Abstract
Purpose
To assess the efficacy of dodecafluoropentane emulsion (DDFPe), a nano droplet emulsion with significant oxygen transport potential, in decreasing infarct volume using an insoluble emboli rabbit stroke model.
Methods
New Zealand White rabbits (n=64; 5.1±0.50 kg) received angiography and embolic spheres in the internal carotid artery occluding branches. Rabbits were randomly assigned to groups in 4-hour and 7-hour studies. Four-hour groups included: control (n=7, embolized without treatment) or DDFPe treatment 30-min before stroke (n=7), or at stroke onset (n=8), 30-min after stroke (n=5), 1-hour after stroke (n=7), 2-hours after stroke (n=5), or 3-hours after stroke (n=6). Seven-hour groups included control (n=6), DDFPe at 1-hour after stroke (n=8), and DDFPe at 6-hours after stroke (n=5). DDFPe dose was 2% w/v (weight/volume) intravenous injection, 0.6 mL/kg, and repeated every 90 minutes as time allowed. Following euthanasia infarct volume was determined using vital stains on brain sections.
Results
At 4-hours, median percent infarct volume decreased for all DDFPe treatment times (pre-treatment=0.30%, p=0.004; onset=0.20%, p=0.004; 30-min=0.35%, p=0.009, 1-hour=0.30%, p=0.01, 2-hours=0.40%, p=0.009, 3-hours=0.25%, p=0.003) compared with controls (3.20%). At 7-hours, median percent infarct volume decreased with treatment at 1-hour (0.25%, p=0.007) but not for 6-hours (1.4%, p=0.49) compared with controls (2.2%).
Conclusions
Intravenous DDFPe in an animal model decreases infarct volumes and protects brain tissue from ischemia justifying further investigation.
Introduction
Many diverse situations involving blood loss, ischemia or hypoxia result in organ and tissue damage causing morbidity and mortality. These situations include common surgical and interventional procedures as well as trauma and natural disease states. These episodes commonly present as myocardial infarctions, as other hypoxic or ischemic syndromes widely distributed throughout the body and extremities, and also as ischemic strokes. Additionally, clinical procedures including surgery and angiography can produce microemboli resulting in silent or sub-clinical cerebral ischemia (1). Neuroprotective compounds, hyperbaric oxygen, hemoglobin-based blood substitutes, other approaches, and liquid perfluorocarbon-based oxygen carriers have shown promise but largely failed to compensate in these situations (2–7). Prompt revascularization and restoration of oxygenated blood flow remain the primary foci of clinical stroke therapy at this time.
Another oxygen transport substance may have therapeutic potential. Due to the highly electrophilic fluorine content and lack of intermolecular attractive forces inherent to perfluorocarbons (PFC), PFC emulsions have the ability to physically dissolve, transport, and deliver significant quantities of oxygen and other electron-rich respiratory gases (8, 9). Sophisticated techniques allow the production of stable PFC emulsions with exceptionally small particles. Such a small-scale droplet allows passage beyond many vascular occlusions that block 8 μm red blood cells and allows perfusion into even the smallest areas of microcirculation and tissues that would not otherwise be oxygenated by an occluded arterial supply.
Dodecafluoropentane emulsion (DDFPe) is a stable emulsion of 250 nanometer droplets that, upon in vitro administration at 37°C, undergoes expansion into the gaseous state (10, 11). This expansion is unique to DDFP among perfluorocarbons. DDFP has a boiling point of approximately 29°C; thus, at 37°C large intermolecular “pockets” open up in the DDFP emulsion droplets such that high concentrations of respiratory gases can be rapidly drawn within. In vitro, the DDFP droplets eventually expand to form microbubbles. However, in vivo, when DDFPe is injected intravenously, it does not expand to true bubble form (11). The intravascular pressure retards full bubble expansion, but fortuitously allows alternation of droplet swelling and contraction as necessary to absorb and release respiratory gases as the droplets travel through the bloodstream without reaching microbubble size. Liquid PFCs do not possess this ability which renders them relatively limited in their gas solubilizing abilities. An in vitro comparison between three PFC emulsions demonstrated markedly superior oxygen delivery for DDFPe in the gaseous state (11). In vivo, DDFPe functions for about 2 hours, and the DDFPe is exhaled through normal respiration without long term retention in the body (12).
