SUMMARY
A limited set of T cell receptor (TCR) variable (V) gene segments are used to create a repertoire of TCRs that recognize all major histocompatibility complex (MHC) ligands within a species. How individual αβTCRs are constructed to specifically recognize a limited set of MHC ligands is unclear. Here we have identified a role for the differential paring of particular V gene segments in creating TCRs that recognized MHC class II ligands exclusively, or cross-reacted with classical and non-classical MHC class I ligands. Biophysical and structural experiments indicated TCR specificity for MHC ligands is not driven by germline encoded pairwise interactions. Rather, identical TCRβ chains can have altered peptide-MHC (pMHC) binding modes when paired with different TCRα chains. The ability of TCR chain pairing to modify how V region residues interact with pMHC helps to explain how the same V genes are used to create TCRs specific for unique MHC ligands.
INTRODUCTION
T cell antigen receptors (TCR) recognize ligands displayed on classical Major Histocompatibility Complex (MHC), non-classical MHC and MHC-like proteins. Classical MHC proteins are the most polymorphic genes in humans (Robinson et al., 2009). Within a population, MHC allele diversity greatly limits the ability of pathogens to escape immune responses (Kosmrlj et al., 2010; Messaoudi et al., 2002). However, the diversity of MHC creates a unique problem for generating host-MHC restricted mature T cell repertoires. Prior to selection, TCR rearrangement has to generate a collection of TCRs which, in aggregate, have the ability to recognize any of the possible MHC classes and alleles present within a species. After selection, individual TCRs must be MHC class specific, allowing a division of labor between MHC class II-reactive helper T cells and MHC class I-reactive cytotoxic T cells (Babbitt et al., 1985; Zinkernagel and Doherty, 1974).
The ability to create TCR repertoires specific for ligands presented by all classes and alleles of MHC requires the generation of individual receptors with a spectrum of MHC ligand binding modes. Remarkably, αβTCRs accomplish this using V gene segments with limited diversity within the complementarity determining region-1 (CDR1) and CDR2 loops, the portions of the TCR which primarily contact the MHC (Davis and Bjorkman, 1988). The majority of TCR diversity occurs within the V(D)J junctional region of the CDR3 loops that sit atop the bound peptide ligand (Rudolph et al., 2006). Thymocytes that express these randomly generated TCRs are prone to react with cells expressing pMHC ligands (Merkenschlager et al., 1997; Zerrahn et al., 1997). To mature, pre-selection thymocytes expressing these TCRs are subjected to the selective pressures of positive selection to ensure T cells are MHC restricted, and negative selection to limit autoimmunity (Fink and Bevan, 1995; Kappler et al., 1987; Kisielow and von Boehmer, 1995; Mathis and Benoist, 2004).
To account for the high frequency at which self-pMHC reactive TCRs are created, it has been hypothesized that TCRs and MHCs have co-evolved to bind one another (Jerne, 1971). The co-evolution of TCRs and MHC could involve the overall shape complementarity of TCRs and MHC, the selection for specific pairwise contacts between V gene residues and MHC (interaction codons) or the selection of amino acids at the tips of CDR loops (such a tyrosine) that can make a variety of chemical bonds (Al-Lazikani et al., 2000; Garcia et al., 2009; Housset and Malissen, 2003; Marrack et al., 2008). Co-evolution models, however, have to account for several facets of ligand recognition. Both MHC class I and MHC class II specific TCRs are created from all TCR V gene families and from individual TCR rearrangements (Garman et al., 1986; Jorgensen et al., 1992). Thus, the identical TCRα or TCRβ residues are capable of binding structurally somewhat dissimilar and extremely polymorphic MHC class I and MHC class II proteins. In addition, crystallographic and biophysical studies show that TCRs use a variety of semi-conserved diagonal docking modes to bind MHC. The only strict “rules of engagement” so far identified are the overall orientation of TCR binding pMHC and the requirement of the CDR3 loop(s) to contact the MHC-bound antigen (Housset and Malissen, 2003; Rudolph et al., 2006).
To decipher why self-reactive TCRs are created at a high frequency, we began studying T cells that develop in mice with limited negative selection. We isolated T cells that recognize the MHC class II molecule IAb presenting the 3K peptide (IAb-3K). Many of these T cells are self-reactive requiring only a few residues of the peptide for recognition and primarily engage either the MHC class II α-chain, or MHC class II β-chain (Dai et al., 2008; Huseby et al., 2006; Huseby et al., 2005). These pMHC reactivity patterns are highly analogous to some self-reactive TCRs, isolated from patients with Multiple Sclerosis and its mouse model, experimental autoimmune encephalomyelitis (EAE), which utilize “unconventional” binding modes (Wucherpfennig et al., 2009). Additionally, many of the IAb-3K reactive TCRs expressed on the T cells that develop when negative selection is limited are highly allo-MHC class II reactive, and two were shown to cross-react with classical MHC class I ligands (Huseby et al., 2005). In contrast, IAb-3K reactive TCRs isolated from conventional, C57BL/6 mice, are self-tolerant, dependent upon multiple residues of the peptide and had a standard rate of allo-MHC class II reactivity. The self-reactive, pMHC cross-reactive T cells and the self-tolerant, pMHC specific TCRs all used Vβ8.1, Vβ8.2 and Vβ14 TCR chains paired with a diverse array of TCR Vα chains. The construction of TCRs with different pMHC specificities and pMHC cross-reactivity patterns from the same V gene segments strongly suggests that control mechanisms must exist to modify how germline TCR residues engage MHC proteins.
To identify mechanisms that control TCR ligand specificity, we isolated and characterized a set of TCRs reactive to IAb-3K carrying the identical TCRβ chain. The TCRs were either self-tolerant or self-reactive, and differed in the ability to cross-react with other classes of MHC ligands. By comparing how each receptor bound the same pMHC complex, we sought to identify how TCR chain-pairing affects MHC specificity. X-ray crystallographic and biophysical experiments indicated that MHC specific TCRs and MHC class cross-reactive TCRs utilize a spectrum of pMHC binding modes within a conventional docking footprint. Our data demonstrate that differential TCR α-chain pairing can result in MHC specific TCRs that have altered TCRβ CDR loop conformations and placements, as well as modified TCRβ-MHC contacts.
RESULTS
Identification of IAb-3K reactive T cells with different pMHC specificities
Why randomly created TCRs are prone to being self-reactive and how this pre-bias is shaped into a foreign antigen specific, self-tolerant T cell repertoire remains unclear. We created a model to identify mechanisms that control TCR specificity by generating mice expressing the Vβ8.2 TCRβ chain of the IAb-3K reactive YAe62 TCR as a transgene (YAe62β mice). YAe62β mice allow for the isolation of IAb-3K reactive TCRs carrying the identical TCRβ chain sequence which develop in either conventional mice and are dependent upon MHC class II proteins for positive selection (defined here as MHC specific), or develop in MHC class II-deficient mice and are dependent upon MHC class I or non-classical MHC class I ligands for positive selection (defined here as MHC cross-reactive). By comparing how these TCRs engage pMHC, we sought to identify how αβTCR chain pairing controls ligand specificity.
