Abstract
The majority of HIV infections occur via mucosal transmission. Vaccines that induce memory T-and B-cells in the female genital tract may prevent the establishment and systemic dissemination of HIV. We tested the immunogenicity of a vaccine that uses human papillomavirus-based gene transfer vectors, also called pseudovirions (HPV PsVs), to deliver SIV genes to the vaginal epithelium. Our findings demonstrate that this vaccine platform induces gene expression in the genital tract in both cynomolgus and rhesus macaques. Intravaginal vaccination with HPV16, HPV45, and HPV58 PsVs delivering SIV Gag DNA, induced Gag-specific antibodies in serum and the vaginal tract and T-cell responses in blood, vaginal mucosa, and draining lymph nodes that rapidly expanded following intravaginal exposure to SIVmac251. HPV PsV-based vehicles are immunogenic, warrant further testing as vaccine candidates for HIV, and may provide a useful model to evaluate the benefits and risks of inducing high levels of SIV-specific immune responses at mucosal sites prior to SIV infection.
Introduction
The female genital tract is unique because of its hormonal responsiveness, commensal bacteria, biochemical processes, and immunological milieu (1,2). These features may contribute to the increased rate of heterosexual male to female HIV transmission when compared to female to male transmission (3). Blocking vaginal transmission of HIV may require vaccines that target the female genital tract and induce local immunity. HIV vaccines based on viral vectors, proteins, or a combination thereof, tested in phase III vaccine efficacy trials in humans, induced mainly systemic immune responses using vaccines delivered by intramuscular inoculation (4-6). While these vaccine modalities induce variable levels of HIV-specific responses in the blood (5,7), little is known about their ability to induce mucosal responses. A limited, but significant, protection from heterosexual transmission has been observed in individuals vaccinated with a combination of the recombinant poxvirus ALVAC-HIV and the gp120 envelope protein (5). This vaccine modality induces low CD8+ and CD4+ T-cell responses, and antibodies to HIV that mediate ADCC but have limited neutralizing activity (5). These findings suggest that a balance of T-cell responses in conjunction with antibodies to the envelope protein may be important. However, in animal models, vaccines that elicit predominantly effector memory CD8+ T-cell responses can also control mucosal SIV infection (8,9). Thus, defining the quantity, quality, and location of protective HIV/SIV vaccine induced immune responses is necessary. We hypothesize that vaccine induced cell-mediated and humoral memory responses, within the interstitial layers of the female genital tract, can curtail the local expansion of HIV/SIV and prevent its systemic dissemination. In the present study, we tested a vaccine delivery platform that specifically targets the vaginal mucosa. A subset of human papillomavirus (HPVs) are sexually transmitted mucosal pathogens that naturally infect cervico-vaginal keratinocytes (10). HPV-VLP-based vaccines are safe and very effective at preventing the HPV infections that cause cervical neoplasia in women (11). HPV capsid proteins, L1 and L2, can self assemble into virus like particles (VLPs) and, when co-transfected with a plasmid containing a gene of interest, L1 and L2 will encapsidate the plasmid forming pseudovirions (PsVs) (12,13). HPV PsVs have been shown to effectively deliver reporter genes to the female genital tract in multiple animal models (14-16). HPV PsVs infection is limited to keratinocytes and requires minor disruption of the epithelium (17). Thus, we treated macaques with progesterone to thin the vaginal epithelium and used mechanical and/or chemical disruption of the epithelium to facilitate efficient HPV PsVs delivery to keratinocytes. Expression of the transgene is robust but transient, lasting approximately seven days in the mouse genital tract (14). Furthermore, HPV PsVs may serve as adjuvants, engaging toll like receptors and facilitating the activation and maturation of antigen presenting cells (18,19).
We have exploited the ability of HPV PsVs to target the female genital tract and used PsVs as vectors to deliver DNA encoding SIV genes to a site of SIV transmission in two non-human primate species. SIV Gag was chosen as our model antigen to initially test the immunogenicity of HPV-PsVs in macaques, as Gag is easily cloned, expressed and secreted. We demonstrate that this vaccination strategy induces local and systemic immune responses in both cynomolgus and rhesus macaques. In addition, HPV PsVs induced mucosal immune responses that rapidly expanded upon vaginal exposure to SIVmac251.
Materials and Methods
Animals, HPV vaccination, and SIV infection
Eight cynomolgus macaques and eight rhesus macaques were used in this study; all animals were housed and cared for under the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care International and were housed at Advanced BioSciences Laboratories in Rockville, MD.
HPV PsVs were produced as previously described (12,13). Briefly, DNA constructs encoding the L1 and L2 capsid of HPV and a smaller SIV Gag, Gag-Pro, RFP or luciferase that could be efficiently encapsidated, were co-transfected into 293TT-cells and the resulting PsVs purified, propagated, and titered. Twenty-eight days prior to vaccination, macaques were given 30mg/kg of Depo-Provera intramuscularly. One week prior to vaccination, macaques were treated with antibiotics to prevent any occult vaginosis. At six and/or eighteen hours prior to vaccination a vaginal application of nonoxynol nine (N9) a non-ionic membrane active surfactant, was administered either as a 10% gel mixed with 4% carboxymethyl cellulose (CMC; Sigma-Aldrich, St. Louis, MO) or a 12.5% foam (VCF; Apothecus Pharmaceutical Corporation; Oyster Bay, NY). Six hours after the last N9 treatment, two of the cynomolgus macaques had the vaginal epithelium lightly abraded with a cytobrush. A standard inoculum of 500μl that consisted of 109-1010 IU of HPV PsVs mixed with CMC was instilled using a positive displacement pipette into the cervix of each animal. Cynomolgus macaques received 2×1010 IU of HPV16 and 45 expressing SIV Gag and RFP given thirty days apart. Six rhesus macaques similarly received 2×1010 IU of HPV16 but 1×109 IU of HPV45 both expressing SIV Gag-Pro after two N9 applications (three macaques received N9 gel and three N9 foam). Three months later, the rhesus macaques were boosted with a third vaccination of 2×1010 HPV58, also expressing SIV Gag-Pro. Two rhesus macaques, used as unvaccinated controls for the study, were given HPV16, HPV45, and HPV58 expressing luciferase at similar doses and timing as animals vaccinated with HPV-Gag-Pro-PsVs. Six weeks post HPV vaccination, rhesus macaques were challenged intravaginally with (105) TCID50 of SIVmac251.
