Abstract
Photodynamic therapy (PDT) for localized microbial infections exerts its therapeutic effect both by direct bacterial killing and also by the bactericidal effects of host neutrophils stimulated by PDT. Therefore, PDT-induced damage to neutrophils must be minimized, while direct photoinactivation of bacteria is maintained to maximize the therapeutic efficacy of antimicrobial PDT in vivo. However, there has been no study in which the cytocidal effect of PDT on neutrophils was investigated. In this study, the cytocidal effects of PDT on neutrophils were evaluated using different antimicrobial photosensitizers to find suitable candidate photosensitizers for antimicrobial PDT. PDT on murine peripheral-blood neutrophils was performed in vitro using each photosensitizer at a concentration that exerted a maximum bactericidal effect on methicillin-resistant Staphylococcus aureus (MRSA), and morphological alteration and viability of neutrophils were studied. Most neutrophils were viable (> 80%) after PDT using toluidine blue-O or methylene blue, while neutrophils showed morphological change and their viabilities were decreased (< 70%) after PDT using other photosensitizers (erythrosine B, rose bengal, crystal violet, Photofrin, new methylene blue, and Laserphyrin). These results suggest that PDT using toluidine blue-O or methylene blue can preserve host neutrophils while exerting a significant therapeutic effect on in vivo localized microbial infection.
INTRODUCTION
Photodynamic therapy (PDT) is a therapeutic modality for diseases caused by unwanted tissues or cells. A photosensitizer accumulates in proliferative tissues such as tumors, and it is activated by visible light and induces the generation of singlet oxygen or other reactive oxygen species (ROS), which damage unwanted cells (1–3). PDT has already been applied in clinical situations for malignant tumors and demonstrated satisfactory results (4, 5).
In recent years, PDT has become an emerging new therapeutic modality for localized microbial infection. Although the antimicrobial effect of PDT was discovered as early as 1900, the potential of PDT was not exploited for several decades due to the discovery and development of antibiotics (6). However, due to the widespread emergence of multiple-drug-resistant bacteria in the 1980s caused by inappropriate or excessive use of antibiotics, PDT has attracted considerable attention as a possible alternative approach. The antimicrobial mechanism of PDT is quite different from that of antibiotics, and PDT might be effective even for multi-drug-resistant bacteria (6–10).
There have been an increasing number of reports, especially since 2009, on therapeutic applications of PDT for microbial infection, indicating a steady increase of interest in antimicrobial PDT. However, although favorable results of in vitro PDT for cultured bacteria have been described in many reports, good results in vivo in animal models of localized infections have only been described in a few reports. Therefore, the conditions for efficacy of PDT in vivo remain unknown in many infection models.
We recently demonstrated that in vivo PDT using Photofrin® for murine methicillin-resistant Staphylococcus aureus (MRSA) -induced arthritis, showed a pronounced biphasic dose response (11, 12). Light doses that were too low and also light doses that were too high were ineffective and there was an optimum fluence of 20 J/cm2 to give the best antibacterial effect. These observations were explained by the role of host neutrophils activated by PDT in killing the bacteria as well as direct bacterial photoinactivation by the PDT generated ROS (12). Low-dose PDT (the product of photosensitizer concentration and light fluence that varies for each photosensitizer) failed to kill the bacteria and failed to activate the neutrophils, while excessively high dose PDT killed the neutrophils as well as the bacteria, and the bacteria were easily able to regrow in the absence of neutrophil host defense (11). Therefore, the cytocidal effects of PDT on neutrophils should be minimized at the same time as the direct bactericidal effects of PDT are maximized to optimize the in vivo therapeutic effect of antimicrobial PDT. Moreover, both the expansion of the therapeutic window and improvement of therapeutic efficacy would be made possible by using a photosensitizer that can kill bacteria but not neutrophils.