YAe62β mice are biased towards creating TCRs reactive to IAb-3K and the related ligand, IAb-P-1K. These results were expected, as the T cell repertoire in mice containing a transgenic TCRβ chain are often biased towards the antigen specificity of the parent TCR (Dillon et al., 1994; Jorgensen et al., 1992). To identify conventional, MHC specific T cells, YAe62β mice were bred onto a MHCwt genetic background. In MHCwt YAe62β mice, T cells expressing IAb-3K and P-1K reactive TCRs were exclusively in the CD4+ subset of the spleen (Figure 1A–F). To identify MHC cross-reactive T cells, YAe62β mice were bred onto mice deficient in MHC class II expression, MHC CL2null (H2-Ab1−/−, Cd74−/−). A high frequency of CD4+ and CD8+ T cells reactive to IAb-3K and IAb-P-1K ligand developed in MHC CL2null YAe62β mice as well. A severe reduction in IAb-3K and P-1K-reactive T cells was observed in MHCnull (B2m−/−, H2-Ab1−/−, Cd74−/−) YAe62β mice indicating the development of these T cells in MHC CL2null mice requires β2M-dependent MHC or MHC-like ligand for selection. Thus, the IAb-3K and P-1K reactive T cells that develop in MHC CL2null mice are reactive to multiple classes of MHC; MHC class II as they react with IAb tetramers and a selecting β2M-dependent classical, non-classical or MHC-like ligand. Experiments indicate that some IAb-3K reactive, MHC cross-reactive TCRs can cross-react with H2-Kb ligands (Huseby et al., 2005; Yin et al., 2011), while others cross-react with the non-classical, MHC-like ligand CD1d (see below). Both IAb-3K and P-1K-reactive T cells were studied as they express semi-overlapping T cell repertoires in MHCwt and MHC CL2null mice, both of which the YAe62β mice are biased to recognize. All of the mice used in this study were heterozygous for the TCR Cα gene to eliminate the possibility that mature T cells co-express two different TCRs.
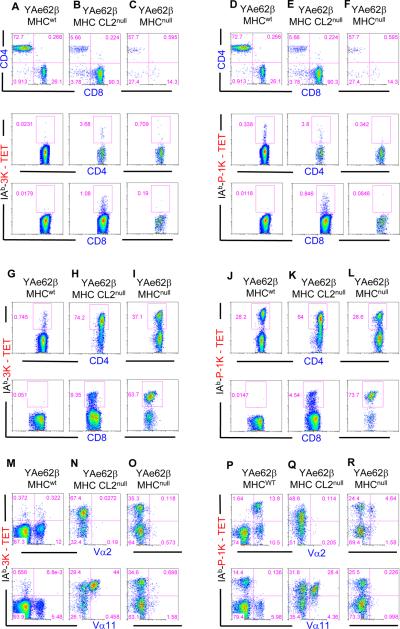
Figure 1.
Development, expansion and TCR Vα gene family segregation of MHC specific and MHC cross-reactive T cells in YAe62β mice.
(A–F) Spleens from (A, D) MHCwt, (B, E) MHC CL2null, (C, F) MHCnull YAe62β were stained with (A–C) IAb-3K or (D–F) IAb-P-1K tetramers and for the expression of CD4, CD8, TCRβ. Each of the three vertical panels show CD4 versus CD8 staining for splenocytes on the indicated MHC background followed by the IAb-3K or IAb-P-1K tetramer expression on the CD4 or CD8 T cells. Data are representative of five independent experiments.
(G–L) MHCwt, MHC CL2null or MHCnull YAe62β mice were infected with (A–C) Vac:IAb-3K, or (D–F) Vac:IAb P-1K, and the CD4+ T cells (top row) and CD8+ T cells (bottom row) from infected mice were analyzed for the ability to be stained with IAb-3K or IAb-P-1K tetramer. Data are representative of five to eight mice per group.
(M–R) MHCwt, MHC CL2null and MHCnull YAe62β mice were infected with (G–I) Vac:IAb-3K, or (J–L) Vac:IAb-P-1K, and the expanded IAb-3K and IAb-P-1K tetramer positive, CD4+ T cells were analyzed for Vα family usage. Vα2+ IAb-3K and IAb-P-1K reactive T cells (top row) are enriched in MHCwt mice, and absent in MHC CL2null mice. Vα11+ IAb-3K and IAb-P-1K T cells (bottom row) are enriched in MHC CL2null mice and absent in MHCwt and MHCnull mice. Data are representative of five independent experiments.
To ensure the MHC specific and MHC cross-reactive T cell populations functioned normally, YAe62β mice were challenged to make an IAb-3K and P-1K T cell response (Figure 1G–L). Mice were immunized with a vaccinia virus expressing the peptide epitope 3K, or P-1K, fused to the carboxyl termini of IAbβ (Vac:IAb-3K, Vac:IAb-P-1K). Because the MHC CL2null mice used in this study are only deficient in IAbβ, the virally expressed IAbβ-3K protein pairs with the endogenous IAbα chain resulting in the cell surface expression of IAb-3K on infected cells. The expansion of IAb-3K and P-1K-reactive T cells in mice on each MHC background indicated the MHC specific and MHC cross-reactive T cells were functional. The generation of IAb-3K reactive T cell responses in MHC CL2null mice was independent of TCRβ transgene expression as non-TCRβ Tg MHC CL2null mice mounted a strong IAb-3K specific CD4+ and CD8+ T cell response following Vac:IAb-3K infection (Figure S1A–F).
Germline encoded TCR sequences and thymic selection impact MHC specificity
If TCR ligand specificity is strictly created by CDR3 rearrangement, then Vα genes should not segregate to either the MHC specific or MHC cross-reactive T cell populations. Strikingly, we observed that TCR Vα gene family usage is a precise indicator of whether YAe62β+ TCRs are MHC specific or MHC cross-reactive. IAb-3K and P-1K reactive CD4+ T cells in MHCwt YAe62β mice primarily expressed TCRs containing the Vα2 gene segment (Figure 1 M–R and Table S1). These Vα2+ T cells were absent in MHC CL2null mice, indicating they require IAb to undergo positive selection. IAb-3K and P-1K reactive CD4+ T cells in MHC CL2null YAe62β mice, however, often contain a Vα11+ gene segment. These Vα11+ T cells were absent in both MHCwt mice and in MHCnull mice. Their absence in MHCwt YAe62β mice strongly suggests they are subject to MHC class II-mediated negative selection, while their absence in MHCnull YAe62β mice indicates these T cells require a β2M-dependent ligand for positive selection.
