T lymphocytes in these severely diseased corneas ranged from 3 to 15 percent with the ratio of CD4+ to CD8+ T cells being about 5 to 1, but again no differences between strains were noted. Likewise F40/80+ macrophages were between 2 and 5% with no differences between strains. There were also no significant differences in inflammatory subsets between strains for those corneas without disease for. However, the total number of CD45+ cells was much lower than from corneas with severe disease and the percentage of Gr-1+, CD11b+ ranged from 2 % to 20% of the total CD45+ cells. The primary cell type in these corneas without disease expressed T lymphocyte markers (the range was 40% to 60%).
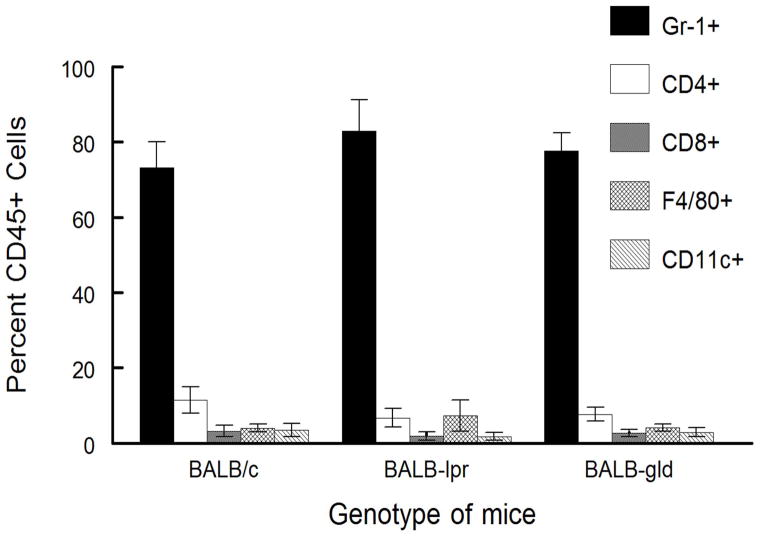
Figure 5.
Inflammatory infiltrate from BALB/c, BALB-lpr, and BALB-gld mice does not indicate strain-specific differences. HSV-infected corneas were removed at days 17 and 23 from mice with severe HSK disease and disaggregated into single-cell suspensions and stained with anti-CD45, CD4, CD8α, Gr-1, CD11b, CD11c, and F4/80 mAb. Cells were analyzed by flow cytometry. Data represents 4 to 6 corneas per group. No significant differences were seen for any particular determination between strains of mice tested.
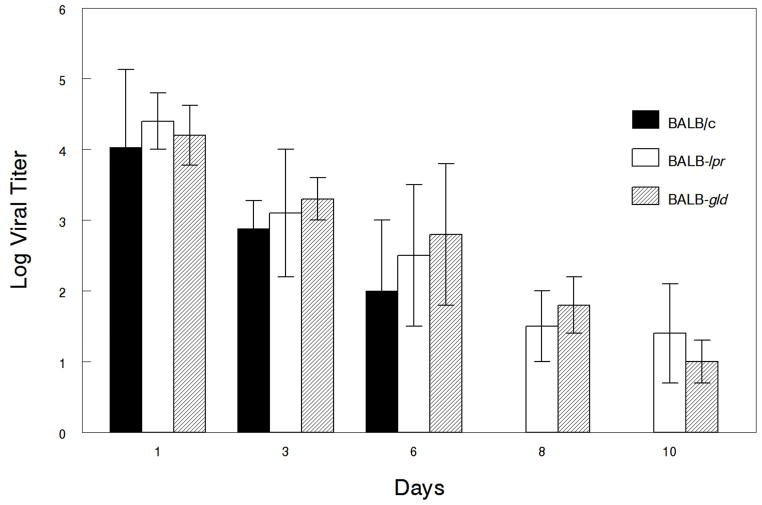
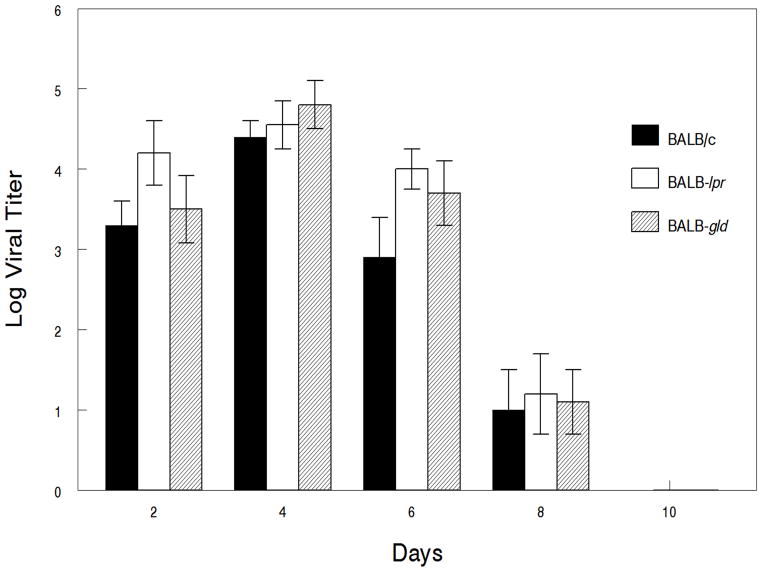
Since it is known that mice expressing the lpr and gld mutations do not mount the same degree of specificity towards foreign antigens as wild-type mice, particularly as they age (28), we also wanted to determine how well these mice were able to clear primary infection with HSV-1. Results indicate that there were no differences in the viral titers of eye swabs from these mice at any of the time points monitored (Fig. 6). Likewise, viral titers from both periocular tissue biopsies and trigeminal ganglia did not reveal any significant differences in viral growth in these tissues (Fig. 7).
