Summary
Purpose
sec-Butyl-propylacetamide (SPD) is a one-carbon homologue of valnoctamide (VCD), a CNS-active amide derivative of valproic acid (VPA) currently in phase II clinical trials. The current study evaluated the anticonvulsant activity of SPD in a battery of rodent seizure and epilepsy models and assessed its efficacy in rat and guinea pig models of status epilepticus (SE) and neuroprotection in an organotypic hippocampal slice model of excitotoxic cell death.
Methods
SPD’s anticonvulsant activity was evaluated in several rodent seizure and epilepsy models including: maximal electroshock (MES), 6Hz psychomotor, subcutaneous (s.c.) metrazol-, s.c., picrotoxin, s.c. bicuculline, audiogenic and corneal and hippocampal kindled seizures following intraperitoneal administration. Results obtained with SPD are discussed in relationship to those obtained with VPA and VCD. SPD was also evaluated for its ability to block benzodiazepine-resistant SE induced by pilocarpine (rats) and soman (rats and guinea pigs) following intraperitoneal administration. SPD was tested for its ability to block excitotoxic cell death induced by the glutamate agonists N-methyl-D-Aspartate (NMDA) and kainic acid (KA) using organotypic hippocampal slices and SE-induced hippocampal cell death using FluoroJade B staining. The cognitive function of SPD-treated rats that were protected against pilocarpine-induced convulsive SE was examined 10-14 days post SE using the Morris water maze (MWM). The relationship between the pharmacokinetic profile of SPD and its efficacy against soman-induced SE was evaluated in two parallel studies following SPD (60 mg/kg, i.p.) administration in the soman SE rat model.
Key Findings
SPD was highly effective and displayed a wide protective index (PI=TD50/ED50) in the standardized seizure and epilepsy models employed. SPD’s wide PI values demonstrate that it is effective at doses well below those that produce behavioral impairment. Unlike VCD, SPD also displayed anticonvulsant activity in the rat pilocarpine model of SE. Thirty minutes after the induction of SE, the calculated rat-ED50 for SPD against convulsive SE in this model was 84mg/kg. SPD was not neuroprotective in the organotypic hippocampal slice preparation; however, it did display hippocampal neuroprotection in both SE models and cognitive sparing in the MWM which was associated with its antiseizure effect against pilocarpine-induced SE. When administered 20 and 40min after SE onset, SPD (100-174mg/kg) produced long-lasting efficacy (e.g., 4-8hr) against soman-induced convulsive and electrographic SE in both rats and guinea pigs. SPD-ED50 values in guinea pigs were 67mg/kg and 92mg/kg at when administered at SE onset or 40min after SE onset, respectively. Assuming linear PK, the PK-PD results (rats) suggests that effective SPD plasma levels ranged between 8-40mg/L (20 min post onset of soman-induced seizures) and 12-50mg/L (40 min post onset of soman-induced seizures). The time to peak (tmax) pharmacodynamic effect (PD-tmax) occurred after the PK-tmax thereby suggesting that SPD undergoes slow distribution to extra-plasmatic sites likely responsible for SPD’s antiseizure activity.
Significance
The results demonstrate that SPD is a broad-spectrum antiseizure compound that blocks SE induced by pilocarpine and soman and affords in vivo neuroprotection that is associated with cognitive sparing. Its activity against SE is superior to diazepam in terms of rapid onset, potency and its effect on animal mortality and functional improvement.
Keywords: New anticonvulsant drug, Status epilepticus (SE), pilocarpine-induced SE rat model, Soman-induced SE rat model, Pharmacokinetic-pharmacodynamic correlation
1.0 Introduction
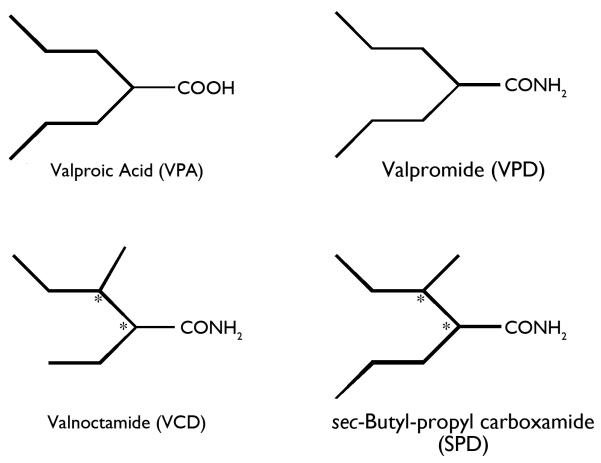
Since 1993, over a dozen new antiepileptic drugs (AEDs) have been brought to the market for the treatment of epilepsy. Each new therapy has provided substantial benefit to the patient in the way of improved seizure control, fewer adverse events, and/or more favorable pharmacokinetics. Despite the availability of several new AEDs, there still remains a significant need for patients with highly refractory epilepsy. In fact, it is estimated that 30% of the patients with epilepsy still suffer from uncontrolled seizures. All new AEDs for the symptomatic treatment of epilepsy have evolved from early evaluation in highly predictive animal seizure and epilepsy models (Bialer et al., 2004; Rogawski, 2006; Bialer & White, 2010). The investigational AED sec-butyl-propylacetamide (SPD, Fig 1), a one-carbon homologue of valnoctamide (VCD, Fig. 1), was submitted to the NINDS, NIH Anticonvulsant Screening Project in September 2008. Over the following 3 years, SPD was evaluated in a battery of rodent electrical and chemoconvulsant seizure models. The results from this investigation, reported herein, suggest that SPD possesses a unique and broad-spectrum antiseizure profile that is comparable to that of valproic acid.
Figure 1.
Chemical structures of valproic acid (VPA), valpromide (VPD), valnoctamide (VCD) and sec-butyl-propyl carboxamide (SPD).
SE is not a disease, but is a manifestation of an underlying central nervous system (CNS) insult or systemic pathology that affects CNS function. SE results when there is a failure of those inherent factors that would normally function to stop seizures. SE can result when there is a decrease in inhibition or an increase in excitation or a combination of both. Based on two large population-based studies in Richmond, VA (DeLorenzo et al., 1996) and Rochester, Minnesota (Hesdorffer et al., 1998) it is estimated that there are between 60,000 and 150,000 episodes of SE and 55,000 SE-related deaths each year. Between 4% and 16% of patients with epilepsy will have at least one episode of SE and one-third of the cases of SE occur as the presenting symptom in patients with a first unprovoked seizure (Hauser, 1990).
The prognosis of the patient with SE depends on the etiology, duration of SE, and age at time of presentation (Goodkin & Riviello, 2010). The mortality rate associated with SE ranges between 4% and 37% and is highly dependent on the age of the person, the presence of an acute precipitant, prior history of epilepsy, and a number of other factors, (see Goodkin and Riviello, 2010 for review and references). Although many people will survive an episode of SE with no, or only limited, untoward effects, SE is life-threatening and is associated with long-term neurologic sequela that include an elevated risk for developing epilepsy and substantial cognitive decline. Although controversial, non-convulsive SE is also associated with high morbidity and mortality (Krumholz et al., 1999).
Treatment of SE is aimed at controlling convulsive seizures as quickly as possible before compensatory mechanisms fail and the patient enters into a “refractory” state. The benzodiazepines (lorazepam and diazepam), phenytoin or its parenteral prodrug phosphenytoin, and phenobarbital are generally considered the first line anti-seizure drugs for the early treatment of SE. Second line anti-seizure drugs include i.v. valproic acid, i.v. levetiracetam, and i.v. lacosamide. SE can quickly become pharmacologically refractory when initial attempts to control the seizures fail despite adequate treatment. The patient with refractory SE is often placed into a drug-induced coma with an i.v. seizure suppressive agent to control both clinical and electrographic seizures. In addition to diazepam and lorazepam, other agents that might be considered for the patient in refractory SE include pentobarbital, midazolam, thiopental, propofol and ketamine. There is a clear need for more effective treatments for refractory SE that displays rapid onset and effective seizure control without producing dose-limiting sedation and respiratory depression. Further, the development of an effective therapy that attenuates refractory SE, offers some neuroprotective potential, and prevents the cognitive decline associated with SE would represent an important advance in the treatment of SE.