Consistent with these interpretations, these Vα11+ T cells were eliminated in chimeric mice which exclusively express MHC class II on bone marrow derived cells (Figure S1G–J). Furthermore, IAb-3K and P-1K reactive Vα11+ and Vα5+ T cell hybridomas generated from MHC CL2null YAe62β mice were self-reactive, while Vα2+ T cell hybridomas were self-tolerant. In addition, several of the Vα11+ TCRs were found to be reactive, albeit with weak affinity, with β2M-dependent, non-classical MHC CD1d ligands (Figures 2A–H, S2). IAb-3K reactive CD8+ T cells in MHC CL2null mice (including the parent YAe62 TCR) often use a Vα4+ and not a Vα2+ TCR, and are cross-reactive with several classical MHC class I ligands (Huseby et al., 2005). Thus, TCR Vα gene family segregation suggests that the generation of MHC specific TCRs can require specific germline encoded TCR residues. Similar to our previous studies, TCR specificity is not dependent upon affinity as MHC cross-reactive TCRs recognizing IAb and CD1d or IAb and H2-Kb have equilibrium affinities for IAb-3K that overlap with MHC specific TCRs (Figures 2 I–L, S2 and Table S2).
Figure 2.
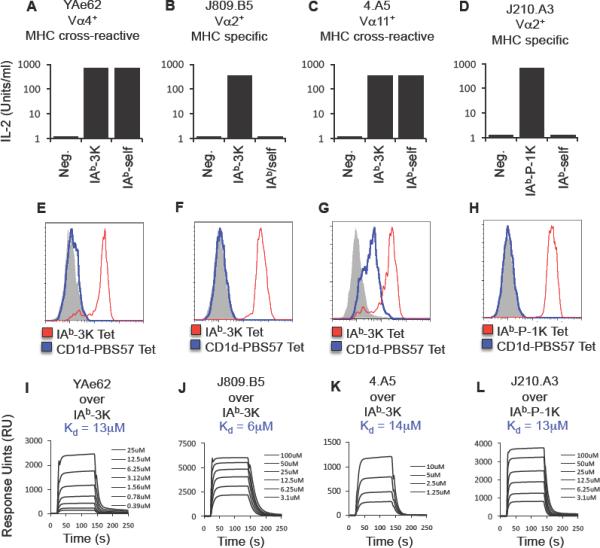
MHC cross-reactive TCRs are self-reactive, have different MHC cross-reactivities, yet have overlapping TCR-pMHC equilibrium affinities with MHC specific TCRs.
(A–D) The IAb-3K reactive T cell hybridomas (A) YAe62, (B) 4.A5 (isolated from an MHC CL2null mouse) and (C) J809.B5 (isolated from an MHCwt mouse) and the (D) IAb-P-1K-reactive T cell hybridoma J210.A3 (isolated from an MHCwt mouse) were stimulated with either antigen presenting cells (APC) expressing no MHC, IAb presenting 3K or P-1K, or IAb presenting endogenous self peptides. Data are average of triplicate wells and are representative of two experiments.
(E–H) The (E) YAe62, (F) 4.A5, (G) J809.B5 or (H) J210.A3 TCRs were expressed on the surface in SF9 cells and stained with IAb-3K or IAb-P-1K tetramer, and CD1d-PBS57 tetramer. Shaded histogram is a negative control tetramer. Data are representative example of three independent experiments.
(I–L) Soluble (I) YAe62, (J) 4.A5, (K) J809.B5 or (L) J210.A3 TCRs were analyzed for equilibrium binding to immobilized IAb-3K or IAb-P-1K using SPR. Sensograms are representative of two independent analyses.
TCRα pairing can modify how TCRβ chains bind pMHC
The segregation of IAb-3K reactive MHC specific and MHC cross-reactive T cells in YAe62β mice based on TCR Vα gene usage suggested that germline encoded TCRα residues are directly contributing to MHC specificity. Thus, it was possible that the observed Vα2 bias in the MHC specific repertoire stemmed from the creation of additional interactions between CDR1α and CDR2α loops with IAb. To identify the side chain residues important for MHC specific TCRs and MHC cross-reactive TCRs to bind IAb-3K, alanine scanning mutagenesis using surface plasmon resonance (SPR) and a TCR multimer staining assay was performed (Figure 3 and Table S3) (Cunningham and Wells, 1989; Govern et al., 2010; Huseby et al., 2006). Similar results were obtained using both methods. By comparing the change in binding affinity (ΔΔG) when a residue is substituted to alanine, the binding affinity contributed by each residue side chain can be calculated.
Figure 3.
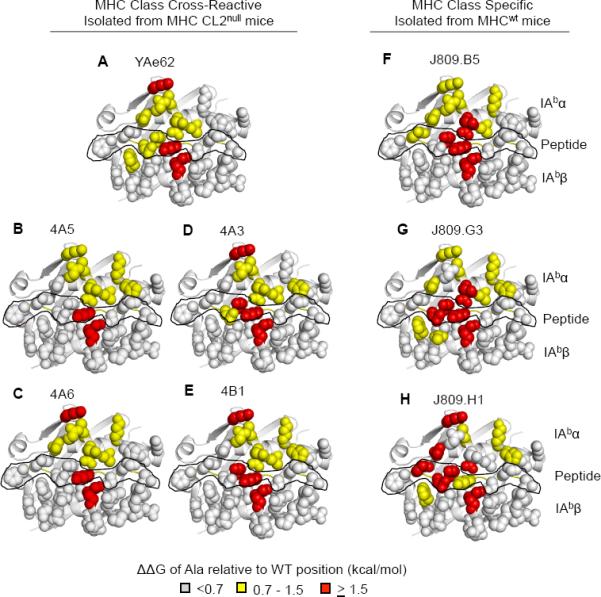
Energetic “Footprint” of MHC cross-reactive TCRs and MHC specific TCRs.
(A–E) MHC side chain contribution for binding relative to wild type IAb-3K for the (A) YAe62 TCR, (B,C) 4A5, 4A6 (Vα11+ TCRs), (D–E) 4A3, 4B1 (Vα5+ TCRs) isolated from MHC CL2null YAe62β mice.
(F–H) MHC side chain contribution of binding relative to wild type IAb-3K for the J809.B5, J809.G3, or J809.H1, (Vα2+ TCRs) isolated from MHCwt YAe62β mice.
Individual IAb-3K alanine substitutions covering the potential TCR binding surface were made in the IAbα chain, IAbβ chain and 3K peptide (see Figure S5). ΔΔG >1.5 kcal/mol are colored red, ΔΔG = 0.7–1.5 kcal/mol colored yellow, ΔΔG <0.7 kcal/mol are colored light gray. The peptide residues are outlined in black. Data are averages of three independent measurements.
Both MHC specific TCRs and MHC cross-reactive TCRs bind IAb with a conventional footprint. This is visualized in Figures 3 and S3 by color-coding IAb-3K residue side chains according to the amount of binding energy contributed to TCR binding. Surprisingly, there is an overall lack in requirements for IAbβ side chain residues, the portion of the MHC that is primarily contacted by the TCRα chain. These data strongly suggests Vα2 residues are not creating specificity through additional contacts with the MHC. The only IAbβ side chain required for high affinity binding for all of the TCRs is R70. For the IAbα residues, primarily contacted by the TCRβ chain, all TCRs relied on IAbα residues K39, L60, N62 and V65 for at least medium affinity binding (>0.8 kcal/mol), whereas none of the TCRs require V72 nor K75. Consistent with observations from previous studies (Huseby et al., 2006), MHC specific TCRs on average rely more on peptide and MHC residue side chains for strong binding (>1.5 kcal/mol) than MHC cross-reactive TCRs (Figures 3, S3 and Table S3). The increase in peptide dependence is similarly observed in polyclonal T cell responses isolated from MHCwt versus MHC CL2null mice (Figure S3).