Figure 6.
Defective expression of Fas and FasL, does not increase the magnitude of corneal viral shedding but does prolong shedding. Eyes of BALB/c wild-type (n=30), BALB-lpr (n=25) and BALB-gld (n=25) mice were infected with 107 pfu of HSV-1, KOS strain and corneas were swabbed on the indicated days and then tittered. No significant differences were seen in the magnitude of viral shedding. Results displayed are means ± S.E.M. for each of the groups of mice indicated.
Figure 7.
Defective expression of Fas and FasL, does not increase the magnitude of viral replication in infected trigeminal ganglia. Eyes of BALB/c wild-type (n=7 mice/time point), BALB-lpr (n=5 mice/time point) and BALB-gld (n=5 mice/time point) mice were infected with 107 pfu of HSV-1, KOS strain and trigeminal ganglia removed and viral titers determined. No significant differences in viral growth were seen between the mouse strains being compared. Results displayed are means ± S.E.M. for each of the groups of mice indicated.
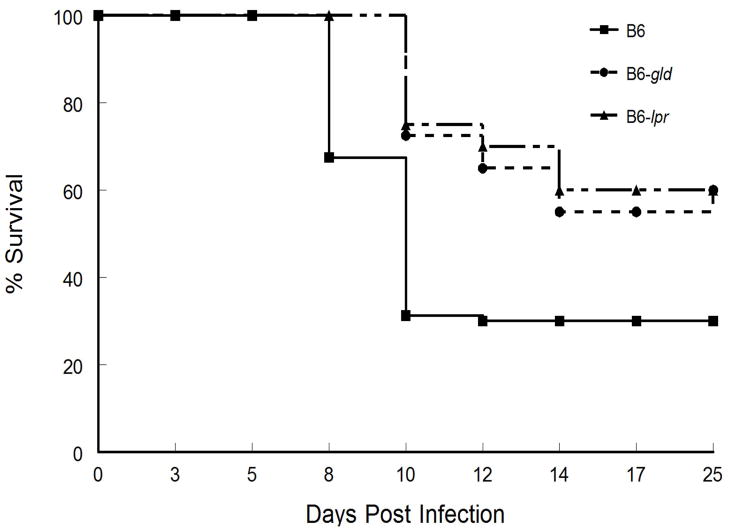
It should be noted however, that even though HSK was worse in mice expressing either the lpr or gld mutations, they had a lower incidence in mortality than did wild-type mice when infected with 5×106 pfu of the McKrae strain of HSV-1, (Fig. 8). In contrast, direct infection of the brain did not reveal any differences in mortality even when less than 100 pfu were injected intra-cranially (data not shown). This is in contrast to what was reported for HSV-2 (29) and suggests that survival of mice with an impaired Fas-FasL interaction is likely due to better control of peripheral infections with a neurovirulent strain of HSV-1 and not an inherently greater susceptibility of brain to infection.
Figure 8.
