Abstract
Purpose
Memory deficits and depression are common in patients with temporal lobe epilepsy (TLE). Previous PET studies have shown reduced mesial temporal 5HT1A receptor binding in these patients. We examined the relationships among verbal memory performance, depression, and 5HT1A receptor binding binding measured with 18FCWAY positron emission tomography (PET) in a cross sectional study.
Methods
We studied 40 patients (24 male; mean age 34.5 ±10.7) with TLE. Seizure diagnosis and focus localization were based on ictal Video-Electoencephalographic recording. Patients had neuropsychological testing with Weschler Adult Intelligence Score III (WAIS III) and Weschler Memory Score III (WMS III) on stable AED regimens at least 24 hours since the last seizure. Beck Depression Inventory (BDI) scores were obtained. We performed interictal PET with [18F]FCWAY, a fluorinated derivative of WAY100635, a highly specific 5HT1A ligand, and structural magnetic resonance imaging (MRI) scans to estimate partial volume and plasma free fraction corrected [18F]FCWAY volume of distribution (V/f1).
Key Findings
Hippocampal V/f1 was significantly lower ipsilateral than contralateral to the epileptic focus (73.7 ± 27.3 versus 95.4 ± 28.4; p<.001). We found a significant relation between both left hippocampal FCWAY V/f1 (r= 0.41; p < 0.02) and left hippocampal volume (r=0.36; p < 0.03) and delayed auditory memory score. On multiple regression there was a significant effect of the interaction of left hippocampal FCWAY V/f1 and left hippocampal volume on delayed auditory memory, but not of either alone. High collinearity was present. In an analysis of variance including the side of the seizure focus, the effect of left hippocampal FCWAY V/f1 but not focus laterality retained significance.
Mean BDI was 8.3 ±7.0. There was a significant inverse relation between BDI and FCWAY V/f1 ipsilateral to the patient’s epileptic focus (r= 0.38 p<0.02) There was no difference between patients with a right or left temporal focus. There was no relation between BDI and immediate or delayed auditory memory.
Significance
Our study suggests that reduced left hippocampal 5-HT1A receptor binding may play a role in memory impairment in patients with TLE.
Keywords: Serotonin Receptors, Temporal Lobe Epilepsy, Memory, Depression, PET Scanning
Introduction
Studies using several ligands have shown reduced serotonin (5-HT) 1A receptor binding in mesial temporal structures of patients with temporal lobe epilepsy (TLE) (Toczek et al 2003, Savic et al 2004, Merlet et al 2004, Giovacchini et al 2005, Ito et al 2007, Didelot et al 2008, Liew et al 2009). Investigators using WAY-100635 labeled with either 11C or 18F found that the degree and distribution of reduced binding was correlated with scores on depression scales (Savic et al 2004, Theodore et al 2007) or a diagnosis of depression on the Standardized Clinical Interview for Depression (SCID) (Hasler et al 2007). One study using 18F-MPPF reported a positive correlation between 5-HT1A receptor binding potential (BP) and Beck Depression Inventory (BDI) score in several regions, including midbrain raphe, contralateral insula and ipsilateral hippocampus (Lothe et al 2008). The contrast in results with studies that found a relation between reduced binding and depression may be due to greater sensitivity of 18F-MPPF than WAY-100635 to extracellular serotonin concentration. Nevertheless, these studies parallel findings in primary major depressive disorders showing alterations in 5-HT1A receptor binding (Parsey et al 2006, Drevets et al 2007).
Imaging studies suggest a role for 5-HT1A in cognitive function as well. 5-HT1A binding is reduced in most PET studies of Alzheimer’s disease (SDAT) compared to controls, but correlations between memory and binding in healthy volunteers have been limited (Borg 2008). 5-HT1A receptor binding measured in post-mortem temporal cortex from patients with dementia was positively correlated with cognition (Elliott and Ballard 2009).