In past studies on PDT for microbial infections, erythrosine B (13), rose bengal (14), crystal violet (15), Photofrin® (11), new methylene blue (14), toluidine blue-O (14), methylene blue (14) and Laserphyrin® (16) have been used as photosensitizers. Some recent studies have shown a favorable therapeutic effect of PDT in vivo using toluidine blue-O or methylene blue in animal models of localized microbial infection (17, 18). In this study, we evaluated and compared the cytocidal effects of PDT using these photosensitizers on murine peripheral-blood neutrophils. Initially, bactericidal effects of PDT using each photosensitizer were evaluated to determine the appropriate concentration of each photosensitizer for the next experiment. Then the cytocidal effect of PDT on neutrophils was examined using each photosensitizer at the previously determined concentration. Based on the results, photosensitizers with weak cytocidal effects on neutrophils but high bactericidal effects are proposed for improved therapeutic efficacy of antimicrobial PDT in vivo.
MATERIALS AND METHODS
This study was conducted in accordance with the guidelines of the Institutional Review Board for the Care of Animal Subjects at the National Defense Medical College.
MRSA strain
MRSA isolated from clinical specimens of a patient in the National Defense Medical College Hospital was used (11, 12). MRSA was grown in brain heart infusion (BHI, Difco Laboratories, Detroit, MI) supplemented with oxacillin with static culture for 12 h followed by shaking culture for 4 h at 37°C under aerobic conditions. Cell density was monitored by absorbance at 600 nm (OD of 0.8 = 1×108 cells/mL).
Photosensitizers
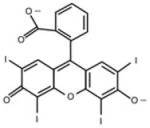
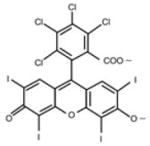
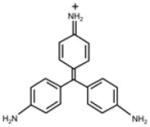
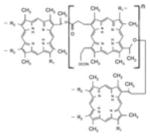
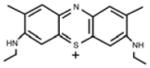
Erythrosine B (ET), rose bengal (RB), crystal violet (CV), new methylene blue (NMB), toluidine blue-O (TB), methylene blue (MB) were all obtained from Sigma-Aldrich (St. Louis, MO, USA), Photofrin® (PHP, Pfizer Japan, Tokyo, Japan) and Laserphyrin® (NPe6, Meiji Seika Pharma, Tokyo, Japan) were used for each experiment (Table 1). The calculated log (P) values (octanol: water partition coefficients) were obtained using ChemBioDraw Ultra (CambridgeSoft, Cambridge, MA, USA). The molecular weight of Photofrin (PHP) is not uniform, therefore, the PHP concentration was indicated by mass concentration in all of the results.
Table 1.
Photosensitizers used
| Erythrosine B (ET) | Rose Bengal (RB) | Crystal Violet (CV) | Photofrin® (PHP) | |
|---|---|---|---|---|
|
|
|
|
|
| FW | 897.88 | 1017.64 | 407.99 | 1231.28 ~ 4883.30 |
| Absorption wavelength | 525 nm | 550 nm | 590 nm | 1st: 396 nm 5th: 630 nm |
| log(P)* | 1.1 | 3.46 | 0.63 | 3.96 |
| Charge | negative | negative | positive | negative |
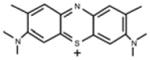
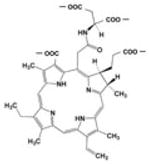
| New Methylene Blue (NMB) | Toluidine Blue O (TB) | Methylene Blue (MB) | Laserphyrin® (NPe6) | |
|
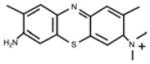
|
|
|
|
| FW | 347.91 | 305.83 | 319.85 | 799.69 |
| Absorption wavelength | 1st: 588 nm 2nd: 628 nm |
1st: 596 nm 2nd: 630 nm |
664 nm | 1st: 403 nm 2nd: 656 nm |
| log(P)* | 2.3 | -1.445 | -0.785 | 1.47 |
| Charge | positive | positive | positive | negative |
log(P): calculated log partition coefficient octanol:water
Measurement of light absorbance of the photosensitizers
Each photosensitizer was dissolved in Hanks’ balanced salt solution (HBSS, Sigma-Aldrich) to a final concentration of 20 μM (except for PHP; final concentration of 10 μg/mL). The absorption spectrum of each solution was measured using a spectrophotometer (U-3300, Hitachi, Tokyo, Japan) from 300 to 800 nm. In each photosensitizer, when the absorption band was single, the wavelength of the absorption peak was used for the irradiation wavelength. When there were two or more absorption bands, the longest wavelength of the absorption peaks was used for the irradiation wavelength (Table 1).
