Abstract
The development of chronic allergic dermatitis in early life has been associated with increased onset and severity of allergic asthma later in life. However, the mechanisms linking these two diseases are poorly understood. Here, we report that the development of oxazolone-induced chronic allergic dermatitis, in a mouse model, caused enhanced ovalbumin-induced allergic asthma after resolution of the former disease. Our findings show that oxazolone-induced dermatitis caused a marked increase in tissue mast cells, which persisted long after the resolution of this disease. Subsequent ovalbumin sensitization and airway challenge of mice that had recovered from dermatitis resulted in increased allergic airway hyperreactivity. The findings demonstrate that the accumulation of mast cells during dermatitis has the detrimental effect of increasing allergic airway hypersensitivity. Importantly, our findings also show that exposure to a given allergen can modify the immune response to an unrelated allergen.
Keywords: Allergy, Fc receptors, IgE, Mast cells/basophils, Skin, Rodent
Introduction
Atopy is a common phenomenon characterized by a predisposition to produce specific IgE antibodies in response to common allergens (1). This predilection occurs primarily in children, and is associated with atopic dermatitis (AD), asthma and rhinoconjuctivitis, collectively known as the atopic diseases. Anaphylaxis has also been described as a related feature, as food anaphylaxis is a more common sequela in children with eczema and children with asthma are at risk for severe episodes of anaphylaxis regardless of the trigger (1). The natural history of these atopic manifestations follows a typical pattern in which AD predates the development of respiratory allergy, a process referred to as the atopic march. It has been suggested that AD is an “entry point” for subsequent allergic disease (2). This hypothesis is supported by the finding that severe AD patients develop exaggerated airway responses to methacholine challenge and airway allergy (3-5). This concept raises the fundamental question of the possible causative role that AD may play in the development of allergic airway inflammation. An answer to this question would be expected to have significant clinical implications. For example, if the atopic march reflects causation, then the recently reported increase in AD might indicate an impending rise in the prevalence of asthma (6, 7). Furthermore, such a link implies that an aggressive, as opposed to symptomatic, approach to treatment of AD may halt the atopic march, thus preventing future asthma.
A common conclusion drawn from multiple studies is that the skin can be the site of potent allergic sensitization. This is particularly true following disruption of skin integrity by mechanical means (8) or by induction of allergic, AD-like, dermatitis. An elegant example is provided in a study in which OVA was applied epicutaneously to tape-stripped skin in order to induce an allergic skin response (9). Subsequent challenge of these mice by inhalation of a single dose of OVA caused a considerably greater response to the inhaled allergen than mice sensitized with saline.
In the present study, we explored the mechanisms contributing to the progression of the atopic march. Given that early development of chronic allergic dermatitis and the later development of allergic asthma are not necessarily linked by a shared allergen, we chose to test the link between these two diseases using two different allergens. Here we found that upon resolution of oxazolone-induced AD in mice, OVA sensitization and challenge caused increased airway responses. This was attributed to increased numbers of MCs in the tissues of OVA-challenged mice following resolution of AD. The findings show that the increase of MCs in chronic allergic dermatitis can have a detrimental effect by promoting subsequent allergic airway hyper-reactivity.
Materials and Methods
Animals
Balb/c, C57BL/6, and the mast cell-deficient KitW-sh/W-sh mice (C57BL/6) were obtained or came from our colonies at Taconic Farms. Age and gender matched animals were used in all individual experiments. Mice were maintained and used in accordance with NIH guidelines and the animal study proposals (A010-04-03) approved by the NIAMS animal care and use committee.
Oxazolone-induced dermatitis
Mice were sensitized on day 0 by application of 15 μl 1% oxazolone (Sigma Aldrich) in acetone on both aspects of both ears. Starting from day 7, mice were challenged in the same manner with 0.5% oxazolone, three times per week for up to 10 challenges. For control mice, treatment was performed with acetone only. Ear thickness was measured by a dial thickness gauge (Mitutoyo).