In vivo fluorescent imaging
A CRi Nuance N-MSI-500-FL multispectral imaging system fitted with a Hawkeye 7” Pro Hardy Borescope (Gradient Lens Corporation, Rochester, NY) using a VC-35 video adapter (Gradient Lens Corporation, Rochester, NY) equipped with a 565 nm long pass filter (Chroma HQ565LP, Rockingham, VT) was used to detect and image the red fluorescent protein (RFP) signal. White illumination light or filtered excitation light at 523 nm from a CRi Maestro light source (CRi, Woburn, MA) was transmitted to the borescope through a fiber bundle. White light images were acquired, followed by fluorescent images. The multi-spectral images from 570-650 nm at 10 nm intervals were co-registered to compensate for motion using ImageJ (http://rsbweb.nih.gov/ij/), and unmixed using the CRi Maestro software. The overlay of resulting RFP and autofluorescene images was created using MIPAV (http://mipav.cit.nih.gov/).
Tissue collection, quantitation of viral RNA and DNA, and immunophenotyping
Mononuclear cells were isolated from blood, axillary, and obturator lymph nodes, rectum, endocervix, and vaginal tissue pre or post vaccination as described (20,21).
SIV RNA was quantified in the plasma by NASBA, as previously described (22) and SIV DNA was quantified in tissues by quantitative PCR (23).
Four and nine-color flow cytometric analysis was performed on mononuclear cells from blood and tissues. Immunophenotype and intracellular cytokine assays to detect SIV specific cells were performed as previously described (20,23). Cells were stained with the following antibodies CD3 (cloneSP34-2), CD4 (clone L200), CD8 (clone RPA-T8), CD95 (clone DX2), IFN-γ (clone B27), TNF-α (clone MAB11), IL-2 (clone MQ1-17H12), and CD107 (clone H4A3) all obtained from BD Biosciences (SanDiego, CA). In addition, the live/dead yellow fixable amine dye was obtained from Invitrogen (Carlsbad, CA), anti-CD28 (clone CD28.2) from eBiosciences (SanDiego, CA), and SIV Gag CM9 APC from Beckman Coulter (Brea, CA). All cells were fixed with 1% paraformaldehyde and acquired on either a FACSCalibur or LSRII. Data analysis was performed with FlowJo (Treestar CA).
ELISpot and Immunohistochemistry Assays
SIV specific T-cells were assessed using an ELISpot kit Mabtech (Mairemont, OH). Peripheral blood cells were stimulated with either SIV Gag or Env overlapping 15mer peptides, Concanavalin A or left unstimulated and added to IFN-γ coated plates for twenty four hours. The plates were developed and the frequency of IFN-γ positive spot forming cells per 106 PBMC was determined after background subtraction.
All slides for immunohistochemistry were stained using the Dako Autostainer (Dako Inc., Carpenteria CA) as previously described (20). The primary antibodies that were used included monoclonal anti-CD4 mouse serum (clone IF6, Vector, Burlingame, CA), monoclonal anti-CD8 mouse serum (clone IA5, Leica Microsystems, Bannockburn, 231 IL), and polyclonal anti- Ki67 rabbit serum (Lab Vision, Fremont, CA). For all primary antibodies, slides were subjected to an antigen retrieval step as previously described (20). Primary antibodies were replaced by normal rabbit IgG (Zymed Inc., South San Francisco CA) or mouse IgG (Dako, Inc., Carpinteria CA) and included with each staining series as the negative control. Binding of the CD4, CD8, and Ki-67 were detected simultaneously using Alexafluor 488-labeled polyclonal goat anti-rabbit IgG and Alexafluor 568-labeled polyclonal goat anti-mouse IgG (Molecular Probes, Eugene OR). Slides were visualized with epi-fluorescent illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss Inc, Thornwood NY). Digital images were captured and analyzed by using a Zeiss Axiocam System and Openlab software (Inprovision Inc., Waltham, MA). The numbers of CD4/Ki67 positive are presented as cells per square millimeter of lamina propria. The numbers of CD8/Ki67 positive are presented as cells per square millimeter of lamina propria.
SIV antibodies in Serum
Serum samples were tested for SIV-specific antibody responses by using an enzyme-linked immunosorbent assay (ELISA)described elsewhere (24,25). Briefly, samples for serum IgA testing were serially diluted and applied to a 96-well half-area plate (Greiner Bio-one), previously coated with 1 μg/ml SIVmac251 purified lysate (Advanced Biotechnologies, Inc.) and blocked with 1% BSA block solution (KPL). After overnight incubation at 4°C, the plate was washed with PBS-Tween, reacted with peroxidase-conjugated anti-monkey IgA antibody (Alpha Diagnostic) and incubated for another hour at room temperature. After washing, TMB (3,3’,5,5’-tetramethylbenzidine) peroxidase substrate solution was added to each well, followed by 20 minutes of incubation at room temperature until the color developed. The reaction was stopped by adding 2 M H2SO4, and the plate was read at 450 nm within 30 min.Antibody titer was defined as the reciprocal of the serum dilution at which the optical density of the test serum was two times greater than that of a naive control macaque serum diluted 1:50. HPV antibodies were detected by ELISA specific for each of the different HPV types as described in Graham et al. (10).
SIV Antibodies in Vaginal Secretions
Vaginal secretions were collected in triplicate using Weck-Cel sponges (Medtronic). To elute secretions, the sponges were incubated for ten minutes on ice in elution buffer and then transferred into a Salivette column (Sarstedt) and centrifuged at 3,000 RPM for thirty minutes at 4°C. For SIV Gag-specific IgA and IgG, serially diluted secretions were applied to a 96-well half-area ELISA plate (Greiner Bio-one), coated overnight at 4°C with 1 μg/ml SIVmac251 purified lysate (Advanced Biotechnologies, Inc.) and blocked. Serial dilutions of Gag-specific IgA or IgG standards of known concentration were prepared as previously described (Manrique ref) and included on each plate. After overnight incubation at 4°C the plate was washed, reacted with peroxidase-conjugated anti-monkey IgA or IgG antibody (Alpha Diagnostic, San Antonio, TX), and incubated for one hour. After washing, TMB (3,3’, 5,5’-tetramethylbenzidine) peroxidase substrate solution (Sigma) was added to each well, incubated in the dark to allow color development, and the reaction stopped. Plates were read using a PowerWave Microplate Spectrophotometer (Biotek). Gen5 software (Biotek) was used to determine the concentration of anti-SIV Gag-specific IgA or IgG in the secretions based on the standard curve. Total IgA or IgG was determined as previously described (Xiao et al. 2010). Data are reported as Gag-specific IgA or IgG divided by the total IgA or IgG concentration in each vaginal secretion.
Statistical Analysis
Comparisons between groups were assessed using either a Wilcoxon rank sum test or a repeated measures analysis of variance. Graphical analysis was performed using Graph Pad Prism.
