Abstract
Cisplatin is a widely used anti-neoplastic agent; however, its major limitation is the development of dose-dependent nephrotoxicity whose precise mechanisms are poorly understood. Here we show that mitochondrial dysfunction is not only a feature of cisplatin nephrotoxicity, but that targeted delivery of superoxide dismutase mimetics to mitochondria largely prevents the renal effects of cisplatin. Cisplatin induced renal oxidative stress, deterioration of mitochondrial structure and function, an intense inflammatory response, histopathological injury, and renal dysfunction. A single systemic dose of mitochondrially-targeted antioxidants, MitoQ or Mito-CP, dose-dependently prevented cisplatin-induced renal dysfunction. Mito-CP also prevented mitochondrial injury and dysfunction, renal inflammation, and tubular injury and apoptosis. Despite being broadly renoprotective against cisplatin, Mito-CP did not diminish cisplatin’s anti-neoplastic effect in a human bladder cancer cell line. Our results highlight the central role of mitochondrially generated oxidants in the pathogenesis of cisplatin nephrotoxicity. Since similar compounds appear to be safe in humans, mitochondrially-targeted antioxidants may represent a novel therapeutic approach against cisplatin nephrotoxicity.
Keywords: nephropathy, cisplatin, oxidative stress, mitochondria, mitochondrial antioxidants
Introduction
Cisplatin is commonly used to treat malignancies. Cisplatin binds to DNA, forming inter- and intra-strand cross-links, resulting in defective DNA templates, arrest of DNA synthesis in rapidly dividing cancer cells[1]. The major limitation of cisplatin chemotherapy is the development of dose-dependent nephrotoxicity in about 30% of patients preventing the administration of high doses to take full advantage of its chemotherapeutic efficacy[2, 3]. Increased oxidative stress and inflammation have been implicated in cisplatin-induced renal tubular cell injury[4–6] as cisplatin accumulates predominantly in tubular cells and undergoes metabolism; however, the precise mechanisms of cisplatin’s renal toxicity are not yet well understood, and efficient approaches to attenuate this dose-limiting side effect are sorely needed [7].
In this study, we used a well-established mouse model of cisplatin-induced nephropathy[5, 6, 8–12] to investigate the role of mitochondrial dysfunction in cisplatin-induced kidney injury. We characterized cisplatin’s effects on renal oxidative stress, the local inflammatory response, and mitochondrial structure and function. Thereafter, we tested the efficacy of two well-characterized membrane-permeable small molecule compounds that deliver superoxide dismutase (SOD) mimetics preferentially into mitochondria in vivo, MitoQ and Mito-CP[13–15].
Material and methods
Animals and drug treatment
All animal experiments conformed to National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism (NIAAA; Bethesda, MD, USA). Six to 8-week-old male C57Bl/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All animals were kept in a temperature-controlled environment with a 12-h light–dark cycle and were allowed free access to food and water at all times, and were cared for in accordance with National Institutes of Health (NIH) guidelines. Mice were sacrificed 72 hrs following a single injection of cisplatin (cisdiammineplatinum(II) dichloride 25 mg/kg i.p.; Sigma). Two mitochondrial antioxidants Mito-Q and Mito-CP were synthesized as described [13, 16]. MitoQ and Mito-CP were stored in ethanol at 50 mg/ml, further diluted in saline and administered at 0.3–10 mg/kg or as described, i.p. once, and starting 1 hour before the cisplatin administration.
Renal function monitoring
Serum levels of creatinine and Blood Urea Nitrogen (BUN) were measured using VetTest 8000 blood chemistry analyzer (Idexx Lab)[5, 12].
Electron Microscopy
Anesthetized animals were perfusion fixed with 1.25 % glutaraldehyde in 0.1 M cacodylate buffer. Kidneys were harvested and processed in standard fashion (Epon embedded) for transmission electron microscopy using a JEOL 1011 Electron Microscope.
Histological examination
Following fixation of the kidneys with 10% formalin, renal tissues were sectioned and stained with periodic acid-Schiff (PAS) reagents for histological examination. Tubular damage in PAS-stained sections was examined under the microscope and scored based on the percentage of cortical tubules showing epithelial necrosis: 0 = normal; 1 = <10%; 2 = 10– 25%; 3 = 26–75%; 4 = >75%. Tubular necrosis was defined as the loss of the proximal tubular brush border, blebbing of apical membranes, tubular epithelial cell detachment from the basement membrane or intraluminal aggregation of cells and proteins as described[5]. For myeloperoxidase (MPO) staning slides were deparaffinized, and hydrated in descending gradations of ethanol, followed by antigen retrieval procedure. Next, sections were incubated in 0.3% H2O2 in PBS to block endogenous peroxidase activity. The sections were then incubated with anti-MPO (Biocare Medical, Concord, CA) or anti-malondialdehyde (Genox, Baltimore, MD, USA) antibodies overnight at 4°C in a moist chamber. Biotinylated secondary antibodies and ABC reagent were added as per the kit's instructions (Vector Laboratories, Burlingame, CA, USA). Color development was induced by incubation with a DAB kit (Vector Laboratories) for 3–5 min, and the sections were counter-stained with nuclear fast red as described [5]. Finally, the sections were dehydrated in ethanol and cleared in xylene and mounted. The specific staining was visualized and images were acquired using microscope IX-81 (Olympus, Center Valley, PA). The morphometric examination was performed in a blinded manner by two independent investigators.
In Situ Enzyme Chemistry
After removal, kidneys were bi-valved and frozen immediately in isopentane cooled in liquid nitrogen. The tissues were cryosectioned (6 µm thick) and stained for NADH and COX activities, as described previously (Mitochondrial DNA and its respiratory chain products are defective in doxorubicin nephrosis [17, 18]
Renal terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) immunohistochemistry
Paraffin sections were dewaxed and in situ detection of apoptosis in the renal tissues was performed by TUNEL assay as per the instructions provided with the kit (Roche Diagnostics, Indianapolis, IN, USA). The nuclei were labeled with Hoechst 33242 [12].
Isolation of mitochondria from tissues
Mitochondria were isolated from the kidneys of mice treated with vehicle, or cisplatin and/or Mito-CP using a tissue mitochondrial isolation kit (Pierce Biotechnology).
Renal DNA fragmentation ELISA
The quantitative determination of cytoplasmic histone-associated DNA fragmentation was determined using an ELISA kit (Roche Diagnostics).
Determination of renal caspase 3/7, poly(ADP-ribose) polymerase (PARP) and myeloperoxidase (MPO) activities, nitrotyrosine (NT) and 4-hydroxynonenal (HNE) content
Renal Caspase 3/7, PARP, MPO activities, nitrotyrosin (NT) and 4-hydroxynonenal (HNE) content in kidney homogenates were determined as described[5, 12, 19].
Real-time PCR analyses
RNA was isolated and prepared for cDNA as described[5, 19]. Realtime PCR was performed in ABI HT7900 instrument using syber green technology. Each amplified sample in all wells was analyzed for homogeneity using dissociation curve analysis. The primers used were previously described[5, 12].
