Summary
Purpose
The adenosinergic system is known to exert an inhibitory affect in the brain and as such adenosine has been considered an endogenous anticonvulsant. Entorhinal cortex (EC) layer II neurons, which serve as the primary input to the hippocampus, are spared in temporal lobe epilepsy (TLE) and become hyperexcitable. Since these neurons also express adenosine receptors, the activity of these neurons may be controlled by adenosine, specifically during seizure activity when adenosine levels are thought to rise. In light of this, we determined if the actions of adenosine on medial EC (mEC) layer II stellate neurons are augmented in TLE and by which receptor subtype.
Methods
Horizontal brain slices were prepared from rats exhibiting spontaneous seizures (TLE) induced by electrical stimulation and compared with age matched control rats. mEC layer II stellate neurons were visually identified and action potentials (AP) evoked by either a series of depolarizing current injection steps or via presynaptic stimulation of mEC deep layers. The effects of adenosine were compared with actions of adenosine A1 and A2A receptor-specific agonists (CPA and CGS 21680) and antagonists (DPCPX and ZM241385) respectively. Immunohistochemical and qPCR techniques were also employed to assess relative adenosine A1 receptor message and expression.
Key Findings
mEC layer II stellate neurons were hyper-excitable in TLE, evoking a higher frequency of AP's when depolarized and generating bursts of AP's when synaptically stimulated. Adenosine reduced AP frequency and synaptically evoked AP's in a dose dependent manner (500 nM – 100 μM); however, in TLE, the inhibitory actions of adenosine occurred at concentrations that were without affect in control neurons. In both cases, the inhibitory actions of adenosine were mediated via activation of the A1 and not the A2A receptor subtype. qPCR and immunohistochemical experiments revealed an up-regulation of the adenosine A1 mRNA and an increase in A1 receptor staining in TLE neurons compared to control.
Significance
Our data indicates the actions of adenosine on mEC layer II stellate neurons is accentuated in TLE due to an up-regulation of adenosine A1 receptors. Since adenosine levels are thought to rise during seizure activity, activation of adenosine A1 receptors could provide a possible endogenous mechanism to suppress seizure activity and spread within the temporal lobe.
Keywords: Adenosine, Temporal Lobe Epilepsy, Entorhinal Cortex, Action Potentials, A1 receptor
Introduction
Temporal lobe epilepsy is a common form of adult epilepsy that involves structures of the limbic system, including the entorhinal cortex (EC). The EC is a six-layered structure that assimilates information from the parahippocampus, prefrontal cortex, and frontal cortex (Apergis-Schoute et al., 2006). Stellate neurons from layer II of the EC provide the major input into the dentate gyrus (DG) via the perforant path (PP) (Steward, 1976), making the EC a central doorway between the cortex and the hippocampus (Witter, 1993; Burwell, 2000). The EC has been implicated in the development of temporal lobe seizures in rodent models (Heinemann et al., 1993; Jones et al., 1992), and in the propagation of seizures in human patients with temporal lobe epilepsy (TLE, Spencer & Spencer, 1994). MRI studies in humans revealed a decreased volume of the EC in patients suffering from TLE (Jutila et al., 2001; Bartolomei et al., 2005), due to a preferential loss of layer III neurons (Du et al., 1993). Superficial layer II neurons remain relatively well spared in TLE and become hyperexcitable (Bear et al., 1996). A number of mechanisms have been proposed for this hyper-excitability including a reduction in inhibitory input (Kobayashi & Buckmaster, 2003) and pro-excitatory alterations in sodium channel gating parameters (Hargus et al., 2011).
Activation of the adenosinergic system by adenosine is known to exert depressant effects on neuronal transmission within the brain (Moore et al., 2003; Dunwiddie & Haas, 1985; Hargus et al., 2009). Four adenosine receptors have been identified to date (A1, A2A, A2B, and A3), however, within the CNS, adenosine A1 and A2a receptors are most widely expressed and it is the A1 subtype that has been primarily implicated in neuroprotection (Wardas, 2002). Binding of adenosine to the A1 receptor leads to activation of an inhibitory G protein (Gi), causing hyperpolarization of the postsynaptic neuron via opening of outwardly rectifying K+ channels (Trussell & Jackson, 1985) and reduced transmitter release in pre-synaptic neurons by inhibiting Ca2+ entry via Cav2 channels at the synapse (Scholz & Miller, 1991).
In view of adenosine's inhibitory mode of action, it is not surprising that adenosine is considered the brain's endogenous anticonvulsant (Siggins & Schubert, 1981; Dunwiddie & Hoffer, 1980; Dragunow, 1986). Adenosine levels rise during epileptic seizures in both animal models of epilepsy and human patients (Berman et al., 2000; During & Spencer, 1992; Daval & Werck, 1991). Delivery of adenosine and adenosine agonists to specific brain loci in animal models of epilepsy leads to a reduction in seizure activity (De Sarro et al., 1999 ;Boison, 2005; Dunwiddie & Worth, 1982) while application of adenosine antagonists increases the frequency and severity of seizures (Dunwiddie & Hoffer, 1980; Mohammad-Zadeh et al., 2005; Mohammad-Zadeh et al., 2007). EC neurons have been shown to express adenosine receptors (Rivkees et al., 1995) and activation of adenosine A1 receptors has been shown to modulate layer II stellate neuron excitability (Li et al., 2010), however, it is unclear if in epileptic neurons the modulation of neuronal excitability by adenosine is altered. In the present study, we determined the actions of adenosine on mEC layer II hyperexcitability using a rat model of spontaneous temporal lobe seizures. We report here that adenosine's inhibitory actions were accentuated in TLE neurons compared with control and were mediated via activation of the A1 receptor. Since we also detected an increase in A1 receptor mRNA and increased immunostaining for the A1 receptor in TLE neurons compared to control neurons, it is likely that these enhanced actions of adenosine in TLE were, in part, due to increased expression of adenosine A1 receptors. The ability of adenosine to exert a greater inhibitory affect on TLE neurons could serve as an important compensatory mechanism by which the hyperexcitability of neurons in TLE is modulated.