Histological staining
At the end of each experiment, mice were euthanized by CO2 inhalation and tissues were collected and placed in 10% neutral buffered formalin. Lungs were inflated with 2 ml formalin for 1 hr prior to harvesting, and then left in formalin overnight with rotation to ensure fixation. Embedding, sectioning and staining were done at American Histolabs.
Flow cytometry
Splenocytes were prepared and stained as previously described (10). Flow cytometry acquisitions were done with FACSCalibur (BD Biosciences) and Cell Quest software. Analysis was with FlowJo software (Treestar Inc.).
Passive systemic anaphylaxis
Anaphylaxis was induced as previously described (11). Briefly, mice were sensitized by i.v. injection of 3 μg DNP-specific IgE (H1-DNP-ε-26.82, 0.2 ml). Twenty four hours later, they were challenged with 250 μg DNP36-HSA. Measurement of body temperature was performed using an implantable electronic transponder as described (11).
Generation of MC and reconstitution of KitW-sh/W-sh mice
Suspended bone marrow cells from C57BL/6 mice were cultured for 4 weeks in the presence of stem cell factor and IL-3 (20 ng/ml) (PeproTech Inc.), as previously described (12, 13). Differentiation of MCs was confirmed by cytometric analysis using cKit and FcεRI as markers. MCs were injected to both ear pinna at a concentration of 107 cells/ml and a total volume of 120 μl. Engraftment was allowed for a minimum of 6 weeks. After disease induction (dermatitis and/or asthma) engraftment was confirmed by toluidine blue staining of tissue sections.
Induction and measurement of allergic airway reactivity
For sensitization, mice were i.p. injected with 50 μg ovalbumin (Sigma Aldrich) in a volume of 0.1 ml on days 0, 3, 7. Starting from day 12, they were challenged intranasally with 20 μg OVA in 30 μl PBS under isoflurane anesthesia (Baxter). Challenges were administered once a week for nine weeks. Starting from 24 hrs after the final OVA challenge, bronchial reactivity was determined. Mice were anesthetized by i.p. administration of 300 μl ketamine-xylazine mixture (1 ml of ketamine (100 mg/ml), 0.5 ml of xylazine (20 mg/ml) and 8.5 ml of PBS). A 19-gauge blunt end needle was inserted into the trachea and the animals were then mechanically ventilated and challenged with increasing doses of aerosolized methacholine using flexiVent (Scireq Scientific Respiratory Equipment Inc.).
Statistical Analysis
For comparison between two populations, two-tailed Student’s t-tests were performed. Airway responses to methacholine were compared using a two-way ANOVA test. Data is presented as mean ± s.e.m. Statistical analysis was done with Prism software (GraphPad Software, Inc.).
Results and Discussion
Increased asthma in mice after recovery from oxazolone-induced dermatitis
Atopic dermatitis (AD)-like disease was elicited by repeated exposures of the ear skin of mice to oxazolone. Disease course could be divided into three phases (Fig. 1A): (I) sensitization, which is a critical step in determining the intensity of subsequent inflammation; (II) acute inflammation manifested as continuously increasing ear swelling; (III) chronic inflammation, starting from approximately the fifth challenge onwards in which ear thickness reaches a plateau. Following withdrawal of oxazolone treatment (Fig. 1A, arrow) ear swelling gradually subsided and stabilized by 20 days post-withdrawal. As shown in Fig. 1B, chronic airway hyper-reactivity was subsequently induced using a protocol of OVA challenges without adjuvant. In this model, MCs have been shown to be key participants and can considerably intensify the inflammatory response to OVA (14). When OVA sensitization was initiated 5 weeks after the last oxazolone challenge (Fig. 1B) airway hyper-reactivity was more severe, with increased resistance (Fig. 1B, left panel) and, to a lesser but nonetheless significant extent, reduced compliance (Fig. 1B, right panel). Having ruled-out a direct effect of oxazolone dermatitis on lung functions (Supplemental Fig, S1A) we hypothesized that it may enhance airway disease by increasing sensitization to OVA. However, OVA-specific IgE was not increased at the post-dermatitis state (Supplemental Fig.S1B) in spite of the diverse cytokines induced by oxazolone (Supplemental Fig. S2A-C), which included IL-4 that is required for full development of dermatitis (Supplemental Fig. S2B).