In addition to the recognized need for better treatments of status arising from traditional causes there are efforts underway to treat insults resulting in status from potential human exposure to chemical nerve agents. In 2006, the NIH-National Institute for Neurological Diseases and Stroke (NINDS)-Anticonvulsant Screening Program (ASP) initiated a multidisciplinary medical countermeasure screening effort designed to identify drugs effective in the treatment of medically refractory SE precipitated by exposure to nerve agents. As a proof of principle, several pilocarpine status models were employed to assess if compounds found effective in these models could also be used to treat status induced by actual chemical nerve agents (e.g. soman).
Beyond the traditional testing offered by the NIH-NINDS-ASP, SPD was evaluated for its ability to block excitotoxin-induced neurotoxicity in an organotypic cultured (rats) hippocampal slice preparation using the glutamate receptor ligands NMDA and kainic acid (KA). SPD was simultaneously examined in vivo in Sprague-Dawley rats for its ability to acutely interrupt diazepam-sensitive and diazepam-resistant convulsive SE-induced by lithium-pilocarpine. Based on the promising results obtained from this evaluation, SPD was submitted to more extensive evaluation to assess its ability to block the cognitive decline associated with pilocarpine-induced SE. SPD was then evaluated for its ability to prevent soman-induced SE at the U.S. Army Medical Research Institute of Chemical Defense (MRICD). The MRICD is well known for their ability to study the arrest of nerve agent (soman)-induced convulsive and non-convulsive SE and neuronal degeneration. In addition, SPD’s pharmacokinetic profile was evaluated following i.p. (60mg/kg) administration to rats and a pharmacokinetic-pharmacodynamic (protection against soman-induced seizures) correlation was performed. The results obtained with SPD provide proof-of-concept that a blinded collaborative multi-faceted approach can identify drugs that are effective against diazepam-resistant and soman-induced SE and preserve cognitive function following SE. The results with SPD using this approach demonstrate that SPD possesses a unique and more favorable profile compared to that obtained with the established anti-seizure drugs diazepam and valproic acid and provide the basis for this report.
2.0 Experimental Section: Materials and Methods
See Supporting Information
3 0 Results
3.1 Anticonvulsant Efficacy of SPD in Seizure and Epilepsy Models
As summarized in Table 1 SPD displayed a broad spectrum of anticonvulsant activity in a variety of seizure and epilepsy models including AGS-susceptible Frings mice, the MES, 6 Hz, scMet, scBic, and scPic seizure tests. SPD also displayed dose-dependent efficacy in two kindling models of partial seizures.
Table 1.
SPD anticonvulsant activity (in comparison to valnoctamide-VCD) in various mouse (ip) & rat (po) models for epilepsy
| Anticonvulsant test | SPD-ED50 (mg/kg) |
95% Confidence interval (CI- mg/kg) |
VCD-ED50 (mg/kg) |
|---|---|---|---|
| Frings Audiogenic Seizures | 20 | 18-22 | -a |
| Maximal electroshock seizure (mice-MES) |
71 | 55-90 | 58 |
| Maximal electroshock seizure (rats- MES) |
ip: 20 po:29 |
15-27 1 -53 |
po: 29 |
| Metrazol-induced seizure (mice-scMet) |
62 | 47-71 | 32 |
| Metrazol-induced seizure (rats-scMet) |
18 | 13–25 | 54 |
| Picrotoxin-induced seizure (mice-Pic) |
17 | 9 – 28 | - |
| Bicuculine-induced seizure (mice-Bic) |
94 | 87 – 103 | - |
| Corneal kindled mouse | 39 | 31-45 | - |
| Hippocampal kindled rats | 19 | 13-28 | ~40 |
| 6 Hz-32mA (mice) | 27 | 24 – 30 | 37 |
| Mice-Neurotoxicity (TD50) | 88 | 81-95 | 77 |
| Rat-Neurotoxicity (TD50) | ip: 49 po: 131 |
43-55 94-175 |
po:58 |
Not tested
In the two kindling models evaluated (i.e., the corneal kindled mouse and the hippocampal kindled rat), SPD produced a dose-dependent reduction in the secondarily generalized seizure (ED50=39 and 19 mg/kg, respectively) and seizure severity as estimated by the Racine seizure score. In the hippocampal kindled rat model, SPD produced a decrease in the seizure score from a pre-drug level of 4.6 to 1.3 at a dose of 32 mg/kg. This effect on seizure severity was also associated with a decrease in the afterdischarge duration from 39 ± 3.0 sec to 25.0 ± 4.0 sec. In the corneal kindled mouse, 60 mg/kg SPD decreased the seizure score from 5 to less than 1. Lastly, SPD was effective against refractory 6 Hz limbic seizures (ED50 =27 mg/kg). Collectively, these results suggest that SPD has the ability to decrease both the focal and the secondarily generalized seizure at doses that are devoid of behavioral toxicity.
In a separate study, SPD was found to produce a marked and significant increase in i.v. metrazol seizure threshold at the two dose-levels tested. At a dose equivalent to the MES-ED50 (70 mg/kg, i.p.) the threshold for first twitch and clonus was increased >200 and >600 fold, respectively. A slightly greater increase in seizure threshold was noted at a dose equivalent to the rotarod TD50 (Table S1).
Importantly, SPD was found to be active following two routes of administration (i.p. and oral) in two different rodent species (mouse and rat) at doses that did not impair motor function. The ED50 values ranged between 17-29 mg/kg. Furthermore, SPD was found to possess a wide safety margin or protective indexes (PI= TD50/ED50) ranging between 4.4-7.7.
3.2 SPD blocks convulsive seizures induced by cholinergic agonist, pilocarpine
Administration of lithium-pilocarpine induces SE characterized by convulsive and non-convulsive seizures that can last for several hours. SE is then followed by a latent phase, characterized by synaptic remodeling and neuronal plasticity, extensive neuronal loss and subsequent cognitive deficits and the precipitation of spontaneous recurrent seizures, the hall mark of epilepsy. From a behavioral perspective, the number and severity of the observed convulsive seizures following pilocarpine administration were similar in the two treatment groups (pilocarpine alone and pilocarpine + SPD). The first convulsive Stage 3 or greater seizure was observed 12 min after pilocarpine administration. Within the succeeding 30 minutes, rats were observed to have 4.8 4.9±0.2 seizures with an inter seizure interval of 3-5 minutes. On average, each convulsive seizure lasted for approximately 60 sec. SPD, administered 30 minafter the first observed stage 3 motor seizure (which was to mark the onset of SE), dose-dependently prevented the expression of further convulsive seizures in the pilocarpine + SPD group (ED50=84 mg/kg; Table 2). In those animals where SPD was observed to halt the convulsive seizure activity, onset was recorded as immediate and complete over the next 90 minutes of observation. The only other AED found to exert an effect when administered under the same experimental conditions was carbamazepine (ED50=50 mg/kg). The other comparator prototypical AEDs tested in this model, clonazepam, diazepam, valproic acid and phenobarbital were all ineffective at the highest dose tested; i.e., 40, 10, 300 and 40 mg/kg, respectively. VCD was equipotent to SPD when given at SE onset but in contrast to SPD VCD lost its activity when administered 30 min after the SE onset (Table 2).
Table 2.