Immunoblot analyses
Kidney tissues were homogenized in mammalian tissue protein extraction reagent (Pierce, Rockford, IL, USA) supplemented with protease and phosphatase inhibitors (Roche Diagnostics). Blots were probed with NOX2 antibody obtained from BioLegend, San Diego, CA. NOX4 monoclonal and polyclonal rabbit antibodies were obtained from Abcam, Cambridge, MA. Blots were incubated with primary antibody at recommended dilution and incubated overnight at 4 °C. After subsequent washing with PBS–Tween 20, the membranes were probed with appropriate secondary antibodies conjugated with HRP (Pierce) and incubated 1 h at room temperature. Then the membranes were developed using a SuperSignal–West Pico substrate chemiluminescence detection kit (Pierce). To confirm uniform loading, membranes were stripped and reprobed with β-actin (Chemicon, Ramona, CA, USA). Immunoblots obtained by using a NOX4 monoclonal antibody showed similar pattern to the ones obtained with the use of a polyclonal antibody, however the polyclonal antibody appeared to be more specific (less additional bands). Quantification of immunoblots were carried out by Quantity one program (BioRad, Hercules, CA) and normalized to actin.
Cell culture of cancer cell line T24, cytotoxicity/cell survival assay
T24, a transitional cell carcinoma from human urinary bladder was purchased from ATCC. The T24 cell line was cultured in McCoy’s medium with 10% foetal calf serum and 1% penicillin–streptomycin as recently described [12]. The effect of cisplatin and Mito-CP, or their combinations on cell survival were assessed using the XTT assay (Cell Proliferation Kit II, Roche Diagnostics, Indianapolis, IN). Cells were seeded into 96 or 24 well culture plates. Twenty-four hours later, cells were treated with different concentrations of cisplatin, Mito-CP, or their combination as described in the figure. Untreated cells were cultured in parallel as a negative control. After 72h of incubation, cells were treated with 50 µL aliquot of the XTT solution (1 mL XTT labeling/20 µL electron-coupling reagent) to each well at the end of the experiment. After 2h of incubation, the absorbance was measured at both 492 nm and a reference wavelength (690 nm) as described [12].
Clonogenic assay
Colony forming (clonogenic) assay of T24 cancer cells treated with vehicle, cisplatin and Mito-CP, or their combinations were performed as described previously [20] and the concentrations of cisplation and Mito-CP are indicated in the figure. Colony forming units were calculated from four experiments in each group.
Sample preparation and EPR measurements
Plasma samples were analyzed without any processing. Frozen kidneys (single, randomly chosen from a pair) were weighted and homogenized in ice-cold 0.1 M phosphate buffer containing 100 µM DTPA (3 µl per 1 mg of tissue). After homogenization, the samples were quickly frozen by immersion in liquid nitrogen and kept at −80 °C until the analysis. Plasma samples or tissue homogenates were thawed and immediately transferred into EPR capillaries and the EPR spectra scanned. For the analysis of total amount of Mito-CP11 ( nitroxide Mito-CP and its reduced form, hydroxylamine Mito-CPH), the aliquots of thawed samples were mixed 1:1 (by vol.) with DMSO and vortexed with 1 mM K3Fe(CN)6 (added by 1:100 dilution of 0.1 M aqueous solution of K3Fe(CN)6) before EPR measurement. EPR spectra were recorded at room temperature using the Bruker EMX spectrometer operating at 9.85 GHz and equipped with a Bruker ER 4119HS-WI high sensitivity resonator. Typical spectrometer parameters were: scan range 80 G, time constant 1.28 ms, sweep time 42 s, modulation amplitude 1.0 G, modulation frequency 100 kHz, microwave power: 20 mW. Micropipettes (50 µl for plasma samples and 100 µl for tissue homogenates) were used as sample tubes. Spectra were averaged over 5 scans. As the EPR signals due to the presence of ascorbyl radical were overlapping with the center line of Mito-CP spectrum, the quantification was done by the comparison of the intensities of the low-field EPR peak of Mito-CP with the Mito-CP solutions of known concentrations, measured under the same conditions. All data are presented as mean values and error bars represent standard deviations.
Statistical analysis
Results are expressed as mean±SEM. Statistical significance among groups was determined by one-way ANOVA followed by Newman-Keuls post hoc analysis using GraphPad Prism 5 software (San Diego, CA). Probability values of P<0.05 were considered significant.
Results
Mitochondrially-targeted antioxidants prevent cisplatin-induced renal dysfunction, histopathological alterations, and tubular mitochondrial injury
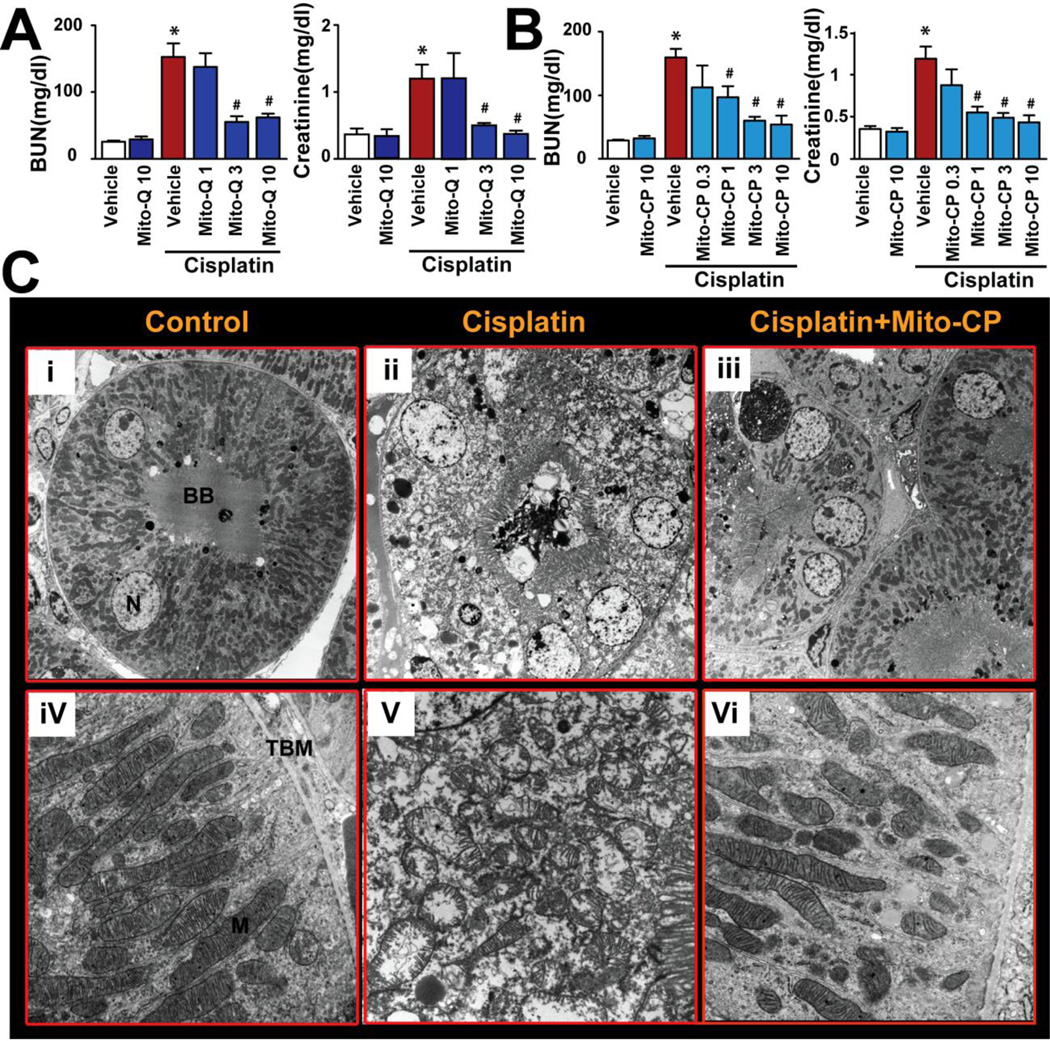
Intraperitoneal administration of a single dose of mitochondrial antioxidants, MitoQ and Mito-CP, 1 h before cisplatin treatment dose-dependently (0.3 to 10 mg/kg) prevented the cisplatin-induced renal dysfunction (attenuated the increase in blood urea nitrogen (BUN) and creatinine values (Fig.1AB)). Based on this result, a fully effective dose of Mito-CP (3 mg/kg i.p.) was used in subsequent mechanistic studies. Next, we evaluated renal cortical tubules with transmission electron microscopy to determine the effects of cisplatin on mitochondrial ultrastructure with or without Mito-CP pre-treatment. Cisplatin induced extensive acute tubular damage (Figures 1C(ii) and 2). Higher magnification (Fig.1Cv) revealed swollen mitochondria with disruption of cristae, suggesting mitochondrial injury. Mito-CP treatment completely prevented cisplatin-induced mitochondrial structural damage and tubular injury (Figs.1Ciii,vi and 2).