MHC specific Vα2+ TCRs were observed to use energetically unique binding energetics at likely TCRβ-MHC contacts, while MHC cross-reactive TCRs used energetically similar TCRβ-MHC binding modes (Figures 4, S4). The TCR Vβ8.2 chain within the parent YAe62 TCR primarily contacts the IAbα chain at αK39, αQ57, and αQ61 using the TCR CDR2β residues Y46, Y48 and E54, along with CDR3β residues W95 contacting αQ61. Since all of the TCRs investigated here carry the same TCR Vβ chain, changes in binding affinity (ΔΔG) at TCR Vβ-pMHC contacts can be solely ascribed to modifications induced by differential TCRα chain pairing. Each of the MHC specific Vα2+ TCRs had differential requirements for the IAb αQ57 or αQ61 side chains relative to the parent YAe62 TCR (Figure 4). The TCRβ residues, Y46, Y48 and E54, were often required for both MHC specific and cross-reactive TCRs to bind IAb-3K. Thus, the generation of TCRs containing the Vα2 gene segment results in receptors which have modified how the identical TCR Vβ gene residues bind pMHC.
Figure 4.
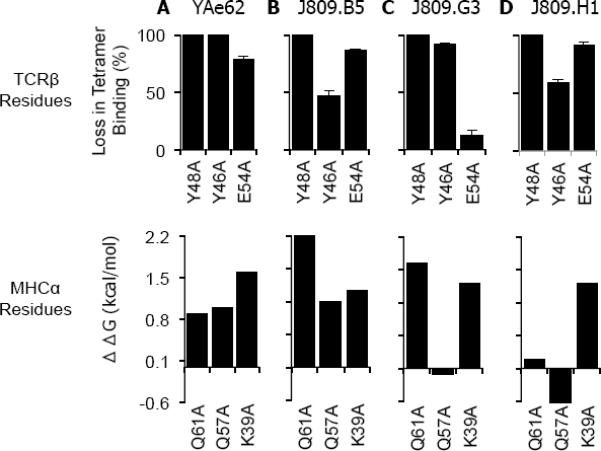
TCRα pairings that create MHC specific TCRs change the binding energetics of the YAe62β chain at IAb α-chain residues αQ61 and αQ57.
(A–D) The (A) YAe62, (B) J809.B5, (C) J809.G3 and (D) J809.H1 TCRs and each TCR carrying alanine substitutions at βY48, βY46 and βE54 were expressed on insect cells and stained with IAb-3K tetramer. Data are percent loss of tetramer binding as compared to the wild type TCRs. Data for the TCR substitution mutations are the average of at least three experiments. Error bars are the standard error of the independent experiments.
(E–H) The ΔΔG of (A) YAe62, (B) J809.B5, (C) J809.G3 and (D) J809.H1 TCRs binding IAb-3K carrying alanine substitutions at IAb αQ57, αQ61 and αK39. The ΔΔG values are those measured by SPR (αQ57 to A, αQ61 to A, Table S1) or calculated from TCR multimer staining (αK39 to A, Figure S5).
Structural comparison of an MHC specific and an MHC cross-reactive TCR
To identify in detail how paring a Vα2 TCR α-chain with the YAe62 β-chain creates pMHC specificity, we determined the 2.7Å crystal structure of the J809.B5 TCR bound to IAb-3K and compared it to the 3.2Å structure of the YAe62 TCR bound to IAb-3K (Table S4) (Dai et al., 2008). The shared use of the identical Vβ chain provides a direct way to evaluate how different Vα pairings control ligand specificity. The overall orientation and size of the interfaces of the two complexes were highly similar (Figure 5A–E). Additionally, the conformation of the TCR Cα, and TCR Cβ domains, and the TCR Vα and TCR Vβ framework regions, superpose closely. The total buried surface area (BSA) for the J809.B5 complex is approximately 1560 Å2, comparable to 1410 Å2 for the parent YAe62 complex. In both cases the TCRβ chain contributes the majority of contacts to IAb-3K, 74% of the total BSA for the J809.B5 TCR, versus 67% for YAe62 TCR (Figure 5 F–G). Some conformational differences were observed for the TCRα subunit, which was expected due to the alternate α-chain usage (Figure 6). Thus, as was observed with the energetic footprint analysis (Figure 3), the J809.B5 TCR does not create self-tolerance from gross structural alterations.
Figure 5.
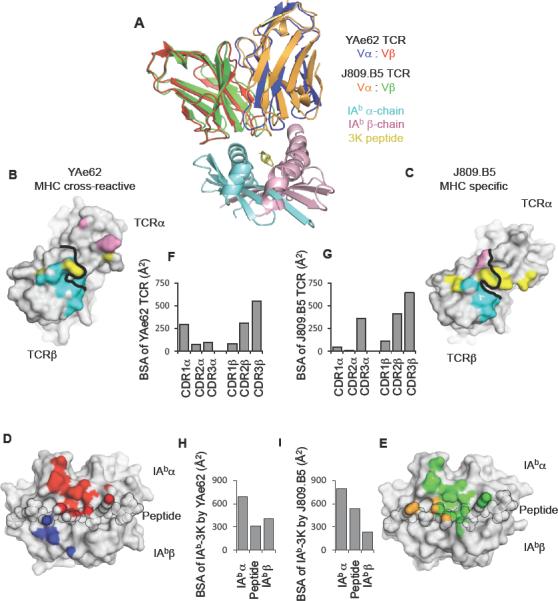
MHC cross-reactive and MHC specific TCRs bind IAb-3K within a similar footprint.
(A) Overlay of YAe62 and J809.B5 TCRs binding IAb-3K. The YAe62 TCR is colored red (TCRβ) and blue (TCRα); the J809.B5 TCR is colored green (TCRβ) and orange (TCRα). IAb-3K is colored cyan (IAbα chain), yellow (peptide) and magenta (IAbβ chain).
(B, C) Projection of IAb-3K binding onto the (B) YAe62 TCR, or (C) J809.B5 TCR. Contacts with the IAbα chain (colored cyan), peptide (colored yellow) and the IAbβ chain (colored magenta). Black line demarcates border of the TCRα subunit from the TCRβ subunit.
(D, E) Projection of the (D) YAe62 TCR or (E) J809.B5 TCR binding onto IAb-3K. YAe62 TCRα contacts are colored blue, YAe62 TCRβ contacts are colored red. The J809.B5 TCRα contacts are colored orange and the TCRβ contacts are colored yellow. The peptide residues are outlined in black.
(F–I) The amount of Buried Surface Area (BSA) contributed by the (F) YAe62 or (G) J809.B5 TCRα and TCRβ loops to the binding reaction with IAb-3K. (H) The amount of BSA contributed by the peptide or MHC chains for the binding reaction with YAe62 TCR or the (I) J809.B5 TCR. Figures were made using PyMol (DeLano).
Figure 6.