Comparative efficacy of SPD, VCD and several prototypical AEDs against benzodiazepine-resistant convulsive seizures in the lithium-pilocarpine model of SE
| Compound Tested |
ED50 - mg/kg (95%CI) | |
|---|---|---|
| 0 min post SE onset | 30 min post SE onset |
|
| SPD | 8/8 protected at 65 mg/kg | 84 (62-103) |
| Valnoctamide (VCD) | 8/8 protected at 65 mg/kg | 0/8 at 80 mg/kg |
| Clonazepam* | 1.3 (0.6-2.5) | >40 mg/kg |
| Carbamazepine* | 45 (38 -52) | 50 (44-56) |
| Diazepam* | 3.0 (1.6 -4.4) | 0/8 at 10 mg/kg |
| Valproic acid* | 366 (23-575) | 0/8 at 300 mg/kg |
| Phenobarbital* | 31 (18-46) | 0/8 at 40 mg/kg |
Data on file with the Anticonvulsant Screening Project, NINDS, NIH. The 0 min post SE onset data are given only as comparators.
3.3 SPD prevents cognitive impairment associated with pilocarpine-induced SE
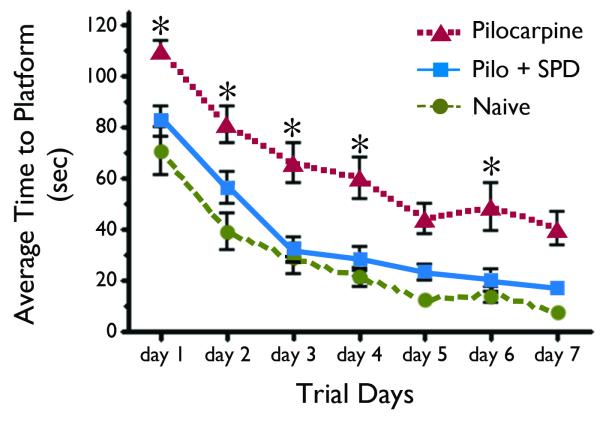
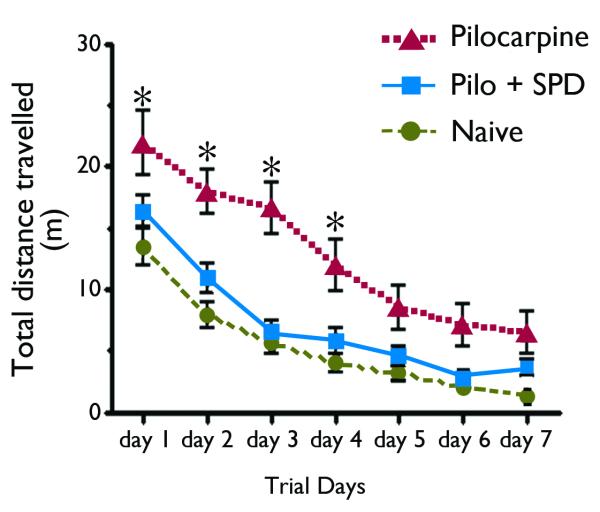
In the Morris water maze spatial memory and learning task, animals in all three treatment groups (non-SE naïve, pilocarpine-SE, and pilocarpine + SPD) groups displayed obvious learning as evidenced by a decrease in their escape latencies over the course of each training session (Figure 2). However, when compared to naïve non-SE and SPD-treated rats, rats in the non-treated pilocarpine-SE group took a longer time to find the platform (Figure 2), experienced a significantly higher number of missed platform encounters and traveled greater distance before finding the platform in both the hidden and visible trials (Figure 3). Together, these results confirm that SE leads to substantial cognitive decline as measured by impaired performance in the Morris water maze. Regardless of the outcome measure employed; time to reach the platform (Figure 2) or total distance travelled (Figure 3), rats that received SPD 30 min after the onset of SE performed better than those that received vehicle only. Indeed, the SPD-treated rats performed as well as naïve control rats; e.g., there was no statistically significant difference between the two groups (p > 0.05, one way ANOVA). The results from this study suggest that SPD treatment at a time when SE is normally refractory to the benzodiazepine diazepam (i.e., 30 min after the onset of SE) prevented the cognitive decline associated with pilocarpine-induced SE.
Figure 2.
SPD prevents lithium-pilocarpine SE-induced cognitive decline. Summarized results representing the average time (Mean ± SEM) required for rats to find the escape platform (latency) of rats trained in the Morris water maze. Trial days one through five consisted of four training sessions with a hidden platform and days six and seven consisted of four trials per day to find the visible platform using the acquired spatial map. There was a progressive decrease in escape latencies over the training days in all 3 groups. Animals in the naïve and drug-treated group (Pilo + SPD) learned to navigate quickly using the visual cues and there was no statistically significant difference between the two groups in their ability to find the escape platform. Pilocarpine-treated SE animals had a significantly higher escape latency and performed poorly in acquisition and retention of spatial memory, in comparison to the naïve control group and the SPD group *, p < 0.05, One-Way ANOVA with Newman-Keuls multiple comparison test.
Figure 3.
SPD treatment 30 min after the first convulsive seizure induced by lithium-pilocarpine decreased the total distance travelled in the Morris water maze. Results are expressed as the Mean ± SEM. Similar to the escape latency, there was a progressive decrease in the distance travelled by the rats in all 3 groups over the course of training. Animals in the naïve and Pilo + SPD group learned to directly swim towards the platform and spent most of their time in the quadrant where the platform was located. In contrast, pilocarpine-treated animals took a significantly longer time and traveled greater distance to find the escape platform (p < 0.05, One-Way ANOVA with Newman-Keuls multiple comparison test; * as compared to naïve and to the Pilo + SPD).
3.4 SPD protects against hippocampal cell death associated with pilocarpine-induced SE
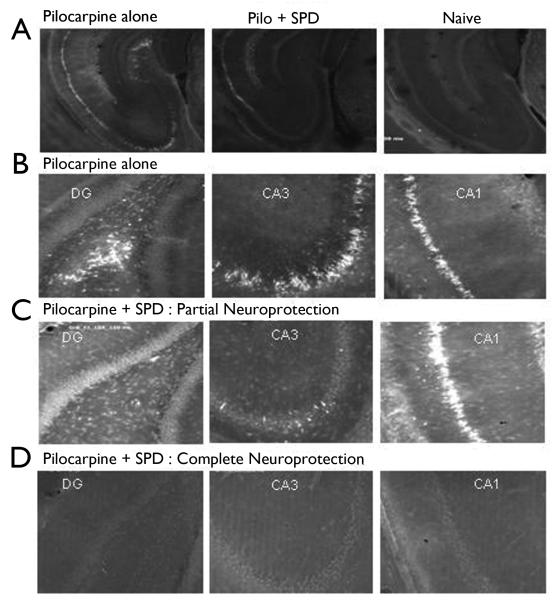
Pilocarpine-induced SE results in marked cell loss in the hippocampus (Figure 4), as evidenced by increased FluroJade B staining in the dentate gyrus (DG), CA1, and CA3 cell layers. Administration of SPD within 30 minutes of SE prevented the hippocampal cell death in a majority of animals (7/15). It is interesting to note that SPD showed variability in its neuroprotective effects: 47% of animals showed complete neuroprotection in all areas of hippocampus. 40% of animals show neuroprotection in CA3 and DG, while showing some damage in CA1 area, while 93% of animals showed complete neuroprotection of dentate hilar neurons. Only 13% of animals showed significant damage in CA1, CA3 or DG (Figure 4 C, D). However, irrespective of the neuronal damage observed, all of the SPD-treated rats showed significantly higher cognitive performance than rats in the pilocarpine SE group. In those animals where SPD was observed to halt the convulsive seizure activity, onset was recorded as immediate and complete over the next 90 min of observation.
Figure 4.
SPD attenuated hippocampal pathology associated with lithium-pilocarpine SE. Images of representative hippocampal sections (n = 24 in each group) stained for FluoroJade-B to determine the extent of cell death in naïve, and treated group of rats. A, B. Pilocarpine-induced convulsive SE resulted in substantial cell loss in the dentate gyrus (DG), CA1 and CA3 hippocampal neurons, as evidenced by increased fluorescence. C, D. SPD, when administered within 30 minutes of the first convulsive seizure (Stage 3 or higher) confered significant neuroprotection in the pyramidal cell layers. C, representative slices from rats showing partial neuroprotection, with significant cell death in CA1 neurons, while DG and CA3 neurons were protected. D, representative slices from animals showing full neuroprotection.