Defective expression of Fas and FasL results in reduced mortality following infection with HSV-1, McKrae strain. Eyes of C57BL/6 wild-type (n= 40 mice), B6-lpr (n= 40 mice) and B6-gld (n= 35 mice) were infected with 5×106 pfu of HSV-1, McKrae strain and observed for mortality. Data displayed were compiled from two independent studies. Mortality for both B6-lpr and B6-gld were significantly less than that for B6 wild-type mice (p<0.05).
We also performed histological analysis of trigeminal ganglia to determine if there were any gross differences in inflammation of these different mouse strains that might explain why mice with the lpr mutation might have worse disease. These ganglia sections did not reveal any significant differences in inflammation (data not shown). Thus those disease symptoms that might be indicative of a neurological disease could not be explained by significant differences in viral titers or degree of inflammation between these mouse strains.
DISCUSSION
One of the prime mechanisms the eye uses to protect itself from T cell–mediated immunopathologic response is the presence of FasL which induces apoptosis in Fas + lymphoid cells (8–11). Our laboratory, as well as several others have shown that lack of functional Fas-FasL-mediated apoptotic ability in the eye most often leads to increased inflammatory responses (8,9), increased corneal allograft rejection (10,30), increased neovascularization (12–14) and the inability to develop systemic tolerance following injection of antigen into the anterior chamber (8). In addition to these responses that are specific to the eye, it is also well established that host T cells eliminate viral infected cells by either the perforin-granzyme pathway (31) or via apoptosis mediated by the interaction of FasL on effector cells with Fas expressed by virally infected cells (32,33). Thus it would seem that mice that are not able to express either functional FasL or their receptor Fas have the potential of expressing a wide variety of abnormalities. These could possibly include being more prone to greater inflammatory responses in the eye due to impaired ability to control entry of inflammatory cells that would normally be subject to apoptosis from engagement of corneal FasL with Fas+ inflammatory cells. One might also hypothesize that they would have difficulty clearing virally infected cells because the Fas-FasL pathway of killing virally infected cells is not available to cytotoxic T cells, which could result in persistence of infectious virus in the cornea.
Histological analysis of the corneas from lpr and gld mice revealed significantly increased inflammatory infiltrate than seen in wild type mice. Thus the entry of inflammatory cells was not controlled well in these mutant mice. This was further demonstrated by studies in which bone marrow chimeras were constructed between BALB/c and BALB-lpr mice. BALB/c mice which possessed bone marrow from BALB-lpr mice displayed significantly increased disease when compared to BALB-lpr mice reconstituted with BALB/c bone marrow. Thus increased disease is associated with Fas expression by bone marrow derived cells and not due to lack of functional Fas expression by potentially Fas expressing resident corneal cells. This suggests that the primary reason for increased HSK in lpr mice is due to reduced control of Fas-expressing inflammatory cells that are not killed by corneal FasL.
However, when inflammation does occur in both wild type and mice expressing mutations in either Fas or FasL, the types of cells infiltrating the cornea are essentially the same with neutrophils being the dominant cell found during severe disease. This illustrates that HSK in lpr and gld mice is mediated by the same types of cells that mediate disease in normal mice and not due to the accumulation or recruitment of some other type of inflammatory cell.
As suggested above, another possible mechanism for increased HSK severity in lpr and gld mice would be persistence of HSV-1 infectious virus in infected corneas. Data comparing infectious HSV-1 in tear films from these mouse strains revealed a slight, though insignificant, prolongation of HSV-1 in the corneas of lpr and gld mice. This could be the result of impaired killing of virally infected corneal cells, which has been shown to be either perforin/granzyme- or FasL-mediated (31). However, several pieces of evidence argue against this hypothesis. First, expression of Fas antigen by corneal cells is below the level of detection by western blot analysis (19), making it unlikely that these cells would be targets of FasL-mediated killing by CTL’s. Second, irradiated BALB-lpr chimeric mice that were reconstituted with normal BALB/c BM cells, which would express the lpr mutant Fas antigen on resident corneal cells did not suffer from significant HSK. Furthermore, when viral shedding was evaluated from these chimeric mice, no differences in HSV-1 titers were detected between the chimeric mice we used. Thus the data does not support the hypothesis that viral persistence plays a significant role in the disease phenotype of mice which have impaired Fas-FasL interactions.
It had also been a possibility that the increased disease seen in lpr mice could have been due to lack of control of neovascularization as vascular endothelium expresses Fas and neovascularization of the cornea has been shown to be controlled by corneal expression of FasL (14). However, we thought that was unlikely as lpr mice express normal Fas on their vascular endothelium (14). By demonstrating that the disease phenotype is associated with lymphoid cells, this further supports the notion that vascular endothelial expression of Fas is not responsible for increased HSK in BALB-lpr mice.