We test this intravenous (IV) emulsion therapy in a rabbit model of acute ischemic stroke caused by permanent angiographic occlusions of branches of the internal carotid artery (ICA). The aim is to determine if neuroprotection can be provided without restoration of blood flow.
Materials and Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee. New Zealand White rabbits (n=95 total) were used in this study.
Surgical and angiographic procedures were described previously (13, 14). Briefly, rabbits were sedated with intramuscular injection of ketamine, 30 mg/kg (Ketaset; Fort Dodge; Fort Dodge, IA) and xylazine, 3 mg/kg (AnaSed; Lloyd Laboratories; Shenandoah, IA) and anesthetized with isoflurane (Novaplus; Hospira Inc.; Lake Forrest, IL). A femoral artery was surgically exposed, and a modified 65-cm angled-tip 3F catheter (SlipCath; Cook Inc.; Bloomington, IN) was advanced using standard angiographic techniques to select the internal carotid artery (ICA).
Sub-selective magnification angiography was performed before embolization and one minute after embolization to document the precise occlusion of the cerebral vasculature (Fig 1). Imaging was performed using a single plane C-arm digital mobile imaging system (OEC 9800; GE Healthcare; Salt Lake City, UT). Embolization with two or three individual microspheres 700–900 μm in diameter (Embosphere Microspheres; BioSphere Medical Inc.; Rockland, MA) flushed into the ICA occluded some branches, usually the middle cerebral artery (MCA) and/or anterior cerebral artery (ACA). Repeat angiography one minute later confirmed vessel occlusion and compromised flow in the ischemic area. To provide uniform deficits, rabbits with other occlusions or angiographic complications were discarded, n=31.
Figure 1.
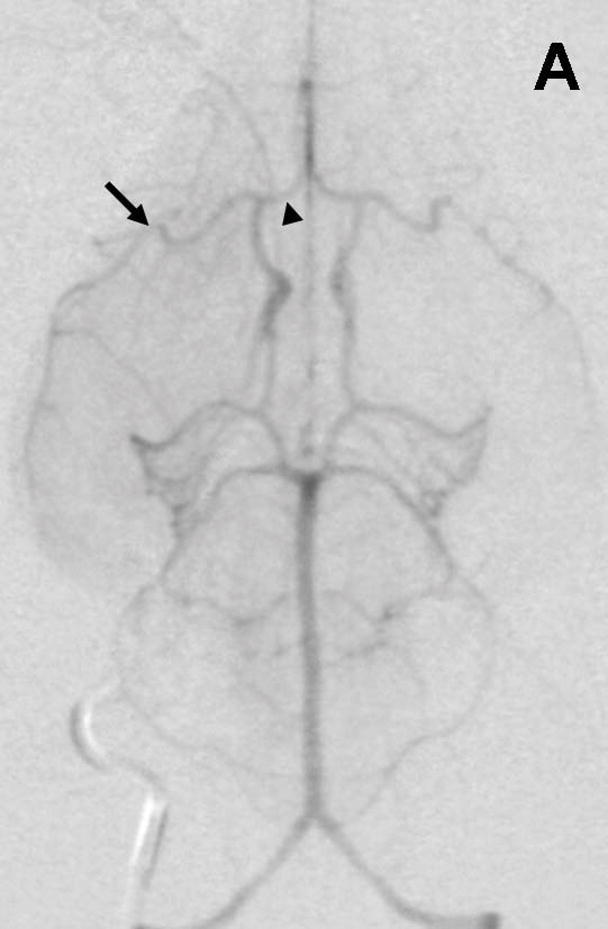
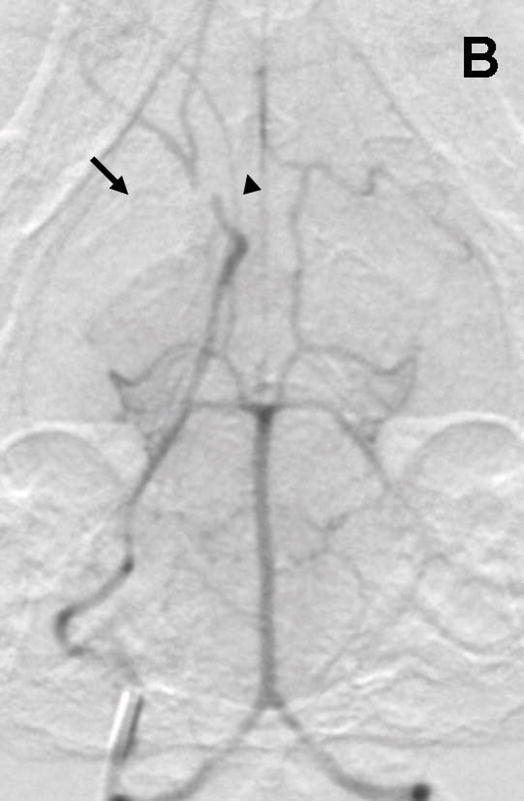
Rabbit Angiography. Subselective magnification angiograms of the internal carotid artery demonstrate (A) the Circle of Willis and the middle cerebral artery (MCA) and anterior cerebral artery (ACA) (arrow and arrowhead, respectively) and (B) occlusion of the MCA and ACA following the injection of three embolic spheres.