Results
Efficient gene delivery and seroconversion to HPV antigens in macaques vaccinated with HPV PsVs
We produced HPV pseudovirions by co-transfecting 293TT-cells, a human cell line, with a DNA plasmid containing the capsid (L1/L2) genes, and either a DNA plasmid encoding RFP, luciferase or SIV Gag genes, as previously described (12,13). Eight cynomolgus macaques were intra-vaginally exposed to 2×1010 infectious units (IU) of HPV16, followed by HPV45 pseudovirions encapsidating red fluorescent protein (RFP) and SIV Gag (Fig. 1A). Efficient gene expression of intra-vaginally delivered HPV PsVs has been observed in progesterone treated macaques, after microtrauma was induced in the vaginal tract (16). Thus, we treated macaques with 30mg/kg of Depo-Provera and compared four methods of transient disruption of the cervico-vaginal epithelium, all of which included nonoxynol nine (N9). N9 is currently in use as an over-the-counter spermicide and is known to disrupt genital epithelium (26,27). N9 was prepared as a 10% gel formulation delivered either once (Gel 1X), twice (Gel 2X), or administered twice in combination with gentle abrasion with a cytobrush (Gel 2X+cytobrush). In addition, we tested a commercially available 12.5% foam application of N9 (foam) delivered twice. Forty-eight hours after vaccination, in vivo imaging detected red fluorescent protein expression from the HPV-RFP constructs in the vaginal tract using an endoscope attached to a Maestro multispectral CCD camera (Fig. 1B). N9 delivered as a gel application, resulted in punctate fluorescence, while N9 foam treatment facilitated diffuse fluorescence throughout the cervico-vaginal tract (data not shown). HPV PsVs vaccination induced seroconversion to HPV antigens and we detected capsid-specific IgG antibodies in the blood of all animals (Fig. 1C).
FIGURE 1.
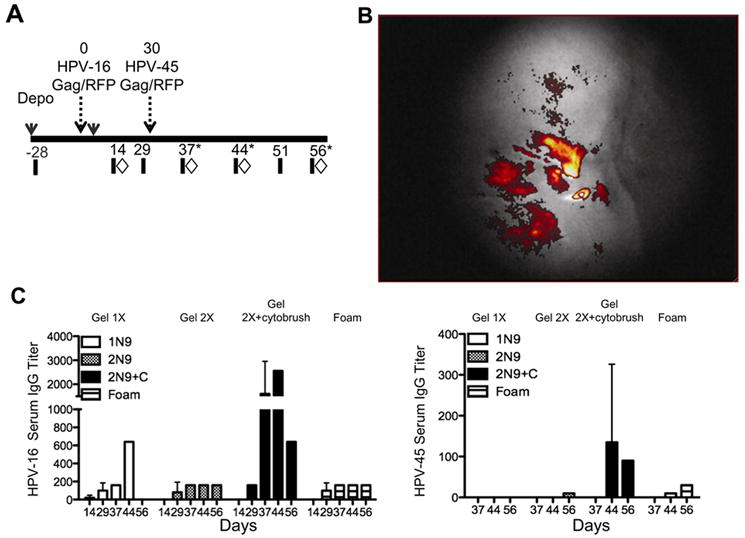
HPV transduces macaque epithelium and induces antibodies to HPV. Cynomolgus macaques were vaccinated intra-vaginally with HPV pseudovirions expressing either RFP or SIV Gag. (A) Schematic showing the design of the study, eight cynomolgus macaques were vaccinated with HPV16 and HPV45, thirty days apart. Blood (black bars) and tissues (diamonds) were sampled pre and post vaccination. In order to obtain sufficient samples to analyze the female genital tract, animals were serially sacrificed at 37, 44, or fifty six days post vaccination (stars). (B) Forty-eight hours post vaccination a camera fitted to an endoscope was used to visualize the cervix and vaginal tract. Successful HPV vaccination was confirmed by the detection of RFP in each animal. Shown is the level of in vivo fluorescence from HPV-RFP PsVs measured in a representative animal. (C) Serum IgG to the HPV16 and 45 capsid L1 measured fourteen-fifty six days post vaccination. The vaginal epithelia were disrupted by 4 methods using nonoxynol 9 (N9). N9 was prepared as a 10% gel and delivered once (Gel 1X white bars), twice, (Gel 2X hatched bars), or twice in combination with abrasion using a cytobrush (Gel 2X+cytobrush, black bars). In addition, we used a 12.5% foam application of N9 also given twice (Foam striped bars).
The magnitude of the antibodies was highest in animals treated with N9 gel in combination with a cytobrush, however increased antibodies to HPV are not related to immune responses to the encapsidated genes (Schiller unpublished observations). Our findings confirm and extend the results from Roberts and colleagues (16), who demonstrated that, after transient disruption, macaque vaginal epithelium is effectively transduced by HPV PsVs, leading to robust in vivo gene expression.
HPV PsVs vaccination induces SIV Gag specific cell mediated and humoral responses in macaques
We next investigated the immunogenicity of intravaginally delivered HPV PsVs. Prior to vaccination the expression of SIV-Gag was confirmed by western blot on cell lysates from 293TT-cells transduced by HPV Gag PsVs. We detected the SIV Gag protein precursor, p55 by antibodies that recognize the recombinant SIV p27 Gag protein, used as a positive control (lane 1 of Fig 2A). Following vaccination, T-cell responses to SIV Gag were measured in PBMCs by ELISpot using 15mer peptides spanning the entire SIV Gag protein. Animals treated with N9 gel mounted T-cell responses of greater magnitude than animals treated with N9 foam (Fig. 2B). Two treatments with N9 gel facilitated the development of high titer Gag specific serum IgG (Fig. 2C). Additionally we measured Gag specific IgA in the serum, very low levels of IgA was detected in the two animals whose epithelia was disrupted with the cytobrush (titers 50 and 100) all other animals were negative (data not shown). Interestingly however, Gag specific IgA in vaginal secretions was equivalent in all groups (Fig. 2D). The small numbers of animals per group (n=2) precluded statistical evaluation of the individual immune responses induced by these four immunization regimens, the data supports the notion that two N9 gel applications may be superior to the foam in its ability to facilitate the induction of systemic serum IgG and T-cell responses.
FIGURE 2.