Cognitive impairment in epilepsy may be related to a variety of factors, including antiepileptic drug therapy, focal cortical atrophy or dysfunction, the effect of repeated seizures themselves, or underlying pathological processes (Hermann et al 2010). However, the role of specific neurotransmitters has not been investigated. In this study, we tested whether left hippocampal 5-HT1A binding and a clinical depression index were related to auditory memory performance in patients with TLE.
Methods
We studied 40 patients (24 male; mean age 34.5 ±10.7) with temporal lobe epilepsy who had been referred to the NINDS Clinical Epilepsy Section for evaluation of intractable seizures. Seizure diagnosis and focus localization were based on ictal Video-Electroencephalographic recording.
Patients had neuropsychological testing with Weschler Adult Intelligence Score III (WAIS III) and Weschler Memory Score III (WMS III) on stable AED regimens at least 24 hours since the last seizure. The WAIS III is a multidimensional assessment of both verbal and nonverbal tasks that generate a Verbal IQ, Performance IQ and a Full Scale IQ. The WMS III was used to assess memory and learning for verbal and visual material in immediate and delayed conditions. We used the Wechsler Memory Scale-III “Prose” memory (LM I&II) with immediate and 30 minute delayed trials (Shamim et al 2009). Subjects self-administered the Beck depression Inventory (BDI).
Imaging studies
No patient experienced seizures for at least 2 days before positron emission tomography (PET) scanning.
We obtained whole brain structural magnetic resonance imaging (MRI) scans on either a 1.5-T Horizon or a 3T Signa scanner (GE Medical Systems, Waukesha, Wisconsin) with standard T-2, Fluid attenuated inversion recovery and T1-weighted pulse sequences. We performed MRI segmentation and measured hippocampal volumes using published methods (Giovacchini et al 2005, Shamim et al 2009).
Dynamic PET scans were acquired for 120 min after bolus injection of approximately 10 mCi of Fluoro-N-(2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl)-N-(2-pyridyl)cyclohexanecarboxamide ([18 F]FCWAY), a fluorinated derivative of WAY100635, on an Advance scanner (GE Medical Systems), with a 68Ga transmission scan for attenuation correction. The scanner provides 35 slices with 4.25-mm separation in three-dimensional mode with septa retracted. Spatial resolution is 6 to 7 mm full-width half-maximum. Tissue radioactivity concentration was corrected for blood volume and acid metabolite uptake. Estimation of distribution volume (V) was performed with data fitted to a 2 compartment 3 parameter model using metabolite-corrected input arterial function. To correct for structural atrophy, we used an MRI-based partial volume correction (PVC) algorithm (Giovacchini et al 2005). To correct for potential antiepileptic drug (AED) effects on ligand protein binding, we used free fraction corrected V (V/f1) as our binding measure. We did not perform correction for nonspecific binding using cerebellum or another region in order to avoid introducing inaccuracies from structural atrophy, or binding heterogeneity (Parsey et al 2005, Theodore et al 2006, Giovacchini et al 2009). Previous studies have shown close agreement between FCWAY v/f1 and binding potential (Bmax/Kd) measurements, and lack of effects of AEDs after free fraction correction (Theodore et al 2006).
Statistical analysis was performed with SPSS Version 19 (IBM Inc Armonk N.Y). Student’s t-Tests were used to compare the effects of dichotomous variables, including side of focus on hippocampal FCWAY V/f1, BDI, and memory indices. Linear regression with colinearity diagnostics was used to assess the relation of continuous variables, and analysis of variance for comparing fixed and continuous variables.
Written informed consent was obtained. The study was approved by the National Institute of Neurological Disorders and Stroke Combined Neuroscience Institutional Review Board and NIH Radiation Safety Committee.
Results
Twenty-two patients had a left, and 18 a right temporal focus. Hippocampal V/f1 was significantly lower ipsilateral than contralateral to the epileptic focus (73.7 ± 27.3 versus 95.4 ± 28.4; p<.001).