PDT in vitro for MRSA to determine appropriate concentration of the photosensitizer
Serial dilutions of each photosensitizer in HBSS solution were made, and MRSA was suspended in each solution (final cell density of 1 × 108 CFU/mL) in a 12-well plate under dark conditions at 37 °C. Each mixed solution was immediately irradiated with a fluence of 20 J/cm2 using a xenon light source (MAX-301, Asahi Spectra, Tokyo, Japan) with a bandpass filter of which the central wavelength was set at the absorption peak wavelength of each photosensitizer. The bandwidth (FWHM) of each filter was kept at 40 nm regardless of the photosensitizer. A power meter (Field Master; Coherent, Santa Clara, CA) was used to measure the light fluence rate, which was set at 40 mW/cm2. Immediately after the irradiation, each mixed solution was subjected to serial dilution and cultured on BHI agar for 24 hours under dark conditions and the numbers of colonies were counted. The number (colony-forming units; CFU) of viable MRSA was calculated on the basis of dilution ratio. From the distribution of CFU, an appropriate concentration of each photosensitizer was defined on the basis of the following criteria: when the surviving CFU showed a biphasic dose response with photosensitizer concentration, the appropriate concentration was determined as that at which the surviving CFU after PDT was the lowest; when the bactericidal response was not biphasic, the appropriate concentration was determined as the lowest concentration at which the CFU after PDT was lower than 100. When the appropriate concentration that met the requirements above could not be optimized, the irradiation energy was reduced from 20 to 5 J/cm2 and the concentration of each photosensitizer was serially decreased.
PDT in vitro for peripheral neutrophils
Murine venous blood was collected from the postcaval veins of C57BL/6NCr mice (8–9 weeks old, 20–25 g in weight, Japan SLC, Hamamatsu, Japan) and neutrophils were collected using a gradient centrifugation method (11). Neutrophils were suspended in 80 μL of each photosensitizer-HBSS solution mixed with 20 μL of 0.4% trypan blue solution (final cell density of neutrophils of 6 × 103/μL). Ten μL of the mixed solution was dropped onto a glass slide and was photoirradiated under microscopic observation. A white light source built into a bright field microscope (CX41; Olympus, Tokyo, Japan) with a bandpass filter was used for irradiation, and the power density was adjusted to 20 mW/cm2 (this was the highest power density that could be obtained using this light source). Morphological changes in neutrophils were visualized using a digital camera (C-3040 ZOOM; Olympus) installed in the microscope.
To quantify the killing of neutrophils, the mixed cell suspension was added to a 96-well plate (100 μL/well) and irradiated with energy of 5, 20 or 50 J/cm2 using a xenon light source with bandpass filters (as described above). After irradiation, 10 μL of mixed suspension was dropped onto a Burker-Türk hemacytometer (Erma, Tokyo, Japan), and trypan blue-stained and unstained neutrophils were counted using the microscope (CX41). The viability of neutrophils (= Trypan blue-unstained neutrophils/Total neutrophils per unit field) was calculated. As a control, viability of neutrophils after incubation (10 minutes) in each mixed solution without irradiation was calculated (0 J/cm2).
Statistical analysis
Data are expressed as means ± SE. One-way analysis of variance followed by Scheffe’s post hoc test was used for data analysis. SPSS ver.16 was used for data analysis. P-values < 0.05 were considered statistically significant.
