Abstract
Deficits in cognitive control, a core disturbance of schizophrenia, appear to emerge from impaired prefrontal gamma oscillations. Cortical gamma oscillations require strong inhibitory inputs to pyramidal neurons from the parvalbumin basket cell (PVBC) class of GABAergic neurons. Recent findings indicate that schizophrenia is associated with multiple pre- and post-synaptic abnormalities in PVBCs, each of which weakens their inhibitory control of pyramidal cells. These findings suggest a new model of cortical dysfunction in schizophrenia in which PVBC inhibition is decreased to compensate for an upstream deficit in pyramidal cell excitation. This compensation is thought to re-balance cortical excitation and inhibition, but at a level insufficient to generate the gamma oscillation power required for high levels of cognitive control.
Introduction
Psychosis (e.g., hallucinations, delusions and disorganized behavior) is the most striking clinical feature of schizophrenia, but impairments in cognition are now recognized as the core domain of dysfunction in the illness [1]. Cognitive deficits are present and progressive years before the onset of psychosis [2], and the degree of cognitive impairment is the best predictor of long-term functional outcome [3]. The range of cognitive deficits in schizophrenia suggests an overarching alteration in cognitive control, the ability to adjust thoughts or behaviors in order to achieve goals [4]. Cognitive control depends on the coordinated activity of a number of brain regions, including the dorsolateral prefrontal cortex (DLPFC) [5], and gamma frequency (30–80 Hz) oscillations in DLPFC neural networks are thought to be a key neural substrate for cognition [6]. Consistent with these observations, when performing tasks that require cognitive control, individuals with schizophrenia exhibit altered activation of the DLPFC [7] and lower power of frontal lobe gamma oscillations [8,9].
Because cortical gamma oscillations require the strong and synchronous inhibition of networks of pyramidal neurons (see [10] for review), deficient GABA neurotransmission in the DLPFC has been hypothesized to contribute to altered gamma oscillations and impaired cognition in schizophrenia [11]. Consistent with this interpretation, manipulations in animal models that reduce GABA-mediated inhibition diminished gamma oscillations [12] and impaired cognitive function [13–16]. In addition, in individuals with schizophrenia, negative modulation of GABAergic neurotransmission exacerbated symptoms [17], whereas positive modulation was associated with increased frontal lobe gamma oscillations during a cognitive control task [18].
However, recent surprising findings regarding the functional properties of certain subtypes of cortical interneurons and new observations regarding cell type-specific alterations in markers of GABAergic neurotransmission in schizophrenia require a new conceptualization of the role of altered cortical GABAergic signaling in the cognitive deficits of schizophrenia. Consequently, here we 1) review recent findings both from cellular physiology experiments and postmortem studies of schizophrenia that demonstrate the limitations of existing circuitry models of cognitive dysfunction in schizophrenia based on earlier data, 2) propose a new pathophysiological model of the role of altered GABA neurotransmission in cortical circuitry dysfunction in schizophrenia, and 3) discuss the key research questions raised by the new data and model.
Deficient cortical GABA synthesis is a conserved feature of schizophrenia
GABAergic signaling is regulated in part by the enzymatic activity of two isoforms of glutamic acid decarboxylase (GAD) which differentially contribute to GABA synthesis. In mice, deletion of the gene for the 67 kDa isoform of GAD (GAD67) results in a 90% reduction of brain GABA levels and is embryonically lethal [19], whereas deletion of the GAD65 gene is associated with only a 20% reduction in total brain GABA [20] and normal survival. Levels of GAD67 mRNA [21] and protein [22,23] have been consistently found to be lower in the DLPFC of subjects with schizophrenia in multiple studies using a variety of techniques. Similar deficits in GAD67 mRNA are also present in other cortical regions including sensory, motor and limbic regions [24–27]. In contrast, cortical expression of GAD65 appears to be normal or only slightly altered in schizophrenia [22,28], and the density of GAD65-labeled axon terminals in the DLPFC is unchanged [29].
The magnitude of the GAD67 deficit in schizophrenia differs substantially across individuals, raising the question of the extent to which the deficit reflects the disease process or co-morbid factors. Because GAD67 expression is activity-regulated [30], lower GAD67 expression in schizophrenia could reflect reduced cortical activity secondary to other factors that accompany a chronic psychiatric illness. However, the variability in GAD67 mRNA levels across subjects with schizophrenia is not attributable to potential confounds such as substance abuse or antipsychotic medications, predictors (e.g., male sex, a family history of schizophrenia, early age of onset), or measures of disease severity (e.g., suicide, lower socioeconomic status, not living independently, and no history of marriage), or duration of illness [23,28]. Thus, lower cortical GAD67 mRNA levels appear to be a conserved feature that is a core common component, and not a consequence, of the disease process of schizophrenia.
However, less GAD67 mRNA and protein does not necessarily support the conclusion that cortical GABA levels are lower in schizophrenia. For example, GAD67 expression could be down-regulated in response to reduced GABA metabolism; indeed, pharmacological inhibition of GABA degradation results in elevated cortical GABA and less GAD67 protein [31]. Unfortunately, current attempts to measure cortical GABA levels in vivo with magnetic resonance spectroscopy (MRS) have produced mixed results in subjects with schizophrenia [32–34]. However, lower GABA levels in the visual cortex in subjects with schizophrenia were correlated with reductions in a behavioral measure of visual inhibition that depends on GABA neurotransmission [34], and frontal lobe GABA levels tended to be correlated with working memory performance in subjects with early-stage schizophrenia [35]; both of these findings support the idea that lower GABA synthesis in schizophrenia results in cognitive impairments. However, because MRS assesses total tissue GABA levels, and not GABA levels in synaptic vesicles or the extracellular space, the relevance of MRS measures to cortical GABA synthesis and transmission remains uncertain. Alternative strategies to measure shifts in the levels of extracellular GABA are emerging and preliminary findings support a positive relationship between the capacity to increase extracellular GABA and physiological correlates (i.e., gamma oscillations) of cognitive control [36]. However, as indicated in the following sections, in vivo methods that can assess the synthesis and release of GABA from particular populations of interneurons may be required.