Previous work from this and other investigators have shown that development of an antibody response against HSV-1 can protect mice from the development of severe HSK (18,34,35). As a consequence we tested antibody responses in BALB/c, BALB-lpr and BALB-gld mice to determine if lpr or gld mice had impaired anti-HSV-1 responses. However, no differences were observed between these strains (data not shown), indicating that the ability to develop an anti-HSV-1 antibody response was not involved.
Taken together, these studies document that mice with impaired Fas-FasL interactions develop significantly increased HSK following infection with HSV-1. Furthermore, the mechanism responsible for increased disease is likely due to increased inflammation of the cornea, which is normally controlled in part by the presence of FasL on resident corneal cells. What do these data suggest concerning therapy to better control HSK? Since the vast majority of people have an intact Fas-FasL system, are there means of potentiating this interaction? Fortunately, there have been a few manuscripts that described methods for potentiating FasL-mediated control of Fas+ cells. One such strategy took advantage of the fact that FasL is very sensitive to cleavage by matrix metalloproteases (36). Thus stabilization of FasL expression by treatment with MMP inhibitors was demonstrated to significantly increase the success of corneal allografts (37). Likewise treatment of mice suffering from choroidal neovascularization with either MMP inhibitors or with apoptotic-inducing soluble FasL significantly reduced neovascularization (38). These studies present possible therapeutic strategies to better control unwanted inflammation and neovascularization using FasL-based therapies.
Acknowledgments
This work was supported by National Institutes of Health Grants EY11885 (PMS), EY21247 (PMS), and EY09083 (DAL) and an unrestricted grant from Research to Prevent Blindness to Department of Ophthalmology.
We wish to acknowledge the technical assistance of Dr. Stephen Ward and Dr. Tammie Keadle and Joy Eslick and Sherri Koehm for assistance with flow cytometry. We also wish to acknowledge Dr. Thomas A. Ferguson for very valuable discussions at the outset of this project.
References
- 1.Pepose JS, Leib DA, Stuart PM, Easty EL. Herpes simplex virus diseases: anterior segment of the eye. In: Pepose JS, Holland GAN, Wilhelmus KR, editors. Ocular Infection and Immunity. Mosby; St. Louis, MO: 1996. pp. 905–932. [Google Scholar]
- 2.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 3.Maertzdorf J, Verjans GM, Remeijer L, van der Kooi A, Osterhaus AD. Restricted T cell receptor beta-chain variable region protein use by cornea-derived CD4+ and CD8+ herpes simplex virus-specific T cells in patients with herpetic stromal keratitis. J Infect Dis. 2003;187:550–558. doi: 10.1086/367991. [DOI] [PubMed] [Google Scholar]
- 4.Divito SJ, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci. 2008;49:1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denniston AK, Kottoor SH, Khan I, Oswal K, Williams GP, Abbott J, Wallace GR, Salmon M, Rauz S, Murray PI, Curnow SJ. Endogenous cortisol and TGF-beta in human aqueous humor contribute to ocular immune privilege by regulating dendritic cell function. J Immunol 2011. 2011;186:305–311. doi: 10.4049/jimmunol.1001450. [DOI] [PubMed] [Google Scholar]
- 6.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 7.Koevary SB. Ocular immune privilege: A review. Clin Eye Vis Care. 2000;12:97–106. doi: 10.1016/s0953-4431(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 8.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD-95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 10.Stuart PM, Griffith TS, Usui N, Pepose JS, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa H, Maruyama K, Streilein JW. CD95 ligand expression on corneal epithelium and endothelium influences the fates of orthotopic and heterotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2004;45:1908–1915. doi: 10.1167/iovs.03-0512. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan HJ, Leibole MA, Tezel T, Ferguson TA. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nature Med. 1999;5:292–297. doi: 10.1038/6509. [DOI] [PubMed] [Google Scholar]
- 13.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Asif M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic TSP-1 and PEDF. Nature Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 14.Stuart PM, Pan F, Plambeck S, Ferguson TA. Fas/Fas ligand interactions regulate neovascularization in the cornea. Invest Ophthalmol Vis Sci. 2003;44:93–98. doi: 10.1167/iovs.02-0299. [DOI] [PubMed] [Google Scholar]