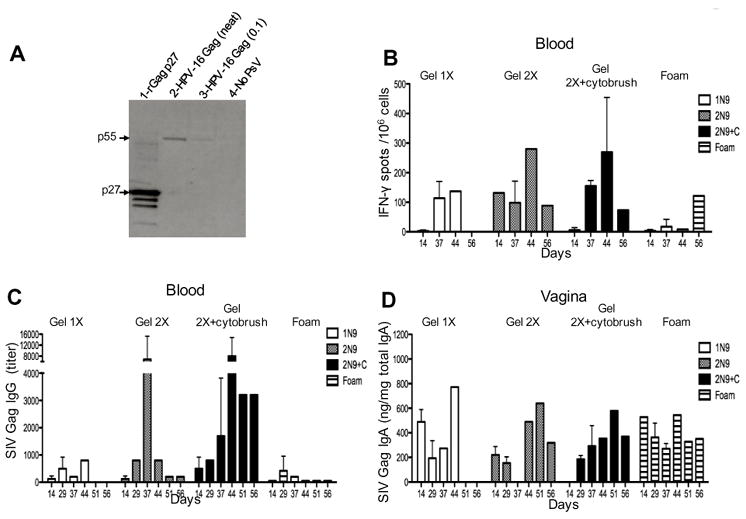
Intravaginal vaccination with HPV PsVs-Gag induces both cell mediated and humoral immune responses to SIV Gag. (A) Western blot showing the expression of the SIV Gag poly-protein (p55) in 293TT-cells following transduction with HPV16–SIV Gag constructs (lanes 2 & 3). Recombinant Gag p27 was loaded as a positive control in lane 1, degradation products of the recombinant protein are seen below the p27 band. Lysates from non-transduced cells were run as a negative control (lane 4). (B) IFN-γ responses measured by ELISpot after stimulation with SIV Gag peptides. (C) SIV Gag serum titers of IgG measured post HPV vaccination. (D) SIV Gag-specific IgA as a function of total IgA in vaginal secretions.
Cytokine production in the blood lymph nodes and female genital tract in cynomolgus macaques
To compare the frequency of SIV specific cells at multiple sites, we serially sacrificed immunized macaques to obtain sufficient mononuclear cells from various tissues. Figure 3A shows representative flow cytometric plots of the frequency of IL-2 produced in unstimulated and Gag stimulated CD4+T-cells from the blood. SIVmac251 specific CD4+T-cell responses were detectable in the blood and the vagina as early as fourteen days post vaccination (Fig. 3B-C, left panels). The responses in blood peaked thirty seven days post vaccination, while vaginal responses were highest fourteen days post vaccination and were boosted in some animals at day forty four (two weeks after HPV45 vaccination) but then waned over time. The magnitude of the CD4+T-cell response was lower in the obturator lymph nodes (Fig. 3D) and was undetectable in the rectal mucosa (data not shown). A similar frequency and pattern was observed for SIV mac251 Gag-specific CD8+T-cell responses (Fig. 3B-3D, right panels).
FIGURE 3.
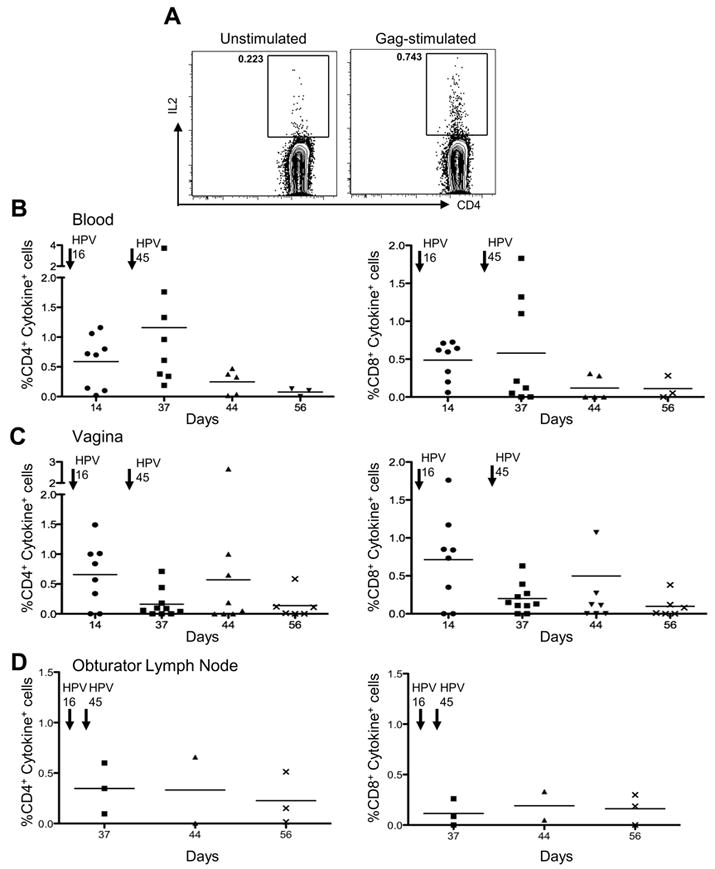
SIV Gag specific cytokine production in systemic and mucosal tissues. (A) Representative flow cytometric plots showing the frequency of IL-2 production in unstimulated or Gag stimulated CD4+ T-cells. (B) PBMCs from HPV-SIVGag vaccinated macaques were stimulated with Gag peptides. The frequency of CD4+ T-cells (left) and CD8+ T-cells (right) producing IFN-γ and IL2 (cytokine+) is shown. Arrows above indicate the approximate time each HPV vaccine was administered. (C) Mononuclear cells were isolated from the cervix and vaginal tract and stimulated with Gag peptides. However, in some animals sufficient cervical mononuclear cells were not obtained to perform stimulations. The frequency of CD4+ T-cells (left) and CD8+ T-cells (right) producing cytokines is shown. (D) At sacrifice, the genital draining obturator lymph nodes were collected from vaccinated macaques. Mononuclear cells were isolated and stimulated with SIV Gag peptides. The frequency of CD4+ T-cells (left) or CD8+ T-cells (right) producing cytokines is shown.
HPV PsVs recruit T-cells to the vaginal mucosa and induces T-cell responses
Next, we tested the immunogenicity of the HPV PsVs in Indian rhesus macaques, we chose to use a second model system to confirm and extend our results. The rhesus macaque is the most commonly used model for HIV vaccine development and there are more reagents to phenotype the immune responses induced in these animals. Six MamuA01+ macaques were treated with Depo-Provera for twenty eight days and immunized sequentially with HPV16, 45, and 58 expressing the entire SIV gag and protease genes (Fig. 4A). Two additional animals (controls) were immunized with HPV16, 45, and 58 expressing the luciferase gene. We elected to disrupt the vaginal epithelium with either N9 delivered twice as a 10% gel (n=4), since our data in cynomolgus macaques suggested a superior immunogenicity of this regimen, or with the 12.5% N9 foam (n=4), since this preparation is widely available, easy to apply, and therefore a more practical approach. In this experiment, we used the Gag-pro SIVmac251/766 construct obtained from a virus variant cloned early in infection following mucosal exposure to SIVmac251. Expression of the uncleaved Gag precursor protein p55 and the cleaved p27 Gag protein from HPV16, HPV45, and HPV58 PsVs encoding SIV/mac251/766 Gag-Pro was confirmed by western blotting (Fig. 4B and data not shown for HPV58). Vaccination with HPV PsVs induced binding antibodies to the HPV capsid in all macaques (data not shown). Because in mice, HPV intravaginal vaccination results in the recruitment of T-cells to the site of vaccination (Cuburu et al. unpublished data), we assessed whether this is also the case in non-human primates. We obtained vaginal biopsies prior to vaccination and at one week after HPV58 vaccination and enumerated the absolute number of CD4+, CD8+, and Ki67+ cells per mm2 of vaginal tissue by immunohistochemistry. HPV vaccination induced an increase in the number of CD4+ cells, as demonstrated in a representative example in Fig. 4C. Quantitation of CD4+ and CD8+ cells demonstrated a significant increased in both CD4+ and CD8+ cells (p=0.0049 and p=0.012 respectively) and in the number of activated and proliferating T-cells that express the nuclear antigen Ki67 (CD4+Ki67+ p=0.040 and CD8+Ki67+ p=0.034) (Fig. 4D and 4E). Next, we assessed humoral responses in vaginal secretions. An increase in SIV Gag specific IgA was detected in N9 foam treated animals at thirty-six days post vaccination and was boosted by HPV58 at 131 days post vaccination (Fig 4F). In contrast, SIV specific IgG was not induced in foam treated animals (Fig 4G). In animals whose vaccination was facilitated by N9 gel, a modest increase was observed in IgA production after the 3rd HPV vaccination at day 145 and SIV specific IgG was also induced at this time point (Fig.4 F and G).