Treatments were initiated according to group schedules using an ear vein catheter access (Instyle-W; Becton Dickinson; Sandy, UT). Four or 7 hours following embolization, rabbits were euthanized with IV 1.5-mL of pentobarbital (Euthasol; Virbac Corp.; Fort Worth, TX).
For treatments, rabbits were randomly assigned to 7 groups in the 4-hour study: 1) control, embolized without therapy (n=7); 2) pre-treatment with DDFPe 30 minutes before embolization (n=7), 3) immediate DDFPe (n=8); 4) DDFPe at 30 minutes after stroke (n=5); 5) DDFPe at 1 hour after stroke (n=7); 6) DDFPe at 2 hours after stroke (n=5); 7) DDFPe at 3 hours after stroke (n=6). The administration of therapy was a slow push intravenous dose of DDFPe (2% w/v DDFP) (NuvOx Pharma; Tucson, AZ), 0.6 mL/kg, at the designated group time and repeated every 90 minutes as time before sacrifice allowed.
To observe the limit of treatment efficacy, a parallel study was performed using a much delayed treatment compared to another control group. Groups were control rabbits (n=6), rabbits treated with DDFPe at 1 hour after stroke with additional doses every 90 minutes (n=8), and rabbits with DDFPe treatment starting at 6 hours after stroke (n=5). These were euthanized seven hours after embolization.
Following euthanasia, the brain was harvested, immediately chilled in saline, and then sliced coronally at 4.0-mm intervals using a chilled brain mold (RBM-7000C; ASI Instruments Inc.; Warren, MI). Brain sections (n=8) were placed in 1% 2, 3, 5-triphenyltetrazolium chloride (Sigma-Aldrich; St. Louis, MO) for 45 minutes at 37°C, fixed in 10% formalin, and digitally photographed (Fig 2). Brain size and areas of infarction were measured using digital analysis (NIH ImageJ) by a technician blinded to treatment groups. Percent infarct volume was calculated as a percent of the whole brain.
Figure 2.