Figure 1. Mitochondrial antioxidants prevent the cisplatin-induced renal dysfunction and mitochondrial injury.
MitoQ and Mito-CP (Panels A and B) ameliorate the cisplatin-induced profound kidney damage as evidenced by the attenuation of the increase in blood urea nitrogen (BUN) and creatinine levels and/or tubular damage (Panel C, electron microscopy) in the kidney 72h after cisplatin administration to mice. Electron microscopy of the renal cortex (Panel C) demonstrates the preservation of mitochondrial structure after mitochondrial antioxidant administration. In sham treated control (i and iv), intact proximal tubular epithelium with preserved mitochondria are shown. In cisplatin-treated kidney (ii) a proximal tubule with extensive acute injury is shown. Note the epithelial swelling and vacuolization accompanied by preservation of the brush border. Higher magnification (v) shows large number of mitochondria with swelling and disruption of their cristae in cisplatin-treated group. Nuclear structure appears maintained. Mito-CP treatment (iii and vi) preserves the tubular epithelium and mitochondrial structures in the cisplatin-treated mouse kidney. Original magnifications: i, ii, and iii - 3,000X; iv, v, and vi - 20,000X (BB : brush border, N: nucleus, TBM: tubular basement membrane, M: mitochondria Results are mean±S.E.M. of 5–9 experiments/group. *P<0.05 vs. Vehicle; #P<0.05 vs. Cisplatin.
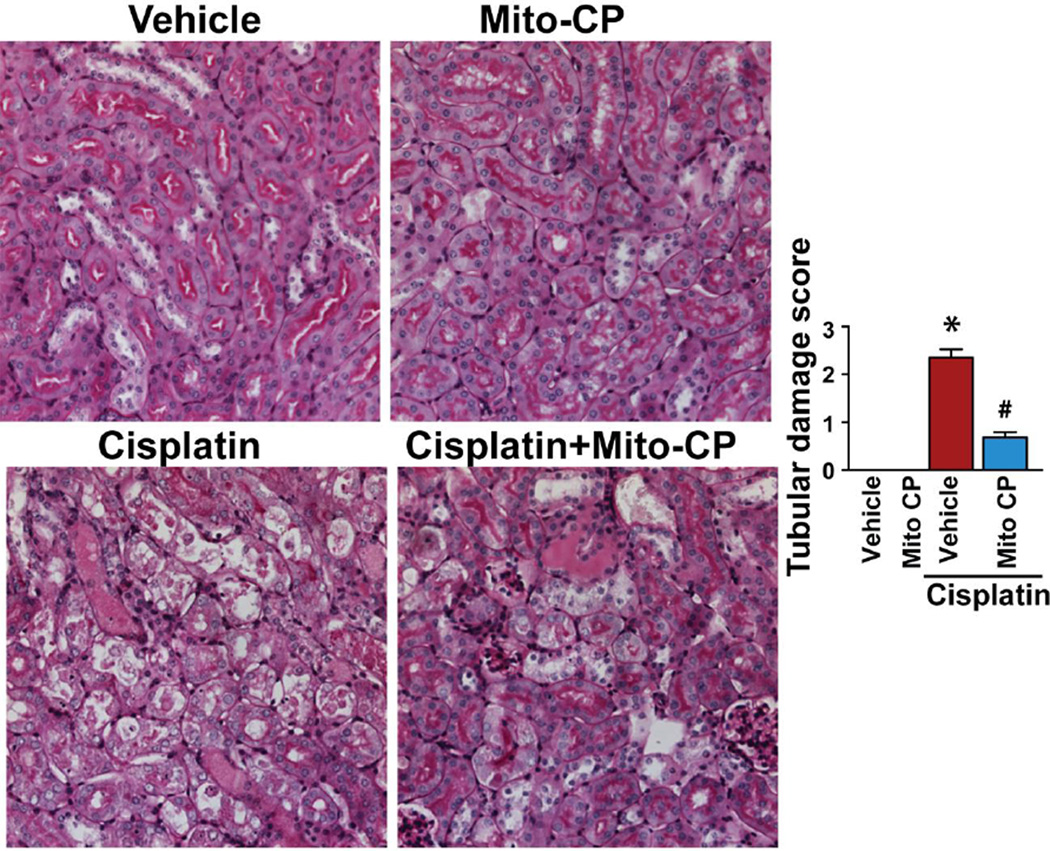
Figure 2. Mito-CP attenuates cisplatin-induced kidney damage.
As shown in the representative images cisplatin induced profound histopathological renal injury 72 h after the administration to mice, evidenced by protein cast, vacuolation and desquamation of epithelial cells in the renal tubules using PAS staining, which were largely prevented by Mito-CP treatment given from 1 hr before the cisplatin injection. The calculation of the tubular damage score is described in methods. Results are mean±S.E.M. of 6–7 experiments/group *P<0.01 vs. Vehicle; #P<0.01 vs. Cisplatin.