GAD67 deficit is prominent in parvalbumin (PV)-positive interneurons
Understanding the functional significance of lower cortical GAD67 levels requires knowledge of the affected class of interneurons. In schizophrenia, GAD67 mRNA levels are markedly lower only in 25–35% of DLPFC interneurons [37,38], and GAD67 mRNA is not detectable in ~50% of the subset of interneurons that express the calcium-binding protein parvalbumin (PV) [39]. Levels of PV mRNA are also lower in schizophrenia [24,40,41], but the densities of neurons labeled for either PV mRNA [39] or protein [42–44] in the DLPFC do not differ from comparison subjects. Together, these findings suggest that the number of cortical PV neurons is not altered in schizophrenia, but that GAD67 is markedly reduced in a subset of these neurons. In contrast, calretinin-containing interneurons, which comprise ~45% of GABA neurons in the primate DLPFC, do not appear to be affected in the illness [11].
Recent in vivo studies using optogenetic techniques have clearly established that activity in PV-positive interneurons is essential for driving cortical gamma oscillations in mice [45,46], although the particular subclass(es) of PV-positive interneurons responsible could not be determined using this approach. Cortical PV-positive interneurons consist of two main types: chandelier and basket cells (Figure 1). The axon terminals of PV-positive chandelier (aka axo-axonic) cells (PVChCs) form distinctive vertical arrays (termed cartridges) that exclusively innervate the axon initial segment (AIS) of pyramidal neurons just proximal to the site of action potential generation. In contrast, PV-positive basket cells (PVBCs) innervate the cell body and proximal dendrites of pyramidal neurons. In the following sections we review recent findings indicating that the nature of the alterations in, and the functional consequences of, schizophrenia-related molecular alterations are markedly different for PVChCs and PVBCs. It is important to note that a number of the recent findings cited below on the biological properties of PVChCs and PVBCs are from studies of the rodent neocortex or hippocampus, and thus, the extent to which they are generalizable to the primate DLPFC remains to be determined.
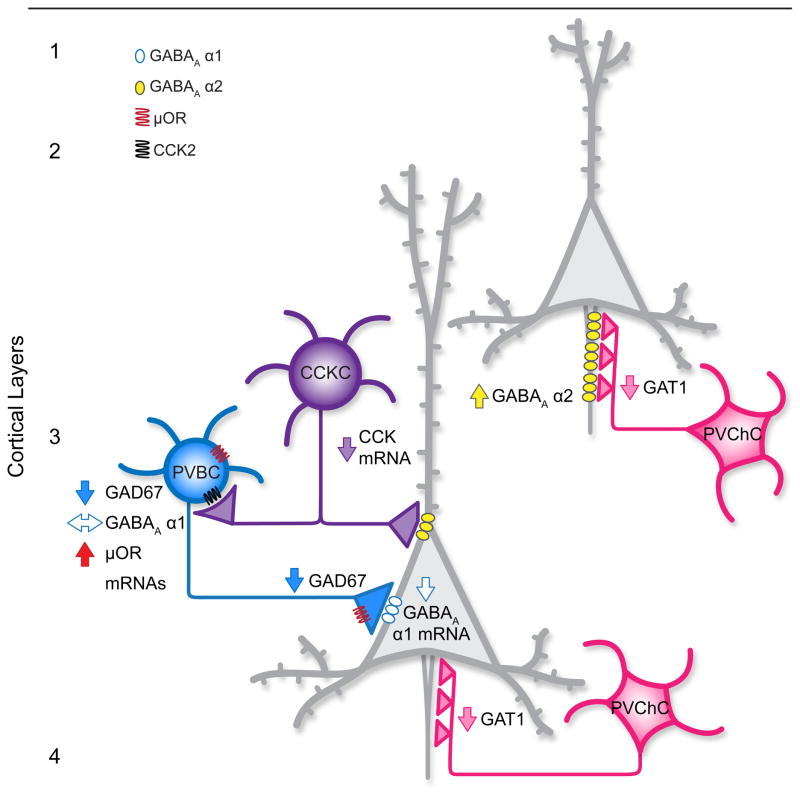
Figure 1.
Schematic summary of alterations in neuronal circuitry in layer 3 of the DLPFC in subjects with schizophrenia. The perisomatic inhibition of pyramidal neurons (gray neurons) by parvalbumin-positive basket cells (PVBCs) is reduced due to 1) lower GAD67 mRNA expression [39] and lower GAD67 protein [23], and hence less GABA synthesis; 2) higher levels of μ opioid receptor expression in PVBCs which reduces their activity and suppresses GABA release [77]; 3) reduced expression of cholecystokinin (CCK) mRNA [28,41] which stimulates the activity of, and GABA release from, PVBCs [79–81]; and 4) less mRNA for, and presumably fewer, postsynaptic GABAA α1 receptors in pyramidal neurons [63]. These alterations are shown only for the pyramidal neuron in deep layer 3, but are likely present throughout layer 3. Chandelier neurons (PVChCs) have decreased GABA membrane transporter 1 (GAT1) protein in their axon terminals [47,48] and increased postsynaptic GABAA α2 receptors in pyramidal neuron axon initial segments [49], suggesting enhanced GABA signaling and increased excitation of pyramidal neurons if these inputs are depolarizing [51,53]. Because relatively few GABAA α2-labeled axon initial segments are detectable in layers deep 3–4 of the adult primate DLPFC [100], perhaps reflecting the postnatal developmental decline in mRNA expression of this subunit in the primate DLFPC [101], it is unclear whether postsynaptic GABAA α2 receptors are increased in deep layer 3 pyramidal neurons in schizophrenia. Levels of GAD67 protein in PVChC axon terminals in schizophrenia are not known.