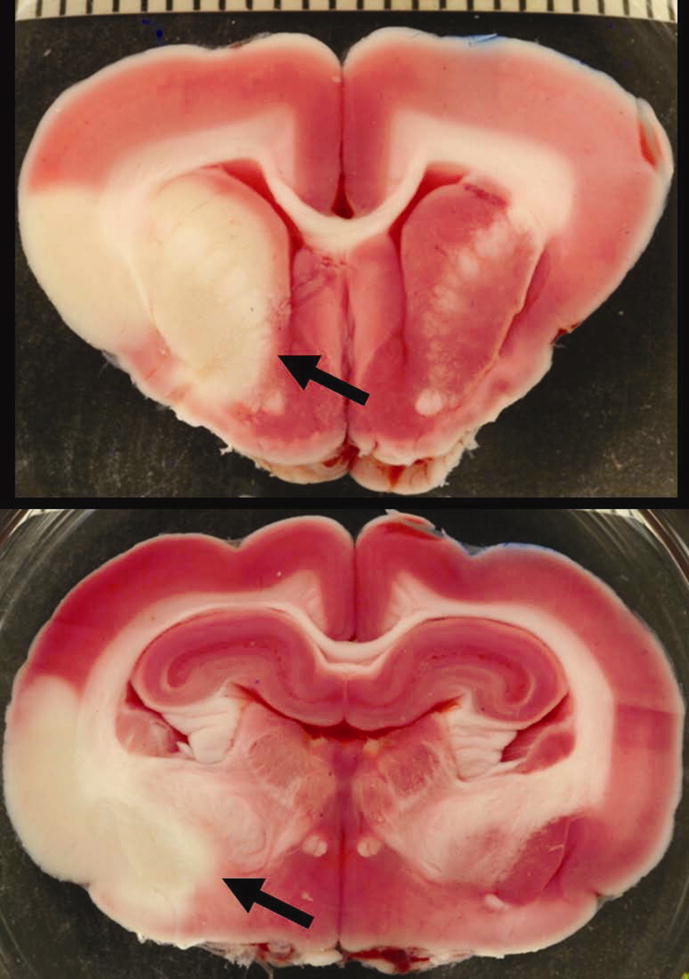
Brain infarction following middle cerebral artery (MCA) and anterior cerebral artery (ACA) embolization. Two sequential sections from a 2, 3, 5-triphenyltetrazolium chloride (TTC) stained rabbit brain clearly display pale areas of infarct (arrows). The scale bar represents millimeters.
Fixed brain sections were embedded in paraffin and sectioned at 4-μm. After a standard hematoxylin and eosin (H&E) stain, sections were analyzed and then scored for intracranial hemorrhage (ICH), defined as extravasations of erythrocytes and fluid into the extracellular space (15). The presence and location of ICH were recorded by a veterinary pathologist blinded to treatment groups.
Treatment with DDFPe was combined into three important groups for analysis: pretreatment 30 minutes before embolization, hyperacute treatment less than one hour following symptom onset, and acute therapy 1 to 3 hours following onset.
Because infarct volumes were not normally distributed, ranks of infarct volume percentages were analyzed with PROC GLM (Kruskal-Wallis equivalent) of SAS (SAS Institute Inc.; Cary, NC). Dunnett-adjusted p-values were used in comparing each DDFPe group to controls. Comparisons of four- and seven-hour control groups, and of treatment groups within the acute and hyperacute treatment subgroups were made using the exact procedures in the software package StatXact (Cytel, Inc.; Cambridge, MA). The incidence of hemorrhage within or outside the stroke area was compared using the chi-square test and Fisher’s exact test.
Results
Ninety-five rabbits underwent the angiographic procedure, 11 resulted in severe vasospasm of the internal carotid artery. Eighty-four rabbits had successful embolization with permanent occlusion of the MCA and/or ACA. Twenty of these also had occlusion of posterior cerebral or superior cerebellar arteries and were discarded, leaving 64 for analysis. All rabbits were successfully maintained at a normal physiologic state of oxygenation and cardiac function throughout the procedure and treatments.