Figure 1. The post-dermatits state confers increased airway hyperreactivity.
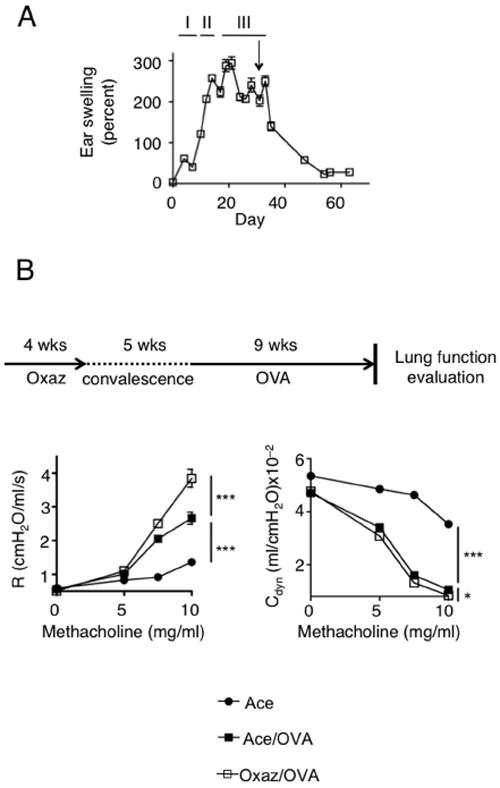
(A) Ear-thickness measurements in Balb/c mice with chronic oxazolone-induced dermatitis. Mice were challenged up to day 30 and then allowed to convalesce. The arrow denotes the time of the last challenge. I, sensitization; II, acute swelling; III, chronic inflammation (plateau) (n=5). (B) Scheme of protocol and measurement of lung function in mice where OVA-induced airway reactivity was initiated 5 weeks following cessation of oxazolone challenges (n=5). (R = resistance and Cdyn = compliance). *, p=0.014; ***, p<0.0001.
We previously reported (15) that, during the course of oxazolone-induced chronic AD in mice, MCs become activated, they migrate from the skin to the draining lymph nodes and spleen, and expand in the tissues. In this setting, MCs were found to suppress inflammation during the late stages of disease by producing IL-2 and supporting Treg function at the site of inflammation. In humans, it is well known that MC numbers increase in the affected skin of individuals with AD (16). Thus, we postulated that the exacerbation of post-AD airway disease, in our mouse model, might be caused by increased numbers of tissue MCs. We evaluated MC numbers in the spleen of mice with dermatitis and found their numbers were increased (data not shown) consistent with prior findings (15). The increased numbers of MCs in tissues remained during convalesence and following challenge with OVA (Figs. 2A, B). Our previous study (15) demonstrated that during dermatitis a significant proportion of splenic MCs produced IL-2. In contrast, five weeks post-dermatitis splenic MCs were no longer producing IL-2 (Fig. 2C) suggesting that these cells were no longer activated, or could be non-responsive. To test the latter possibility we conducted a systemic anaphylactic challenge of dermatitis-induced mice five weeks after convalescence. As shown in Fig. 2D, an enhanced anaphylactic response was observed for dermatitis-induced mice relative to those treated with solvent alone and this enhanced response was still present after five weeks of convalescence. This demonstrated that MCs are not readily cleared from the tissues and remain fully capable of responding to a subsequent challenge. Moreover, examination of the spleen of mice challenged with oxazolone, allowed to convalesce and subsequently challenged with OVA disclosed more MCs than in the spleen of mice challenged with OVA alone (Fig. 2E). This demonstrates that the development of dermatitis is the cause of the marked increase in tissue MCs seen in post-dermatitis airway challenged mice.
Figure 2. Long-term persistence of increased MC numbers and enhanced systemic anaphylaxis in post-dermatitis mice.