3.5 SPD did not prevent excitotoxic cell death induced by NMDA or Kainic acid (KA) in organotypic hippocampal slice cultures
In hippocampal slice cultures, exposure to NMDA (10 μM) or KA (20 μM) induced substantial cell death as determined by significant PI uptake after 24 hr. SPD was added to the slice cultures at 10 μM and 100 μM to assess the neuroprotective effect against each glutamate receptor agonist. No significant difference in the extent of cell death was observed with this compound with either concentration against either NMDA or KA (n=8).
3.6 SPD blocks electrographic and convulsive seizures induced by soman in rats and guinea pigs
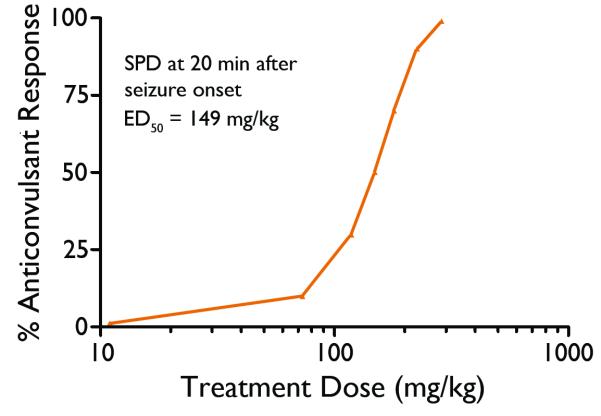
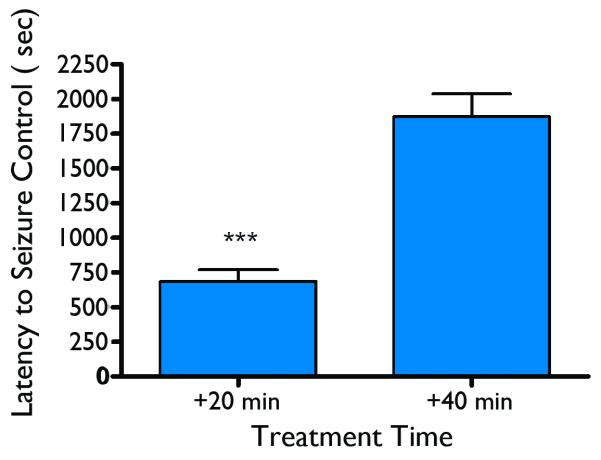
As discussed above, SPD was found to attenuate conclusive SE in the lithium-pilocarpine-SE rat model which closely resembles nerve agent-induced SE. In the rat nerve agent seizure model, SPD was administered at various doses along with the standard medical countermeasures at treatment delays of 20 or 40 min after the onset of soman-induced seizures to determine the effective dose for termination of soman-induced seizures. SPD was capable of stopping soman-induced seizures at both treatment delay times. The ED50 for seizure control at the 20-min treatment delay was calculated by probit analysis to be 149 mg/kg (p=0.06) (Figure 5) an ED50 dose for the 40-min treatment delay could not be calculated. Following administration of SPD at the 20-min treatment the average latency for seizure termination at the 20-min treatment delay time was 11.4± 1.4 min (SEM) while the seizure termination latencies for the 40-min treatment delay time group averaged 31.3±2.7min (SEM). The seizure termination latencies of the 20-min treatment delay group were significantly shorter than those of the 40-min treatment delay group (Figure 6). In these initial tests of SPD, the drug was suspended in 0.5% methyl cellulose. Following some pilot formulation studies, additional testing of SPD in rats was conducted using the multisol vehicle (a pure solution rather than a suspension). In limited tests at the 20-min treatment delay, the anticonvulsant ED50 = 71 mg/kg, p<0.05, and seizure termination latency = 389 sec (6.8 min). When compared to the study conducted using methylcellulose as the vehicle, results obtained using the multisol vehicle resulted in significant reductions in both measures of approximately 50%.
Figure 5.
Anticonvulsant dose-response curve of SPD administered 20 min after the onset of soman-induced seizures in rats.
Figure 6.
Latency for seizure control – the time from when SPD was administered to rats until the last epileptiform event could be detected on the EEG record. Asterisks indicate a significantly (p < 0.001) shorter latency at the 20-min treatment time than the 40-min treatment time.
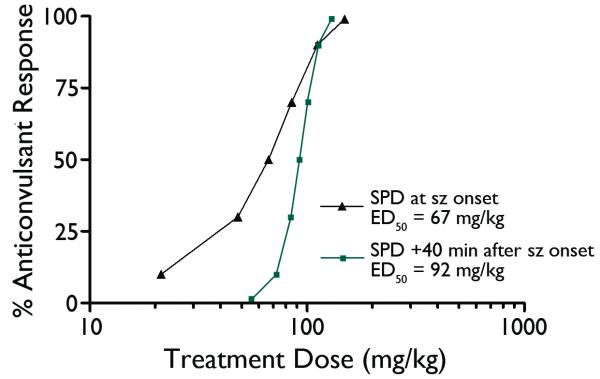
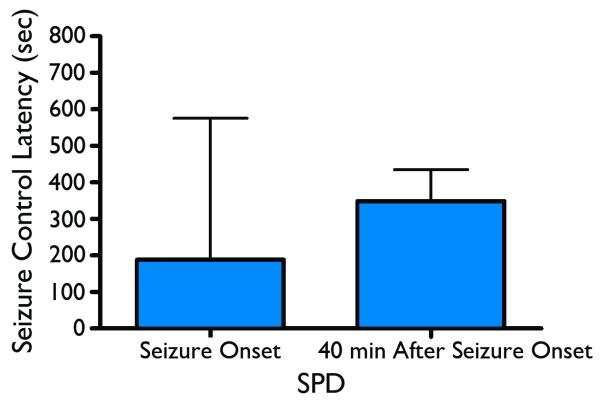
When SPD was tested in the guinea pig soman status model SPD administered at seizure onset was found to be effective with an ED50=67mg/kg; p=0.06; mean time seizure termination = 3.3 min from injection to spike termination. SPD administered at 40 min after seizure onset produced an anticonvulsant effect with an ED50= 92mg/kg; p=0.01; mean time to seizure termination equivalent to 5.8 min from injection to spike termination. These ED50 curves and seizure termination latencies are displayed in Figures 7 and 8. Figure 9 depicts the rapid anticonvulsant effect of SPD in the guinea pig model when given 40 min after SE onset.
Figure 7.
Probit-derived dose-effect curves for the anticonvulsant action of SPD at the two different test times in the guinea pig model.
Figure 8.
Latency for seizure control – the time from when SPD was administered to guinea pig until the last epileptiform event could be detected on the EEG record. Asterisks indicate a significantly (p < 0.001) shorter latency at the 0 min treatment time than the 40-min treatment time.
Figure 9.
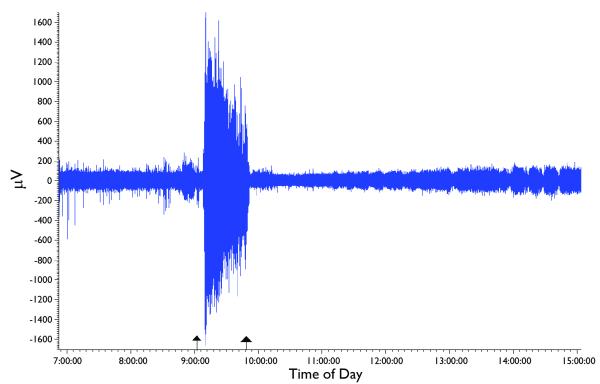
An example of the rapid anticonvulsant effect of SPD in the guinea pig model. Shown is a compressed EEG file of one animal, with time of day on the x-axis. This animal was administered soman at 9:02:54 AM (left arrow); seizures began at 9:08:19 and were allowed to continue for 40 min. SPD, 100 mg/kg (i.p.), was administered at 9:49:04 (right arrow) and seizure activity promptly terminated at 9:51:30 with no further indication of seizure activity throughout the rest of the recording session.