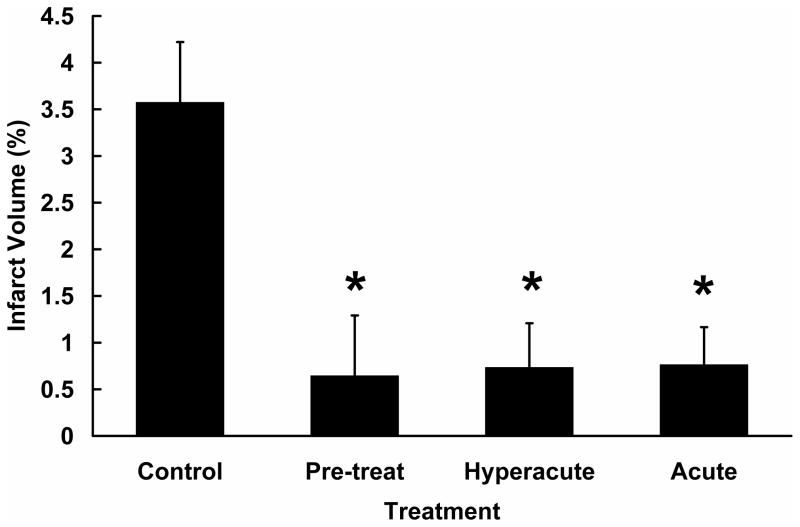
In the 4-hour study (Table 1), median percent infarct volumes were decreased (p=0.001, exact Mann-Whitney test) for all rabbits treated, N=38, with DDFPe (0.30%) compared with controls (3.20%). The hyperacute group median (Fig 3), N=13, was significantly reduced (0.30%) compared to controls, p=0.021 (Dunnett-adjusted comparison of ranks, unadjusted p=0.008). The acute group median, N=18, was also reduced, 0.30%, p=0.005 (Dunnett-adjusted comparison of ranks, unadjusted p=0.002). The individual groups within the hyperacute and acute categories did not differ from each other, p=0.54 and p=0.92 (exact Kruskal Wallis test), respectively.
Table 1.
Influence of dodecafluoropentane emulsion (DDFPe) treatment start time on percent infarct volume at 4 hours. Pre-treat represents DDFPe administration starting 30 minutes before embolization.
| DDFPe treatment start time | N | Mean ±Standard Error, % | Median, % | P-value (unadjusted) | P-value (Dunnett-adjusted) |
|---|---|---|---|---|---|
| Control | 7 | 3.57 ± 1.41 | 3.20 | - | - |
| Pre-treat | 7 | 0.64 ± 0.37 | 0.30 | 0.008 | 0.04 |
| Immediate | 8 | 0.75 ± 0.35 | 0.20 | 0.010 | 0.05 |
| 30-min | 5 | 0.70 ± 0.32 | 0.40 | 0.083 | 0.32 |
| 1-hour | 7 | 1.03 ± 0.59 | 0.30 | 0.012 | 0.06 |
| 2-hours | 5 | 0.72 ± 0.50 | 0.40 | 0.028 | 0.12 |
| 3-hours | 6 | 0.48 ± 0.28 | 0.25 | 0.008 | 0.04 |
P-values compare each treatment time to untreated controls.
Figure 3.
Infarct volume at 4 hours vs. dodecafluoropentane emulsion (DDFPe) treatment time. Categorization of treatment times to model various clinical scenarios, pre-treatment, hyperacute, and acute therapy, demonstrates improved outcomes compared to control. Whether DDFPe is used as a pre-treatment (30 minutes before embolization), a hyperacute treatment (0 to 30 minutes), or an acute treatment (1 to 3 hours), stroke volumes are significantly reduced. *P≤0.021, Dunnett-adjusted comparison of ranks.
In the 7-hour study (Table 2), control infarct volumes were similar to the 4-hour controls, mean=3.88%, median=2.2%, p=0.70 (exact Mann-Whitney test). The hour 1 therapy animals had 7 of 8 values at or below the lowest control value, while the hour 6 therapy animals showed 3 of 5 at or below the lowest control value.
Table 2.
Influence of dodecafluoropentane emulsion (DDFPe) treatment start time on percent infarct volume at 7 hours.
| DDFPe treatment start time | N | Mean ±Standard Error, % | Median, % | P-value (unadjusted) | P-value (Dunnett- adjusted) |
|---|---|---|---|---|---|
| Control | 6 | 3.88 ± 1.41 | 2.20 | - | - |
| 1-hour | 8 | 1.02 ± 0.69 | 0.25 | 0.007 | 0.01 |
| 6-hours | 5 | 3.92 ± 2.21 | 1.40 | 0.49 | 0.71 |
P-values compare each treatment start time to untreated controls.