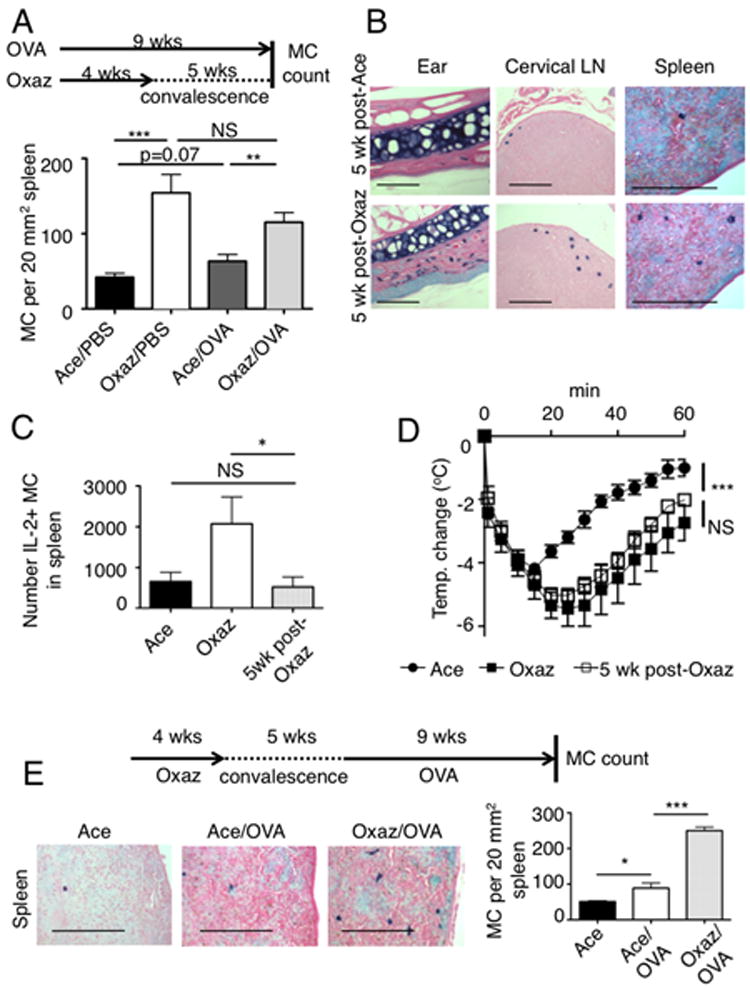
(A) Protocol scheme and measurement of MC numbers in Balb/c mice after 5 weeks of post-dermatitis convalescence and concomitant 9 weeks of OVA treatment, as shown (n=5). **, p=0.0039; ***, p=0.0006. (B) Toluidine- blue (TB) staining for MCs in indicated tissues, 5 weeks after cessation of either vehicle (acetone) or oxazolone. Bars, 150 μm. (C) Numbers of IL2 producing (IL-2+) MCs in the spleen of mice with dermatitis (Oxaz) or after recovery (5wk post-Oxaz). *, p=0.03. (D) Passive systemic anaphylaxis in mice with oxazolone-induced dermatitis or following their recovery (post-dermatitis). After challenge, body temperature was recorded using a subcutaneous probe (n=5). ***, p<0.0001. (E) Representative picture (left) of toluidine blue stained MCs in the spleens of mice after sequential oxazolone-dermatitis and OVA-airway hyper-reactivity induction, solvent (Ace) challenge only, or OVA-airway hyper-reactivity induction only. Quantitation of MC numbers in spleens (n=5). *, p=0.017; ***, p<0.0001. Bars, 150 μm.