FIGURE 4.
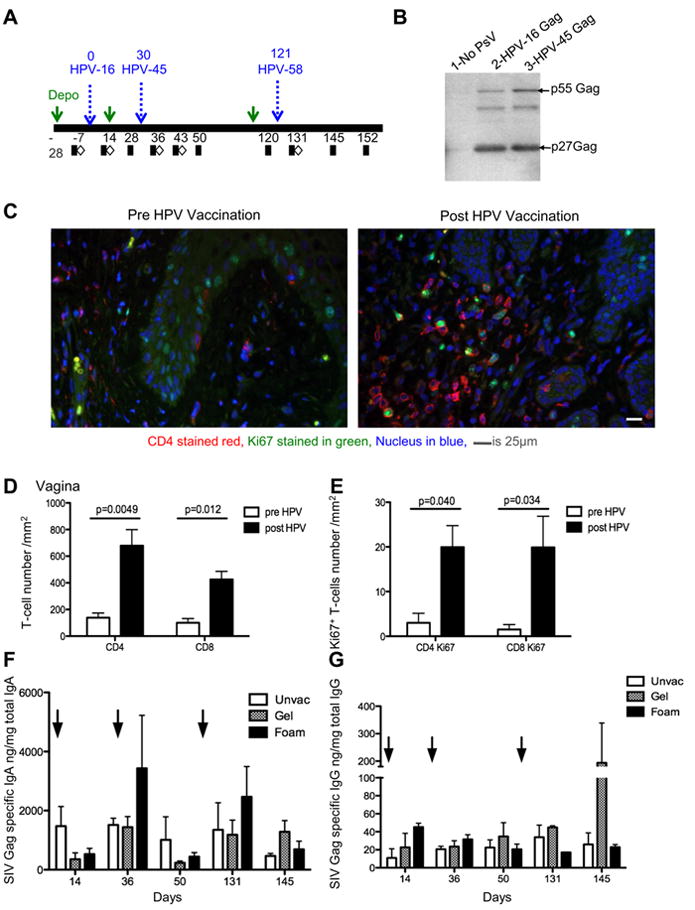
HPV PsVs are immunogenic in rhesus macaques, recruit CD4+ and CD8+ T-cells to the site of vaccination and induce vaginal humoral responses. (A) Schematic showing the vaccination and sampling schedule in rhesus macaques. Macaques were vaccinated on days 0, 30, and 121 with HPV16, 45, and 58, respectively. Blood (black squares) and tissues (white diamonds) were collected pre and post HPV vaccination. (B) Western blot showing the expression of SIV Gag polyprotein p55 and processed p27 protein in 293TT-cells transduced with HPV16 and HPV45 Gag-Pro constructs (lanes 2 & 3). Non-transduced 293TT lysates was used as a negative control (lane 1).
(C) Vaginal biopsies were obtained prior to and one-week post HPV vaccination, and paraffin embedded. A representative example of immunohistochemical staining performed on embedded tissue, stained for CD4 (red) Ki67 (green) and nuclear material stained by dapi (blue) prior to (left) and post (right) HPV vaccination. (D) The absolute number of CD4+ and CD8+ T-cells enumerated from vaginal biopsies pre (white bars) and post vaccination (black bars). A repeated measure analysis of variance demonstrated that the difference is statistically significant (p=0.0049 and p=0.012). (E) The absolute number of Ki67+ CD4+ and Ki67+ CD8+ cells in vaginal biopsies pre and post HPV vaccination. A repeated measure analysis of variance demonstrated that the difference is statistically significant (p=0.040 and p=0.034) (F) SIV Gag-specific IgA as a function of total IgA in vaginal secretions. (G) SIV Gag-specific IgG as a function of total IgG in vaginal secretions.
To characterize the quality and quantity of the cellular immune responses elicited by the HPV PsVs-Gag-pro vaccines, we performed immunophenotyping and functional assays of SIV specific T-cells in the blood of the immunized rhesus macaques.
CD95+ (antigen experienced) CD8+ T-cells were gated and the frequency of the SIV specific Gag CM9 tetramer positive cells assessed. Detectable Gag specific CM9 tetramer responses were observed after the first HPV16 PsVs vaccination; however the frequency of tetramer positive cells was only marginally increased following immunization with the HPV45 PsVs (Fig. 5A). This is likely due to the lower dose of HPV45 PsVs (1×109) IU as opposed to (2×1010) IU of HPV16PsVs, which was delivered as a result of difficulties in propagating the HPV45 PsVs. A further boost with 2×1010 IU of HPV58 PsVs induced a marked increase in tetramer positive cells in animals that had been treated with either the N9 gel or the foam (Fig. 5A).
FIGURE 5.