Microscopic hemorrhage rates were similar in all groups (N=44) in the 4-hour study, both in the stroke area (p=0.85) and outside the stroke (p=0.32). Hemorrhage within stroke was seen in controls 14% (N=7), DDFPe pretreatment 14% (N=7), immediate DDFPe 14% (N=7), 30-min 20% (N=5), 1-hour 0% (N=7), 2-hours 20% (N=5), 3-hours 0% (N=6). Hemorrhage outside of stroke was 14%, 57%, 28%, 0%, 14%, 20%, and 17% respectively.
The control rabbits at 7-hours had a numerically greater overall hemorrhage rate compared to 4-hour controls but not to a significant level (83% vs. 29%, p=0.10). The incidence of hemorrhage within stroke trended downward with treatment with DDFPe at 1 hour and every 90 minutes until 7-hour sacrifice (p=0.06) compared to control. Hemorrhage within stroke was seen in controls 67% (N=6), DDFPe at 1 hour 0% (N=6), and DDFPe at 6 hours 60% (N=5). Hemorrhage outside of stroke occurred in 50%, 20%, and 33% respectively and did not differ between groups (p=0.82).
One DDFPe dose, N=11, two doses, N=25, three doses, N=7, four doses, N=8, and zero dose controls, N=13, rabbits all survived to scheduled sacrifice without apparent adverse event.
Discussion
The search for a neuroprotectant to use in acute stroke has been a high priority for many years. The parallel search for blood substitutes has included hemoglobin substitutes and perfluorocarbons in liquid form. Numerous studies for their use in hypoxia and ischemia have encountered side effects and severe complications and all have failed to translate into successful human therapy. Several oxygen free radical scavengers and other novel techniques have shown great promise in small animal strokes, usually in mouse or rat models. None has yet translated into therapy of human stroke (4). Here, we test a novel oxygen transport approach in an embolic stroke model without the possibility of thrombolysis. This rabbit model of stroke is similar to a model used in the successful development of tPA stroke therapy (16). Although this model is more expensive than rats and mice, its advantage in scale may be important and it must be noted that other success has translated into human results. This included predicting failure of the SAINT Trial of the antioxidant NXY-059 (17, 18).
Blood has a limited capacity to deliver oxygen, in large part requiring red blood cells to transit capillaries. With decreased blood flow or occlusion this limitation becomes critical, causing infarction with nearly immediate cell death in some areas and ischemic damage without immediate cell death in others. This threatened area is penumbra. In many strokes an ischemic penumbra of potentially viable brain tissue might be saved if oxygen could be delivered there.
Previous therapies including liquid perfluorocarbon-based oxygen carriers have largely failed to compensate for oxygen deficits. However, DDFPe as a gas at body temperature transports many times more oxygen per weight volume than liquid PFCs (11). The IV dose of DDFPe is less than 1/100th of other PFC based agents. The nano-sized droplets and bubbles pass, like tPA, through spaces smaller than red blood cells and transport oxygen to ischemic areas blocked from whole blood flow. Other PFC agents require larger doses and are retained within the body long-term. In human pharmacokinetic studies IV DDFPe as a single smaller dose is well tolerated, and is rapidly cleared by exhalation without significant residual or side effect (12). In rats and pigs larger doses act for up to 2 hours (19).
When given intravenously, DDFPe may “pause the clock” on the treatment window for several hours, acting as a bridge to further acute stroke therapies which might be delayed far beyond current therapeutic time windows. This rabbit study shows clear benefit in decreased stroke volume compared with untreated controls, not only when given prior to occlusion or in the hyperacute time period, from 0 to 30 minutes, but also with delays of 1 to 3 hours. While prior administration could model preventive therapy in high-risk procedures and 0–30 minute therapy model iatrogenic ischemic episodes, the later groups model the usual stroke therapy which is more delayed (20). The continued improved outcome at 3 hours here is very promising in clinical terms, since the most common human therapy, IV tPA, begins to lose efficacy in this time frame, and endovascular recanalization, which can be performed up to 6 hours after onset, is limited to major medical centers. This 3 hour improvement raises the possibility of DDFPe actually reversing non-lethal damage in addition to halting further damage. The 7-hour model shows that the damage has progressed too far for statistically significant therapeutic benefit with these small sample sizes at 6 hour administration. Importantly, this model shows that administration at 1-hour can be carried successfully to 7 hours with multiple doses, a point beyond most current thrombolysis protocols now in use. Prolonged success may also be possible. However, safety of multiple large doses is unproven in humans and problematic in dogs where rapid doses of DDFPe caused pulmonary hypertension and severe symptoms (21).