Alterations in PVChCs in schizophrenia: Increasing pyramidal cell excitation?
In schizophrenia, the density of PVChC axon cartridges immunoreactive for the GABA membrane transporter 1 (GAT1) is reduced in DLPFC layers 2–4 [47,48] (Figure 1). In layers 2 and superficial 3 of subjects with schizophrenia, the lower density of GAT1-labeled cartridges is inversely correlated with an increase in the density of AISs that are immunoreactive for the α2 subunit of the GABAA receptor [49] (Table 1). Consistent with these findings, GABAA α2 subunit mRNA levels are elevated in the same laminar location in schizophrenia [50]. The decrease in pre-synaptic GAT1 protein and increase in postsynaptic GABAA α2 receptor protein levels were interpreted as coordinated compensations to a PVChC deficit in GABA synthesis in schizophrenia which, by reducing GABA re-uptake and increasing the probability of receptor binding, respectively, would strengthen GABA signaling [11]. However, although GAD67 mRNA expression has been shown to be lower in PV neurons [39], GAD67 mRNA and protein levels have not been assessed specifically in PVChCs or their axon cartridges in schizophrenia.
Table 1.
Comparison of the properties of parvalbumin-positive chandelier cells (PVChCs) and basket cells (PVBCs) in schizophrenia.
| Properties | PVChCs | PVBCs | Refs |
|---|---|---|---|
| Location of synapses on pyramidal neuron | Axon initial segment (AIS) | Soma and proximal dendrites and spines | |
| Predominant GABAA receptor α subunit | α2 | α1 | [57] |
| Alterations in the DLPFC in schizophrenia | GAD67 mRNA and protein levels unknown | ↓ GAD67 protein in axon terminals in layer 3 | [23] |
| ↓ density of GAT1-positive axon cartridges in layers 2–4 | ↔ density of GAT1-positive axon terminals in layer 3 | [48] | |
| density of PV-positive axon cartridges unknown | ↓ density of PV-positive axon terminals in layer 3 | [65] | |
| ↑ GABAA α2 mRNA in layer 2 | ↓ GABAA α1 mRNA in layer 3 | [50] | |
| ↑ density of GABAA α2- positive AIS in layers 2- superficial 3 | ↓ GABAA α1 mRNA selectively in layer 3 pyramidal cells | [49,63] | |
| ↑ μ opioid receptor mRNA | [77] |
Given the presumed potent inhibitory regulation of pyramidal neurons by PVChCs, deficient GABA neurotransmission from PVChCs was previously postulated to be the neural substrate for the lower frontal lobe gamma band power observed during cognitive control tasks in schizophrenia [11]. However, recent findings raise questions about this interpretation. For example, rather than providing the strong hyperpolarization of pyramidal neuron networks required for gamma oscillations, recent findings indicate that the synaptic inputs from neocortical chandelier cells are actually depolarizing in some cases. For example, stimulation of neocortical chandelier cells can initiate spikes in postsynaptic pyramidal cells [51]. This excitatory effect may be due to much lower levels of the K+-Cl−co-transporter 2 (KCC2), which extrudes chloride, at the AIS relative to the soma or dendrites [51,52]. The resulting higher intracellular levels of chloride in the AIS would lead to the flow of chloride out of, rather than into, the AIS when GABAA receptors are activated, depolarizing the membrane. Indeed, under experimental conditions that preserve the physiological intracellular chloride concentration, PVChC inputs depolarize postsynaptic pyramidal neurons, whereas inputs from PVBCs are, as expected, hyperpolarizing [51,53].
Other recent findings also raise questions about the role of PVChCs in the generation of gamma oscillations. For example, gamma oscillations require a fast decay of the inhibitory postsynaptic current in pyramidal cells, and the duration of this postsynaptic inhibitory current depends on the subunit composition of the GABAA receptors that mediate it [54]; the kinetics of the α2 subunit-containing GABAA receptors post-synaptic to PVChCs appear to be too slow to drive a circuit at gamma frequency [10]. In addition, the firing of PVChCs in the rodent hippocampus is not strongly coupled to the gamma oscillation cycle [55], but is strongly coupled to the much slower cycle of theta oscillations (reviewed in [56]).
Alterations in PVBCs in schizophrenia: Decreasing pyramidal cell inhibition?
The PV neurons with reduced GAD67 mRNA expression in schizophrenia do include PVBCs, as lower GAD67 protein levels have been found in PVBC axon terminals (identified by excluding PVChCs axon cartridges) in DLPFC layers 3–4 in subjects with schizophrenia [23] (Figure 2). The cell type-specificity of this finding was supported by the observation that the GAD67 protein deficit in these terminals was ~10× greater than in total DLPFC gray matter from the same subjects [23]. Thus, although not conclusive, the existing data suggest that the GAD67 deficit in schizophrenia may be specific to, or at least particularly pronounced in, PVBCs. In contrast, GAT1 protein levels do not appear to be altered in the axon terminals of PVBCs in schizophrenia [47], suggesting that GABA uptake is not reduced in these terminals as it is in PVChC axon cartridges.