- 15.Richardson BC, Lalwani ND, Johnson KJ, Marks RM. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- 16.Laurence J, Mitra D, Steiner M, Staiano-Coico L, Jaffe E. Plasma from patients with idiopathic and human immunodeficiency virus-associated thrombotic thrombocytopenic purpura induces apoptosis in microvascular endothelial cells. Blood. 1996;87:3245–3254. [PubMed] [Google Scholar]
- 17.Suhara T, Fukuo K, Sugimoto T, Morimoto S, Nakahashi T, Hata S, Shimizu M, Ogihara T. Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. J Immunol. 1998;160:4042–4047. [PubMed] [Google Scholar]
- 18.Keadle TL, Morrison LA, Morris JL, Pepose JS, Stuart PM. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J Virol. 2002;76:3615–3625. doi: 10.1128/JVI.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart PM, Yin XT, Plambeck S, Pan F, Ferguson TA. The role of Fas ligand as an effector molecule in corneal graft rejection. Eur J Immunol. 2005;35:2591–2597. doi: 10.1002/eji.200425934. [DOI] [PubMed] [Google Scholar]
- 20.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J Immunol. 2005;174:4098–4104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 21.Stuart PM, Summers B, Morris JE, Morrison LA, Leib DA. CD8+ T cells control corneal disease following ocular infection with HSV-1. J Gen Virol. 2004;85:2055–2063. doi: 10.1099/vir.0.80049-0. [DOI] [PubMed] [Google Scholar]
- 22.Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PA, Pepose JS. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Ackland-Berglund CE, Leib DA. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J Virol. 2000;74:3598–3604. doi: 10.1128/jvi.74.8.3598-3604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiss BJ, Smith TJ, Leib DA, Morrison LA. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J Virol. 2000;74:11137–11144. doi: 10.1128/jvi.74.23.11137-11144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divito SJ, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci. 2008;49:1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen QD, Uy HS, Merchant A, Medina CA, Baltatzis S, Zhao T, Zhao ZS, Cantor H, Foster CS. Effect of Fas and Fas ligand deficiency in resistance of C57BL/6 mice to HSV-1 keratitis and chorioretinitis. Invest Ophthalmol Vis Sci. 2001;42:2505–2509. [PubMed] [Google Scholar]
- 27.Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Levi-Strauss M. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol. 2004;172:2118–125. doi: 10.4049/jimmunol.172.4.2118. [DOI] [PubMed] [Google Scholar]
- 28.Laouar Y, Ezine S. In vivo CD4+ lymph node T cells from lpr mice generate CD4–CD8–B220+TCR-β low cells. J Immunol. 1994;153:3948–3955. [PubMed] [Google Scholar]
- 29.Ishikawa T, Yamada H, Oyamada A, Goshima F, Nishiyama Y, Yoshikai Y. Protective role of Fas-FasL signaling in lethal infection with herpes simplex virus type 2 in mice. J Virol. 2009;83:11777–11783. doi: 10.1128/JVI.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamagami S, Kawashima H, Tsuru T, Yamagami H, Kayagaki N, Yagita H, Okumura K, Gregerson DS. Role of Fas-Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplant. 1997;64:1107–1111. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- 31.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu ZX, Govindarajan S, Okamoto S, Dennert G. Fas-mediated apoptosis causes elimination of virus-specific cytotoxic T cells in the virus-infected liver. J Immunol. 2001;166:3035–3041. doi: 10.4049/jimmunol.166.5.3035. [DOI] [PubMed] [Google Scholar]
- 34.Deshpande SP, Zheng M, Daheshia M, Rouse BT. Pathogenesis of herpes simplex virus-induced ocular immunoinflammatory lesions in B-cell-deficient mice. J Virol. 2000;74:3517–3524. doi: 10.1128/jvi.74.8.3517-3524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu K, Dou J, Yu F, He X, Yuan X, Wang Y, Liu C, Gu N. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine. 2011;29:1455–1462. doi: 10.1016/j.vaccine.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- 37.Stuart PM, Pan F, Yin XT, Haskova Z, Plambeck S, Ferguson TA. Effect of metalloprotease inhibitors on corneal allograft survival. Invest Ophthal Vis Sci. 2004;45:1169–1173. doi: 10.1167/iovs.03-0932. [DOI] [PubMed] [Google Scholar]
- 38.Roychoudhury J, Herndon JM, Yin J, Apte RS, Ferguson TA. Targeting immune privilege to prevent pathogenic neovascularization. Invest Ophthalmol Vis Sci. 2010;51:3560–3566. doi: 10.1167/iovs.09-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]