METHODS
TLE model preparation
All animal experiments were conducted in accordance with the guidelines established by the National Institutes of Health guide for the Care and Use of Laboratory Animals and were approved by the University of Virginia's Institute of Animal Care and Use Committee. Adult male Sprague-Dawley rats were implanted with a pair of stainless steel electrodes unilaterally to the left hemisphere. One week following surgery, rats were stimulated through the electrode to induce limbic status epilepticus (SE). At least two months after the induction of SE, animals were evaluated for the presence of spontaneous temporal lobe seizures, as the seizure pattern and frequency typically stabilizes by this time (Bertram & Cornett, 1993). Details regarding electrode implantation, stimulation and seizure monitoring are listed in Supplementary Data S1.
Entorhinal cortex slice preparation
Horizontal brain slices (300 μM) were prepared from Sprague Dawley rats (250-400 grams) with TLE or aged matched controls. Animals were euthanized with isoflurane, decapitated, and their brains were rapidly removed and submerged in ice cold artificial cerebral spinal fluid (ACSF) containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 0.5 L-ascorbic acid, 2 pyruvate, 10 glucose, and 25 NaHCO3 (oxygenated with 95% O2 and 5% CO2). Slices were prepared using a Vibratome (Vibratome 1000 Plus), transferred to a chamber containing oxygenated ACSF, incubated at 37° C for 35 min, and then stored at room temperature. For recordings, slices were held in a small chamber superfused with heated (32°C) oxygenated ACSF at 3 mL/min. For electrophysiology experiments, mEC layer II stellate neurons were visually identified by infra-red video microscopy (Hamamatsu, Shizouka, Japan) using a Zeiss Axioscope microscope (Zeiss, Oberkochen, Germany). Whole-cell current clamp recordings were performed using an Axopatch 700B amplifier (Molecular Devices) using pCLAMP 10 software (Molecular Devices) and a Digidata 1322A (Molecular Devices). Electrodes were fabricated from borosilicate glass using a Brown-Flaming puller (model P97, Sutter Instruments Co). Electrodes (3.0 – 3.5 MΩ) were filled with (in mM): 120 Kgluconate, 10 NaCl, 2 MgCl2, 0.5 K2EGTA, 10 HEPES, 4 Na2ATP, 0.3 NaGTP (pH adjusted to 7.2 with KOH). APs were evoked using current injection steps from -20 pA to 470 pA in 10 pA steps for 300 ms at 5 sec inter-pulse intervals. AP analysis details are listed in supplementary data S2. APs were also evoked using a stimulating electrode (WPI, Sarasota, FL, USA) placed in layer III of the mEC. A 400 μs stimulus of varying current amplitude (1 to 3.2 mA) was applied every 15 sec via a digital stimulator (Digitimer Ltd, Hertfordshire, UK). In order to consistently evoke APs the stimulus amplitude was increased 1.5 times from threshold.
qPCR experiments
qPCR experiments were carried out from three control rats and three TLE rats. Details regarding the procedure can be found in supplementary data (Data S3).
Immunohistochemistry experiments
Confocal images were captured of mEC layer II using rabbit anti-adenosine A1R (1:200, Chemicon) and anti-NeuN (1:500, Millipore). Details regarding the procedure can be found in supplementary data (Data S4).
Drugs
Adenosine, N6-cyclopentyl-adenosine (CPA), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), CGS-21680 hydrochloride, ZM 241385, and adenosine deaminase (ADA) were obtained from Sigma Aldrich (St. Louis, MO, USA) and prepared as 1000 × stock solutions in DMSO. Drugs were then diluted to working concentrations directly preceding experiments. DMSO concentration did not exceed 0.02%.
Data Analysis
Data represent means ± standard error of the mean (S.E.M). Statistical significance was determined using a Student's t-test (unpaired) or a standard one way ANOVA followed by Tukey's or Dunn's post hoc test for parametric data or the Rank Sum test for non-parametric data (SigmaStat, Jandel).