Measurements of intracranial hemorrhage rates 4 hours after stroke were similar in all groups. The trend for increased rates of hemorrhage in control rabbits at 7 hours suggests that this time window of several hours after onset is important in the development of microscopic bleeding. Particularly encouraging is the absence of ICH in the 7-hour group treated with DDFPe from 1 hour (15, 22). This raises the possibility of a protective aspect in this therapy, but needs to be confirmed with larger numbers of animal studies (23).
In addition to both ischemic and hemorrhagic acute strokes, clinical applications might also include pretreatment of high-risk cardiac and carotid surgeries or neurovascular or cardiac interventions providing a few hours of improved tissue oxygenation during iatrogenic ischemic episodes. Many strokes, cognitive deficits, or myocardial infarctions caused by transient clot, bubbles, or hypoxia might be completely avoided. Both gaseous emboli and hypoperfusion episodes associated with surgery and vascular or cardiac interventions are transient phenomena and may require no additional therapy after DDFPe treatment. Since human single dose experience appears safe, this testing could quickly progress.
In addition to the need to fully investigate the time course of effectiveness of DDFPe, another limitation of the present study is the lack of therapeutic dosage testing. These studies used established dose levels for sonographic imaging and optimization of therapeutic dose levels in rabbits and humans is required. Although considerable benefit was demonstrated at the chosen dosage and time points, further studies that compare other artificial oxygen carriers and fully characterize the treatment effects are needed. Moreover, the use of DDFPe must be examined in a thromboembolic stroke model as a combination treatment with intravenous tPA thrombolysis, intra-arterial interventions, or sonothrombolysis using microbubbles and ultrasound. Here both safety and synergistic or additive effects will be appraised. If continued preclinical research overcomes these limitations, human feasibility testing in acute stroke can rapidly advance.
Further research will be required to optimize human dosage, timing, efficacy, and safety. This will be facilitated by the previous study of DDFPe as an ultrasound contrast agent tested in more than 2,000 patients and its approval as an ultrasound contrast agent by the European Agency for the Evaluation of Medicinal Products (EMEA, now known as the European Medicines Agency, EMA) (24, 25). The current single dose is smaller than that used as a human contrast agent, and dose optimization for therapeutic uses and safety testing of multiple doses have not yet been performed. While reports as a contrast agent were very positive, development stopped for economic reasons and DDFPe is not commercially available at this time.
Intravenous DDFPe protects brain tissue from ischemia possibly by decreasing the degree of hypoxia. It decreases infarct volumes in stroke and the effect can be sustained for several hours with repeated doses. Safety in humans has been demonstrated. Further animal studies and rapid development as a therapeutic oxygen delivery agent during times of stroke, blood loss, ischemia, hypoxia, and in some preventative situations such as high-risk procedures are warranted.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health [R01HL82481].
Jeff Hatton is acknowledged for his important participation.
Footnotes
This work was presented, in part, at SIR 2011 Annual Meeting.