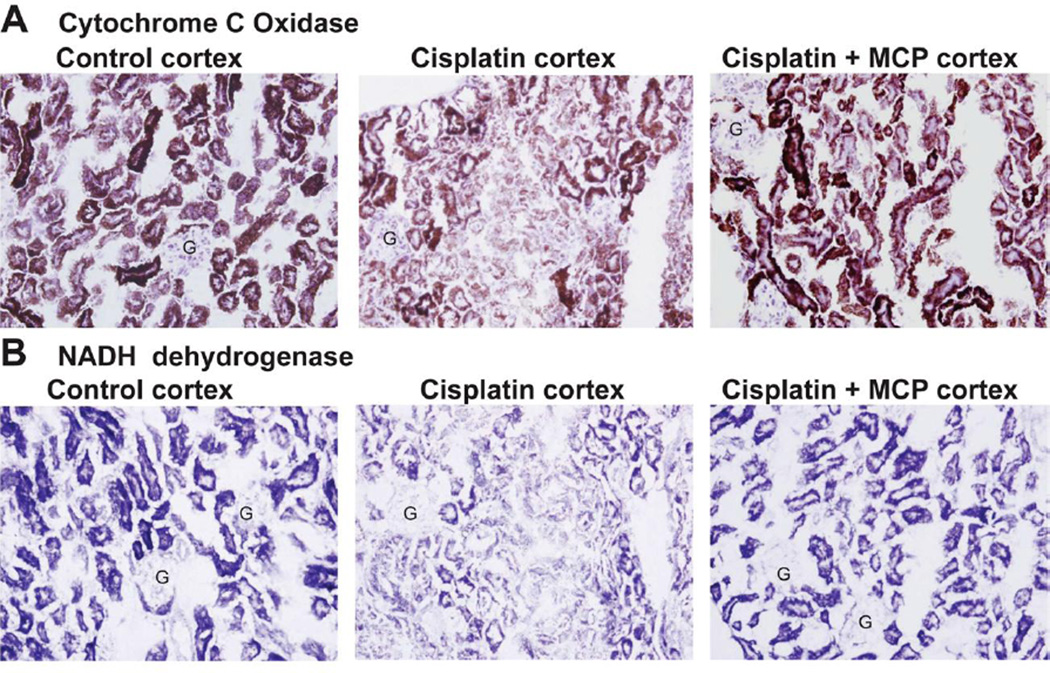
To determine the localization and severity of mitochondrial dysfunction within the kidney, we measured the in situ activity of the electron transport chain enzyme complex component, cytochrome c oxidase (COX; Fig.3A). In this enzymatic assay, incubation of tissue sections with substrate that is combined to the cytochrome c oxidase enzyme (oxygen acting as acceptor) produces a series of reactions resulting in brown staining at the site of enzyme activity. The degree of brown staining therefore reflects cytochrome c oxidase activity, which was found to be high in cortical tubules and dramatically reduced 72 hrs after cisplatin administration.
Figure 3. Mito-CP attenuates cisplatin-induced mitochondrial dysfunction.
In situ enzyme chemistry. Panel A, Staining (brown) for cytochrome c oxidase enzyme activity on sections of snap-frozen kidneys 72 h after vehicle, cisplatin and cisplatin+MCP treatment. Enzymatic activity is greatly decreased after cisplatin treatment in the cortex which was prevented by MCP treatment (representative images of n=5/condition are shown). (G = glomerulus) (original magnification 40X). Panel B, NADH dehydrogenase enzyme activity (blue staining) 72 h after vehicle, cisplatin and cisplatin+MCP treatment (representative of n=5/condition). (G = glomerulus) (original magnification 40X).
Just as prior reports on cisplatin nephrotoxicity had identified cortical tubules as the most histologically affected region of the kidney[21–23], our study of in situ mitochondrial function also demonstrated that the most prominently affected tubules were in the cortex. Mito-CP pre-treatment prevented the decrease in mitochondrial enzyme activity. To confirm these results, we repeated the in situ study with another enzyme component of the mitochondrial electron transport chain, NADH dehydrogenase (Fig.3B). This technique produced analogous results to the COX assay, again revealing severe mitochondrial dysfunction in cortical tubules that was nearly completely prevented by Mito-CP. These results provide strong evidence of cisplatin disrupts the normal structure and function of renal tubular mitochondria and that systemic delivery of mitochondrially targeted antioxidants can largely prevent these pathological alterations within the kidney.
Mito-CP attenuates cisplatin-induced oxidative and nitrative stress
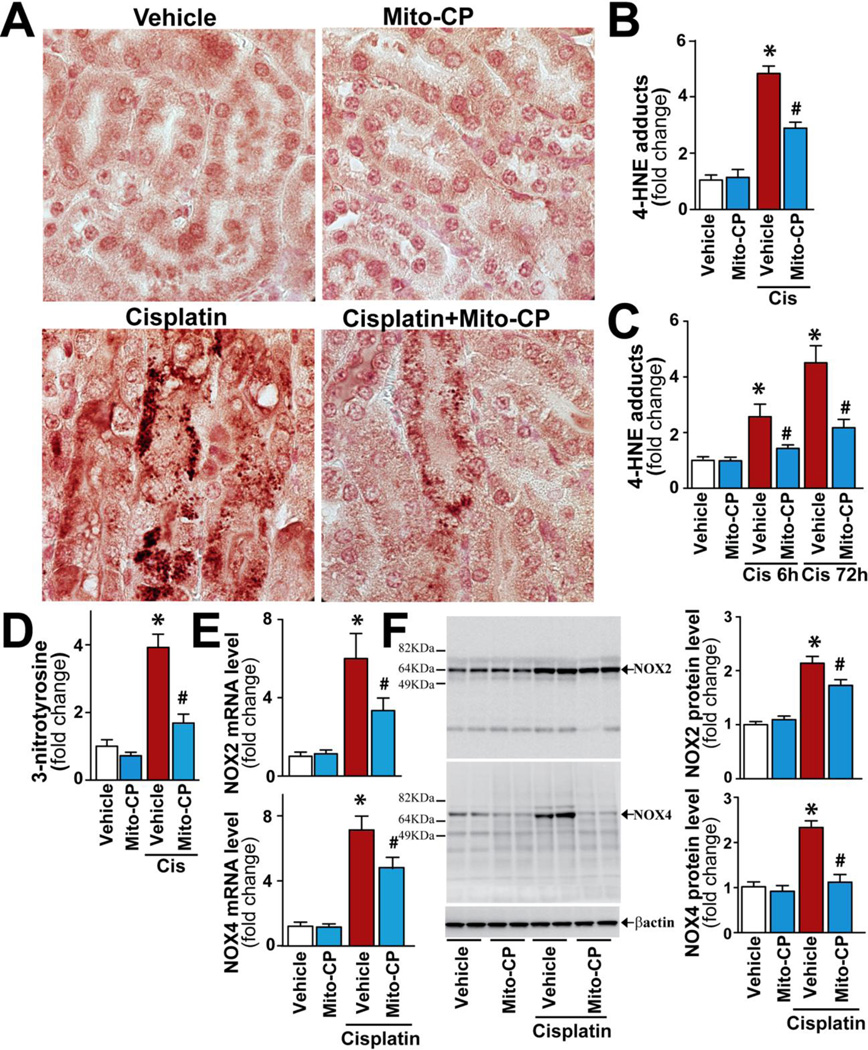
While several cell culture studies have implicated tubular mitochondria as a source of oxidant stress following cisplatin[4, 24, 25], less work has been focused on testing and exploiting this hypothesis in vivo. We found that cisplatin exposure significantly increased renal oxidative/nitrative stress as evidenced by enhanced accumulation of malondialdehyde (MDA, a marker of lipid peroxidation/oxidative stress; red-brown staining) in damaged tubular cells (Fig.4A), elevated HNE and nitrotyrosine levels in kidney homogenates (Fig.4B,D) and elevated HNE in isolated kidney mitochondria (Fig.4C). Mito-CP inhibited cisplatin-induced oxidative stress both in the kidney tissues (Fig.4A,B,D) and in isolated kidney mitochondria (Fig.4C). Mito-CP also inhibited the late/secondary expression of mRNA and protein of ROS generating NOX2 and NOX4 induced by cisplatin (Fig.4E,F).
Figure 4. Mito-CP attenuates cisplatin-induced oxidative and nitrative stress.