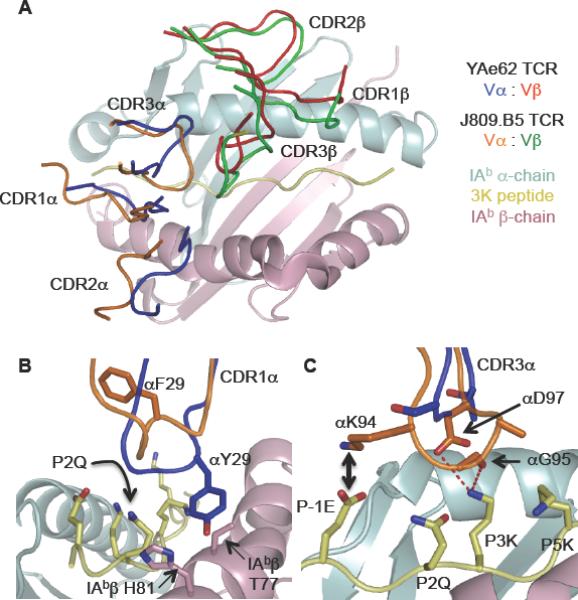
The J809.B5 TCRα chain induces a TCR rigid body movement and replaces the YAe62 TCRα contacts to MHC with TCRα contacts to the peptide.
(A) A rigid body movement rotates the J809.B5 TCRβ chain 1–2 Å towards the peptide; J809.B5 TCRβ (green), J809.B5 TCRα (orange), YAe62β (red) and YAe62α (blue).
(B) The YAe62 CDR1 αY29 (blue) contacts IAb βT77 and βH81 whereas as the J809.B5 CDR1α (orange) makes no contact with the IAbβ chain.
(C) The J809.B5 CDR3α (orange) makes extensive contacts with the P-1E and P3K residues of the peptide whereas the YAe62 CDR3α (blue) makes very few. Salt bridge (black arrow) and hydrogen bonds (red dashed lines) between J809.B5 CDR3α and the 3K peptide are shown. Figures were made using PyMol (DeLano).
Alternative TCRα to pMHC contacts by the J809.B5 TCR versus the YAe62 TCR explains the change in peptide recognition of J809.B5. The majority of the YAe62 TCRα contacts with IAb-3K involve CDR1α Y29 engaging the IAbβ chain residues T77 and H81 (Figure 6B, blue). This MHC-dominant binding mode allows the YAe62 TCR to be less-sensitive to alanine substitutions at several residues of the peptide. In contrast, the J809.B5 TCRα does not make any substantial contacts with the IAbβ chain (Figure 6B, orange), consistent with the energetic binding data (Figure 3). However, the J809.B5 CDR3α residues make extensive interactions with the peptide, including salt bridges and hydrogen bonds to the peptide side chains at P-1E, P3K and P5K, (Figure 6C, orange), explaining why these peptide residues contribute 1.1, >2.2 and >2.2 kcal/mol, respectively, of binding affinity (Table S2).
The J809.B5 TCR binds IAb-3K with an altered TCRβ-pMHC binding mode
Despite having the identical TCRβ sequence, the TCRβ chains within the J809.B5 and YAe62 TCRs bind IAb-3K differently. The J809.B5 Vα2 chain pairing results in the TCRβ binding IAb-3K with a modified CDR3β loop conformation, and a rigid body movement that rotates the TCRβ chain 1–2 Å towards the center of the interface (Figure 6A, compare J809.B5 CDR3β (green) with YAe62 CDR3β ( red)). In conjunction with the modified TCRβ-pMHC binding mode a set of TCRα to TCRβ inter-chain contacts are observed between TCR CDR1α, CDR2α and CDR3α residue side chains (orange) with the CDR3β loop (green) (Figure 7A). Most prominent are contacts between CDR1α Y31 and CDR2α R50 with CDR3 Jβ D97, and between CDR3 Jα A96 with CDR3 Jβ W95, contacts that are not present in the MHC cross-reactive YAe62 TCR.
Figure 7.
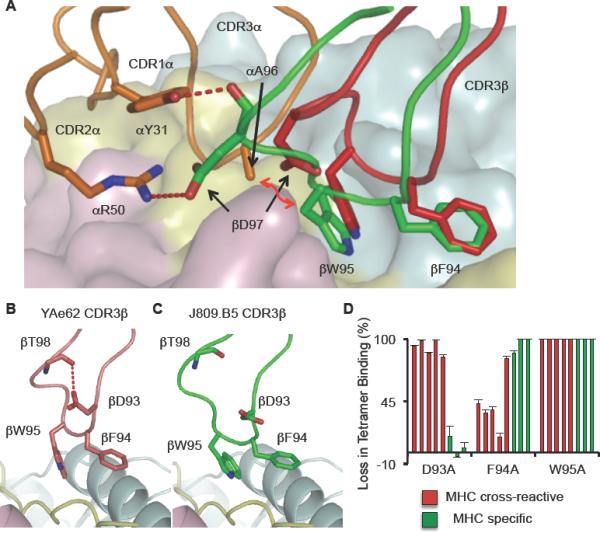
TCRα chains can induce a conformational change to the TCRβ CDR3 loop.
(A) The J809.B5 chain (green) is in an altered conformation as compared to the identical CDR3β chain within the YAe62 TCR (red). The J809.B5 CDR3β chain creates a hydrogen bond (red dashed lines) with J809.B5 CDR1 αY31, CDR2 αR50 and a Van der Waals contact (red arrow) with CDR3 JαA96.
(B, C) Within the (B) YAe62 TCR an internal hydrogen bond is created between CDR3 βD93 and the main chain of βT98. This internal hydrogen bond is absent within the J809.B5 TCR.
(D) The MHC cross-reactive and MHC specific TCRs and each TCR carrying alanine substitutions at βD93, βF94 and βW95 were expressed on insect cells and stained with IAb-3K tetramer. The MHC cross-reactive TCRs (red bars) are displayed in the order: YAe62, 4.A3, 4.B1, 4.A5 and 4.A6. The MHC specific TCRs (green bars) are displayed in the order: J809.B5, J809.G3 and J809.H1. Data are percent loss of tetramer binding as compared to the wild type TCRs. Data for the TCR substitution mutations are the average of at least three experiments. Error bars are the standard error of the independent experiments.
Differential TCRα chain pairing results in TCRs with altered ligand recognition at key TCRβ contacts of the MHC and peptide. A favorable contact between IAb αQ61 and a cluster of residues contributed by CDR1β, CDR2β, and CDR3β (Figure S5) results in a high affinity interaction for the J809.B5 TCR (Figure 2). For the YAe62 TCR, this contact is less favorable, because the YAe62 Vα T97 residue (not shown) forces IAb αQ61 to adopt an alternate rotamer that makes different contacts with CDR3β (Figure S5). The side chain rotamer changes at IAb αQ61 and P5K of the peptide allows the J809.B5 Vβ W95 to insert into a pocket created by the P5K, IAb αQ61 and αN62 residues despite having a different CDR3β conformation. Overall, the J809.B5 TCR creates pMHC specificity by increasing the number of CDR3α contacts with peptide, and using a modified set of TCRβ contacts with both the peptide and MHC.