Conflicts/Disclosures: Dr. Unger has patents and license to produce DDFPe. A patent has been applied for using this stroke therapy by Drs. Culp, Skinner, and Unger. Drs. Unger and Johnson are shareholders in NuvOx Pharma and Dr. Johnson is an employee of NuvOx Pharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jurga JJ, Nyman J, Tornvall P, et al. Cerebral microembolism during coronary angiography. Stroke. 2011;42:1475–1477. doi: 10.1161/STROKEAHA.110.608638. [DOI] [PubMed] [Google Scholar]
- 2.Kim HW, Greenburg AG. Artificial oxygen carriers as red blood cell substitutes: a selected review and current status. Artif Organs. 2004;28:813–828. doi: 10.1111/j.1525-1594.2004.07345.x. [DOI] [PubMed] [Google Scholar]
- 3.Pignataro G, Simon P, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 4.Donnan GA. The 2007 Feinberg Lecture: a new road map for neuroprotection. Stroke. 2008;39:242–248. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- 5.Fergusson DA, McIntyre L. The future of clinical trials evaluating blood substitutes. JAMA. 2008;299:2324–2326. doi: 10.1001/jama.299.19.jed80027. [DOI] [PubMed] [Google Scholar]
- 6.Vinukonda G, Csiszar A, Hu F, et al. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain. 2010;133:2264–2280. doi: 10.1093/brain/awq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liesz A, Zhou W, Mracskó É, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 8.Reiss JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 9.Remy B, Deby-Dupont G, Lamy M. Red blood cell substitutes: Fluorocarbon emulsions and haemoglobin solutions. Br Med Bull. 1999;55:277–298. doi: 10.1258/0007142991902259. [DOI] [PubMed] [Google Scholar]
- 10.Correas JM, Quay SC. EchoGen emulsion: a new ultrasound contrast agent based on phase shift colloids. Clin Radiol. 1996;51S1:11–14. [PubMed] [Google Scholar]
- 11.Johnson JLC, Dolezal MC, Kerschen A, Matsunaga TO, Unger EC. In vitro comparison of dodecafluoropentane (DDFP), perfluorodecalin (PFD), and perfluoroctylbromide (PFOB) in the facilitation of oxygen exchange. Artif Cell Blood Sub. 2009;37:156–162. doi: 10.1080/10731190903043192. [DOI] [PubMed] [Google Scholar]
- 12.Correas JM, Meuter AR, Singlas E, Kessler DR, Worah D, Quay SC. Human pharmacokinetics of a perfluorocarbon ultrasound contrast agent evaluated with gas chromatography. Ultrasound Med Biol. 2001;27:565–570. doi: 10.1016/s0301-5629(00)00363-x. [DOI] [PubMed] [Google Scholar]
- 13.Culp BC, Brown AT, Erdem E, Lowery J, Culp WC. Selective intracranial magnification angiography of the rabbit: basic techniques and anatomy. J Vasc Interv Radiol. 2007;18:187–192. doi: 10.1016/j.jvir.2006.12.720. [DOI] [PubMed] [Google Scholar]
- 14.Brown AT, Skinner RD, Flores R, et al. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol. 2010;21:903–909. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores R, Hennings LJ, Lowery JD, Brown AT, Culp WC. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Invest Radiol. 2011;46:419–24. doi: 10.1097/RLI.0b013e31820e143a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 17.Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfate N-oxide (generic NCY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2002;33:1411–1415. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- 18.Diener HC, Lees KR, Lyden P, et al. SAINT I and II Investigators. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren CEG, Bergoe GW, Tyssebotn IM. Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artif Cell Blood Sub. 2006;34:473–486. doi: 10.1080/10731190600769271. [DOI] [PubMed] [Google Scholar]
- 20.McKhann GM, Grega MA, Borowicz LM, Jr, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery, an update. Stroke. 2006;37:562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 21.Grayburn PA, Erickson JM, Escobar J, Womack L, Velasco CE. Peripheral Intravenous Myocardial Contrast Echocardiography Using a 2% Dodecafluoropentane Emulsion: Identification of Myocardial Risk Area and Infarct Size in the Canine Model of Ischemia. J Am Coll Cardiol. 1995;26:1340–1347. doi: 10.1016/0735-1097(95)00306-1. [DOI] [PubMed] [Google Scholar]
- 22.Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46:202–207. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao BQ, Suzuki Y, Kondo K, Ikeda Y, Umemura K. Combination of a free radical scavenger and heparin reduces cerebral hemorrhage after heparin treatment in a rabbit middle cerebral artery occlusion model. Stroke. 2001;32:2157–2163. doi: 10.1161/hs0901.095640. [DOI] [PubMed] [Google Scholar]
- 24.The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit, 27 July 1998, CPMP/1342/98.
- 25.The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit, London, 22 May 2001, Doc. Ref: EMEA/37043/00.