RESULTS
Appropriate concentration of each photosensitizer
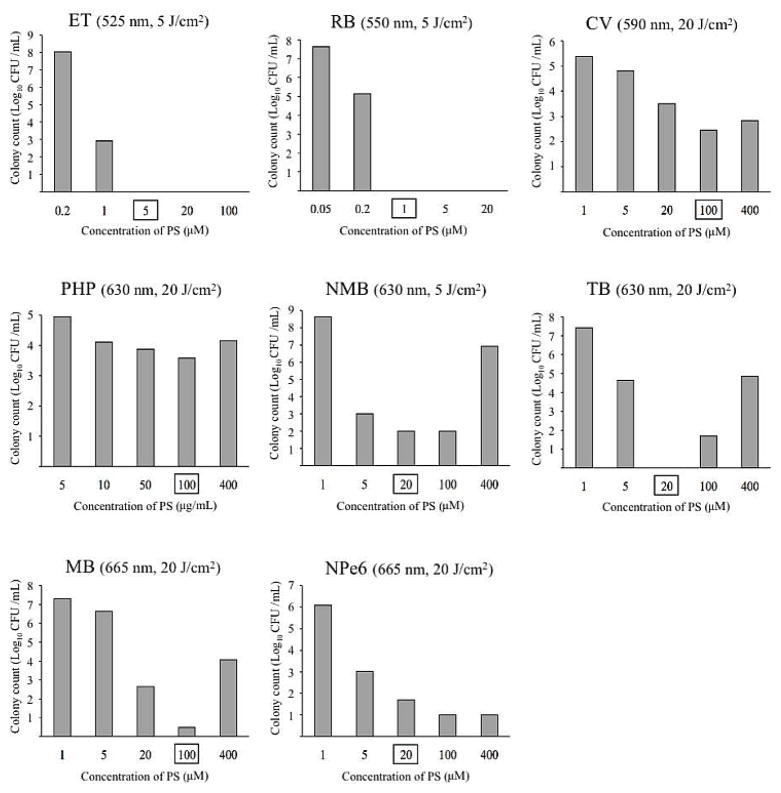
Figure 1 shows the surviving CFU of MRSA after in vitro PDT using the eight photosensitizers. Because two different fluences were used (5 and 20 J/cm2) it is difficult to compare all 8 photosensitizers, however it can readily be seen that ET and RB were the most effective, while CV and PHP were the least effective. The remaining 4 photosensitizers (NMB, TB, MB, NPe6) had intermediate levels of effectiveness. Many photosensitizers (CV, PHP, NMB, TB and MB) showed a biphasic dose response with the most killing occurring at a concentration lower than the maximum tested. The reason for this observation is thought to be due to self-shielding by the photosensitizer in solution. In other words at high photosensitizer concentrations light is absorbed non-productively by photosensitizer in solution which is not bound to bacteria preventing light from reaching photosensitizer which is bound to bacteria. The selected concentrations of the photosensitizers according to the specifications outlined above were determined to be 5 μM for ET, 1 μM for RB, 100 μM for CV, 100 μg/mL for PHP (corresponding to 168 μM HP equivalent), 20 μM for NMB, 20 μM for TB, 100 μM for MB and 20 μM for NPe6 (Fig. 1, values highlighted by squares).
Figure 1.
Colony-forming units (CFU) of methicillin-resistant Staphylococcus aureus after photodynamic therapy in vitro using each photosensitizer (Log10 CFU/mL). The values on the right side of the name of each photosensitizer are the applied wavelength and fluences of photoirradiation. The square-rounded values are the estimated appropriate concentration of each photosensitizer.