The J809.B5 TCR and YAe62 TCR structural comparison demonstrates that one control mechanism which impacts ligand specificity is the ability to modify how TCR CDR loop residues bind pMHC. To determine if additional αβTCR chain pairings result in receptors with an altered contribution of specific CDR3β residues for binding IAb-3K, we expressed IAb-3K reactive TCRs carrying alanine substitutions at the CDR3β residues D93, F94 and W95 and measured how these substitutions impacted ligand binding. The parent YAe62 TCR creates an H-bond between the CDR3β D93 side chain and the CDR3β backbone at T98 (Figure 7B). The disruption of this contact eliminates the ability of the YAe62 TCR to bind IAb-3K (Figure 7D). In contrast, this H-bond is not present in the J809.B5 TCR due to the CDR3β conformational change and thus, the D93 to alanine substitution has minimal effect on binding IAb-3K (Figure 7C, D). Analysis of the other TCRs revealed that all MHC cross-reactive TCRs required CDR3β D93 for binding to IAb-3K, while this residue was not required for any MHC specific TCRs. The MHC specific TCRs are more dependent upon CDR3β F94 for binding, while the W95 residue is required for all of the TCRs. Taken together, these results indicate that differential αβTCR chain pairings result in MHC specific TCRs which have changed how the TCR CDR3β chain engages IAb-3K.
DISCUSSION
The identical set of TCR V genes are used to create TCRs that are specific for either classical or non-classical MHC class I or MHC class II ligands. Although there are reports of some TCR V genes being preferentially used in either CD4+ or CD8+ T cell populations (DerSimonian et al., 1991; Jameson et al., 1990; Sim et al., 1996), none of the V genes are precluded from creating MHC class I or MHC class II specific receptors (Garman et al., 1986; Jorgensen et al., 1992; Valkenburg et al.). Thus, TCRs are able to utilize a limited set of TCR V gene residues to create receptors that bind the highly diverse set of ligands. The dichotomy that individual TCR chains can be incorporated into receptors that are able to bind a wide range of polymorphic MHC ligands, while complete receptors can specifically engage a limited set of MHC ligands, strongly suggests αβTCR chain pairings limit MHC cross-reactivity. The isolation of MHC specific and MHC cross-reactive, IAb-3K reactive TCRs carrying the identical TCRβ chain allowed us to identify mechanisms by which TCRs become MHC specific.
Several mechanisms likely allow TCRαβ chain pairing to create receptors that are specific for MHC ligands. These could include the binding requirements of each TCR chain for the pMHC, modification of CDR loop conformational structures, CDR loop positioning or induced alterations of the TCR or pMHC complexes that arise during binding (Jorgensen et al., 1992). Not surprisingly, the self-tolerant, MHC specific TCRs carrying the YAe62β chain required binding energy to be contributed from multiple residues of the peptide at likely TCRα contacts. However, MHC specific TCRs did not require additional MHC contacts at potential TCRα binding sites. Within the J809.B5 TCR-pMHC structure, no contacts were observed between the CDR1α and CDR2α loops with IAb-3K. Our data further demonstrated that TCRα pairings, which created MHC specific TCRs, had a modified TCRβ binding reaction with pMHC. Thus, TCRα chains did not influence pMHC specificity by requiring additional MHC class or allele specific contacts. This was a surprising observation given the strong Vα2 gene segregation to MHC specific TCR, and suggested this skewing was not based on TCR Vα gene residues providing contacts with the pMHC.
The phenomena of TCR V gene skewing is well documented in graft rejection, responses to viruses, autoimmunity and MHC restriction (Fink et al., 1986; Urban et al., 1988; Winoto et al., 1986). Three major TCR V gene skewings occur; the use a particular TCR V gene family with diverse CDR3 sequences, the selection for TCRs which carry a particular CDR3 N-D-J sequence motif, and those T cell responses which select the identical TCRα or TCRβ sequence (Turner et al., 2006). The investigations of the molecular basis of TCR Vα skewing have focused on identifying particular TCR residues that mediate or stabilize pMHC binding, and have found heavily CDR3 dependent binding TCRs, as well as TCRs which require multiple contacts between germline CDR1 and CDR2 residues with MHC (Borg et al., 2005; Maynard et al., 2005). Experiments here suggest an additional role for particular TCR V gene residues in allowing or restraining MHC cross-reactivity, and thus creating MHC ligand specificity.
The two IAb-3K-reactive TCRs (4.A5 and 4.A6) which cross-react with CD1d-PBS57 use the identical or near identical CDR3α sequence as the Jα50+ non-canonical CD1d-reactive TCR 143.3 (Behar et al., 1999), and a set of Vα10+ non-canonical CD1d-reactive TCRs (Uldrich et al., 2011). The recent structure of a Vα10-Jα50 TCR bound to CD1d indicates these TCRs bind CD1d with a relatively similar docking mode as the canonical Vα14-Jα18 NKT TCRs (Uldrich et al., 2011). This TCR-CD1d docking footprint is highly divergent from the docking site of TCRs on MHC class II proteins. These binding site differences suggest the 4.A5 and 4.A6 TCR CDR loops are either sufficiently flexible, like the parent YAe62 TCR, to adopt two distinct conformations allowing similar amino acids to bind different classes of MHC ligands or these TCRs are in the same confirmation and bind MHC class II and CD1d with different strategies (Colf et al., 2007; Yin et al., 2011). The conservation of the CDR3α sequences of non-canonical TCRs binding CD1d strongly suggest the Jα50 sequence of the 4.A5 and 4.A6 TCRs is contributing to the MHC class cross-reactivity. In addition, the strong bias to express a Vα11+ TCR suggests the germline residues are also involved in creating reactivity for CD1d and or IAb-3K.
In contrast to the Jα50+ MHC cross-reactive TCRs, the three MHC specific Vα2+ TCRs studied in detail were generated from two different Jα family members and had CDR3α lengths of 12, 13 and 14 amino acids. Surprisingly, the Vα2+ TCRs did not require additional binding affinity to be contributed from MHC contacts at potential TCRα binding sites. Within the J809.B5 TCR structure, the CDR1α and CDR2α residues do not contact the pMHC. Instead germline encoded residues of the Vα2 loops along with CDR3α residues make direct contacts with the TCRβ chain. These TCRα to TCRβ inter-chain contacts may create or stabilize the altered TCR CDR3β conformation. Thus, germline encoded TCR V sequences may aid the generation of pMHC ligand specificity by providing inter-chain contacts which influence the placement or conformations of paired TCR chain loops.