RESULTS
Medial EC layer II neurons are hyperexcitable In TLE
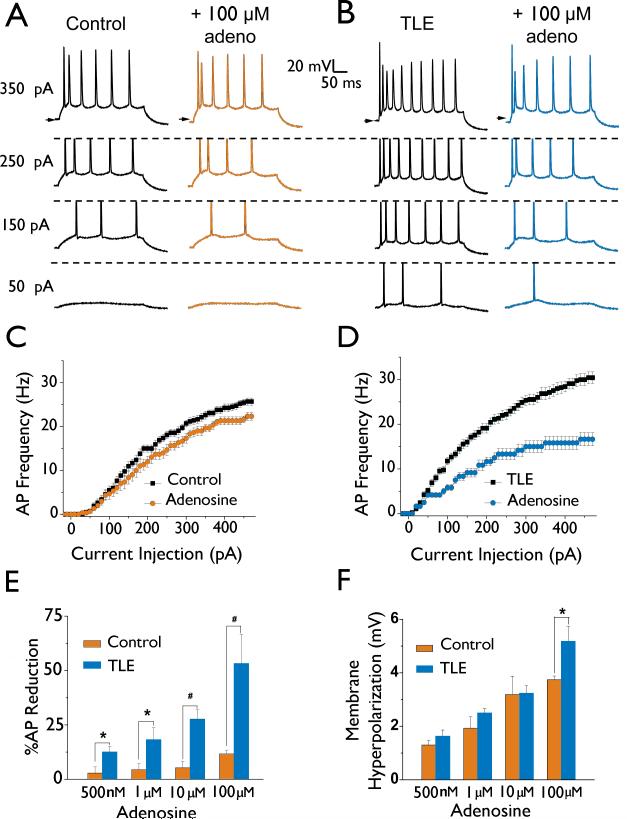
mEC layer II stellate neurons were identified based upon their location within the mEC, their distinct “star like” morphology and their characteristic action potential (AP) waveform (Hargus et al., 2011; Alonso & Klink, 1993). Consistent with previous findings (Hargus et al., 2011), mEC layer II stellate neurons in our rat model of chronic TLE were significantly hyperexcitable (Figure 1). AP firing frequencies were increased from 25.6 ± 0.9 Hz (n = 15) in control to 31.1 ± 1.1 Hz (n = 18, P < 0.05) in TLE when compared at a current injection step of 470 pA (Figure 1C & D). Analysis of AP properties revealed that TLE neurons also had significantly depolarized resting membrane potentials (RMP), hyperpolarized AP thresholds, increased input resistances, and increased AP upstroke velocities as compared to controls (Table 1).
Figure 1. mEC layer II stellate neurons In TLE are hyperexcltable and more sensitive to Inhibition by adenosine than control neurons.
A and B: Membrane properties and neuronal excitability were recorded from both control (A) and TLE (B) mEC layer II stellate neurons (left traces). APs were evoked using current injection steps from -20 to 470 pA. Shown are the responses to 50, 150, 250, and 350 pA current injections. Membrane properties and excitability were again recorded in control (A) and TLE (B) neurons following a 5 minute bath application of 100 |jM adenosine (right traces). C and D: Current-frequency plots showing reduction of AP frequency by adenosine (100 μM) in control neurons (C) and TLE neurons (D). E: Bar chart representing a concentration dependent inhibition of AP frequency measured at a depolarizing current injection step of 470 pA for both control and TLE neurons. Note the profound inhibition of AP frequency in TLE at all doses tested. F: Bar chart demonstrating the average dose-dependent effect of adenosine application on membrane hyperpolarization. Data points represent means ± S.E.M. * P<0.05, # P<0.005.
Table 1.
Membrane properties of mEC layer II stellate neurons.
| RMP (mV) | Ri (MΩ) | Threshold (mV) | Width (ms) | Amplitude (mV) | Upstroke velocity (mV/ms) | n | |
|---|---|---|---|---|---|---|---|
| Control | -61.0 ± 0.3 | 60.9 ± 1.3 | -45.8 ± 0.5 | 1.10 ± 0.06 | 97.4 ± 1.0 | 223.1 ± 6.3 | 19 |
| TLE | -59.1 ± 0.3‡ | 73.3 ± 2.8‡ | -49.3 ± 0.7‡ | 1.13 ± 0.06 | 97.1 ± 1.1 | 258.3 ± 10.7# | 17 |
P<0.001
P<0.01.
Suppression of AP firing by adenosine is enhanced in TLE
Bath application of adenosine (500 nM, 1 μM, and 10 μM) had little effect on AP frequency in control neurons (Figure 1A, C & E). AP firing frequencies at a current injection step of 470 pA were reduced by 2.4 ± 2.4 % (n = 6), 5.8 ± 2.6 % (n = 6), and 6.7 ± 2.8 % (n = 5) after bath application of 500 nM, 1 μM, and 10 μM adenosine, respectively (Figure 1E). Only at a concentration of 100 μM adenosine were AP firing frequencies significantly decreased in control neurons by 10.4 ± 3.3 % (n = 10, P < 0.05). Adenosine did significantly hyperpolarize the RMP of control neurons in a dose-dependent manner (Figure 1F).
In contrast, the inhibitory actions of adenosine on current injection evoked AP discharge frequency were more pronounced in TLE neurons; significantly reducing AP discharge frequency at all concentrations tested when compared to control neurons (Figure 1E). Analysis of AP firing frequencies at a current injection step of 470 pA revealed dose dependent reductions by 12.6 ± 2.5 % (500nM; n = 4) , 18.1 ± 5.7 % (1 μM; n = 4), 25.8 ±3.3 % (10 μM; n = 11, P < 0.01 ) and 53.5 ± 13.4 % (100 μM; n = 5, P < 0.01, Figure 1B, D, E). As with control neurons, adenosine also hyperpolarized the resting membrane potential to values similar to those recorded in control except at a concentration of 100 μM where the effects on TLE neurons were significantly greater (Figure 1F).