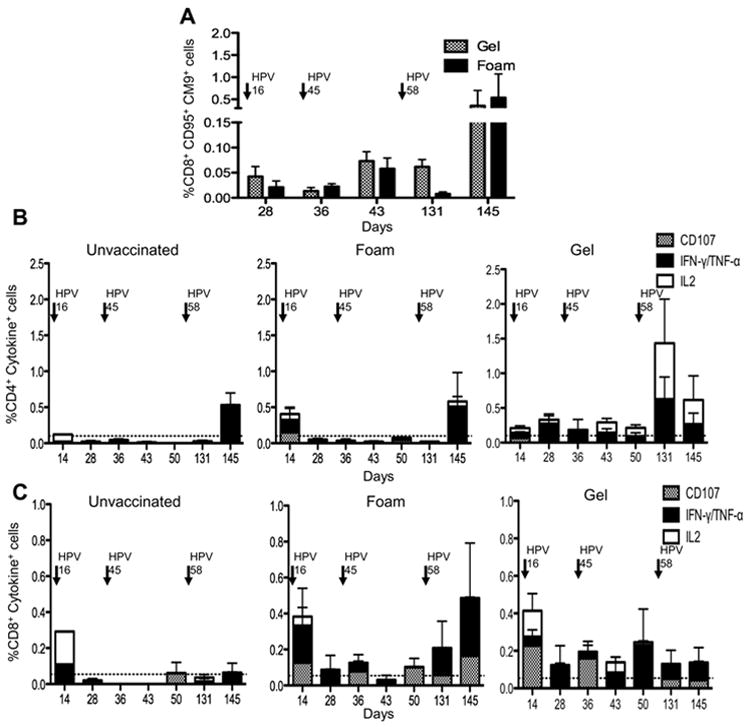
SIV specific Memory T-cell responses to HPV PsVs (A) Antigen experienced CD95+CD28 (+/-) CD8+ T- cells from PBMC’s were gated and the frequency of SIVGagCM9+ cells assessed post HPV vaccination. Animals treated with N9 foam are depicted in hatched bars, while N9 gel treated animals are in black bars. Arrows above indicate the times when each HPV vaccine was administered. (B) Intracellular cytokine staining indicating the frequency of CD107+(hatched), IL-2 (white), IFN-γ and/or TNF-α (black) producing CD4+ T-cells from Gag stimulated PBMC’s in unvaccinated (left) foam treated (middle) and gel treated (right) animals. (C) Intracellular cytokine staining indicating the frequency of CD107+, IL-2+, IFN-γ and/or TNF-α producing CD8+ T-cells from Gag stimulated PBMC’s.
Antigen experienced T-cells are characterized as central memory or effector memory cells, with central memory cells releasing IL-2 upon stimulation, while effector memory cells are more likely to produce markers of degranulation, such as CD107. Using these molecules, along with IFN-γ and TNF- α production, we characterized the cytokine profile of the SIV Gag specific memory (CD95+ CD28+/-) cells induced by HPV PsVs vaccination. We found that the majority of memory circulating cells induced by this vaccine modality are mono-functional (i.e. secrete only one cytokine). While limited cytokine production was observed after HPV16 PsVs and -45 PsVs vaccinations, HPV58 PsVs caused a robust increase in the frequency of SIVmac251 specific CD4+ cytokine producing cells in both the gel and foam treated animals after background subtraction, and these CD4+ T-cells produced either IL-2 or IFN-γ/TNF-α (Fig. 5B). Of interest, the phenotype of the cells differed between the groups, vaccination in the presence of the gel resulted in higher IL-2 production (Fig. 5B). Gag specific CD8+ memory cells produced either CD107 or IFN-γ/ TNF-α and negligible IL-2 (Fig. 5C). HPV58 PsVs vaccination boosted CD8+T-cell cytokine production in the N9 foam treated animals but not in the N9 gel treated animals (Fig. 5C). The low frequency of cells obtained from vaginal biopsies in rhesus macaques precluded reliable longitudinal analysis of T-cell phenotype and function at this site. Altogether, these data show that in rhesus macaques, this vaccine modality recruits and/or expands CD4+ and CD8+ T-cells at the site of vaccination and induces effector memory CD8+ T-cell responses and central and effector memory CD4+ immune responses in the blood.
HPV PsVs Vaccination does not exacerbate SIVmac251 replication but primes SIV specific responses in the vaginal mucosa, lymph nodes and the blood
The aim of mucosal HIV vaccines is to induce a population of virus-specific resident memory B and T-cells at the portal of entry, that are able to prevent the systemic spreading of the virus. However, a possible caveat, in the case of HIV infection, is that the recruitment and activation of CD4+ T-cells may result in exacerbation of virus replication. To investigate the kinetics of the expansion of SIV specific responses and a possible exacerbation of infection, the six vaccinated and two control rhesus macaques were exposed to a high dose (105 TCID50) of SIVmac251 by the vaginal route at six weeks after the final HPV58 PsVs vaccination and euthanized at one or two weeks post infection as indicated in Fig. 6A. We quantified plasma viral RNA, and viral DNA levels in vaginal tissue collected at euthanasia. We did not observe differences in the levels of viral RNA in plasma one or two weeks post infection (Fig. 6B). Similarly, we observed no difference in the levels of SIV DNA in the vaginal tissues of vaccinated or unvaccinated animals collected two weeks post infection (Fig. 6C). Statistical analysis that included two additional naive controls challenged intravaginally with the same stock of SIVmac251 at the same dose, but at a different time, supported a lack of a significant difference in virus levels in plasma between vaccinated and unvaccinated animals. We next investigated the virus-specific immune responses in multiple tissues at one week or two weeks post SIVmac251 infection. We measured SIV Gag CM9 tetramer CD8+ T-cell responses in the blood, genital draining obturator lymph node, in intraepithelial and lamina propria lymphocytes from the vagina, endocervix, and from the rectal mucosa. Fig. 6d, top panel, shows representative staining of Gag CM9 tetramer positive cells from each tissue. Gag specific responses remained at low levels one week post challenge (data not shown) but expanded by two weeks post infection in blood, female genital tract, and genital draining obturator lymph node, but not in the rectum (Fig. 6D). Suggesting, the focal immune response induced by HPV in the female genital tract and draining sites had been expanded upon exposure to SIV. To assess the functionality and to confirm the extent of the secondary responses to SIVmac251 in vaccinated macaques, we performed an IFN-γ ELISpot in blood, obturator and axillary lymph nodes, using both the Gag peptides (included in the vaccine) and Envelope peptides, (not included in the vaccine). We observed significantly higher Gag specific IFN-γ responses in vaccinated animals when compared to unvaccinated controls in the genital draining obturator lymph node p=0.034 (Fig. 6E, left). As expected, no Env responses were observed in all tissues tested (Fig. 6E, right). Furthermore, similar to what was observed by Gag CM9 tetramer staining, the blood and obturator lymph node had a greater magnitude of response compared with a distal site (i.e. the axillary node).
FIGURE 6.