Morphological alteration of neutrophils during irradiation
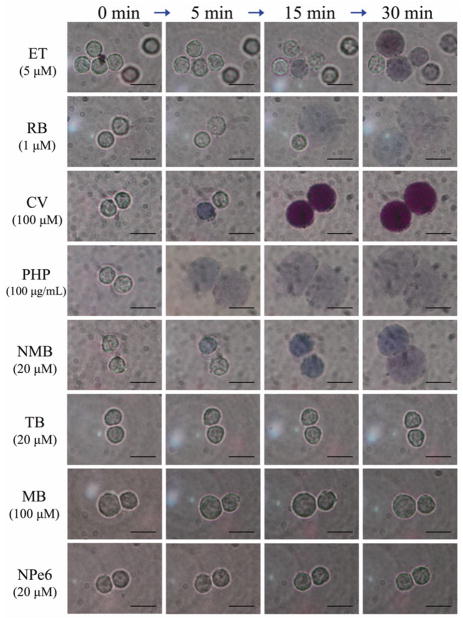
When ET, RB, CV, PHP or NMB were used as photosensitizers via irradiation on microscope slides, neutrophils began to be stained with trypan blue and showed a swollen morphological change during photoirradiation (Fig. 2). On the other hand, neutrophils did not show any remarkable morphological changes during photoirradiation using TB, MB or NPe6 (Fig. 2).
Figure 2.
Morphological alteration of neutrophils during photoirradiation using each photosensitizer. Bar = 10 μm. Total irradiation time was 30 min in each case.
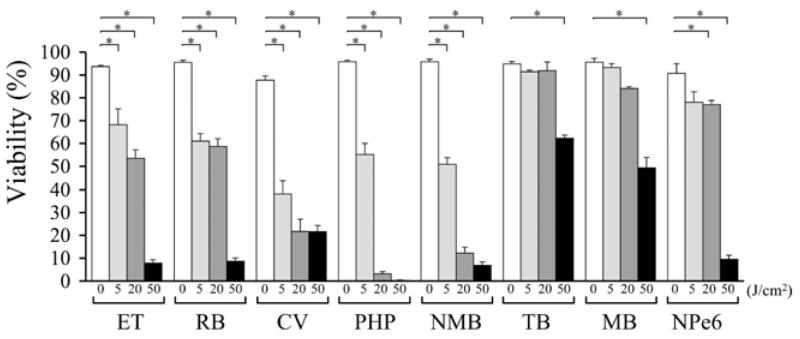
Viability of neutrophils after PDT
Viability of neutrophils after PDT using ET, RB, CV, PHP or NMB was significantly lower than dark control (< 70%) at each fluence tested (Fig. 3). After PDT using TB or MB, the viability of neutrophils was maintained at a high level (> 80%) with fluences of 5 or 20 J/cm2. However, with a fluence of 50 J/cm2, the viability of neutrophils after PDT was significantly lower than dark control (Fig. 3). After PDT using NPe6, the viability of neutrophils was maintained at 75% with a fluence of 5 J/cm2; however, it was significantly lower than dark control with fluences of 20 or 50 J/cm2 (Fig. 3).
Figure 3.
Viability of neutrophils after photodynamic therapy using each photosensitizer at fluences of 0, 5, 20 and 50 J/cm2. *P < 0.05
DISCUSSION
Two of the antimicrobial photosensitizers, TB and MB, did not show a significant cytocidal effect on neutrophils. In the case of TB or MB, the cytocidal effect was weaker than that of other photosensitizers even when high fluences were applied. The other photosensitizers, especially PHP and NMB, demonstrated a strong cytocidal effect on neutrophils.
While TB, MB are hydrophilic with a negative log (P) value (19, 20), CV, ET, NPe6, NMB, RB, and PHP are amphiphilic with progressively higher positive log (P) values (20–26). The cell membrane of a mammalian cell is composed of double-layered phospholipids, and hydrophilic molecules therefore cannot permeate the cell membrane, while amphiphilic and hydrophobic molecules can easily permeate the cell membrane and be promptly transported into the cell (27). However, bacteria also have a cell membrane composed of double-layered phospholipids but encased in a cell wall. It is likely that more hydrophilic photosensitizers are relatively better at inactivating bacteria, perhaps because they can at least bind to the cell wall if not penetrate the cell membrane, while more hydrophobic photosensitizers can penetrate the cell membrane of the much bigger mammalian cells but are excluded by the cell wall of bacteria. This study showed that other properties of photosensitizers such as electric charge, molecular weight and absorption wavelength seemed not to be correlated with strength of cytocidal effect on neutrophils.