Endogenous levels of adenosine have been reported in brain slice preparation (Dunwiddie & Diao, 2000). To reduce endogenous adenosine levels, slices were incubated for 10 mins with adenosine deaminase (ADA), the enzyme responsible for the breakdown of adenosine. ADA incubation (10 or 20 mg/mL) had no significant effect on membrane properties or AP discharge frequency in mEC layer II stellate neurons from control tissue (n = 4) or from our model of TLE (n = 4, data not shown), suggesting that endogenous adenosine within the slice were not influencing the actions of exogenously applied adenosine.
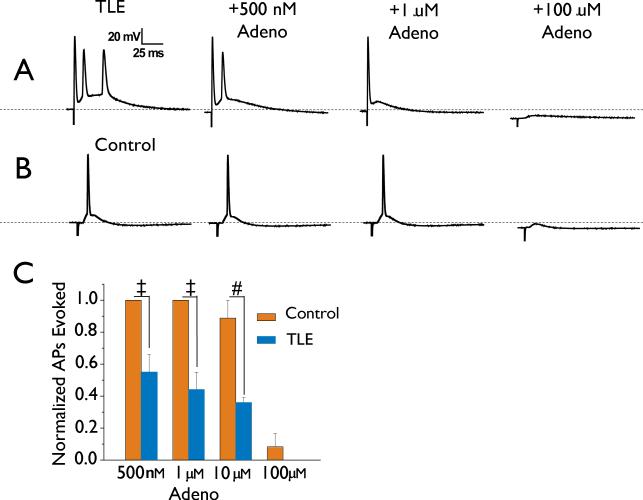
Adenosine inhibition of synaptically stimulated APs is enhanced in TLE
Brief stimulation of mEC layer III evoked depolarizing potentials that produced a single AP in control mEC layer II stellate neurons (Figure 2B). In contrast, stimulation evoked depolarizing events in TLE neurons had larger depolarizing amplitudes and longer durations that were associated with AP bursts of 3.0 ± 0.22 APs (n = 18; P < 0.001 compared with control; Figure 2A). Bath application of 500 nM, 1 μM and 10 μM adenosine had little effect on synaptically evoked responses in control neurons and only at a higher concentration of 100 μM adenosine was the evoked AP abolished (in 11 of 12 neurons tested; P < 0.01; Figure 2B, C). Again, TLE neurons were more sensitive to the actions of adenosine and caused significant reductions in the number of APs evoked by synaptic stimulation (Figure 2A & C). Again, application of ADA (10 and 20 mg/mL) had no effect on presynaptic stimulation evoked APs recorded in mEC layer II stellate neurons from control tissue (n = 3) or in TLE (n = 3; data not shown), indicating that endogenous adenosine was not affecting either evoked AP activity or influencing the actions of exogenously applied adenosine.
Figure 2. Action potential bursts evoked in TLE neurons by synaptic stimulation are profoundly suppressed by adenosine.
Stimulation of mEC layer III consistently evoked bursts of APs in mEC layer II neurons in TLE (A, left trace). In contrast, only a single AP could be evoked from control neurons (B, left trace). The profound inhibitory effects of adenosine in TLE neurons (500 nM, 1 μM and 100 μM) are shown on the top panel of A. In B, the same concentrations were applied to control neurons, but had little effect. Dashed lines represent -60 mV. In C, bar chart shows the enhanced concentration dependent effect of adenosine on TLE neurons compared to control. Bars represent means ± S.E.M. # P < 0.005, t P < 0.001.
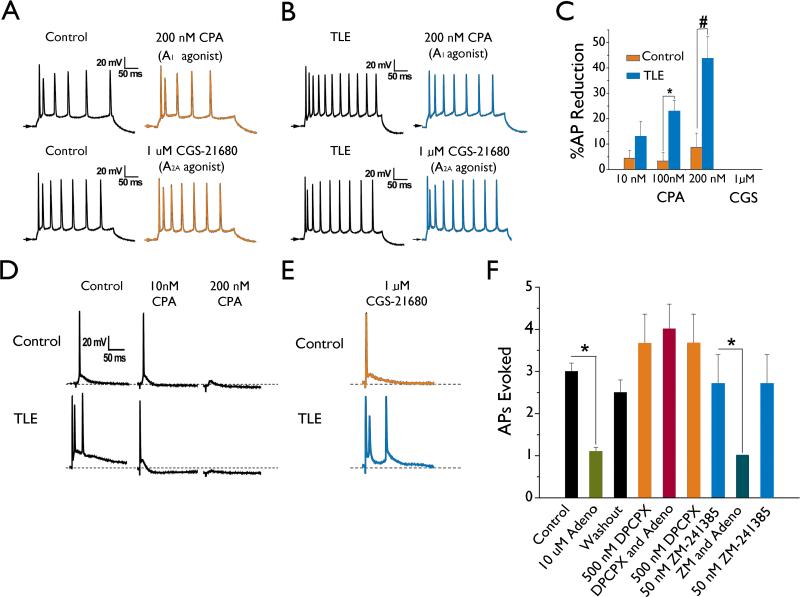
Inhibition of membrane excitability by adenosine is mediated via activation of the A1 receptor
The modulatory effects of adenosine within the CNS and hippocampus have been shown to involve interactions with predominantly the A1 and A2A receptor subtypes (Dunwiddie & Masino, 2001;Rebola et al., 2005). In order to determine the adenosine receptor subtype responsible for the actions of adenosine on mEC layer II stellate neurons, adenosine A1 and A2A receptor agonists and antagonists were tested. In a similar manner to adenosine, the A1 receptor agonist N6-cyclopentyl-adenosine (CPA) elicited minimal effects on AP discharge frequency in mEC layer II stellate control neurons. Concentrations of 10, 100, and 200 nM CPA reduced AP discharge frequency by 4.4 ± 3.0 % (n = 7), 3.3 ± 3.3 % (n = 3), and 8.7 ± 5.6 % (n = 6, Figure 3A, C), respectively at a current injection step of 470 pA. In contrast, CPA had a pronounced effect in TLE neurons with concentrations of 10, 100, and 200 nM CPA significantly reducing AP frequency by 13.0 ± 5.9 % (n = 4, P < 0.05), 20.8 ± 6.5 % (n = 3, P < 0.05), and 44.3 ± 1.3 % (n = 3, P < 0.001), respectively (Figure 3B, C).