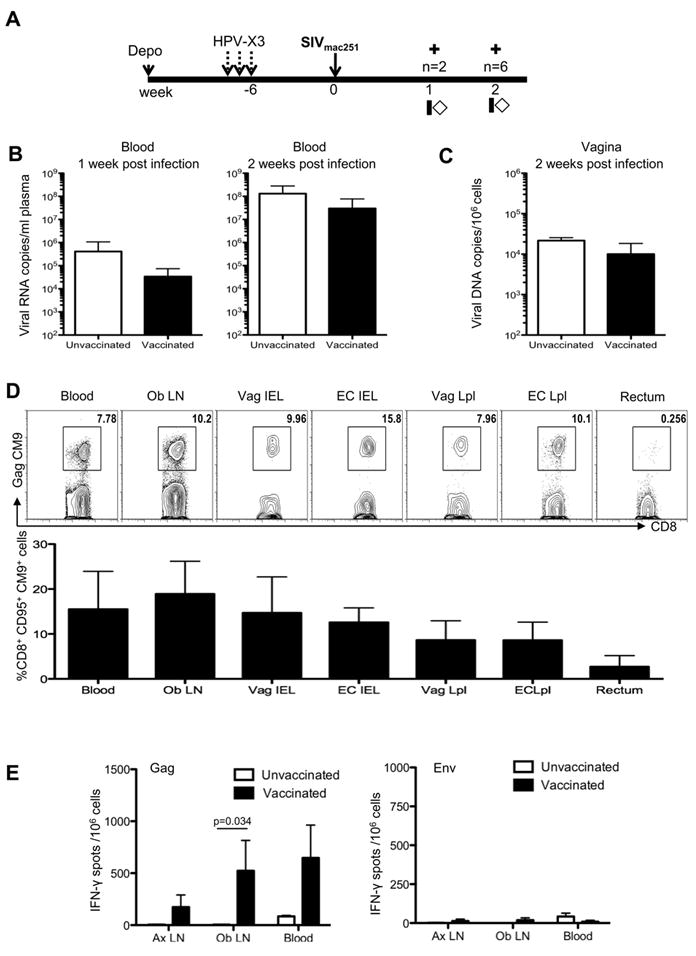
Intravaginal exposure to SIV induces an expansion of vaccine-primed immune responses and does not exacerbate virus replication. (A) Study design showing SIV exposure six weeks post the final HPV vaccine. Animals were sacrificed at one or two weeks post infection (indicated by crosses), and blood and tissues collected at sacrifice. (B) Plasma viral load in unvaccinated and vaccinated macaques one and two weeks post infection. (C) Cell associated viral load in the vagina quantified as the number of SIV DNA copies/106 cells. (D) Representative flow cytometric plots (top panel) showing the frequency of SIVGagCM9 positive memory CD8+ T-cells in the blood, genital draining obturator lymph node, cervix, vagina, and rectum two weeks post challenge. Both intraepithelial and lamina propria CD8+T-cells were obtained from the female genital tract. The bottom panel shows the average frequency of SIV specific Gag CM9 CD95+CD8+ T-cells from vaccinated animals. (E) Average IFN-γ producing spot forming mononuclear cells from axillary, obturator lymph nodes, and blood after stimulation with overlapping peptides spanning SIV Gag (left) or SIV Env (right) from vaccinated and unvaccinated controls, two weeks post challenge. A repeated measure analysis of variance demonstrated that the difference in IFN- γ production from the obturator lymph node between vaccinated and non-vaccinated animals is statistically significant (p=0.034).
Discussion
Most HIV infections worldwide occur by mucosal routes and recent studies demonstrate that mucosal exposure of humans to HIV, and of macaques to SIV, result in the transmission of a single or few viral variants (28-30). The founder viruses cross the mucosal epithelium, replicate in the lamina propria, and seed distal sites producing a chronic viral infection. Mucosal immune responses may be needed to curb the initial local expansion of the founder virus and prevent systemic viral spread. This hypothesis would require a vaccine to induce durable protective memory immune responses at the relevant mucosal site. We tested the immunogenicity of a novel mucosal vaccine, HPV PsVs, as this vaccine modality targets the cervico-vaginal epithelium (15,16). We demonstrated that HPV PsVs, used as vehicles for the delivery of SIV DNA, induced SIVmac251 specific cellular and humoral immune responses at the genital tract, in the genital draining lymph nodes, and in blood. The immunogenicity of HPV PsVs may be related to its ability to activate and mature dendritic cells, which in turn, facilitates the induction of Th1 responses (18). In murine models, gene expression from HPV PsVs is observed in antigen presenting cells in the lamina propria of the intestines after oral exposure to HPV PsVs (31). The ‘adjuvant effect’ of HPV PsVs has been demonstrated where stronger T-cell responses and antibodies were elicited using PsVs as opposed to naked DNA in the respiratory, gastrointestinal, and female genital tract (14,31,32). Furthermore, intravaginal vaccination in mice (Cuburu et al. unpublished observations), and in macaques as reported here, results in the recruitment of both CD4+ and CD8+ T-cells to the site of vaccination, increasing the absolute number of T-cells in the cervico-vaginal mucosa. The HPV Gag PsVs induced mucosal immune response is likely underestimated as vaccination may induce focal responses that may be missed by studying pinch biopsies and tissue sections. In addition SIV specific responses are determined as a percentage of isolated T-cells that does not account for the recruitment or increase in absolute number of cells or the distribution of vaccine induced cells within the female genital tract. HIV/SIV specific antibodies in the female genital tract may represent a first line of defense against infection. In humans, IgG has been shown to be the dominant immunoglobulin isotype in the vaginal tract and is transported across epithelial cells via the neonatal Fc Receptor (33). In addition, HIV specific IgG is predominant in vaginal secretions from infected women (34,35). In our studies we measured Gag specific IgA and IgG in rhesus macaques post HPVPsV-SIV vaccination and detected both subtypes in the vaginal tract. Surprisingly, we observed increased IgA as compared to IgG levels in vaginal secretions. HPV PsVs are potent inducers of IgA, however further investigation is needed to determine if the predominance of Gag specific IgA in our studies is related to the vector, route of administration, or hormonal environment.
We challenged immunized macaques with a high dose of SIVmac251 which precludes the assessment of relative vaccine efficacy, since our goal was to test the expansion of HPV-Gag-pro PsVs –induced responses in tissues after vaginal exposure to SIV. Importantly, we have not observed exacerbation of SIV replication despite the recruitment or expansion of a significant number of activated CD4+T-cells in the female genital tract. Whether the extent and rate of expansion of SIV-specific immune responses will be sufficient to prevent SIV replication and spread to distal sites, or will favor virus immune escape, needs to be tested using repeated low doses of SIVmac251, whereby few virus variants are transmitted, similar to heterosexual HIV transmission in women. Furthermore, the inflammatory profile, length of the eclipse phase (36), initial innate and adaptive responses may vary in low dose versus high dose models. Indeed, we have observed diverse outcomes in macaques given the same vaccine regimen when half of the animals were challenged with a single high dose and the other half with lower repeated doses of SIVmac251 (Vaccari et al. unpublished observations).