The pairing of the YAe62β chain with Vα2 TCRα chains, creating MHC specific TCRs, resulted in the Vβ8.2 portion of the TCRs to engage IAb-3K with diverse interactions. The Vβ8.2 gene segment contains CDR2β residues Y46, Y48 and E54, which have been found to make pairwise contacts with the IA residues α39K, α57Q and α61Q (Garcia et al., 2009; Maynard et al., 2005); a proposed “interaction codon” that guides TCR recognition of pMHC. Most of the TCRs studied here used all three of the CDR2β residues Y46, Y48 and E54 in binding IAb-3K, consistent with the findings that the same V gene residues are often used to bind pMHC (Burrows et al., 2010; Rudolph et al., 2006). In addition, the MHC IAbα side chains of the interaction codon, α39K, α57Q and α61Q, were not always required by the MHC specific TCRs to bind IAb-3K. These observations are inconsistent with the model that MHC reactivity arises from structurally encoded specific pairwise contacts between TCRs and MHC (Garcia et al., 2009). Rather, our data indicates individual TCR CDR loop residues can engage pMHC with an array of interactions, which can be influenced by the paired TCR chain. TCR-pMHC binding specificity will likely be impacted by allelic variations of TCRα and TCRβ sequences as well (Gras et al., 2010). The variable requirements for centrally located, often recognized MHC side chains have been noted for TCR binding to MHC class I as well (Ding et al., 1998; Rudolph et al., 2006). The highly conserved, MHC class I residues Q65, T69 and Q155 are invariably contacted by most bound TCRs, yet they also contribute variable amounts of binding energy for individual TCRs (Burrows et al., 2010).
How often TCRα to TCRβ contacts impact ligand specificity is unknown. All TCRs make extensive TCRα to TCRβ inter-chain contacts which can occur between residues of the two CDR3 loops, and between residues of CDR3 with CDR1 and CDR2. Analyses of the few structures of TCRs which carry an identical TCRα or TCRβ sequence support the idea that TCRα to TCRβ interactions can modify how TCRs bind MHC ligands. Though still specific for the same antigen, the pairing of a Vβ7+ versus a Vβ8.2+ TCRβ chain with the canonical Vα14Jα18 NKT TCRα chain resulted in residues of the CDR3α chain to adopt different rotamers (Pellicci et al., 2009). Localized side chain rotamer changes at the TCR-pMHC binding site were similarly observed for a set of human TCRs, the TK3 receptor and altered versions carrying TCRβ micropolymorphisms (Gras et al., 2010). Consistent with the hypothesis that TCR chains can modify the ligand specificity of the paired chain, TCRs containing a TCRα chain from the 149.42 TCR specific H2-Kb + OVA can be incorporated into TCRs specific for H2-Db + influenza (Valkenburg et al., 2010). However, the mechanism which allows the 149.42 TCRα chain to bind an alternate unique ligand is currently unknown. In addition, molecular modeling experiments suggest TCR inter-chain contacts will contribute to the specificity of human TCRs reactive to influenza as well (Zhong et al., 2007). TCRα induced changes to TCRβ were not, however, observed in the structures of the 2C TCR and the highly similar, high affinity variant M6 bound to H2-Ld (Colf et al., 2007). Thus, the impact of chain pairing on how each TCR α or β chain binds pMHC ligands can span from no effect all the way to the rigid body movements and CDR3 conformational changes observed for the J809.B5 and YAe62 TCRs.
The strength of the interaction of the TCR with MHC ligands does not appear to regulate MHC specificity, as MHC specific and MHC cross-reactive TCRs had similar equilibrium affinity for IAb-3K. These data are consistent with our previous findings indicating that peptide cross-reactive TCRs and peptide specific TCRs having similar equilibrium affinities (Huseby et al., 2006; Huseby et al., 2005). In other systems, cross-reactive pMHC ligands have been identified for a multitude of different T cells. The affinities or avidities for the cross-reactive ligands have been demonstrated to be weak or strong (Krogsgaard and Davis, 2005; Stone et al., 2009). For the MHC class cross-reactive ligands described here, the 4.A5 and 4.A6 TCRs have a weak interaction with the non-classical MHC class I ligand, CD1d-PBS57, though it is possible that other bound ligands may facilitate stronger affinity interactions with these TCRs (Wun et al.). In contrast, the YAe62 TCR is strongly allo-reactive to self peptides presented by H-2k MHC class I proteins, and recognizes a H2-Kb ligand with an equilibrium affinity of 15μM (Huseby et al., 2005; Yin et al., 2011). These data indicate TCR recognition of the cross-reactive MHC ligand does not have an intrinsically strong or weak affinity. TCR-pMHC affinity certainly dictates the threshold of whether a T cell functionally engages a cross-reactive ligand; however, our data strongly suggest TCR specificity for unique MHC ligands in not based on affinity.
Despite intense research and an increasing number of TCR-pMHC structures, the reason why TCRs are prone to being self-reactive and how specificity arises for foreign antigens is largely lacking in detail. We have demonstrated that differential TCRα paring can alter how TCRβ chains engage MHC, and influence the pMHC specificity of the TCR. The ability to affect TCR-MHC ligand specificity by subtle binding site reorganization and loop conformational changes through differential chain pairing likely enhances the efficiency of creating an αβTCR repertoire that can bind all the possible MHCs present within the species, while simultaneously providing a mechanism for limiting the self-reactive and cross-reactive nature of TCRs.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, β2-microglobulin (b2m−/−), Invariant chain−/− (Cd74−/−), and TCR Cα−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). H-2Ab1−/− (Class II−/−) mice were purchased from Taconic (Germantown, NY). MHCwt: TCR Cα+/− C57BL/6 mice; MHC CL2null: TCR Cα+/−, Cd74−/− H-2Ab1−/−, C57BL/6 mice; MHCnull: TCR Cα+/−, B2m−/−, Cd74−/− H-2Ab1−/−, C57BL/6 mice. Tcrb Tg mice were established by expressing rearranged PCR cloned TCRs using the human CD2 promoter (Zhumabekov et al., 1995). The Tg TCRβ chain is expressed on >99% of all CD4+ and CD8+ T cells. All mice were maintained in a pathogen-free environment in accordance with institutional guidelines in the Animal Care Facility at the University of Massachusetts Medical School.
Recombinant Vaccinia viruses and immunization of mice
Recombinant vaccinia viruses expressing the peptide epitope 3K or P-1K fused to the carboxyl termini of full length IAbβ (Vac:IAb-3K or Vac:IAb-P-1K) were constructed using standard techniques. Mice were infected IP with 107 PFU Vac:IAb-3K or Vac:IAb-P-1K. The 3K peptide is FEAQKAKANKAVD, numbered P-2 to P11, the P-1K peptide is FKAQKAKANKAVD.
Calculation of ΔG and ΔΔG
Free energy of binding (ΔG) was calculated using the equation:
where R is the gas constant 1.987 cal/(mol*K) and T is Temperature in degrees Kelvin. To calculate the amount of binding energy contribution by a parental side chain, the free energy of the parental side chains was compared to an alanine substitution at the given position. Thus, the parental side chains' contribution was determined as:
Crystallization and data collection
J809.B5 TCR and IAb-3K proteins were mixed at 10 mg/ml each and crystallized by hanging-drop vapor diffusion at room temperature over 12% PEG 4000, 100mM sodium citrate and 100mM sodium cacodylate, at pH 5.0. Crystals about 25μm × 25μm × 100μm typically formed within a few days. For data collection, crystals were transferred to crystallization buffer containing 25% (w/v) glycerol and were flash-cooled by plunging into liquid nitrogen. X-ray diffraction data were collected from a single crystal at 100°K using 1.10 Å radiation at the National Synchrotron Source X25 undulator beamline at Brookhaven National Laboratory. Diffraction data were indexed, integrated, and scaled using HKL2000 (Otwinowski and Minor, 1997). Unit cell parameters and data collection statistics are shown (Table S4). See Supplemental data for structural determination and analysis. Coordinates and structure factors for the J809.B5 TCR-IAb-3K complex will be available from the Protein Data Bank under accession number, 3RDT.