Figure 3.
The actions of adenosine on mEC layer II stellate neurons are mediated via activation of the A1 receptor subtype and not A2A. A and B: AP discharge frequency in control (A) and TLE (B) neurons were both reduced by bath application of the A1 receptor specific agonist CPA (200 nM; top trace), but unaffected by application of the A2A receptor specific agonist CGS-21680 (1 μM; bottom trace). Traces shown are examples of AP's evoked in response to a 470 pA depolarizing current injection. C: Bar chart representing the dose-dependent CPA-induced reduction in AP frequency at 470 pA. D: Synaptically evoked APs in EC layer II stellate neurons from control (top traces) and TLE (lower traces) tissue were both affected by application of the A1 receptor specific agonist CPA (middle traces) but unaffected by application of the A2A receptor specific agonist CGS-21680 (E). Note the increased sensitivity of TLE neurons to lower doses of CPA as compared to controls. Arrows and dotted line represent -60 mV. F: Adenosine inhibits evoked APs in TLE tissue by activation the A1 and not the A2A receptor subtype. Adenosine (10 μM) application reversibly inhibited presynaptically evoked APs in TLE tissue. These inhibitory actions of adenosine were abolished by application of the A1 receptor specific antagonist DPCPX (500 nM), but not affected by application of the A2A receptor specific antagonist ZM-241385 (50 nM). Bars represent means ± S.E.M. * P < 0.05, # P<0.005.
Synaptically evoked APs were also sensitive to the A1 receptor agonist CPA. In control neurons, 10 nM CPA had little effect on AP firing and only inhibited the AP in 4 of 6 neurons tested at a concentration of 200 nM CPA (Figure 3D; n = 6). In contrast, TLE neurons were more sensitive to the actions of CPA (Figure 3D). Application of 10 nM CPA reduced evoked AP frequency from 3 APs to a single AP in 2 of 3 neurons tested, 100 nM CPA completely abolished the evoked APs in 2 neurons and reduced the number of APs evoked from 3 to 1 in another neuron. All evoked APs were completely abolished at 200 nM CPA (Figure 3D; n = 3, P < 0.01). The actions of adenosine and CPA were not reproduced by bath application of the A2A receptor selective agonist CGS-21680 (1 μM; Figure 3 E; n = 3) on either APs evoked by depolarizing current injections or synaptically evoked APs.
To further confirm a role for the A1 receptor in mediating the actions of adenosine, the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and the A2A receptor antagonist ZM-241385 were applied prior to and during application of adenosine (Figure 3F) in TLE neurons. In the presence of DPCPX (500 nM) the inhibitory effects of adenosine (10 μM; n = 10) on synaptically evoked APs were abolished (n = 4). In contrast, the inhibitory effects of adenosine were not affected by the A2A receptor antagonist ZM-241385 (50 nM; n = 3).
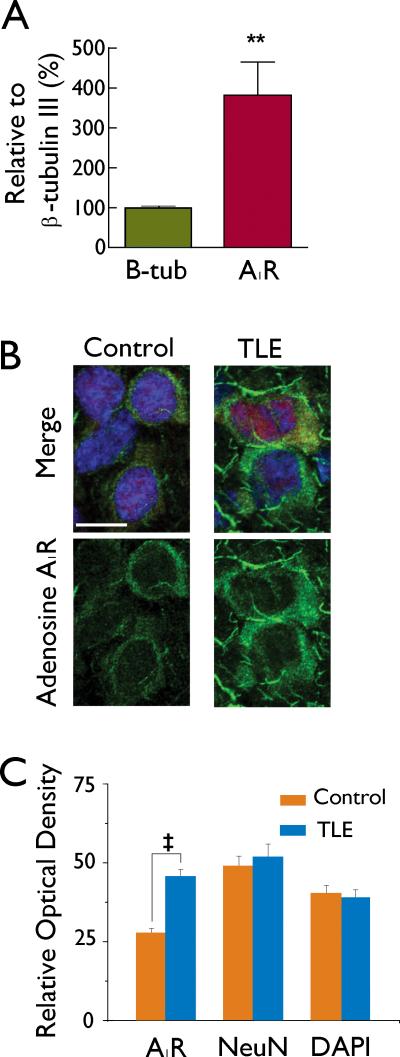
Adenosine A1 receptor mRNA and immunostaining is increased in TLE
In order to determine if the changes in the efficacy of adenosine in modulating neuronal excitability were due to increases in adenosine receptor message or expression, qPCR for the A1 receptor was performed on excised mEC layer II tissue from control and TLE brains. When normalized to β-tubulin III, there was a 378 ± 87% (n=9) increase in adenosine A1R message in mEC layer II in TLE tissue as compared to controls (P < 0.01; Figure 4A). To determine if increases in mRNA were associated with increases in adenosine A1 receptor expression levels, immunohistochemistry experiments were performed (Figure 4 B, C). Staining of the A1 receptor was intense around the peri-somatic region indicating membrane expression (Figure 4B). In comparison to control, staining intensity of the A1 receptor was increased approximately 1.6 fold in TLE tissue (P<0.001, Figure 4B, C). Staining intensities for the neuronal marker NeuN and the nuclear marker DAPI were not significantly different between control and TLE tissue, and NeuN was used to normalize A1 receptor staining. Ratios of A1/NeuN were increased from 0.71 ± 0.05 (n = 41) in control tissue to 1.17 ± 0.09 (n = 22, P < 0.001) in TLE tissue. The increase in A1 receptor staining in TLE sections indicate an up-regulation of the A1 receptor in TLE and could be involved in mediating the greater inhibitory actions of adenosine on membrane excitability in TLE neurons reported here.