3.7 Effect of SPD on Soman-induced Histopathology in Rats
Following treatment with SPD, animals were categorized as either having seizure turned off (regardless of SPD dose) or not having seizures turned off. As depicted in Table 3, brains from these animals showed that seizure control prevented neuropathology, while animals that had seizures continued to display extensive neuropathology.
Table 3.
Number of animals and degree of neuropathology (mean neuropathology score) as a function of soman induced seizure control by SPD in rats
| No Neuropathology | Neuropathology | |
|---|---|---|
| Seizure Controlled | 38 (X = 0) | 2 (X=11.5) |
| Seizure Not Controlled | 2 (X = 0) | 29 (X = 17.5) |
Chi Square = 55.67, df = 3, p<0.0001
3.8 SPD pharmacokinetics in rats
The pharmacokinetics (PK) of SPD was studied following intraperitoneal administration (60 mg/kg) to naïve rats (not treated with soman). SPD’s water solubility is 1.5 mg/mL, therefore SPD was administered to rats in a solution of propylene glycol, alcohol and water for injection 5:1:4, where it solubility was increased to 27 mg/mL. The plasma concentration–time plots of SPD are presented in Figure S1. SPD PK parameters, calculated by non-compartmental analysis, are summarized in Table 3. SPD total clearance (CL) of 0.3 L/h was mainly metabolic with only 0.1% of the dose or CL being excreted unchanged in the urine. In rats SPD displayed a 7-fold higher CL than VCD and due to its higher lipophilicity SPD volume of distribution (V) was 3-times more than that of VCD (Blotnik et al., 1996). As a consequence of these opposite trends in CL and V, SPD half-life (t1/2) was similar to that of VCD. The dose was chosen as the intermediate dose among SPD various ED50 values, assuming linear pharmacokinetics. Rat liver blood flow is 60-70 ml/min (Altman and Dittmer, 1974). Assuming that SPD metabolism occurs primarily in the liver and that SPD blood-to-plasma ratio is about one, shows that SPD liver extraction ratio (E) is E=CL/Q=CLm/Q=20/65=0.3. If these rat data can be extrapolated in humans it may indicate that SPD might be slightly susceptible to hepatic first pass effect following oral dosing.
3.8 SPD pharmacokinetic-pharmacodynamic (PK-PD) correlation (rats)
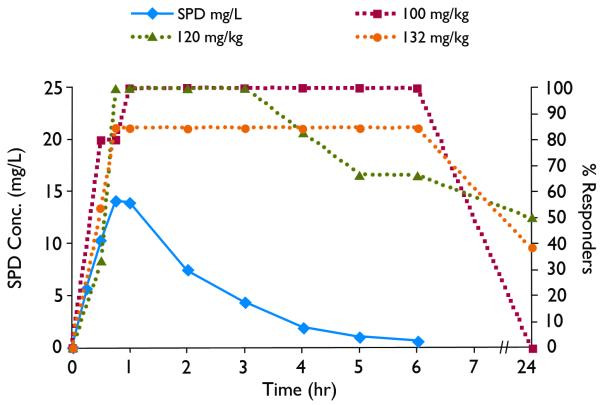
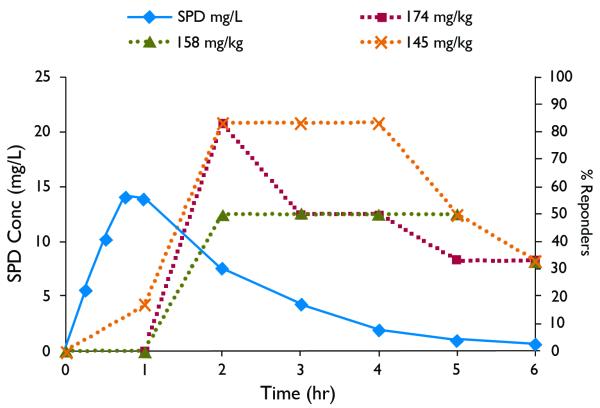
A pharmacokinetic-pharmacodynamic (PK-PD) correlation between SPD plasma profile and SPD activity in terminating soman-induced seizures at delay time of 20 min and 40 min is presented in Figures 10 and 11 When SPD (100-132 mg/kg) was administered at 20 min post (soman-induced) seizure onset, the maximal effect was reached at 30 min and lasted for 4-6hr (Fig. 10). When SPD (145-158 mg/kg) was administered at 40 min post (soman-induced) seizure onset, the maximal effect was reached at 1h and lasted for 3-5hr. Assuming linear PK the PK-PD correlation depicted in Figures 10 and 11 shows that SPD effective plasma levels ranged between 8-40 mg/L (20 min post seizure onset) and 12-50 mg/L (40 min post seizure onset). The time to peak effect (PD-tmax) occurred after the PK-tmax and may indicate slow distribution of SPD to the extra-plasmatic active site responsible for SPD activity. This slower distribution to active site may contribute to the fact that SPD effect (responders rate) declined significantly slower than SPD plasma levels and in a few rats at the 20min post seizure onset lasted for 24h.
Figure 10.
Pharmacokinetic-pharmacodynamic (PK-PD) correlation. Solid line indicates plasma concentrations following ip administration of SPD (60mg/kg) to rats. Dashed lines indicate % responders to SPD administered 20min after seizure onset, measured as the number of “protected” rats (rats without soman-induced seizures) divided by total number of rats tested at each time point.
Fig. 11.
Pharmacokinetic-pharmacodynamic PK-PD correlation. Solid line indicates plasma concentrations following ip administration of SPD (60mg/kg) to rats. Dashed lines indicate % responders to SPD administered 40min after seizure onset, measured as the number of “protected” rats (rats without soman-induced seizures) divided by total number of rats tested at each time point.
4.0 Discussion
4.1 SPD Displays Broad-Spectrum Antiseizure Activity in Rodent Seizure and Epilepsy Models
In the present investigation, we describe the acute antiseizure activity of a VPA amide analogue (or a VCD one-carbon homologue); i.e., SPD in a battery of traditional seizure and epilepsy models often employed in the search for novel AEDs. The results obtained from these investigations demonstrate that SPD, like VPA, possesses a broad-spectrum anticonvulsant profile in models of focal and generalized seizures. Moreover, SPD was found to be effective in the 6 Hz seizure model of pharmacoresistant epilepsy. Although little can be said about the molecular mechanism through which SPD exerts its acute antiseizure effects, the results from the anticonvulsant testing conducted thus far would support the conclusion that it exerts its effects through an ability to prevent seizure spread and elevate seizure threshold. This conclusion is based on the marked effect exerted by SPD in the mouse and rat MES test (seizure spread) and its ability to elevate seizure threshold in the i.v. Metrazol seizure threshold test. Further evidence supporting this conclusion is provided by the results obtained in the two kindling models of partial seizures; i.e., the hippocampal kindled rat and the corneal kindled mouse. In both of these animal models, SPD prevented the expression of secondarily generalized seizures (i.e., decreased the Racine seizure score from 5 to 1 or less). Consistent with this observation, SPD was found to decrease the afterdischarge duration in the hippocampal kindled rat; an indirect measure of seizure spread. The results at the whole animal level are not necessarily surprising given the close structural similarity between SPD and VPA. Ongoing studies at the cellular level will hopefully provide some insight into the specific molecular mechanisms underlying the pronounced antiseizure activity of SPD.