Cisplatin significantly increased renal malondialdehyde (red-brown) staining in damaged tubular cells (Panel A), HNE protein adduct and 3-nitrotyrosine levels in the kidney tissues (Panels B and D) or isolated mitochondria (panel C), as well as mRNA and protein expressions for NOX2/4 (Panels E and F), indicating enhanced oxidative/nitrative stress 72 h (or 6 h if indicated) following its administration to mice. These changes could be largely prevented by treatment with Mito-CP. Results are mean±S.E.M. of 6–15/group. *P<0.05 Vehicle; #P<0.05 vs. Cisplatin.
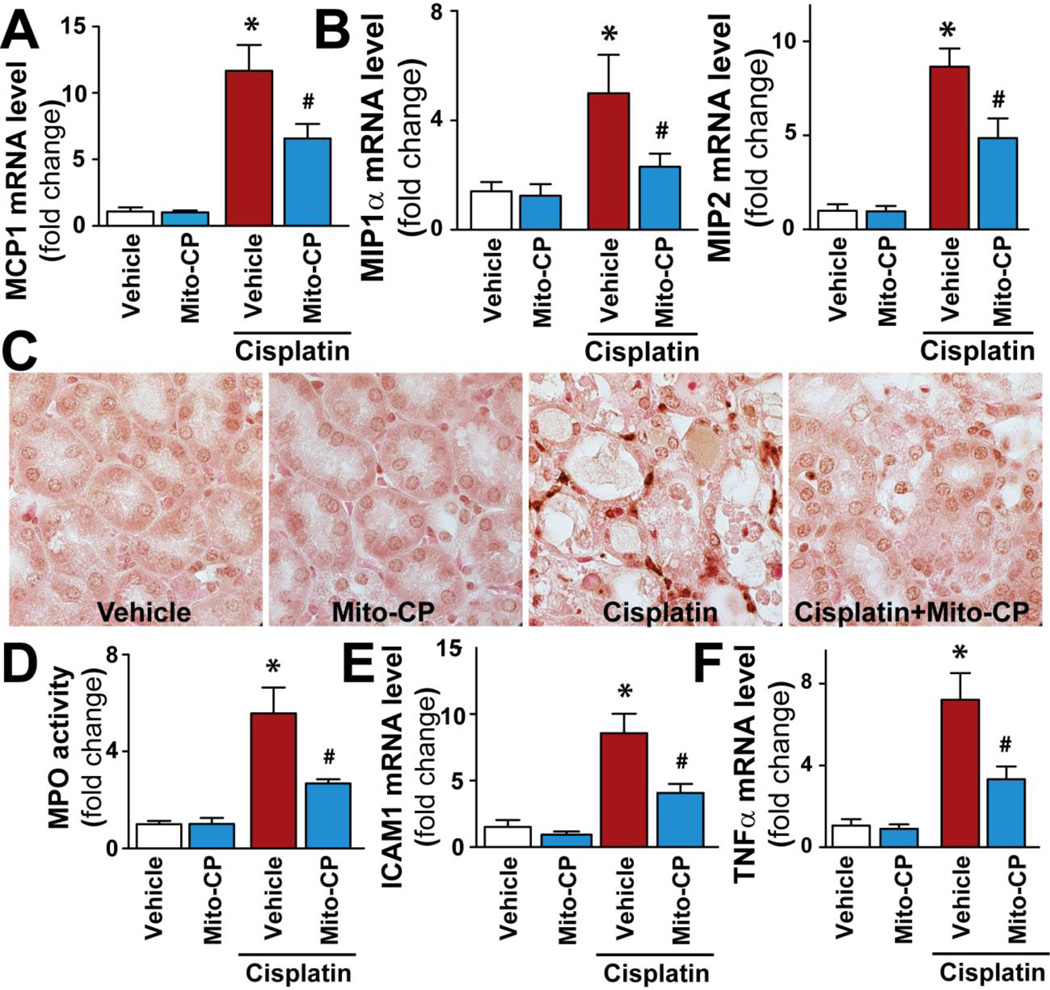
Mito-CP mitigates cisplatin-induced acute and late inflammatory response
Since pro-inflammatory chemokines/cytokines and expression of adhesion molecules are critical mediators of renal dysfunction following cisplatin exposure[6, 9, 10, 26, 27], we investigated the effects of Mito-CP on these processes as well. Cisplatin markedly increased the expression of pro-inflammatory chemokines MCP-1, MIP1/2 (Fig.5A,B), pro-inflammatory cytokine TNF-α (Fig.5F), the adhesion molecule ICAM-1 (Fig.5E), and renal myeloperoxidase staining and activity (an indicator of leukocyte infiltration; Figure 5CD). These changes were all significantly reduced by pre-treatment with Mito-CP.
Figure 5. Mito-CP attenuates cisplatin-induced inflammation.
Cisplatin significantly increased mRNA expression of pro-inflammatory chemokines MCP-1 (Panel A), MIP-1α and MIP-2 (Panel B), myeloperoxidase staining and activity (Panels C and D), adhesion molecule ICAM-1 and pro-inflammatory cytokine TNF-α mRNA expressions (Panels E and F) in the kidneys 72 h following its administration to mice, indicating enhanced inflammatory response. These changes could be largely prevented by treatment with Mito-CP. Results are mean±S.E.M. of 6–16/group. *P<0.05 Vehicle; #P<0.05 vs. Cisplatin.
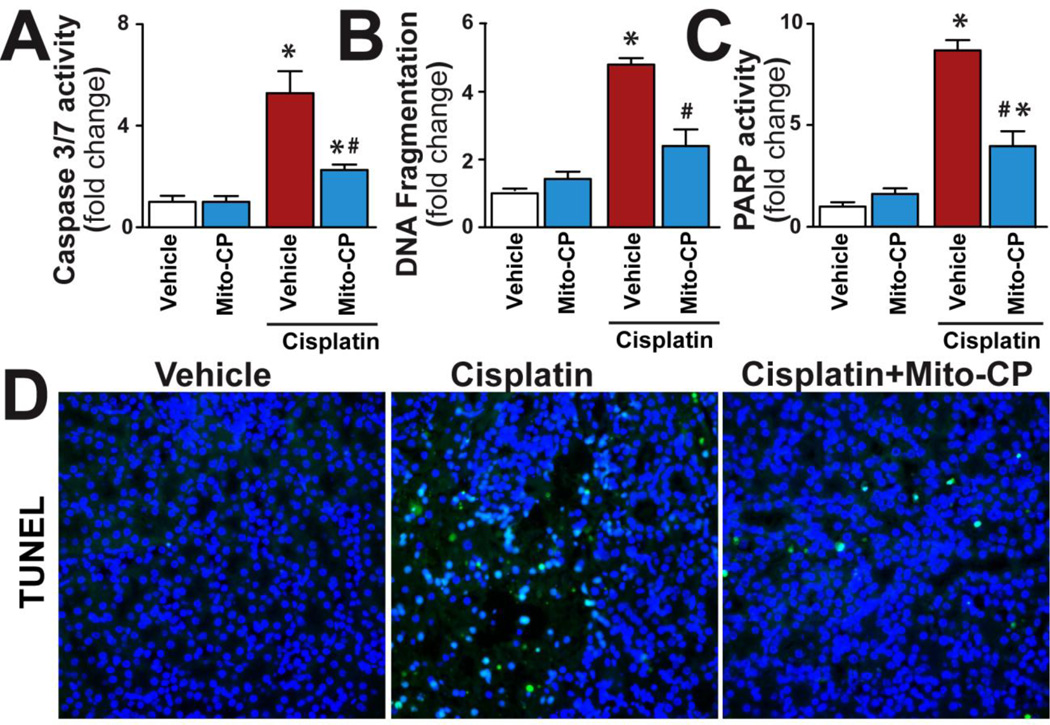
Mito-CP attenuates cisplatin-induced cell death in the kidney
Cisplatin significantly increased apoptotic (caspase 3/7, DNA fragmentation, TUNEL) and poly(ADP-ribose) polymerase-dependent cell death (Fig.6A-D) in the kidneys, both of which were prevented by Mito-CP.