While HPV PsVs targeting of the vaginal mucosa makes this approach attractive, the intravaginal delivery of HPV PsVs and hormonal treatment is cumbersome, in addition, effective vaccination may require disruption of the epithelium with agents like N9 that can exacerbate HIV infection (37). Thus, this vaccination regimen, in its current form, may be somewhat impractical for clinical studies. However, vaccine administration in the early follicular phase of the cycle may eliminate the need for hormonal treatment. In addition the N9 foam is easy to use, inexpensive, readily available, and could be self administered prior to a gynecological visit. Thus a clinical trial with HPV-PsV is feasible. Furthermore, adolescent girls have extensive cervical ectopy and the exposed simple columnar epithelium may be susceptible to HPV PsVs infection under less stringent conditions eliminating the need for epithelia disruption. In addition, alternate mucosal routes or other alterations in the mode of delivery of HPV PsVs could be explored. In this study, we have used HPV16, 45, and 58 PsVs, some of these serotypes or closely related ones, are in the HPV vaccine. Thus, neutralizing antibodies to these serotypes would preclude their efficacy in HPV VLP vaccinated women. However, since HPV PsVs mediated genital transduction is not species restricted, animal papillomavirus serotypes, that are not cross-neutralized by VLPs in current HPV vaccines, could be selected for the development of a human HIV vaccine.
To date, there have been three large Phase II/III HIV clinical vaccine trials and all have used systemically administered vaccine candidates (4-6). Among those, the recombinant canarypox ALVAC-HIV vaccine, administered together with HIV envelope proteins in the Thai RV144 trial, afforded 31% protection against mucosal heterosexual HIV infection (5). Similarly, non-human primate vaccine studies using ALVAC-SIV and SIVgp120 protein have protected some vaccinees from repeated low dose SIVmac251 challenges (24) (Pegu et al. unpublished observations). These vaccine regimens induce limited T-cell responses and high levels of non-neutralizing antibodies, however the correlates of protection have not been defined allowing only limited speculation regarding vaccine-elicited immune responses that may protect from HIV infection. An effective HIV vaccine may need to elicit mucosal high-titer antibodies and high-frequency T-cell responses, as opposed to only one of the aforementioned responses. In this study, HPV PsVs elicited both CD4+ and CD8+ T-cell responses, and low-level Gag antibody responses. Importantly, immune responses were induced locally at the mucosal site at risk for lentivirus transmission. Adding SIV envelope antigens to induce antibodies against the surface of the virus, as well as other accessory/regulatory genes (38) will be a crucial next step in the evaluation of this vaccine modality. Thus, the data presented here warrants the further testing of the efficacy of this vaccine approach in protection from a dose of SIVmac251 that results in transmission of a few virus variants to mimic HIV transmission to humans.
Acknowledgments
We thank David Venzon for statistical evaluation of the data. Brandon Keele and George Shaw for the Gag-Pro sequence of the M766/SIVmac251 variant, Gary Nabel for the SIV Gag DNA sequence. We also thank Kathryn Mckinnon for flow cytometric support, Teresa Habina for editorial assistance, Drs. Weiss and Treece for their exemplary veterinary care.
This study was funded from the NIH intramural budget of Dr. Franchini and Dr. Schiller.
Footnotes
Disclosures The authors have no conflicts of interest. Barney Graham, John Schiller, Christopher Buck, Jeffery Roberts, and Rhonda Kines are named in a patent application related to this technology.
References
- 1.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 2.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padian NS, Shiboski SC, Jewell NP. Female-to-male transmission of human immunodeficiency virus. JAMA. 1991;266:1664–1667. [PubMed] [Google Scholar]
- 4.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del RC, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de SM, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 6.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van GF, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del RC, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118:S12–S17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 12.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 14.Graham BS, Kines RC, Corbett KS, Nicewonger J, Johnson TR, Chen M, LaVigne D, Roberts JN, Cuburu N, Schiller JT, Buck CB. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal Immunol. 2010;3:475–486. doi: 10.1038/mi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JN, Kines RC, Katki H, Lowry DR, Schiller JT. Effect of Pap Smear Collection and Carrageenan on Cervicovaginal HPV16 Infection in a Rhesus Macaque Model. 2011 doi: 10.1093/jnci/djr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 19.Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 20.Gordon SN, Weissman AR, Cecchinato V, Fenizia C, Ma ZM, Lee TH, Zaffiri L, Andresen V, Parks RW, Jones KS, Heraud JM, Ferrari MG, Chung HK, Venzon D, Mahieux R, Murphy EL, Jacobson S, Miller CJ, Ruscetti FW, Franchini G. Preexisting infection with human T-cell lymphotropic virus type 2 neither exacerbates nor attenuates simian immunodeficiency virus SIVmac251 infection in macaques. J Virol. 2010;84:3043–3058. doi: 10.1128/JVI.01655-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevceva L, Kelsall B, Nacsa J, Moniuszko M, Hel Z, Tryniszewska E, Franchini G. Cervicovaginal Lamina Propria Lymphocytes: Phenotypic Characterization and Their Importance in Cytotoxic T-Lymphocyte Responses to Simian Immunodeficiency Virus SIV(mac251) J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano JW, Williams KG, Shurtliff RN, Ginocchio C, Kaplan M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol Invest. 1997;26:15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- 23.Vaccari M, Mattapallil J, Song K, Tsai WP, Hryniewicz A, Venzon D, Zanetti M, Reimann KA, Roederer M, Franchini G. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J Virol. 2008;82:9629–9638. doi: 10.1128/JVI.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, Tryniszewska E, Lewis MG, Vancott TC, Hirsch V, Woodward R, Gibson A, Grace M, Dobratz E, Markham PD, Hel Z, Nacsa J, Klein M, Tartaglia J, Franchini G. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Patton DL, Kidder GG, Sweeney YC, Rabe LK, Hillier SL. Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina) Am J Obstet Gynecol. 1999;180:1080–1087. doi: 10.1016/s0002-9378(99)70598-3. [DOI] [PubMed] [Google Scholar]
- 28.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 29.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi W, Liu J, Huang Y, Qiao L. Papillomavirus pseudovirus: a novel vaccine to induce mucosal and systemic cytotoxic T-lymphocyte responses. J Virol. 2001;75:10139–10148. doi: 10.1128/JVI.75.21.10139-10148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Fayad R, Wang X, Quinn D, Qiao L. Human immunodeficiency virus type 1 gag-specific mucosal immunity after oral immunization with papillomavirus pseudoviruses encoding gag. J Virol. 2004;78:10249–10257. doi: 10.1128/JVI.78.19.10249-10257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu FX. Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin Immunol. 2000;97:59–68. doi: 10.1006/clim.2000.4910. [DOI] [PubMed] [Google Scholar]
- 35.Belec L, Dupre T, Prazuck T, Tevi-Benissan C, Kanga JM, Pathey O, Lu XS, Pillot J. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson D, Tholandi M, Ramjee G, Rutherford GW. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect Dis. 2002;2:613–617. doi: 10.1016/s1473-3099(02)00396-1. [DOI] [PubMed] [Google Scholar]
- 38.Hel Z, Tsai WP, Tryniszewska E, Nacsa J, Markham PD, Lewis MG, Pavlakis GN, Felber BK, Tartaglia J, Franchini G. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J Immunol. 2006;176:85–96. doi: 10.4049/jimmunol.176.1.85. [DOI] [PubMed] [Google Scholar]