4.2 Anticonvulsant effects of SPD in animal models of SE
SE is initially treated with a benzodiazepine such as diazepam or lorazepam. Both are extremely effective when given early in SE; however, the benzodiazepines loose their efficacy when given after 30 minutes of spontaneous self-sustaining seizures; e.g., animals that experience prolonged SE quickly develop pharmacoresistant SE if treatment is not initiated within a short period of time (Jones et al., 2002). There is very little data concerning the treatment of refractory SE.
SPD was found to be a highly effective antiseizure drug in two distinctly different models of SE; i.e., the lithium-pilocarpine convulsive SE model and the soman-induced SE model. The results obtained in these two models demonstrate that SPD has the capability to arrest ongoing seizure activity when administered after 30 min in the lithium-pilocarpine-SE model and after 20 or 40 min in the soman-induced SE model.
4.2.1 Comparative effect of SPD in lithium-pilocarpine SE Model
As summarized in Table 1, SPD clearly differentiated itself in the lithium pilocarpine SE convulsive model from other AEDs that have been evaluated under identical conditions; i.e. diazepam, clonazepam, valproic acid, phenobarbital, and VCD. The only other traditional AED that has displayed a similar antiseizure effect in the lithium-pilocarpine model is carbamazepine. Carbamazepine halted the progression of convulsive SE when administered 30 min after the first observable seizure with an ED50 of 50 mg/kg. However, carbamazepine’s antiseizure effect is not correlated with a similar degree of neuroprotection and/or cognitive sparing (Alex and White, unpublished data) to that observed with SPD (discussed below).
4.2.2 SPD activity in soman-induced SE in rats and guinea pigs
The time required for seizure control in the soman-rat model following SPD treatment at the 20-min treatment delay time was 11.4 min. In general, SPD provided faster seizure control against soman-induced seizures than the anticholinergics or benzodiazepines under comparable conditions (McDonough & Shih, 1993; Shih et al., 1999). Also, the increase in the latencies for seizure control with a longer treatment delay (e.g., 40 min vs 20 min) is commonly observed with other drugs in this model. However, it seems clear that the vehicle in which SPD was prepared (0.5% methyl cellulose vs multisol) had a significant effect on the ED50 and latency for seizure control in the rat model; e.g., the multisol vehicle significantly enhanced the pharmacodynamic effect of the drug. Based on our previous studies (McDonough & Shih, 1993; McDonough et al., 2000; Shih et al., 1999), very few drugs show the rapid anticonvulsant effectiveness against soman-induced seizures in rats or guinea pigs at long (40 min) treatment delays as was seen with SPD. Similar to the neuroprotective effects seen in the lithium-pilocarpine SE model, control of soman-induced seizures with SPD successfully protected animals against the severe neuropathology that is typically observed in the brains of animals exposed to nerve agents (Carpentier et al., 1990; Lallement et al., 1994; McDonough et al., 1995).
SPD is a water-insoluble compound and therefore in the rat study it was suspended in 0.5% methyl cellulose (MC). The i.p. injection of a MC SPD suspension into an animal using anything <18 gauge needle proved problematic. While other solvents were not tested in the rats it can be presumed that administering SPD in a pure solution (multisol, like in the guinea pig study) could only help absorption and improve the SPD anticonvulsant (pharmacodynamic) response. In summary, SPD was effective against generalized SE seizures induced by the nerve agent soman in the rat and guinea pigs model when treatment was delayed 20 min and 40 min after seizure onset. SPD showed a unique ability to immediately stop soman-induced devastating seizures even at the 40-min treatment delay. In contrast to other AEDs that have been studied using this model, SPD was successful (even after a 40 min delay time) in terminating soman-induced seizures.
It was noted that many of the animals treated with SPD had an initial anticonvulsant effect (i.e., the seizures stopped), but that later in the day, or more typically, by the recording session the next morning, the seizures had returned. This occurred almost exclusively when SPD was formulated with the 0.5% methylcellulose. The rats in which the seizures were terminated by SPD displayed no neuropathology, while animals in which the treatment was not successful in stopping the seizures displayed significant levels of neuropathology in those brain areas susceptible to nerve agent-induced damage.
4.3 SPD attenuates hippocampal sclerosis
Prolonged SE and chronic epilepsy is associated with marked hippocampal sclerosis, spontaneous seizures, and progressive cognitive decline (Loscher & Brandt, 2010; Mazarati et al, 2004). Furthermore, the frequency, duration and severity of seizures is variably associated with greater risk of cognitive impairment (Meador, 2002). Using an acute animal model of SE, the NIH-NINDS-ASP was able to identify a novel drug candidate; i.e., SPD, that possesses substantial antiseizure activity against benzodiazepine-resistant SE that was subsequently shown to prevent SE-induced hippocampal sclerosis in both the lithium pilocarpine and soman models and cognitive impairment in the lithium-pilocarpine model of SE.
Diazepam has been found to be effective in preventing neuronal damage in SE (Ben-Ari et al., 1980; Pitkanen et al., 2005). However, our studies have shown that diazepam (20 mg/kg, i.p.) is most effective, when given early after the onset of pilocarpine-induced SE and the neuroprotective effect wanes after 30 min of uninterrupted SE (Alex & White, unpublished data). It is also important to note that diazepam’s apparent neuroprotective properties are almost exclusively associated with its ability to interrupt SE; i.e., insult modification. In the current study, SPD (130 mg/kg, i.p.) when administered after 30 min of pilocarpine-induced SE, significantly reduced the hippocampal neuronal loss in a majority of animals when sacrificed 3-4 weeks after the initial insult. Bolanos et al. (1998) have shown that VPA (600 mg/kg, twice a day, for 1 month) reduced cell death in CA1 damage, but did not protect CA3 or dentate hilar neurons. Considering all the available data so far, a single dose of SPD exhibits superior neuroprotection over VPA and other standard AEDs when administered shortly after SE induction (Brandt et al., 2006; Bolanos et al., 1998; Klitgaard et al., 2001; Francois et al., 2006; Zheng et al., 2010). As discussed in the preceding paragraph, the ability of SPD to prevent hippocampal neuronal cell loss was most likely due to its acute anticonvulsant effect (insult modification); and as discussed below, not a result of any inherent neuroprotective properties.
In contrast to MK-801 which is neuroprotective in vitro in organotypic hippocampal slice cultures and in vivo when administered 30 min after SE onset (Kristensen et al., 2001; Lee et al., 1997), SPD was only neuroprotective in vivo. In contrast to SPD, MK-801 did not display substantial antiseizure effects when administered 30 min after SE onset. These results suggest that the neuroprotective action of MK-801 is related to its activity as a NMDA receptor antagonist; whereas, the neuroprotective effects observed in SPD-treated rats is more likely the result of its marked antiseizure activity in vivo. These results demonstrate the benefit associated with early intervention of SE with a highly effective antiseizure agent.
4.4 Early Treatment with SPD Mitigates Lithium Pilocarpine SE-induced cognitive impairment
Previous investigations have demonstrated that pilocarpine-induced SE induces neuronal loss, particularly in the hippocampal subfields CA1, CA3 and dentate hilus, chronic spontaneous seizures, and long term deficits in learning, memory (Mello, et al., 1993; Cunha et al., 2009). Place navigation in Morris water maze requires place representations or cognitive maps, and the hippocampus is thought to be critical for computing place representations. The present study evaluated the ability of SPD to prevent the short- and long- term consequences associated with experimentally-induced SE; e.g., hippocampal sclerosis and SE-induced learning deficits.