Figure 6. Mito-CP attenuates cisplatin-induced renal cell death.
Cisplatin significantly increased various markers of apoptotic and PARP-dependent cell death in kidneys (Panels A-D) 72 h following its administration to mice, which could be largely prevented by treatment with Mito-CP. Panel D shows example of representative TUNEL staining colocalized with nuclear Hoechst staining (the TUNEL positive nuclei are light blue). Results are mean±S.E.M. of 6–8/group. *P<0.05 Vehicle; #P<0.05 vs. Cisplatin.
The development of cisplatin-induces intrarenal oxidative stress, inflammation, tubular cell death, and renal dysfunction is time-dependent
Consistently with our recent observations[12], an intraperitoneal dose of cisplatin induced timedependent increase in 4-hydroxynonenal (HNE), a marker of lipid peroxidation, in the kidney detectable 6 h after its administration which coincided with acute pro-inflammatory chemokine response and increased expression of the adhesion molecule ICAM-1 (not shown, see [12]). 3-Nitrotyrosine (3-NT), a marker of reactive nitrogen species (e.g., peroxynitrite) generation and/or protein nitration [28], also increased from 6–12 hours following cisplatin administration in the kidneys[12]. The expression of mRNAs of reactive oxygen species (ROS) generating NAD(P)H oxidase isoforms NOX2 and NOX4 occurred only from 24–48 h following cisplatin administration[12]. This again coincided with the increased leukocyte infiltration in the kidneys and markedly increased TNF-α levels and associated second wave of ROS/RNS[12]. The peak of delayed inflammatory response also correlated with increased apoptotic and necrotic cell death in the kidneys and renal dysfunction[12]. These results suggest that increased ROS generation and oxidant-induced damage in the kidneys precedes the inflammatory response and dysfunction.
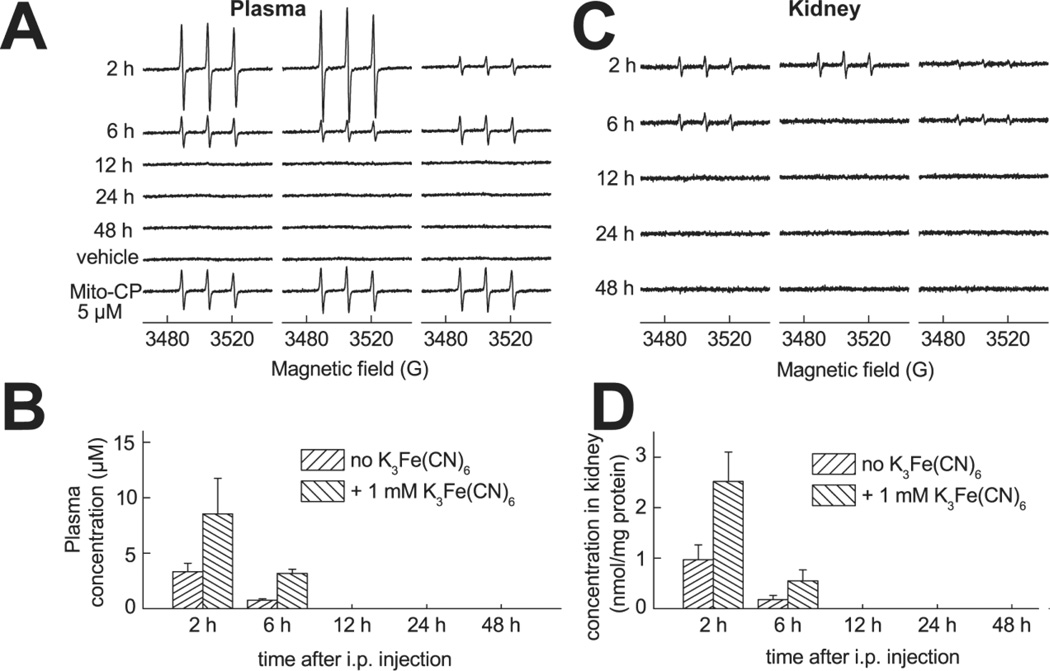
Pharmacokinetics of Mito-CP: EPR analyses
After injection of Mito-CP11 it undergoes a partial reduction in the blood to an EPR-silent hydroxylamine, as evidenced by the increase in the EPR signal intensity caused by the addition of ferricyanide. Ferricyanide oxidized hydroxylamines back to an EPR-active form. In plasma, Mito-CP is cleared within 12 hours after injection (Fig.7A,B) The lack of the EPR signal at ≥ 12 h is not due to the reduction to EPR-silent hydroxylamine, as ferricyanide is unable to restore the signal intensity. In the kidney tissue, Mito-CP is present mostly in the reduced form up to 6h after injection (Fig.7C,D). At ≥ 12 h after injection no EPR signal was detected in any kidney tissue sample even after the addition of ferricyanide (Fig.7C,D).
Figure 7. Mito-CP pharmacokinetics in plasma and kidney.
EPR analyses of the pharmacokinetics of Mito-CP11 in mice plasma and kidney after i.p. injection of Mito-CP11 at the dose of 10 mg/kg (Panels A-D). Representative EPR spectra of plasma and tissue homogenates mixed with DMSO and 1 mM K3Fe(CN)6 are shown in panels (A) and (C), respectively. The results of quantitative analysis are shown in panels B (concentration of Mito-CP11 in plasma) and D (concentration of Mito-CP11 in kidney homogenates, normalized to the protein concentration).