It is well-known that PDT is able to stimulate many aspects of the mammalian immune response including neutrophils, dendritic cells, macrophages, T-lymphocytes and mast cells (28). These studies have been mainly directed towards understanding how PDT can stimulate anti-tumor immunity by enhancing the uptake of tumor-associated antigens by dendritic cells and their priming of T-cells (29). It is also known that neutrophils play an important role in the anti-tumor effects of PDT, which is abrogated if they are eliminated (30). However it was not generally realized that PDT-stimulated neutrophils also played an important role in the anti-bacterial effect of PDT until our studies concerning bacterial arthritis mentioned above (11, 12).
The effectiveness of different photosensitizers in killing bacteria (especially Gram-negatives) can vary markedly (31) and now in addition the effectiveness of the cytocidal effect against neutrophils must be taken into account in designing antimicrobial PDT protocols. Both TB and MB have long been used as bactericidal photosensitizers against Gram-negative bacteria as well as Gram-positive bacteria (32). The peak absorption wavelength of MB is 664 nm while 596 nm of TB, which might enable deeper treatment due to better light penetration compared with other photosensitizers (33). In conclusion, PDT using toluidine blue-O or methylene blue showed minimal damage to neutrophils under optimal concentrations of the photosensitizers for killing MRSA. Therefore, further investigations of the in vivo therapeutic efficacy of PDT using TB or MB should be performed.
Acknowledgments
This work was supported by a Grant-in-Aid for Challenging Exploratory Research (23659628) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to Yuji Morimoto. Liyi Huang and Michael R. Hamblin were supported by US NIH grant R01AI050875 to MRH and Tianhong Dai by an Airlift Research Foundation Extremity Trauma Research Grant (grant 109421).
References
- 1.Macdonald IJ, Dougherty TJ. Basic Principles of photodynamic therapy. J Porphyr Phthalocyanines. 2001;5:105–129. [Google Scholar]
- 2.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–156. [PubMed] [Google Scholar]
- 3.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 4.Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38:364–370. doi: 10.1002/lsm.20354. [DOI] [PubMed] [Google Scholar]
- 5.Mimura S, Narahara H, Otani T, Okuda S. Progress of photodynamic therapy in gastric cancer. Diagn Ther Endosc. 1999;5:175–182. doi: 10.1155/DTE.5.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 7.Malik Z, Gozhansky S, Nitzan Y. Effects of photoactivated haematoporphyrin derivative on bacteria and antibiotic resistance. Microbios Lett. 1982;21:103–112. [Google Scholar]
- 8.Nitzan Y, Gutterman M, Malik Z. Effect of photoactivated hematoporphyrin derivative on the viability of Staphylococcus aureus. Curr Microbiol. 1983;8:279–284. [Google Scholar]
- 9.Venezio FR, DiVincenzo C, Sherman R, Reichman M, Origitano TC, Thompson K, Reichman OH. Bactericidal effects of photoradiation therapy with hematoporphyrin derivative. J Infect Dis. 1985;151:166–169. doi: 10.1093/infdis/151.1.166. [DOI] [PubMed] [Google Scholar]
- 10.Martinetto P, Gariglio M, Lombard GF, Fiscella B, Boggio F. Bactericidal effects induced by laser irradiation and haematoporphyrin against Gram-positive and Gram-negative microorganisms. Drugs Exp Clin Res. 1986;12:335–342. [PubMed] [Google Scholar]