Hippocampal neuronal preservation has a direct relation to the functional outcome in patients with epilepsy (Meador, 2002). While, transient disruption of cognitive encoding may occur with paroxysmal focal or generalized epileptic discharges, epileptogenesis-related neuronal plasticity, reorganization, and sprouting may contribute to the progressive cognitive decline following brain injury induced by SE and other brain insults (Pitkanen et al., 2002; Hamed, 2009). There are only limited studies that compare the long-term functional outcome of SE and behavioral responses. Data from experimental models of SE indicate that AEDs preventing hippocampal damage usually afford protection against SE-induced behavioral responses (Bolanos et al., 1998; Cha et al., 2002). Reduction of cell loss in VPA-treated rats has been correlated with an increased performance in visuospatial learning and memory tasks in KA-induced SE rats, whereas phenobarbital did not prevent the hippocampal damage nor the cognitive impairment associated with SE (Bolanos et al., 1998). However, in another study VPA did not improve the performance of rats in Morris water maze (Brandt et al., 2006) and it has been suggested that the VPA-induced cognitive deficits may be due to its negative effect on hippocampal neurogenesis (Umka et al., 2010). Interestingly, clinical studies have not demonstrated any postitive effect with VPA on epileptogenesis or on cognition in patients with traumatic brain injury (Temkin et al. 1999). Topiramate a broad-spectrum AED is neuroprotective, but only partially prevented pilocarpine-induced cognitive impairment (Cha et al., 2002; Frisch et al., 2007). Compared to the available data obtained with a number of currently available AEDs, SPD prevented visuospatial learning and memory deficits induced by pilocarpine-SE, without causing any motor or behavioral toxicity. Again, the present results support the value of early treatment of SE with an effective antiseizure drug such as SPD in preventing neuronal death and ultimately preserving cognitive function. Regardless of the mechanism responsible for this outcome, the goal is to provide the patient with therapies that improve their overall quality of life.
5.0 Summary
SPD was found to be a highly effective anticonvulsant in a battery of well-defined acute and chronic seizure models and may represent a novel alternative for the symptomatic treatment of epilepsy. Corroborating results obtained in two different SE models (i.e., lithium-pilocarpine and soman) using two different species (rats and guinea pigs) demonstrate that SPD is extremely effective in preventing pilocarpine or soman-induced benzodiazepine-resistant seizures while offering neuroprotection and cognitive sparing. These effects suggest that SPD may offer long-term functional benefit following SE.
In the interest of developing a truly antiepileptogenic therapy, it must be recognized that effective suppression of all early seizures, even nonclinical seizures, may interfere with the process of epileptogenesis. Thus, control of symptomatic seizures following SE or other brain injuries with an effective drug therapy may also contribute to disease modification or antiepileptogenesis (Dichter, 2009). SPD, a novel VPA amide derivative, was effective in blocking behavioral seizures induced by pilocarpine or soman. The fact that SPD is a very close homologue of VCD that has >40 years of clinical experience in Europe contributes to SPD’s promising clinical potential as an anti-nerve gas agent and new antiepileptic and CNS drug that is effective following oral and parenteral administration.
Lastly, the early identification of the marked antiseizure effect of SPD in both the lithium-pilocarpine and soman models of SE was the result of a unique collaborative effort among different government agencies (the NIH and the U.S. Army’s MRICD) and the actual owners of the candidate compounds represented by either academia or various pharma groups, or in this case The Hebrew University of Jerusalem, is an example of a new type of collaboration. Such partnerships are a testament to how future drug development will likely be performed. Crafting “win-win” relationships with previously unlikely partners is becoming more common and even essential for the successful translation of new therapeutics.
Supplementary Material
Table 4.
PK parameters of SPD obtained following ip administration (60 mg/kg) to rats
| Parameter | SPD (ip) |
|---|---|
| t1/2 (h) | 0.84 |
| CL/F (L/h/kg) | 1.9 |
| AUC (mg/L×h) | 32.1 |
| AUMC (mg/L×h2) | 61 |
| MRT (h) 1.9 Vz/F (L/kg) | 2.3 |
| Cmax (mg/L) | 14.1 |
| tmax (h) | 0.75 |
| fe (%) | 0.1 |
t1/2, half-life; CL, total clearance; CL/F, extravascular (oral) clearance calculated after extravascular (ip) dosing; AUC, area under plasma drug concentration-time curve; AUMC, area under the first moment curve; MRT, mean residence time; Vz, volume of distribution based on linear terminal slope; Vz/F, apparent volume of distribution calculated after extravascular dosing; Cmax, peak plasma drug concentration; tmax, time to reach Cmax; fm, fraction metabolized of the systemically available drug ; fe, fraction of the systemically available drug excreted unchanged in the urine.
Acknowledgements
Studies conducted at the Univ. of Utah were supported by NINDS, NIH Contract No. NO1-NS-4-2359 (HSW, ABA, KSW, ALP). The studies conducted at MRICD were supported by an Inter-Agency Agreement between NIH/NINDS (Y1-O6-9613-01) and USAMRICD (A120-B.P2009-2) and the Defense Threat Reduction Agency – Joint Science and Technology Office, Medical S&T Division. The views expressed in this paper are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government.
Footnotes
Disclosure/ Conflict of Interest Dr. Meir Bialer has received in the last three years speakers or consultancy fees from Bial, CTS Chemicals, Desitin, Janssen-Cilag, Johnson & Johnson, Medgenics, Rekah, Sepracor, Teva, UCB Pharma and Upsher-Smith. In the last five years, the author received research grants from Jazz Pharmaceuticals, Johnson & Johnson and The Epilepsy Therapy Development Project and has been involved in the design and development of new antiepileptics and CNS drugs as well as new formulations of existing drugs.
Dr. H. Steve White is the Director of the University of Utah Anticonvulsant Drug Development Program and Principal Investigator of the NINDS, NIH Contract that funded the University of Utah Research reported herein. Dr. White also wishes to disclose that in the last three years he has served as a paid consultant to Johnson & Johnson Pharmaceutical Research and Development, GlaxoSmithKline, Valeant Pharmaceuticals, Eli Lilly & Co., and Upsher-Smith Laboratories, Inc., is a member of the UCB Pharma Speakers Bureau, the NeuroTherapeutics Pharma Scientific Advisory Board, has received research funding from NeuroAdjuvants, Inc.. Lastly, Dr. White is one of two scientific co-founders of NeuroAdjuvants, Inc., Salt Lake City, UT.
None of the other authors has any conflict of interest to disclose.