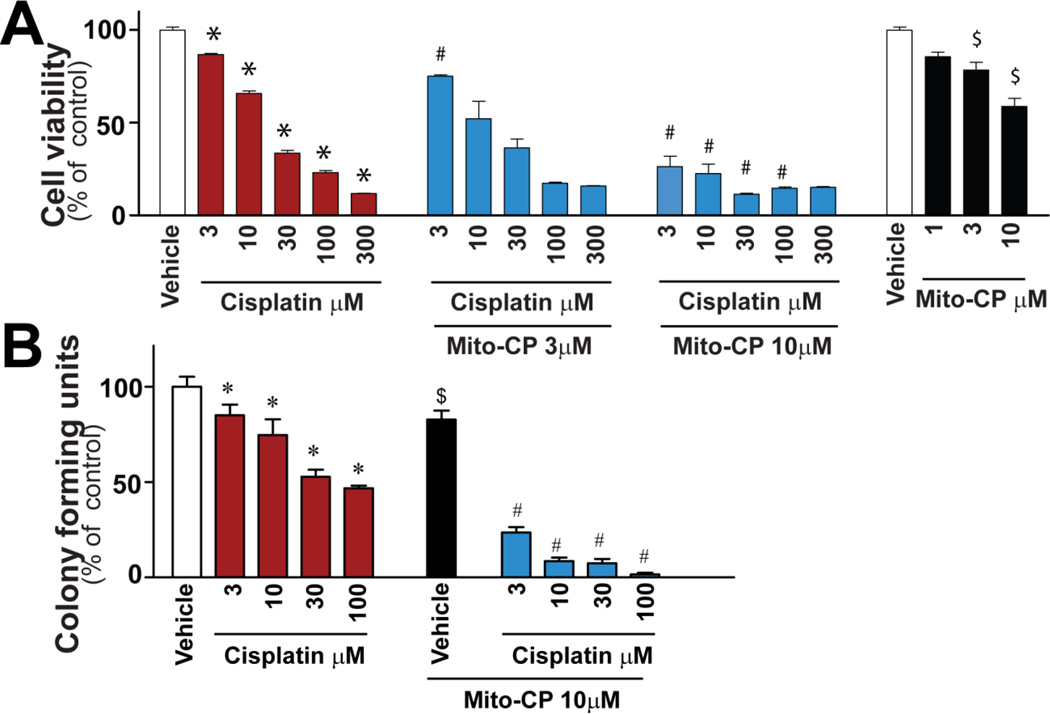
Mito-CP does not inhibit cisplatin-mediated T24 cancer cell death
Several renoprotective strategies against cisplatin may be less applicable clinically because of reduced anti-neoplastic effect[7]. Since one of the major indications for cisplatin is for treatment of urinary tract cancers, we determined if Mito-CP interferes with the therapeutic effect of cisplatin in the human bladder carcinoma cell line (T24)(Fig.8). Cisplatin induced concentration-dependent cell death in cancer cells measured by XTT assay after 72h (Fig.8A), which was actually further enhanced by Mito-CP (Fig.8A). Furthermore, Mito-CP also promoted the cisplatin-induced decrease of tumor cell colony formation (Fig.8B). These results are consistent with recently published studies in other cancer cells[29, 30]. Therefore, Mito-CP did not diminish the chemotherapeutic efficacy of cisplatin. In contrast, Mito-CP actually enhanced this effect of cisplatin.
Figure 8. Mito-CP enhances cisplatin-mediated T24 cancer cell death.
Cisplatin induced concentration-dependent cell death in T24 cancer cells (A) or decrease of tumor colony forming units (B) which were enhanced in the presence of Mito-CP, which by itself was able to attenuate slightly the cell viability in cancer cells. Results are mean±S.E.M. of 4/group. *P<0.05 vs. Vehicle; #P<0.05 vs. corresponding Cisplatin, $P<0.05 vs. Vehicle.
Discussion
In this study we investigated whether mitochondrially–targeted antioxidants could protect against cisplatin-induced kidney injury using a preclinical mouse model of cisplatin-induced nephropathy. Previous mechanistic studies using this model have variously implicated tubular apoptosis and cell-cycle changes, mitochondrial injury, oxidant stress, and local inflammation as key upstream events that result in renal dysfunction[5, 7, 12], but the inter-relationships among these have remained unclear. We hypothesized that cisplatin induces mitochondrial oxidant stress, which in turn, results in mitochondrial injury and dysfunction, tubular cell death, and local inflammation that all culminate in renal dysfunction. To test this, we administered membrane-permeable small molecule SOD mimetics that target mitochondria 1 hr before cisplatin challenge and observed improvements in each of these pathogenic events including, most importantly, global renal function These findings suggest that mitochondrial ROS may be an indispensable mediator of cisplatin nephrotoxicity.
In agreement with prior reports, we found that cisplatin induced marked tubular injury, increased inflammatory cell infiltration, oxidative/nitrative stress and impaired renal function[5, 6, 8–12]. As revealed by transmission electron microscopy, one of the most prominent features of cisplatin-induced tubular damage and nephropathy was the swelling of mitochondria with disruption of cristae practically in all tubular cells. Two independent studies of in situ electron transport chain activity not only confirmed widespread mitochondrial dysfunction, but localized the mitochondrial lesion to cortical tubules. Most critically, an intervention designed to target mitochondrial oxidant production largely prevented the functional and structural lesions in tubular mitochondria, suggesting that cisplatin’s ability to induce mitochondrial production of oxidants is key to its ability to injure mitochondria.
Increased oxidative stress was one of the earliest features we observed during the development of cisplatin-induced nephropathy, which was followed by a marked inflammatory cell infiltration and a secondary wave of ROS generation[12]. The secondary ROS mechanism likely involves the phagocyte NAD(P)H oxidase isoform gp91phox/NOX2, as well as NOX4/renox (the NAD(P)H oxidase isoform considered to be a major source of ROS generation in the kidneys under pathological conditions) since the expression of these ROS generating enzymes was significantly increased in the kidneys from the second day of cisplatin administration[12]. The increased NOX2 expression was consistent with enhanced leukocyte infiltration in the kidneys of cisplatin-treated mice around damaged tubular structures[12]. Cisplatin-induced ROS generation may also promote the expression of adhesion molecules through the activation of NF-κB. Indeed, we found increased expression of adhesion molecule ICAM-1 and enhanced chemokine signaling (MCP-1, MIP1-α/2) in the kidney of cisplatin-treated mice. These chemokines may further facilitate migration and adhesion of inflammatory cells to the activated endothelium or damaged tubular cells. These activated immune cells then may release various chemokines, cytokines ROS/RNS further amplifying the inflammatory cascade and injury[28, 31, 32]. We also detected marked increases in mRNA expressions of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) consistent with exacerbated inflammatory processes. Inflammation may also enhance oxidative stress, and these processes are interrelated leading to a concerted activation of various mitochondrial and other (e.g PARP-1-dependent) cell death pathways, as demonstrated in the cisplatin-induced nephropathy model (see also Figure 9).
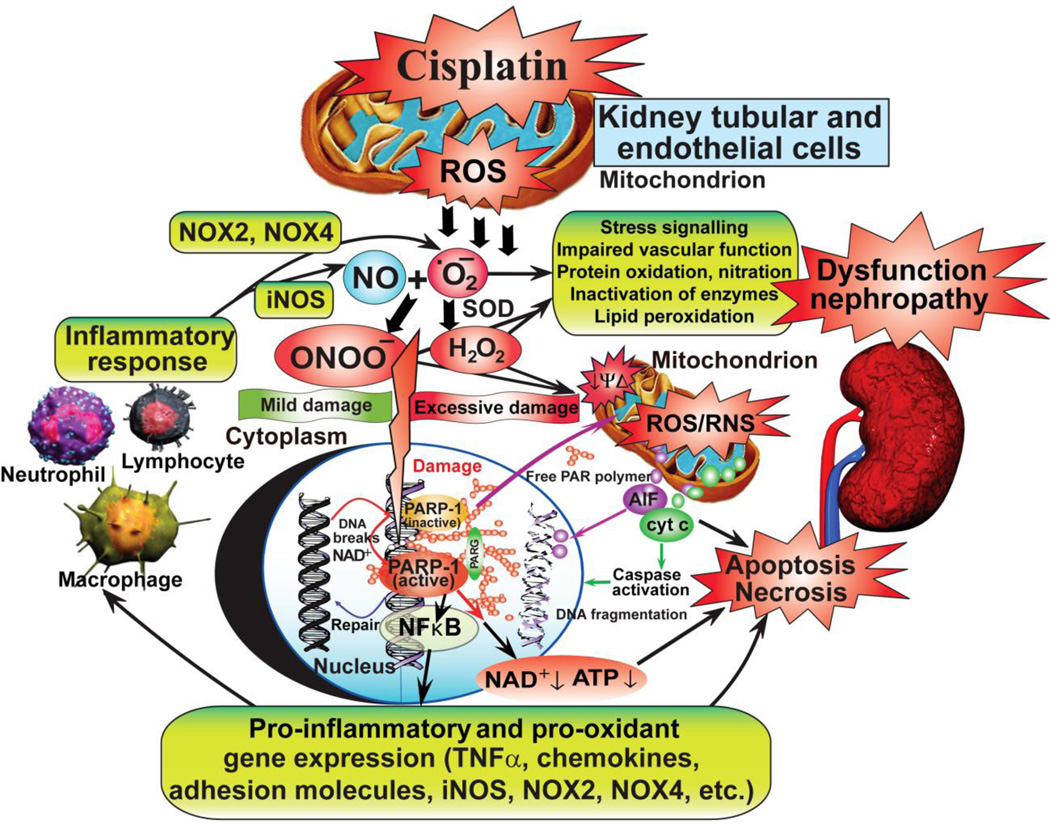
Figure 9. Cisplatin-induced mitochondrial reactive oxygen species generation triggers inflammatory response, cell death, and kidney dysfunction/nephropathy.