- 11.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Morimoto Y. Influence of intra-articular neutrophils on the effects of photodynamic therapy for murine MRSA arthritis. Photochem Photobiol. 2010;86:403–409. doi: 10.1111/j.1751-1097.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hamblin MR, Morimoto Y. Photodynamic therapy using intra-articular Photofrin for murine MRSA arthritis: biphasic light dose response for neutrophil-mediated antibacterial effect. Lasers Surg Med. 2011;43:221–229. doi: 10.1002/lsm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57:680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 14.Demidova TN, Hamblin MR. Photodynamic inactivation of Bacillus spores, mediated by phenothiazinium dyes. Appl Environ Microbiol. 2005;71:6918–6925. doi: 10.1128/AEM.71.11.6918-6925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docampo R, Moreno SN, Muniz RP, Cruz FS, Mason RP. Light-enhanced free radical formation and trypanocidal action of gentian violet (crystal violet) Science. 1983;220:1292–1295. doi: 10.1126/science.6304876. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Usuda J, Okunaka T, Furukawa K, Honda H, Sakaniwa N, Suga Y, Hirata T, Ohtani K, Inoue T, Maehara S, Kubota M, Yamada K, Tsuitsui H. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med. 2006;38:371–375. doi: 10.1002/lsm.20346. [DOI] [PubMed] [Google Scholar]
- 17.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragàs X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies. Lasers Surg Med. 2010;42:384–390. doi: 10.1002/lsm.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng SP, Teng LJ, Chen CT, Lo TH, Hung WC, Chen HJ, Hsueh PR, Tsai JC. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg Med. 2009;41:391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 20.Hirao A, Sato S, Saitoh D, Shinomiya N, Ashida H, Obara M. In vivo photoacoustic monitoring of photosensitizer distribution in burned skin for antibacterial photodynamic therapy. Photochem Photobiol. 2010;86:426–430. doi: 10.1111/j.1751-1097.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 21.Vermathen M, Vermathen P, Simonis U, Bigler P. Time-dependent interactions of the two porphyrinic compounds chlorin e6 and mono-L-aspartyl-chlorin e6 with phospholipid vesicles probed by NMR spectroscopy. Langmuir. 2008;24:12521–12533. doi: 10.1021/la802040v. [DOI] [PubMed] [Google Scholar]
- 22.Sârbu C, Casoni D, Kot-Wasik A, Wasik A, Namieœnik J. Modeling of chromatographic lipophilicity of food synthetic dyes estimated on different columns. J Sep Sci. 2010;33:2219–2229. doi: 10.1002/jssc.201000099. [DOI] [PubMed] [Google Scholar]
- 23.Suschek CV, Briviba K, Bruch-Gerharz D, Sies H, Kröncke KD, Kolb-Bachofen V. Even after UVA-exposure will nitric oxide protect cells from reactive oxygen intermediate-mediated apoptosis and necrosis. Cell Death Differ. 2001;8:515–527. doi: 10.1038/sj.cdd.4400839. [DOI] [PubMed] [Google Scholar]
- 24.Indig GL, Anderson GS, Nichols MG, Bartlett JA, Mellon WS, Sieber F. Effect of molecular structure on the performance of triarylmethane dyes as therapeutic agents for photochemical purging of autologous bone marrow grafts from residual tumor cells. J Pharm Sci. 2000;89:88–99. doi: 10.1002/(SICI)1520-6017(200001)89:1<88::AID-JPS9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Z, Hansen CB, Allen TM, Miller GG, Moore RB. Distribution of photosensitizers in bladder cancer spheroids: implications for intravesical instillation of photosensitizers for photodynamic therapy of bladder cancer. J Pharm Pharm Sci. 2005;8:536–543. [PubMed] [Google Scholar]
- 26.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem. 2009;9:974–983. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 27.Renkin EM. Cellular and intercellular transport pathways in exchange vessels. Am Rev Respir Dis. 1992;146:S28–31. doi: 10.1164/ajrccm/146.5_Pt_2.S28. [DOI] [PubMed] [Google Scholar]
- 28.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7:75–91. doi: 10.1586/eci.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vree WJ, Essers MC, de Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–2911. [PubMed] [Google Scholar]
- 31.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol Sci. 2004;23:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against Gram-positive and Gram-negative microorganisms. Lasers Surg Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 33.König K, Bockhorn V, Dietel W, Schubert H. Photochemotherapy of animal tumors with the photosensitizer methylene blue using a krypton laser. J Cancer Res Clin Oncol. 1987;113:301–303. doi: 10.1007/BF00396390. [DOI] [PubMed] [Google Scholar]