Because we previously found (15) that MCs dampen chronic AD, we asked whether MCs can function as regulatory and effector cells in dermatitis and airway disease, respectively. MC-deficient (KitW-sh/W-sh) and appropriate control C57BL/6 mice (colony established from F1 wild type progeny of a KitW-sh/+ mating) were subjected to oxazolone-induced dermatitis. Control C57BL/6 mice demonstrated milder inflammation relative to KitW-sh/W-sh mice, consistent with our prior results (15). In a separate experiment, induction of airway disease revealed increased airway hyper-reactivity in control C57BL/6 mice when compared to KitW-sh/W-sh (Fig. 3B). This suggests that MCs, which dampen chronic AD (15), conversely promote the induction of OVA-mediated airway hyper-responsiveness. To formally demonstrate that this transition from regulatory to effector MCs could occur in the same mouse, we performed an adoptive (intra-dermal (i.d.)) transfer of MCs in KitW-sh/W-sh and afterwards we induced oxazolone-dermatitis in these mice as well as in non-reconstituted KitW-sh/W-sh mice. Disease induction was followed by five weeks of convalescence and subsequent OVA-induced airway hyper-reactivity. As shown in Fig. 3C, MC-reconstituted KitW-sh/W-sh mice showed reduced inflammation during the late stages of oxazolone-induced dermatitis when compared to their non-reconstituted counterparts. No protection was observed at early stages of disease. In contrast, OVA-induced airway hyper-reactivity was markedly increased in MC-reconstituted KitW-sh/W-sh mice (Fig. 3D) when compared to non-reconstituted littermates. These findings demonstrate that, whereas MCs play a protective role in one disease, they are conversely able to promote the severity of another disease. The findings are consistent with the view that induction of a TH2 response in one disease may well predispose one to another disease, as previously reported for lyn-/- mice (10, 17). Examination of the tissues of MC-reconstituted KitW-sh/W-sh mice revealed that i.d. injected MCs not only engrafted the skin but were also found in the cervical LNs and the spleen (Fig. 4A, B), consistent with our previous findings (15). These dynamics were associated with increased inflammation, as judged by airway inflammation (Fig. 4A) and by increased numbers of eosinophils in the bronchoalveolar lavage fluid (Fig. 4C). While some MCs could be found in the trachea of WT mice (14) (Fig. 4D), we observed no MC engraftment in the lungs or large airways of MC-reconstituted mice (Fig. 4A, B) demonstrating that the dermatitis-induced effect on airway hyper-reactivity is mediated distal to the site of inflammation.