We, the authors, confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Alex AB, Saunders GW, Dalpé-Charron A, Reilly CA, Wilcox KS. CGX-1007 prevents excitotoxic cell death via actions at multiple types of NMDA receptors. Neurotoxicology. 2011;32:392–399. doi: 10.1016/j.neuro.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman PL, Dittmer DS. Federation of American Societies for Experimental Biology. 2nd ed vol. 3. Bethesda, MD: 1974. Biological Data Book; pp. 1702–1710. [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Bersudsky Y, Applebaum J, Gaiduk Y, Sharony L, Mishory A, Podberezsky A, Agam G, Belmaker RH. Valnoctamide as a valproate substitute with low teratogenic potential in mania: a double-blind, controlled, add-on clinical trial. Bipolar Disord. 2010;12:376–382. doi: 10.1111/j.1399-5618.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92:89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bialer M, Twyman RE, White HS. Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav. 2004;5:866–872. doi: 10.1016/j.yebeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- Bliss C. The statistics of bioassay with special reference to the vitamins, in Vitamin Methods. Academic Press; New York: 1952. [Google Scholar]
- Bolanos AR, Sarkisian M, Yang Y, Hori A, Helmers SL, Mikati M, Tandon P, Stafstrom CE, Holmes GL. Comparison of valproate and phenobarbital treatment after status epilepticus in rats. Neurology. 1998;51:41–48. doi: 10.1212/wnl.51.1.41. [DOI] [PubMed] [Google Scholar]
- Brandt C, Gastens AM, Sun M, Hausknecht M, Löscher W. Treatment with valproate after status epilepticus: effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology. 2006;51:789–804. doi: 10.1016/j.neuropharm.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Carpentier P, Delamanche IS, Le Bert M, Blanchet G, Bouchaud C. Seizure-related opening of the blood-brain barrier induced by soman: possible correlation with the acute neuropathology observed in poisoned rats. Neurotoxicology. 1990;11:493–508. [PubMed] [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002;51:217–232. doi: 10.1016/s0920-1211(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Cunha AO, Mortari MR, Liberato JL, dos Santos WF. Neuroprotective effects of diazepam, carbamazepine, phenytoin and ketamine after pilocarpine-induced status epilepticus. Basic Clin Pharmacol Toxicol. 2009;104:470–477. doi: 10.1111/j.1742-7843.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, Garnett L, Fortner CA, Ko D. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- Dichter MA. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol. 2009;66:443–447. doi: 10.1001/archneurol.2009.10. [DOI] [PubMed] [Google Scholar]
- Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Statisical logic in the monitoring of reactions to therapeutic drugs. Methods Inf Med. 1971;10:237–245. [PubMed] [Google Scholar]
- François J, Koning E, Ferrandon A, Nehlig A. The combination of topiramate and diazepam is partially neuroprotective in the hippocampus but not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2006;72:147–163. doi: 10.1016/j.eplepsyres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Frisch C, Kudin AP, Elger CE, Kunz WS, Helmstaedter C. Amelioration of water maze performance deficits by topiramate applied during pilocarpine-induced status epilepticus is negatively dose-dependent. Epilepsy Res. 2007;73:173–180. doi: 10.1016/j.eplepsyres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Goodkin H, Riviello J., Jr . Status Epilepticus. In: Wyllie E, editor. Wyllie’s treatment of epilepsy: principles and practice. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 469–485. [Google Scholar]
- Hamed SA. The aspects and mechanisms of cognitive alterations in epilepsy: the role of antiepileptic medications. CNS Neurosci Ther. 2009;15:134–156. doi: 10.1111/j.1755-5949.2008.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Rich SS, Annegers JF, Anderson VE. Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163–1170. doi: 10.1212/wnl.40.8.1163. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50:735–741. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 2002;50:301–312. doi: 10.1016/s0920-1211(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, Bialer M, Shimshoni JA, Devor M, Yagen B. Synthesis and evaluation of antiallodynic and anticonvulsant activity of novel amide and urea derivatives of valproic acid analogues. J Med Chem. 2009;52:7236–7248. doi: 10.1021/jm901229s. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: the preclinical profile of a new class of antiepileptic drugs? Epilepsia. 2001;42(Suppl 4):13–18. [PubMed] [Google Scholar]
- Kristensen BW, Noraberg J, Zimmer J. Comparison of excitotoxic profiles of ATPA, AMPA, KA and NMDA in organotypic hippocampal slice cultures. Brain Res. 2001;917:21–44. doi: 10.1016/s0006-8993(01)02900-6. [DOI] [PubMed] [Google Scholar]
- Krumholz A. Epidemiology and evidence for morbidity of nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16:314–322. doi: 10.1097/00004691-199907000-00003. discussion 353. [DOI] [PubMed] [Google Scholar]
- Lallement G, Pernot-Marino I, Baubichon D, Burckhart MF, Carpentier P, Blanchet G. Modulation of soman-induced neuropathology with an anticonvulsant regimen. Neuroreport. 1994;5:2265–2268. doi: 10.1097/00001756-199411000-00015. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chou JY, Lee KH, Choi BJ, Kim SK, Kim CY. MK-801 augments pilocarpine-induced electrographic seizure but protects against brain damage in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:331–344. doi: 10.1016/s0278-5846(97)00004-3. [DOI] [PubMed] [Google Scholar]
- Lennox WJ, Harris LW, Talbot BG, Anderson DR. Relationship between reversible acetylcholinesterase inhibition and efficacy against soman lethality. Life Sci. 1985;37:793–798. doi: 10.1016/0024-3205(85)90513-2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Res. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Lothman EW. Seizure circuits in the hippocampus and associated structures. Hippocampus. 1994;4:286–290. doi: 10.1002/hipo.450040311. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Salerno RA, Perlin JB, Kaiser DL. Screening and characterization of antiepileptic drugs with rapidly recurring hippocampal seizures in rats. Epilepsy Res. 1988;2:367–379. doi: 10.1016/0920-1211(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin R, Klitgaard H, Matagne A, Wasterlain CG. Anticonvulsant effects of levetiracetam and levetiracetam-diazepam combinations in experimental status epilepticus. Epilepsy Res. 2004;58:167–174. doi: 10.1016/j.eplepsyres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–132. [PubMed] [Google Scholar]
- McDonough JH, McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999;73:473–478. doi: 10.1007/s002040050637. [DOI] [PubMed] [Google Scholar]
- McDonough JH, McMonagle JD, Shih TM. Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol. 2010;33:279–283. doi: 10.3109/01480540903483417. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Shih TM. Pharmacological modulation of soman-induced seizures. Neurosci Biobehav Rev. 1993;17:203–215. doi: 10.1016/s0149-7634(05)80151-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Van Shura KE, LaMont JC, McMonagle JD, Shih TM. Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic and Clinical Pharmacology and Toxicology. 2008:27–34. doi: 10.1111/j.1742-7843.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58:S21–26. doi: 10.1212/wnl.58.8_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Noraberg J. Organotypic brain slice cultures: an efficient and reliable method for neurotoxicological screening and mechanistic studies. Altern Lab Anim. 2004;32:329–337. doi: 10.1177/026119290403200403. [DOI] [PubMed] [Google Scholar]
- Orlof M, Williams H, Pfeiffer C. Timed intravenous infusion of Metrazol and strychnine for testing anticonvulsant drugs. Proc Soc Exp Biol Med. 1949:254–257. doi: 10.3181/00379727-70-16891. [DOI] [PubMed] [Google Scholar]
- Pessah N, Bialer M, Wlodarczyk B, Finnell RH, Yagen B. Alpha-fluoro-2,2,3,3-tetramethylcyclopropanecarboxamide, a novel potent anticonvulsant derivative of a cyclic analogue of valproic acid. J Med Chem. 2009;52:2233–2242. doi: 10.1021/jm900017f. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Nissinen J, Nairismägi J, Lukasiuk K, Gröhn OH, Miettinen R, Kauppinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- Racine R. Modification of seizure activity by electrical stimulation. II. Motor Seizures. Electroencephalogr Clin Neurophysiol. 1972;3:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res. 2006;68:22–28. doi: 10.1016/j.eplepsyres.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics : concepts and applications. Lippincott William & Wilkins; Philadelphia: 2009. [Google Scholar]
- Shih TM, Koviak TA, Capacio BR. Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev. 1991;15:349–362. doi: 10.1016/s0149-7634(05)80028-4. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–17. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, Newell DW, Nelson P, Awan A, Winn HR. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999;91:593–600. doi: 10.3171/jns.1999.91.4.0593. [DOI] [PubMed] [Google Scholar]
- Toman JE, Everett GM, Richards RK. The search for new drugs against epilepsy. Tex Rep Biol Med. 1952;10:96–104. [PubMed] [Google Scholar]
- Umka J, Mustafa S, ElBeltagy M, Thorpe A, Latif L, Bennett G, Wigmore PM. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166:15–22. doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- White HS, Johnson M, Wolf HH, Kupferberg HJ. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazol seizure models. Ital J Neurol Sci. 1995;16:73–77. doi: 10.1007/BF02229077. [DOI] [PubMed] [Google Scholar]
- White HS, Patel S, Meldrum BS. Anticonvulsant profile of MDL 27,266: an orally active, broad-spectrum anticonvulsant agent. Epilepsy Res. 1992;12:217–226. doi: 10.1016/0920-1211(92)90076-6. [DOI] [PubMed] [Google Scholar]
- Woodbury LA, Davenport VD. Design and use of a new electroshock seizure apparatus, and analysis of factors altering seizure threshold and pattern. Arch Int Pharmacodyn Ther. 1952;92:97–107. [PubMed] [Google Scholar]
- Zheng Y, Moussally J, Cash SS, Karnam HB, Cole AJ. Intravenous levetiracetam in the rat pilocarpine-induced status epilepticus model: behavioral, physiological and histological studies. Neuropharmacology. 2010;58:793–798. doi: 10.1016/j.neuropharm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.