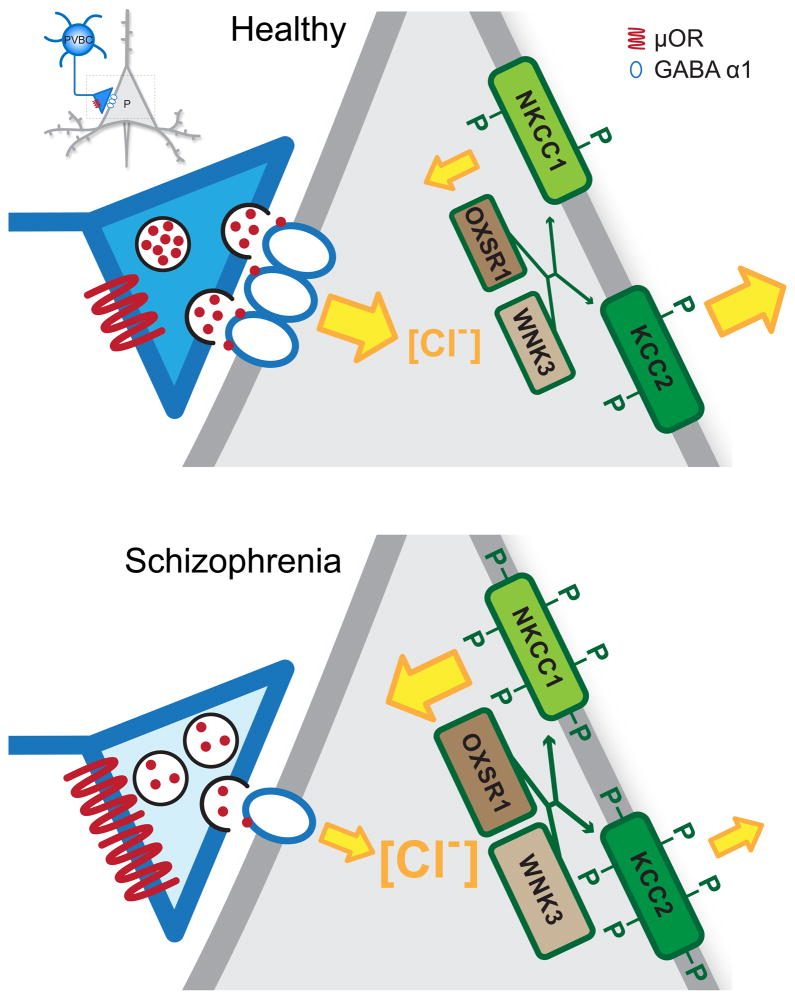
Figure 2.
Summary of regulators of GABA neurotransmission from a parvalbumin-positive basket cell (PVBC) terminal to a layer 3 pyramidal cell (gray neuron, insert) in the DLPFC from a healthy subject (top) and a subject with schizophrenia (bottom). In both panels, the size and orientation of the yellow arrows indicates the magnitude and direction of chloride (Cl−) ion flow mediated by the Cl− transporters N+-K+-Cl-cotransporter 1 (NKCC1) and K+-Cl-cotransporter 2 (KCC2) and Cl− channels in α1-containing GABAA receptors. In healthy adult neurons, intracellular Cl− concentration is low due to low levels of NKCC1 and high levels of KCC2. The binding of GABA (red dots) to α1-containing GABAA receptors triggers Cl− entry and membrane hyperpolarization. In schizophrenia, lower levels of GAD67 (light blue shading) in PVBC axon terminals [23] lead to less GABA synthesis. The release of GABA is further suppressed by greater signaling through up-regulated μ opioid receptors (μOR) [77], and the effects of GABA are reduced due to fewer postsynaptic α1-containing GABAA receptors [63]. Higher levels of two kinases (OXSR1 and WNK3) [69] are thought to lead to increased phosphorylation (green P) of both Cl− transporters and consequently increased NKCC1 activity and decreased KCC2 activity, producing a greater intracellular Cl− concentration. Thus, upon activation of the α1-containing GABAA receptors, Cl− influx is reduced and GABA neurotransmission is less hyperpolarizing.
In addition to the evidence for lower GABA synthesis in PVBCs, postsynaptic findings in pyramidal cells also support a role for weaker inhibitory inputs from PVBCs in the pathophysiology of DLPFC dysfunction in schizophrenia. These inputs are principally mediated by α1-containing GABAA receptors in hippocampal pyramidal cells [57,58]. Most [24,28,50,59], but not all [60], well-controlled studies have reported lower levels of GABAA α1 subunit mRNA in the DLPFC of schizophrenia subjects, with this decrease most prominent in layers 3 and 4 [50]. Expression of the GABAA receptor β2 subunit, which preferentially assembles with α1 subunits [61], was also lower selectively in layers 3–4 [50]. In contrast, other GABAA receptor subunits were unchanged in layer 3 (or showed a different laminar pattern of altered expression) [50,59,62]. Within DLPFC layer 3, cellular level analyses revealed that α1 subunit mRNA expression was markedly lower in pyramidal cells (Figure 2), but was unaltered in interneurons [63]. However, whether these expression changes are associated with alterations in the number of membrane-bound GABAA receptors [64] containing α1 and β2 subunits remains to be determined.
Lower pyramidal cell α1 subunit expression could indicate a lower number of inputs from PVBC axons in schizophrenia, an interpretation consistent with the lower density of PV-immunoreactive axon terminals in the same laminar location [65]. Whether this deficit reflects fewer PVBC terminals or fewer PV-containing axonal projections from the thalamus remains unresolved [65]. Interestingly, genetic reductions of GAD67 in PV interneurons during development result in less PVBC axonal arborization and synapse formation [66]. Thus, the undetectable levels of GAD67 mRNA in ~50% of DLPFC PV interneurons in schizophrenia [39], if present early in life, could lead to fewer PVBC axon terminals, and thus fewer synapses needing GABAA α1 receptors in postsynaptic cells. However, diminished PVBC axonal arbors would be expected to affect all postsynaptic cells, including other interneurons [67]. Since α1 subunit mRNA levels in interneurons were not altered in schizophrenia [63], the reductions in both GAD67 and GABAA α1 levels are likely to be attributable to some other common factor.