Figure 4.
mEC layer II adenosine A1 receptor expression is increased in TLE. A: qPCR of the adenosine A1 receptor isoform on excised mEC layer II tissue from control and TLE tissue reveals a 387 % increase in the adenosine A1 receptor subtype as compared to control levels. B: Immunolabelling intensity for the adenosine A1 receptor (green, lower traces) are increased in mEC layer II TLE tissue as compared to control tissue while immunolabelling intensity for NeuN (red, shown in Merge) and DAPI (blue, shown in Merge) remain unchanged. Scale bar is 20 μM. C: Bar chart showing the cumulative relative optical density of immunostaining for all cells examined. Bars represent means ± S.E.M. t P<0.001.
DISCUSSION
Medial entorhinal cortex layer II stellate neurons comprise the main input to the hippocampus via the perforant path, are spared in both humans with TLE and animal models of TLE (Bear et al., 1996) and become hyperexcitable in TLE (Hargus et al., 2011). This hyperexcitability is thought to lead to an increase in the excitatory drive into the DG and hippocampus (Buckmaster & Dudek, 1997) contributing to seizure initiation and spread. mEC layer II neurons could, therefore, represent an important site at which endogenous mechanisms to suppress the increased neuronal excitability are employed to prevent seizures from initiating or reducing seizure severity once fully evoked (Hosseinmardi et al., 2007). Here we report that adenosine can suppress the hyperexcitability of mEC layer II stellate neurons observed in TLE, exhibiting inhibitory effects on membrane excitability at concentrations that were ineffective in control neurons. These inhibitory events were reproduced by the A1 selective agonist CPA and blocked by the A1 selective antagonist DPCPX. In contrast, the A2A selective agonist CCG-21680 had no affect on membrane excitability and the actions of adenosine were not inhibited by the A2A selective antagonist ZM-241385, indicating that the inhibitory actions of adenosine were mediated via the A1 receptor and not the A2A receptor. Furthermore, we report an increase in A1 receptor mRNA levels and show increased somatic staining of the A1 receptor in TLE compared to control, indicating an acquired up-regulation of A1 adenosine receptors in epilepsy. Increased A1 receptors in TLE could account for the accentuated actions of adenosine in TLE neurons compared to control neurons. Nevertheless, we can not exclude the possibility that in addition to the increase in A1 receptor numbers, an enhancement of G-protein coupling to the adenosine receptors to downstream effectors is also enhanced in TLE (Daval & Werck, 1991a). Importantly, these findings suggest that in an animal model of chronic limbic epilepsy, adenosinergic control of mEC layer II stellate neurons is enhanced and may, therefore, serve as an endogenous compensatory mechanism to suppress membrane hyperexcitability, preventing seizure initiation or reducing seizure duration within the temporal lobe.
Adenosine's anticonvulsant actions have been previously documented using a variety of different animal models of seizures (Dunwiddie & Haas, 1985; Dunwiddie & Worth, 1982; Boison et al., 1999). In support of our findings, these actions were shown to be primarily mediated via activation of the A1 receptor (Zeraati et al., 2006; Mohammad-Zadeh et al., 2005; De Sarro et al., 1999). In contrast, activation of A2a receptors were found to cause mostly proconvulsant actions (Hosseinmardi et al., 2007; Zeraati et al., 2006), with a few exceptions (De Sarro et al., 1999; Huber et al., 2002). One possibility for this discrepancy is the partially loss or down regulation of adenosine A2A receptors as a result of seizures previously reported following hippocampal kindling (Aden et al., 2004). Furthermore, since A2A receptors have a lower affinity for adenosine than the A1 receptors (Fredholm et al., 2001) it is possible that A2A receptor activation is not achieved at concentrations of adenosine applied in the different studies. Although entorhinal cortex neurons are thought to express both A1 and A2A receptors (Hosseinmardi et al., 2007; Rivkees et al., 1995; Lopes et al., 2004) the use of an A2A agonist (CGS-21680) and antagonists (ZM -241385) at concentrations sufficient to activate/antagonize the specific receptors in our study (Jarvis et al., 1989; Poucher et al., 1995) had no effect on membrane excitability in either control or TLE neurons.