Cisplatin initially triggers oxidative stress in the mitochondria of kidney proximal tubular and endothelial cells, which is followed by secondary wave of ROS/RNS generation and inflammatory response. The secondary ROS most likely also involves the phagocyte NAD(P)H oxidase isoform gp91phox/NOX2, as well as NOX4/renox (isoform considered to be an important source of ROS in the kidney under pathological conditions). The cisplatin-induced ROS/RNS generation also induces oxidative DNA injury and rapid activation of the nuclear enzyme poly(ADP)ribose polymerase-1 (PARP-1) with consequent tubular and/or endothelial cell death, activation of pro-inflammatory transcription factors such as NF-κB. Inflammation may further amplify oxidative/nitrative stress, and these interrelated processes eventually culminate in more concerted renal tubular and endothelial cell demise (both apoptotic and necrotic), secondary hypoxia, kidney dysfunction and failure.
A single dose of Mito-CP, which was cleared from the serum and kidney within 12 h following its administration, not only attenuated the cisplatin-induced early and delayed ROS generation and mitochondrial injury, but also blunted the secondary wave of inflammation and associated cell death. Together, these observations strongly indicate that early mitochondrial ROS generation triggers the deleterious cascade of inflammation and tissue injury and suggest fruitful areas for future exploration. First, if tubular mitochondrial ROS production is an early and necessary pathogenic event following systemic cisplatin, how does its accumulation in tubular cells trigger mitochondrial oxidant generation? Second, mitochondrial fragmentation following cisplatin also appears to be an important event for tubular cytotoxicity to occur[8]. Considered in the context of the present results, prevention of mitochondrial ROS may attenuate the tendency for mitochondria to fragment following cisplatin. Conversely, fragmentation may accelerate the breakdown of the electron transport chain, resulting in ROS. Related to this, cisplatin inhibits fatty acid oxidation in tubular mitchondria, and transgenic expression of the metabolic transcription factor, peroxisome proliferation-activated receptor-alpha (PPAR-α), in proximal tubular cells, prevents cisplatin-induced lipid peroxidation and nephrotoxicity[33]. Induction of anti-oxidant genes downstream of PPAR-α or prevention of the secondary wave of inflammation may contribute to its beneficial effects[34]. Third, previous human trials using anti-oxidants to prevent cisplatin toxicity have yielded mixed results[35, 36]. We speculate that these approaches may not have efficiently targeted the subcellular source of oxidants. Finally, pro-oxidant function of cisplatin may also contribute to its anti-neoplastic effect; therefore, our demonstration that Mito-CP actually sensitized a human bladder cancer cell line to cisplatin lends further support to the development of similar compounds for clinical use. Further studies will be needed to understand how this sensitization occurs.
In summary, mitochondrial ROS generation may initiate the deleterious cascade of events induced by cisplatin, culminating in tubular cell death and diminished renal function (the simplified mechanisms of cisplatin-induced nephrotoxicity/nephropathy are summarized in Figure 9). Thus, mitochondrially-targeted antioxidants represent a novel approach to prevent this devastating complication of chemotherapy, thereby also increasing its therapeutic window. The current work not only establishes an in vivo mechanism implicating cortical tubular mitochondrial ROS following cisplatin—a pathway previously tested primarily in cultured cells—but also provides justification for development of this preventative strategy for clinical use. This is particularly exciting as mitochondrially-targeted antioxidants such as MitoQ are already being evaluated in humans for various therapeutic indications with no undue toxic effects (see ClinicalTrials.gov). Our findings may also be applicable in other forms of acute kidney injury, such as ischemia-reperfusion, where several investigators have demonstrated a pathogenic role for mitochondrial dysfunction[37–39].
Highlights.
Cisplatin (CIS) is a widely used anticancer drug which induces nephrotoxicity
Mitochondrial ROS generation initiates CIS-induced kidney injury and inflammation
Mitochondrially-targeted antioxidants prevent CIS-induced kidney injury
Acknowledgements
This study was supported by the Intramural Research Program of NIH/NIAAA (to P.P.) and by National Institutes of Grants RO1CA152810 (BK). Authors are indebted to Dan Brown and Lena Ellezian for help with electron microscopy sample preparation, Dr. George Kunos (the Scientific Director of NIAAA) for providing key resources, and the American Society of Nephrology (Carl Gottschalk award to S.M.P.). Dr. Pacher dedicates this study to his beloved mother Iren Bolfert, who died from complications of chemotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–379. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW. Cancer therapy and renal injury. J Clin Invest. 2002;110:743–745. doi: 10.1172/JCI16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 8.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121 doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay P, Horvath B, Kechrid M, Tanchian G, Rajesh M, Naura AS, Boulares AH, Pacher P. Poly(ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free radical biology & medicine. 2011;51:1774–1788. doi: 10.1016/j.freeradbiomed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med. 2011;11:106–114. [PubMed] [Google Scholar]
- 15.Smith RA, Hartley RC, Murphy MP. Mitochondria-Targeted Small Molecule Therapeutics and Probes. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 16.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebrecht D, Setzer B, Rohrbach R, Walker UA. Mitochondrial DNA and its respiratory chain products are defective in doxorubicin nephrosis. Nephrol Dial Transplant. 2004;19:329–336. doi: 10.1093/ndt/gfg564. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan D. Theory and practice of Histotechnology. 1980:308. [Google Scholar]
- 19.Mukhopadhyay P, Horvath B, Rajesh M, Matsumoto S, Saito K, Batkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Hasko G, Pacher P. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–195. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol. 2004;287:F990–F998. doi: 10.1152/ajprenal.00206.2004. [DOI] [PubMed] [Google Scholar]
- 22.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 24.Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997;280:638–649. [PubMed] [Google Scholar]
- 25.Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med. 2003;142:178–186. doi: 10.1016/S0022-2143(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 26.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–F618. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 28.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferators-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 2009;76:1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469–F480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 35.Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink-Bakker A, Zwinderman AH, Cleton FJ, Osanto S. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer. 2004;40:1713–1723. doi: 10.1016/j.ejca.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, Biroccio A, Leonetti C, Jandolo B, Cognetti F, Bove L. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927–931. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 37.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol. 2006;290:F35–F42. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]