In addition to lower levels of these pre- and post-synaptic mediators of PVBC inputs to DLPFC layer 3 pyramidal neurons, several other findings suggest that inhibitory inputs from PVBCs are weaker in schizophrenia. First, the extent to which DLPFC pyramidal neurons can be hyperpolarized may be lower in schizophrenia. The strength of the postsynaptic response to GABA depends on the driving force for the influx of chloride when GABAA receptors are activated. Intracellular chloride levels are determined by the balance of activity between the chloride transporters N+-K+-Cl−-cotransporter 1 (NKCC1) and KCC2 which mediate chloride uptake and extrusion, respectively [68]. The expression of these transporters is not altered in the DLPFC of subjects with schizophrenia [69,70], but two kinases OXSR1 (oxidative stress response kinase) and WNK3 [with no K (lysine) protein kinase] that phosphorylate both chloride transporters are markedly over-expressed in schizophrenia [69]. Interestingly, protein levels of OXSR1 are most prominent in layer 3 pyramidal neurons in the DLPFC [69]. Assuming that the elevated levels of OXSR1 and WNK3 expression represent greater kinase activity, then increased phosphorylation of the chloride transporters would decrease the activity of KCC2 and increase the activity of NKCC1, resulting in elevated chloride levels in layer 3 pyramidal neurons in schizophrenia (Figure 2). As a result, the gradient for chloride entry associated with activation of GABAA receptors would be reduced, resulting in less hyperpolarization of layer 3 pyramidal neurons when GABA is released from PVBCs.
Second, other molecular alterations in schizophrenia point to both reduced activity of, and a suppression of GABA release from, PVBCs. For example, μ opioid receptors are present on the perisomatic region and axon terminals of hippocampal PV neurons [71,72]. Stimulation of the perisomatic μ opioid receptors activates G protein-coupled inwardly rectifying potassium channels, hyperpolarizing the cell body [73,74]; stimulation of μ opioid receptors on axon terminals suppresses vesicular GABA release [75,76]. Consistent with the role of PVBC firing and GABA neurotransmission in generating gamma oscillations, stimulation of μ opioid receptors disrupts neural network activity at gamma frequencies in the hippocampus [55]. In the DLPFC of subjects with schizophrenia, μ opioid receptor transcript levels are higher than in healthy comparison subjects (Figure 1), a difference not attributable to psychotropic medications or other factors co-morbid with schizophrenia [77]. In contrast, other markers of opioid signaling (e.g., δ and κ opioid receptors, proenkephalin, prodynorphin) are not altered in the illness [77,78]. Thus, by both reducing the activity of PVBCs and suppressing GABA release from their axon terminals, elevated levels of μ opioid receptors in schizophrenia could serve as yet another means of weakening PVBC inhibition of pyramidal neurons (Figure 2).
Third, the activity of PVBCs is also regulated by inputs from cholecystokinin (CCK)-positive basket cells via CCK2 receptors. CCK stimulates the activity of, and GABA release from, PV cells in the hippocampus [79–81]. Thus, the observed down-regulation of CCK mRNA expression in schizophrenia [28,41] might also contribute to weaker PVBC inputs to pyramidal neurons (Figure 1). Interestingly, recent studies in monkey DLPFC indicate that the terminals of CCK basket neurons (at least the subclass that also expresses the cannabinoid 1 receptor) principally contain GAD65, and not GAD67, protein [82]. Thus, because cortical expression of GAD65 appears to be normal or only slightly altered in schizophrenia [22,28], GABA neurotransmission may not be altered in CCK neurons; the positive correlation between the deficits in GAD67 and CCK mRNAs in schizophrenia [22,28] may reflect their shared downregulation in different cell types in response to an upstream reduction in cortical excitation (Box 1).
Box 1. Balancing cortical excitation and inhibition in schizophrenia.
The dynamic balance between excitation and inhibition (E/I balance) allows activity to propagate through local cortical networks without either dying out or increasing uncontrollably [94]. E/I balance is maintained in the face of perturbations in circuit activity [102], at least in part, by reciprocal, sustained adjustments in the levels of excitatory and inhibitory synaptic transmission through a process termed synaptic scaling or homeostatic synaptic plasticity [94,103,104]; that is, the amplitudes of excitatory and inhibitory postsynaptic currents are independently adjusted via scaled changes in the neurotransmitter content of synaptic vesicles and in the density of postsynaptic receptors (eg. [104] and reviewed in [104,105].
In schizophrenia, current findings suggest the hypothesis that the pre- and postsynaptic strength of PV basket cell inputs to pyramidal neurons is a downstream response to maintain E/I balance in the face of persistently lower DLPFC network excitatory activity. The idea of an upstream deficit in cortical excitatory activity is supported by evidence that schizophrenia is associated with an intrinsic deficit in excitatory drive to layer 3 pyramidal neurons. The density of dendritic spines, which reflect the number of glutamatergic synapses to pyramidal cells, is significantly decreased in schizophrenia, and this deficit is most pronounced in DLPFC deep layer 3 and present to a milder degree in superficial layer 3 [106,107]. In addition, the somal size [108,109] and dendritic tree [106] of layer 3 pyramidal neurons is smaller in schizophrenia, suggestive of a developmental disturbance in these neurons. Consistent with this interpretation, in cortical regions with layer 3 pyramidal neurons that are smaller and have lower spine density in schizophrenia (e.g., the superior temporal gyrus) [109,110], gray matter volume is lower at baseline in high risk youth [111] and progressively declines in those who subsequently convert to psychosis [112,113].
The idea that an intrinsic deficit in excitatory drive to layer 3 pyramidal cells is an upstream event is supported by the findings that the expression of certain gene products [e.g., Duo, cell division cycle 42 (Cdc42)] that regulate dendritic spine formation and maintenance are altered in schizophrenia, and these alterations are strongly correlated with DLPFC layer 3 spine deficits [114]. The particular prominence of the deficit in dendritic spines on layer 3 pyramidal neurons may reflect the layer specificity of certain molecular abnormalities. For example, Cdc42 effector protein 3 (Cdc42EP3) mRNA, which is preferentially expressed in layer 3 of human DLPFC [115], is upregulated in schizophrenia [116]. The activation of Cdc42 by glutamate stimulation is thought to inhibit Cdc42EP3 activity which in turn dissociates the complex of septin filaments in spine necks, enabling the movement from the parent dendrite of molecules required for synaptic potentiation [116]. Thus, lower levels of Cdc42 and higher levels of Cdc42EP3 might lead to a reduced capacity for glutamatergic stimuli to open the septin filament barrier in the spine neck, resulting in impaired synaptic plasticity and spine loss.