Epileptic seizures are associated with an increase in adenosine levels in both animal models of epilepsy (Berman et al., 2000) and in human patients (During & Spencer, 1992). An increase in adenosine availability coupled with an increase in the receptor density would further potentiate the inhibitory actions of adenosine. In human patients with TLE both increases (Angelatou et al., 1993), and decreases (Glass et al., 1996) in A1 receptor expression have been reported in the temporal neocortex. In animal model studies, both kindling (Rebola et al., 2003) and kainic-acid (Ekonomou et al., 2000) induced seizures were associated with a decrease in adenosine A1 receptor density within the rat hippocampus, an another area associated with neuronal loss. Since A1 receptors are mostly expressed on pyramidal neurons within the hippocampus, this discrepancy in A1 receptor density could be an influenced by the significant loss of pyramidal neurons within the hippocampus in TLE (Engel, 1996). In contrast, mEC layer II neurons are spared in both animal models of TLE and humans (Bear et al., 1996; Du et al., 1993) and would, therefore, retain their A1 receptors. Here we report a nearly 3.8-fold increase in A1 receptor mRNA that was normalized to tubulin III to account for neuronal loss and a nearly 1.6-fold increase in A1 receptor immunostaining suggesting a significant increase in A1 receptor expression in TLE.
In summary, we report here that adenosine has profound inhibitory effects on neuronal excitability in mEC layer II stellate neurons from TLE neurons compared to control neurons. A potential mechanism for this enhanced inhibitory effect could be the up-regulation of adenosine A1 receptors in TLE, the receptor subtype primarily responsible for suppression of neuronal activity. Since the EC is heavily implicated in the initiation and generation of epileptic seizures, these studies provide a rationale for the selective targeting of adenosine A1 receptors, through receptor agonists or allosteric enhancers (Pietra et al., 2010) as a new treatment strategy for the treatment of temporal lobe epilepsy.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health-National Institutes of Neurological Disorders and Stroke grants R21NS061069 (MKP & EHB), The Epilepsy Foundation Predoctoral Research Fellowship and 1F31NS064694 NINDS (NJH). We would like to thank John Williamson for expert technical assistance.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest.
Ethical Publication
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Data S1: Induction of TLE Procedure
Data S2: Action Potential Analysis
Data S3: qPCR Experimental Procedure
Data S4: Immunohistochemistry Experimental Procedure.
Reference List
- Aden U, O'Connor WT, Berman RF. Changes in purine levels and adenosine receptors in kindled seizures in the rat. Neuroreport. 2004;15:1585–1589. doi: 10.1097/01.wnr.0000133227.94662.c9. [DOI] [PubMed] [Google Scholar]
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- Angelatou F, Pagonopoulou O, Maraziotis T, Olivier A, Villemeure JG, Avoli M, Kostopoulos G. Upregulation of A1 adenosine receptors in human temporal lobe epilepsy: a quantitative autoradiographic study. Neurosci Lett. 1993;163:11–14. doi: 10.1016/0304-3940(93)90217-9. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute J, Pinto A, Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci. 2006;24:135–144. doi: 10.1111/j.1460-9568.2006.04894.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Khalil M, Wendling F, Sontheimer A, Regis J, Ranjeva JP, Guye M, Chauvel P. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: An electrophysiologic and volumetric study. Epilepsia. 2005;46:677–687. doi: 10.1111/j.1528-1167.2005.43804.x. [DOI] [PubMed] [Google Scholar]
- Bear J, Fountain NB, Lothman EW. Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol. 1996;76:2928–2940. doi: 10.1152/jn.1996.76.5.2928. [DOI] [PubMed] [Google Scholar]
- Berman RF, Fredholm BB, Aden U, O'Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 2000;872:44–53. doi: 10.1016/s0006-8993(00)02441-0. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett J. The Ontogeny of Seizures in A Rat Model of Limbic Epilepsy - Evidence for A Kindling Process in the Development of Chronic Spontaneous Seizures. Brain Res. 1993;625:295–300. doi: 10.1016/0006-8993(93)91071-y. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: From therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Tseng JL, Aebischer P, Mohler H. Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer. Exp Neurol. 1999;160:164–174. doi: 10.1006/exnr.1999.7209. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol. 1997;77:2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: Corticocortical connectivity. Parahippocampal Region. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Daval JL, Werck MC. Autoradiographic changes in brain adenosine A1 receptors and their coupling to G-proteins following seizures in the developing rat. Dev Brain Res. 1991;59:237–247. doi: 10.1016/0165-3806(91)90104-q. [DOI] [PubMed] [Google Scholar]
- De Sarro G, De Sarro A, Di Paola ED, Bertorelli R. Effects of adenosine receptor agonists and antagonists on audiogenic seizure-sensible DBA/2 mice. Eur J Pharmacol. 1999;371:137–145. doi: 10.1016/s0014-2999(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Dragunow M. Adenosine - the brains natural anticonvulsant. Trends in Pharmacol Sci. 1986;7:128–130. [Google Scholar]
- Du F, Whetsell WO, Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Regulation of extracellular adenosine in rat hippocampal slices is temperature dependent: Role of adenosine transporters. Neuroscience. 2000;95:81–88. doi: 10.1016/s0306-4522(99)00404-2. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus - evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Hoffer BJ. Adenine-nucleotides and synaptic transmission in the in vitro rat hippocampus. B J Pharmacol. 1980;69:59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Worth T. Sedative and Anticonvulsant Effects of Adenosine-Analogs in Mouse and Rat. J Pharmacol Exp Thera. 1982;220:70–76. [PubMed] [Google Scholar]