Figure 3. Introduction of MCs into the skin results in a dual effect of protection from dermatitis and exacerbated asthma.
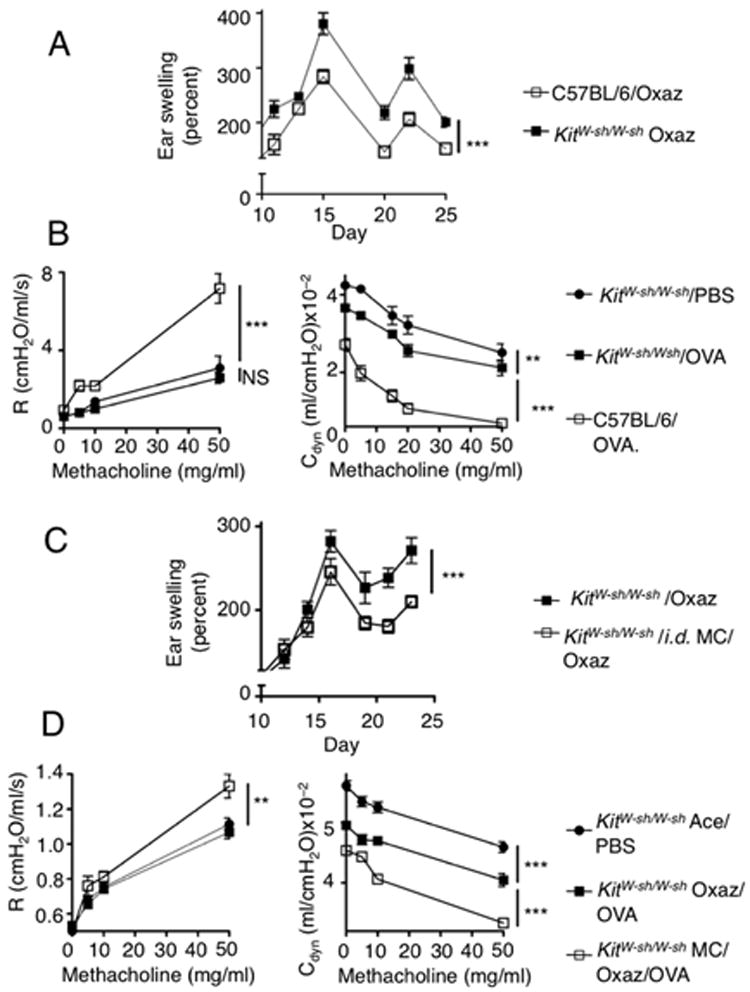
(A) C57BL/6 and KitW-sh/W-sh mice were subjected to oxazolone challenges and ear swelling responses were compared (n=5) ***, p<0.0001. The graph shows the range of ear swelling (Y axis) with time (X axis) previously shown (15) to be the point in the disease where MCs are involved. (B) Concomitantly, OVA sensitization and challenging was started in littermates of each group and lung function (R = ressitance and Cdyn = compliance) was assessed by a methacholine study (n=5). **, p=0.0086; ***, p<0.0001;. (C) KitW-sh/W-sh mice were i.d. injected with bone marrow-derived MCs. Six weeks later, mice were sensitized and subsequently challenged with oxazolone (n=7). ***, p<0.0001. (D) After 5 weeks of recovery from dermatitis, sensitization to OVA was initiated in a manner similar to the experimental design depicted in Fig. 1B. At the end of asthma induction, lung functions were evaluated as in (3B) (n=5). **, p=0.0008; ***, p<0.0001.
Figure 4. MC distribution in tissues after oxazolone-dermatitis and OVA-induced asthma in i.d. reconstituted KitW-sh/W-sh mice.
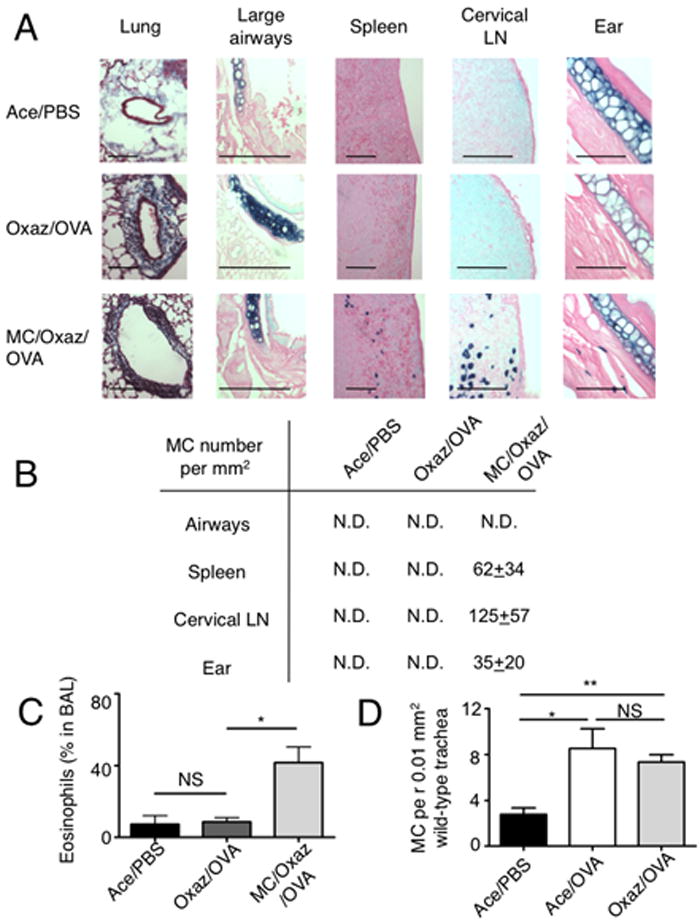
(A) Tissues were harvested from the indicated three groups of mice and embedded in paraffin after the OVA challenge. Lung samples were stained with Masson’s trichrome to demonstrate inflammatory infiltrate within peri-bronchiolar collagen. Large airways, spleens, cervical LN and ear tissues were stained for MCs, with toluidine-blue. Bars, 100 μm. (B) Quantitation of MCs in tissues of mice with asthma was done under a light microscope and is presented as mean±sem per mm2. N.D., not detected. For comparison, the basal MC numbers in unchallenged i.d. reconstituted mice were: airways, N.D; spleen, 4.5±1.5; cervical LN, 218±59, ear, 27±6 (n=5). (C) : Quantitation of eosinophils in bronchoalveolar lavage (BAL) fluid of mice in (A, B) (n=3). *, p=0.02. (D) MCs in trachea tissue of wild type Balb/c mice, enumerated following toluidine blue staining under a light microscope (n=5). *, p=0.016; **, p=0.0011.