Thus, intrinsic deficits in dendritic spines resulting in lower activity of layer 3 pyramidal neurons might represent an upstream pathology that induces a number of homeostatic responses (Figure 1) to reduce PVBC inhibition of pyramidal neurons in order re-balance network levels of excitation and inhibition.
Role of PVBCs in lower DLPFC gamma oscillation power in schizophrenia
The findings summarized above suggest that a number of molecular alterations in different cell types converge to weaken PVBC inputs to pyramidal neurons in DLPFC layer 3 in schizophrenia. Interestingly, PVBCs play a critical role in the generation of cortical gamma band oscillations [10,45,46]. For example, the α1-containing GABAA receptors that are postsynaptic to PVBCs inputs in hippocampal pyramidal cells produce currents with a decay period appropriate for gamma oscillations [10]. In addition, electrophysiological findings in the hippocampus indicate that PVBC firing is more strongly coupled to the gamma oscillation cycle than is the firing of PVChCs or CCK basket cells [55]. Furthermore, gamma oscillations are significantly reduced by stimulation of the presynaptic μ opioid receptors that suppress GABA release from PVBCs, but not from PVChCs or CCK basket cells [55].
Thus, the multiple alterations that weaken PVBC inhibition of pyramidal neurons could provide the neural substrate for lower power of frontal lobe gamma band oscillations during cognitive control tasks [8,9]. The presence of such abnormalities in both the first episode [9] and chronic [8] phases of the illness are consistent with the idea that the alterations in GABA markers observed in postmortem studies are not a consequence of the treatment or chronicity of the illness [23]. Furthermore, the tendency for these alterations to be most prominent in layer 3 is consistent with evidence that circuitry in this laminar location of the primate neocortex is critical for both gamma oscillations [83] and for delay-dependent cognitive control tasks [84]. These findings, though, raise the question of whether the multiple pre- and post-synaptic factors that lower PVBC inhibition could each represent a different type of primary pathology, any of which could lead to impaired gamma oscillations and the resulting cognitive control deficits in schizophrenia. However, given the frequency of these findings in individuals with schizophrenia [23,63,69,77], and their co-occurrence in the same individuals, it seems more likely that together they represent convergent consequences or compensations to some common factor that is upstream in the disease process.
PV interneurons and cortical excitatory-inhibitory balance in schizophrenia
One possible upstream factor might be the dendritic spine deficit on layer 3 pyramidal neurons [85] which could lead to a net reduction in local DLPFC excitatory activity and impaired gamma oscillations. According to the PING (pyramidal-interneuron network gamma) model of gamma oscillations, PVBCs are recruited by phasic, glutamatergic inputs from pyramidal neurons, and PVBCs provide strong and fast (i.e., GABAA α1-receptor mediated) feedback inhibition to pyramidal neurons [86]. The divergent connections of neocortical PVBCs [87] results in the simultaneous hyperpolarization of a distributed group of pyramidal neurons, and the fast and synchronous decay of this inhibition permits the simultaneous firing of the pyramidal cells at gamma frequency (see [10] for a review). The strength of the excitatory inputs to PVBCs from neighboring pyramidal cells is likely to be lower in schizophrenia since these pyramidal cells, due to a deficit in dendritic spines and presumably fewer excitatory inputs, are thought to be less active (Box 1). Lower expression of the NR2A subunit of glutamatergic NMDA receptors in DLPFC layer 3 PV neurons in schizophrenia [88] could also contribute to reduced strength of excitatory inputs to PVBCs; interestingly, computational modeling suggests that lowering the slow excitatory current from NMDA receptors (relative to the fast excitatory current provided by AMPA receptors) increases gamma band power [89], suggesting that a downregulation of NMDA receptors might represent a compensatory response in PVBCs.
The net reduction in network excitatory activity due to layer 3 pyramidal neuron spine deficits (Box 1) might then evoke homeostatic mechanisms to reduce the inhibition of these pyramidal cells. From this perspective, all of the molecular alterations described above (Figures 1 and 2) that weaken PVBC inhibition of pyramidal neurons could be understood as compensatory responses to lower pyramidal cell inhibition and to restore excitatory-inhibitory balance (E/I balance) in DLPFC circuitry (Box 1). The idea that altered excitation is upstream of altered inhibition is supported by recent findings that genes related to glutamate signaling (and not those related to GABA signaling) appear to mediate schizophrenia susceptibility [90].
If neocortical chandelier cells are, in fact, a potent source of a slow depolarizing current in cortical pyramidal cells, then each of the protein alterations at PVChC inputs to pyramidal cells (e.g., lower presynaptic GAT1 and higher postsynaptic GABAA α2 receptors; Figure 1) would, respectively, prolong the duration and increase the strength of the excitatory postsynaptic current in the AIS. Thus, in contrast to our prior interpretation that these changes in PVChC inputs are compensations to augment pyramidal cell inhibition [11], the idea that PVChCs are excitatory suggests that the pre- and post-synaptic changes in their inputs to pyramidal neurons could provide a means to increase the type of slow, NMDA-like depolarization of pyramidal cells that is thought to be essential for the DLPFC neural network activity associated with cognitive control tasks [91]. Thus, the well-documented alterations in PVChC synapses in schizophrenia might provide another means to increase excitation and restore E/I balance. Such a mechanism might explain the increase in frontal gamma oscillations during a cognitive control task seen in patients with schizophrenia following treatment with a GABAA α2 receptor agonist [18].