- During MJ, Spencer DD. Adenosine - A potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Sperk G, Kostopoulos G, Angelatou F. Reduction of A1 adenosine receptors in rat hippocampus after kainic acid-induced limbic seizures. Neurosci Lett. 2000;284:49–52. doi: 10.1016/s0304-3940(00)00954-x. [DOI] [PubMed] [Google Scholar]
- Engel J. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Glass M, Faull RL, Bullock JY, Jansen K, Mee EW, Walker EB, Synek BJ, Dragunow M. Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain Res. 1996;710:56–68. doi: 10.1016/0006-8993(95)01313-x. [DOI] [PubMed] [Google Scholar]
- Hargus NJ, Bertram EH, Patel MK. Adenosine A1 receptors presynaptically modulate excitatory synaptic input onto subiculum neurons. Brain Res. 2009;1280:60–68. doi: 10.1016/j.brainres.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus NJ, Merrick EC, Nigam A, Kalmar CL, Baheti AR, Bertram EH III, Patel MK. Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons. Neurobiol Dis. 2011;41:361–376. doi: 10.1016/j.nbd.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Zhang CL, Eder C. Entorhinal Cortex Hippocampal Interactions in Normal and Epileptic Temporal-Lobe. Hippocampus. 1993;3:89–97. [PubMed] [Google Scholar]
- Hosseinmardi N, Mirnajafi-Zadeh J, Fathollahi Y, Shahabi P. The role of adenosine A1 and A2a receptors of entorhinal cortex on piriform cortex kindled seizures in rats. Pharmacol Res. 2007;56:110–117. doi: 10.1016/j.phrs.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Huber A, Guttinger M, Mohler H, Boison D. Seizure suppression by adenosine A(2A) receptor activation in a rat model of audiogenic brainstem epilepsy. Neurosci Letts. 2002;329:289–292. doi: 10.1016/s0304-3940(02)00684-5. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS 21680, a selective A2a adenosine receptor agonist directly labels A2a receptors in rat brain. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- Jones RSG, Heinemann UFH, Lambert JDC. The entorhinal cortex and generation of seizure activity - Studies of Normal Synaptic Transmission and Epileptogenesis Invitro. Epilepsy Res. 1992;8:173–180. doi: 10.1016/b978-0-444-89710-7.50027-6. [DOI] [PubMed] [Google Scholar]
- Jutila L, Ylinen A, Partanen K, Alafuzoff I, Mervaala E, Partanen J, Vapalahti M, Vainio P, Pitkanen A. MR volumetry of the entorhinal, perirhinal, and temporopolar cortices in drug-refractory temporal lobe epilepsy. Am J Neuroradiol. 2001;22:1490–1501. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan S, Yan J, Li B, Chen F, Xia J, Yu Z, Hu Z. Adenosine modulates the excitability of layer II stellate neurons in entorhinal cortex through A1 receptors. Hippocampus. 2010 doi: 10.1002/hipo.20745. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. B J Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Zadeh M, Amini A, Mirnajafi-Zadeh J, Fathollahi Y. The role of adenosine A1 receptors in the interaction between amygdala and entorhinal cortex of kindled rats. Epilepsy Res. 2005;65:1–9. doi: 10.1016/j.eplepsyres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mohammad-Zadeh M, Mirnajafi-Zadeh J, Fathollahi Y, Javan M, Ghorbani P, Sadegh M, Noorbakhsh SM. Effect of low frequency stimulation of perforant path on kindling rate and synaptic transmission in the dentate gyrus during kindling acquisition in rats. Epilepsy Res. 2007;75:154–61. doi: 10.1016/j.eplepsyres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra D, Borghini A, Breschi MC, Bianucci AM. Enhancer and competitive allosteric modulation model for G-protein-coupled receptors. J Theoretic Biol. 2010;267:663–675. doi: 10.1016/j.jtbi.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PW, Jones G, Coll MG. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2A selective adenosine receptor antagonist. Br J Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- Rebola N, Rodrigues RJ, Lopes LV, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localized in glutamatergic nerve terminals of the rat hippocampus. Neuroscience. 2005;133:79–83. doi: 10.1016/j.neuroscience.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine Receptors in rat-brain with emphasis on localization in the hippocampal-formation, cerebral-cortex, cerebellum, and basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurons. J Physiol. 1991;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Schubert P. Adenosine depression of hippocampal-neurons in vitro - an intracellular study of dose-dependent actions on synaptic and membrane-potentials. Neurosci Lett. 1981;23:55–60. doi: 10.1016/0304-3940(81)90186-5. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal-lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of projections from entorhinal area to hippocampal formation of rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Jackson MB. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci USA. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardas J. Neuroprotective role of adenosine in the CNS. Pol J Pharmacol. 2002;54:313–326. [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal hippocampal system - a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Dasilva FHL, Lohman AHM. Functional-organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Zeraati M, Mirnajafi-Zadeh J, Fathollahi Y, Namvar S, Rezvani ME. Adenosine A1 and A2A receptors of hippocampal CA1 region have opposite effects on piriform cortex kindled seizures in rats. Seizure. 2006;15:41–48. doi: 10.1016/j.seizure.2005.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.