In summary, the work presented herein provides a murine model for the atopic march with several characteristics of the syndrome in humans. First, accumulation of MCs during chronic allergic dermatitis was observed consistent with reports in human disease (16). While in our model of oxazolone-induced AD the accumulation of MCs was protective (15), these cells were also able to enhance anaphylaxis as reported in some humans with AD (1). Second, post-dermatitis mice developed more severe airway disease, which is consistent with the well described observations of increased incidence and severity of asthma following AD (18). Finally, exposure to one allergen caused increased responsiveness to another allergen suggesting potential epigenetic regulation, consistent with a role of the epigenome in increasing susceptibility and/or severity of allergic disease (19). Thus, the atopic model described herein, provides a mechanistic link between the dermatitis-driven MC expansion and the increased risk and/or severity of asthma. It appears that, whereas the expansion of MCs in AD is beneficial at late stages of this disease, the cost is an increased risk for development or severity of airway hyper-reactivity.
Supplementary Material
Acknowledgments
We are extremely grateful to M. Yu and S. J. Galli (Stanford) for their kind generosity in training A.Y.H. on murine models of airway hyper-reactivity. We also acknowledge the invaluable help of the Flow Cytometry Section, the Laboratory Animal Care and Use Section, and the Light Imaging Section of the Office of Science and Technology, NIAMS.
Footnotes
This research was supported by the Intramural Research Programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health. A.Y.H. was also supported by the American Physician Fellowship and the Morasha Program of the Israel Science Foundation.
References
- 1.Gold MS, Kemp AS. Atopic disease in childhood. Med J Aust. 2005;182:298–304. doi: 10.5694/j.1326-5377.2005.tb06707.x. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Galli E, Gianni S, Auricchio G, Brunetti E, Mancino G, Rossi P. Atopic dermatitis and asthma. Allergy Asthma Proc. 2007;28:540–543. doi: 10.2500/aap2007.28.3048. [DOI] [PubMed] [Google Scholar]
- 4.Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Abramson MJ, Hill DJ, Dharmage SC. Do boys do the atopic march while girls dawdle? J Allergy Clin Immunol. 2008;121:1190–1195. doi: 10.1016/j.jaci.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Spergel JM. Atopic march: link to upper airways. Curr Opin Allergy Clin Immunol. 2005;5:17–21. doi: 10.1097/00130832-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 7.Burgess JA, Lowe AJ, Matheson MC, Varigos G, Abramson MJ, Dharmage SC. Does eczema lead to asthma? J Asthma. 2009;46:429–436. doi: 10.1080/02770900902846356. [DOI] [PubMed] [Google Scholar]
- 8.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34:2100–2109. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 9.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O’Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, Watford W, Meylan F, Diesner SC, Li L, Schnermann J, Proia RL, Rivera J. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 13.Razin E, Ihle JN, Seldin D, Mencia-Huerta JM, Katz HR, LeBlanc PA, Hein A, Caulfield JP, Austen KF, Stevens RL. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132:1479–1486. [PubMed] [Google Scholar]
- 14.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, Rivera J. Mast cell IL-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–571. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell EB, Crow J, Williams G, Platts-Mills TA. Increase in skin mast cells following chronic house dust mite exposure. Br J Dermatol. 1986;114:65–73. doi: 10.1111/j.1365-2133.1986.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 17.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005;25:231–246. v. doi: 10.1016/j.iac.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.North ML, Ellis AK. The role of epigenetics in the developmental origins of allergic disease. Ann Allergy Asthma Immunol. 2011;106:355–361. doi: 10.1016/j.anai.2011.02.008. quiz 362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