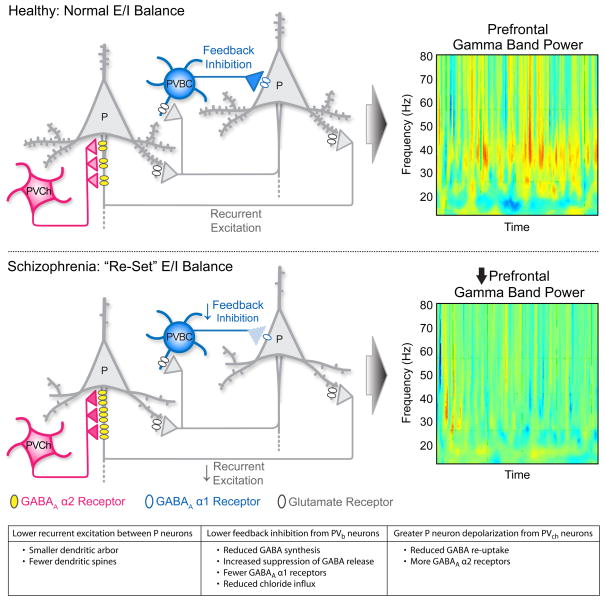
This hypothesis, that lower excitatory activity in layer 3 pyramidal neurons leads to multiple compensatory responses to reduce inhibition from PVBCs and increase excitation from PVChCs, requires answers to additional questions regarding the nature of the alterations in PVBCs and PVChCs in schizophrenia (see Box 2). However, it does raise interesting interpretations and predictions for clinical observations in schizophrenia. First, although the compensatory changes in PV basket cell activity are predicted to rebalance excitation and inhibition, the new level of E/I balance in the DLPFC of subjects with schizophrenia would lack the strength of both excitation and inhibition required for generating sufficient levels of gamma band power to support cognitive control (Figure 3). For example, cell type-specific experimental manipulations in mice have demonstrated that lowering either AMPAreceptor mediated excitatory inputs to PV-positive neurons [92] or inhibitory output from PV neurons [45,46] (but not inhibitory inputs to PVBCs [93]) reduces gamma band power. The deleterious effects of lower levels of both excitation and inhibition in the PVBC-pyramidal neuron circuit on generating gamma oscillations might be expected to be most evident under task conditions demanding high levels of cognitive control, as demonstrated in experimental studies of subjects with schizophrenia [8,9].
Box 2. Outstanding questions.
Are protein levels of GAD67 altered in the axon terminals of PVChCs? The idea that PV basket cell inhibition is reduced to compensate for an intrinsic deficit in pyramidal cell excitability (see Box 1), and that PVChCs are excitatory, makes the prediction that GAD67 levels and GABA synthesis are either normal or increased in the axon terminals of PVChCs; that is, PVChCs are predicted to be among the ~50% of PV neurons that do not lack detectable GAD67 mRNA in schizophrenia.
Are protein levels of GAT1 altered in the axon terminals of PVBCs? Although changes in GAT1 levels have not been reported in experimental models of homeostatic synaptic plasticity, the idea that changes in PVBCs and PVChCs are compensatory to reduce inhibition and increase excitation of pyramidal cells, respectively, predicts that the ratio of GAD67/GAT1 is increased in PVChC axon cartridges and decreased in PVBC terminals in schizophrenia, a prediction which can now be tested with new methods of quantifying protein levels in specific populations of axon terminals [23,82].
Is the deficit in PV mRNA expression in schizophrenia [24,40,41] present in PVBCs and/or PVChCs? Because the absence of PV facilitates repetitive GABA release and augments gamma oscillations [117], the current model predicts that PV protein levels would be lower in the axon cartridges of PVChCs and unchanged in PVBC terminals in schizophrenia.
Are markers of excitatory inputs to PVBCs lower in schizophrenia as an additional means of reducing their inhibitory output? Unfortunately, because molecular markers that differentiate PVBCs from PVChCs are still unknown, it is not yet possible to determine if AMPA and NMDA receptor subunits are specifically down-regulated in PVBCs in schizophrenia.
Are levels of the vesicular GABA transporter (vGAT), which regulates the loading of GABA into synaptic vesicles, altered in PVBCs? Because changes in vGAT contribute to homeostatic synaptic plasticity in experimental systems [118], the current model predicts that vGAT would be lower in the axon terminals of PVBCs.
Figure 3.
Connectivity between pyramidal (P) neurons and parvalbumin-positive basket (PVBC) and chandelier (PVChC) cells in DLPFC layer 3. Reciprocal connections formed by the local axon collaterals of pyramidal neurons provide recurrent excitation, whereas the excitatory inputs from pyramidal neurons to PV basket cells furnish feedback inhibition. These connections are critical for generating gamma band oscillations, and the strengths of these connections are adjusted to maintain normal E/I balance in the healthy brain (top panel). In schizophrenia (bottom panel), lower spine density in layer 3 pyramidal neurons is hypothesized to result in lower network excitation, evoking a compensatory reduction in feedback inhibition of pyramidal neurons from PVBCs (less presynaptic GAD67; fewer postsynaptic GABAA α1 receptors) and increased depolarization of pyramidal neurons by PVChs (less presynaptic GABA membrane transporter 1; more postsynaptic GABAA α2 receptors). The resulting “re-set” of E/I balance at a lower level of both excitation and inhibition renders the circuit less able to generate normal levels of gamma band power, resulting in impaired cognition. Heat maps are reproduced, with permission, from [8] © (2006) Proceedings of the National Academy of Sciences.
Second, the re-set E/I balance in individuals with schizophrenia, at lower levels of both excitation and inhibition, would have less dynamic range for adjusting excitation and inhibition in the face of new forces that alter one or the other. That is, homeostatic synaptic plasticity, the capacity to scale the strength of all excitatory and inhibitory inputs to a neuron in response to large scale changes in network activity [94], would be limited. This reduced capacity of the circuitry to respond to challenges altering either excitation or inhibition might explain 1) the tendency for the symptoms of schizophrenia to worsen in the face of stress-induced changes in prefrontal activity [95]; 2) the worsening of cognitive deficits [2] that is temporally associated with the normal adolescence-related pruning of excitatory synapses, which is most prominent in layer 3 of the DLPFC [96]; and 3) the increased liability to, and severity of, schizophrenia associated with marijuana use [97], which can suppress cortical GABA release [98].