Nomenclature and amino acid numbering
Vβs and Vαs are named and their amino acids numbered according to the IUIS/Arden compilation (Arden et al., 1995).
Supplementary Material
Highlights
MHCII-reactive TCRs can cross-react with classical and non-classical MHCI ligands
Specific and cross-reactive TCRs bind MHCII with a conventional footprint
MHC specific TCRs utilize divergent interactions to bind the same pMHC complexes
Differential αβ TCR chain pairing can result in modified TCRβ-pMHC binding
ACKNOWLEDGEMETNS
The authors would like to thank Angela Bean, Michelle Paczosa and Dr. Vijay Vanguri for assistance with some experiments and helpful discussions. This work was supported by a Searle Scholars Award to E.S.H. and NIH grants to E.S.H. (RAI088495A and RC1 DK086474 NIH/NIDDK, PI Kang), NIH grants to L.J.S. (R01-AI-38996 and U19-AI-57391, PI Rothman), and a NIH training grant to B.D.S. (T32 AI 007349). E.S.H. is a member of the UMass DERC (grant DK32520). The following tetramer and biotinylated monomer was obtained through the NIH Tetramer Facility: (CD1d-PBS57).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Supplemental Data for additional Experimental Procedures.
The authors declare no conflict of interest.
REFERENCES
- Al-Lazikani B, Lesk AM, Chothia C. Canonical structures for the hypervariable regions of T cell alphabeta receptors. J Mol Biol. 2000;295:979–995. doi: 10.1006/jmbi.1999.3358. [DOI] [PubMed] [Google Scholar]
- Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- Borg NA, Ely LK, Beddoe T, Macdonald WA, Reid HH, Clements CS, Purcell AW, Kjer-Nielsen L, Miles JJ, Burrows SR, et al. The CDR3 regions of an immunodominant T cell receptor dictate the 'energetic landscape' of peptide-MHC recognition. Nat Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- Burrows SR, Chen Z, Archbold JK, Tynan FE, Beddoe T, Kjer-Nielsen L, Miles JJ, Khanna R, Moss DJ, Liu YC, et al. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proc Natl Acad Sci U S A. 2010;107:10608–10613. doi: 10.1073/pnas.1004926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMol molecular graphics system. DeLano Scientific; San Carlos, California: 2002. [Google Scholar]
- DerSimonian H, Band H, Brenner MB. Increased frequency of T cell receptor V alpha 12.1 expression on CD8+ T cells: evidence that V alpha participates in shaping the peripheral T cell repertoire. J Exp Med. 1991;174:639–648. doi: 10.1084/jem.174.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Jameson SC, Fink PJ. V beta 5+ T cell receptors skew toward OVA+H-2Kb recognition. J Immunol. 1994;152:1790–1801. [PubMed] [Google Scholar]
- Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- Fink PJ, Matis LA, McElligott DL, Bookman M, Hedrick SM. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986;321:219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol. 2009;10:143–147. doi: 10.1038/ni.f.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman RD, Ko JL, Vulpe CD, Raulet DH. T-cell receptor variable region gene usage in T-cell populations. Proc Natl Acad Sci U S A. 1986;83:3987–3991. doi: 10.1073/pnas.83.11.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc Natl Acad Sci U S A. 2010;107:8724–8729. doi: 10.1073/pnas.1000966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S, Chen Z, Miles JJ, Liu YC, Bell MJ, Sullivan LC, Kjer-Nielsen L, Brennan RM, Burrows JM, Neller MA, et al. Allelic polymorphism in the T cell receptor and its impact on immune responses. J Exp Med. 2010;207:1555–1567. doi: 10.1084/jem.20100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housset D, Malissen B. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity? Trends Immunol. 2003;24:429–437. doi: 10.1016/s1471-4906(03)00180-7. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Crawford F, White J, Marrack P, Kappler JW. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Kaye J, Gascoigne NR. A T cell receptor V alpha region selectively expressed in CD4+ cells. J Immunol. 1990;145:1324–1331. [PubMed] [Google Scholar]
- Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- Jorgensen JL, Esser U, Fazekas de St Groth B, Reay PA, Davis MM. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992;355:224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, Pereyra F, Carrington M, Walker BD, Chakraborty AK. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465:350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard M, Davis MM. How T cells `see' antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Graf D, Lovatt M, Bommhardt U, Zamoyska R, Fisher AG. How many thymocytes audition for selection? J Exp Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, et al. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Waller MJ, Fail SC, McWilliam H, Lopez R, Parham P, Marsh SG. The IMGT/HLA database. Nucleic Acids Res. 2009;37:D1013–1017. doi: 10.1093/nar/gkn662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- Sim BC, Zerva L, Greene MI, Gascoigne NR. Control of MHC restriction by TCR Valpha CDR1 and CDR2. Science. 1996;273:963–966. doi: 10.1126/science.273.5277.963. [DOI] [PubMed] [Google Scholar]
- Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol. 2006;6:883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, Kyparissoudis K, Kjer-Nielsen L, Vivian JP, Cao B, et al. A semi-invariant Valpha10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12:616–623. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, Ando DG, Sercarz EE, Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988;54:577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Valkenburg SA, Day EB, Swan NG, Croom HA, Carbone FR, Doherty PC, Turner SJ, Kedzierska K. Fixing an irrelevant TCR alpha chain reveals the importance of TCR beta diversity for optimal TCR alpha beta pairing and function of virus-specific CD8+ T cells. Eur J Immunol. 2010;40:2470–2481. doi: 10.1002/eji.201040473. [DOI] [PubMed] [Google Scholar]
- Winoto A, Urban JL, Lan NC, Goverman J, Hood L, Hansburg D. Predominant use of a V alpha gene segment in mouse T-cell receptors for cytochrome c. Nature. 1986;324:679–682. doi: 10.1038/324679a0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Call MJ, Deng L, Mariuzza R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr Opin Immunol. 2009;21:590–595. doi: 10.1016/j.coi.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 34:327–339. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Huseby E, Scott-Browne J, Rubtsova K, Pinilla C, Crawford F, Marrack P, Dai S, Kappler JW. A Single T Cell Receptor Bound to Major Histocompatibility Complex Class I and Class II Glycoproteins Reveals Switchable TCR Conformers. Immunity. 2011;35:23–33. doi: 10.1016/j.immuni.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- Zhong W, Dixit SB, Mallis RJ, Arthanari H, Lugovskoy AA, Beveridge DL, Wagner G, Reinherz EL. CTL recognition of a protective immunodominant influenza A virus nucleoprotein epitope utilizes a highly restricted Vbeta but diverse Valpha repertoire: functional and structural implications. J Mol Biol. 2007;372:535–548. doi: 10.1016/j.jmb.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.