It is important to note that an alternative model, that an upstream GAD67 deficit in PV neurons (due to NMDA receptor hypofunction in these neurons) leads to a disinhibition of pyramidal cells, has support from pharmacological animal models of schizophrenia [99]. However, the resulting increase in cortical network activity would be expected to evoke compensatory responses to enhance inhibition, but as summarized above, schizophrenia is accompanied by multiple molecular changes each of which would decrease PVBC-mediated inhibition in DLPFC circuitry.
The current findings and suggested model for interpreting these findings focus on only a limited portion of cortical circuitry; a full accounting of the pathophysiology underlying cognitive control deficits in schizophrenia requires both better knowledge of the patterns of connectivity within the DLPFC and more sensitive methods for assessing the functional integrity and compensations of these connections in the illness. In addition, the use of appropriate animal models as proof-of-concept tests of the hypothesized upstream versus downstream relationships between cellular level alterations in schizophrenia are essential. Such future advances will provide a more informed substrate for designing rational interventions to enhance cognitive function in people with schizophrenia.
Acknowledgments
The work conducted by the authors and cited in this review was supported by National Institutes of Health (NIH) grants MH084053 and MH043784. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the National Institute of Mental Health.
Footnotes
Disclosure statement
D.A.L. currently receives investigator-initiated research support from Bristol-Myers Squibb (BMS), Curridium Ltd and Pfizer and in 2009–2011 served as a consultant in the areas of target identification and validation and new compound development to BioLine RX, BMS, Merck and SK Life Science.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichenberg A, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. [PubMed] [Google Scholar]
- 4.Lesh TA, et al. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharm. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Howard MW, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 7.Minzenberg MJ, et al. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho RY, et al. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minzenberg MJ, et al. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharm. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis DA, et al. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 12.Lodge DJ, et al. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enomoto T, et al. Reducing prefrontal gamma-aminobutyric Acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Gruber AJ, et al. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paine TA, et al. Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABA(A) receptors. Neuropsychopharm. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawaguchi T, et al. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 17.Ahn K, et al. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharm. 2011;36:677–683. doi: 10.1038/npp.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asada H, et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asada H, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Comm. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Burgos G, et al. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 23.Curley AA, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto T, et al. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson M, et al. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Woo TU, et al. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 27.Impagnatiello F, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benes FM, et al. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 30.Benson DL, et al. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- 31.Mason GF, et al. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 32.Ongur D, et al. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto N, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JH, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto N, et al. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol. 2009;24:639–645. doi: 10.1002/hup.1070. [DOI] [PubMed] [Google Scholar]
- 36.Frankle WG, et al. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharm. 2009;34:624–633. doi: 10.1038/npp.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 38.Volk DW, et al. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellios N, et al. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Fung SJ, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 42.Woo TU, et al. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 43.Beasley CL, et al. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 44.Tooney PA, Chahl LA. Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:273–278. doi: 10.1016/j.pnpbp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohal VS, et al. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo TU, et al. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierri JN, et al. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 49.Volk DW, et al. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 50.Beneyto M, et al. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabadics J, et al. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 52.Baldi R, et al. Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur J Neurosci. 2010;32:1319–1325. doi: 10.1111/j.1460-9568.2010.07361.x. [DOI] [PubMed] [Google Scholar]
- 53.Woodruff AR, et al. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capogna M, Pearce RA. GABA(A,slow): causes and consequences. Trends Neurosci. 2011;34:101–112. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Gulyas AI, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nusser Z, et al. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doischer D, et al. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akbarian S, et al. GABAA receptor subunit gene expression in human prefrontal cortex: Comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 60.Duncan CE, et al. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatry Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maldonado-Aviles JG, et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharm. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benes FM, et al. Up-regulation of GABA-A receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 65.Lewis DA, et al. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: Evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 66.Chattopadhyaya B, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melchitzky DS, et al. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: Laminar, regional and target specificity of Type I and Type II synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- 68.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 69.Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011;68:21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyde TM, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- 72.Stumm RK, et al. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 73.Wimpey TL, Chavkin C. Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron. 1991;6:281–289. doi: 10.1016/0896-6273(91)90363-5. [DOI] [PubMed] [Google Scholar]
- 74.Glickfeld LL, et al. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28:1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capogna M, et al. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lupica CR. Delta and mu enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Volk DW, et al. Cortical opioid markers in schizophrenia and across development. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peckys D, Hurd YL. Prodynorphin and kappa opioid receptor mRNA expression in the cingulate and prefrontal cortices of subjects diagnosed with schizophrenia or affective disorders. Brain Res Bull. 2001;55:619–624. doi: 10.1016/s0361-9230(01)00525-1. [DOI] [PubMed] [Google Scholar]
- 79.Foldy C, et al. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 80.Karson MA, et al. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54:117–128. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karson MA, et al. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fish KN, et al. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buffalo EA, et al. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011;108:11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 85.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology Reviews. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 86.Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 87.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bitanihirwe BK, et al. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rotaru DC, et al. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–126. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenwood TA, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuchs EC, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 93.Wulff P, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 95.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bourgeois JP, et al. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 97.van Os J, et al. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 98.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cruz DA, et al. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- 101.Hashimoto T, et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kilman V, et al. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 107.Kolluri N, et al. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 108.Pierri JN, et al. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 109.Sweet RA, et al. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 110.Sweet RA, et al. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharm. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: A cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi T, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 113.McIntosh AM, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Hill JJ, et al. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 115.Arion D, et al. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci. 2007;25:1843–1854. doi: 10.1111/j.1460-9568.2007.05396.x. [DOI] [PubMed] [Google Scholar]
- 116.Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: Implications for dendritic spine deficits. Biol Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vreugdenhil M, et al. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 118.De Gois S, et al. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]