We found no difference between males and females in age at scan, seizure onset, or BDI.
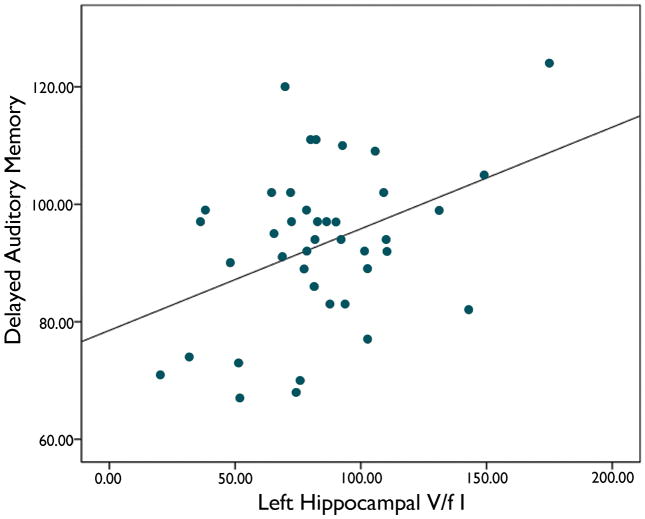
We found a significant relation between left hippocampal FCWAY V/f1 and delayed auditory memory score (r= 0.41; p < 0.02) (figure 1). Patients with a left temporal focus had lower verbal IQ (92.7 ± 12.8 versus 104.5 ± 16.0; P<0.02) and non-significant trends toward lower immediate (89.8 ± 16.4 versus 100.3 ± 16.2) and delayed auditory memory (89.7 ± 13.3 vs 97.3 ± 13.9). However, in an analysis of variance including the side of the seizure focus, the effect of left hippocampal FCWAY V/f1 was still present, while the seizure focus lateralization was not significant. The relation between left hippocampal FCWAY V/f1 and immediate auditory memory was not significant (p<0.07). Right hippocampal FCWAY V/f1 was not related to either memory measure.
Figure 1.
Delayed auditory memory scores showed a significant relationship with 5HT1A receptor plasma free-fraction corrected volume of distribution (V/f1) in left hippocampus, independent of the side of the epileptic focus.
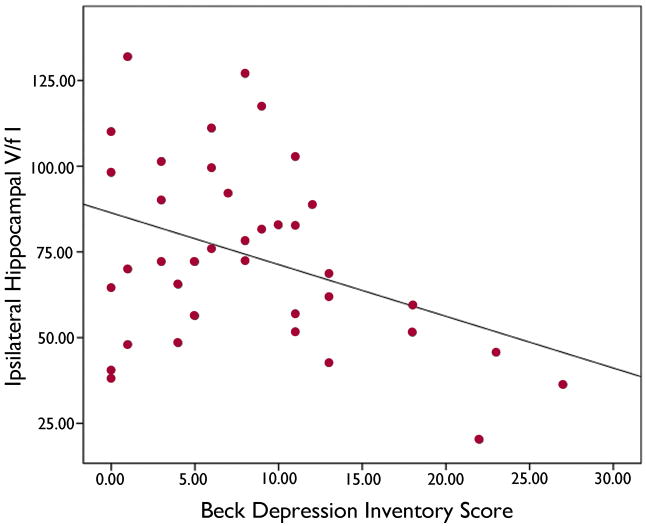
The presence of a structural abnormality on MRI did not affect delayed auditory memory score. There was a significant relation of left hippocampal volume to delayed auditory memory (r=0.36; p < 0.03). In a multiple regression, neither left hippcampal volume (p=0.11) nor left hippocampal FCWAY V/f1 (p=0.11) was significant, although the regression itself was (r=0.43; p < 0.03). However, there was significant multi-collinearity between the two variables. There was a trend toward a relationship between left hippocampal volume and left hippocampal FCWAY V/f1 (r=2.7; 0.05<p<0.10). Mean BDI was 8.3 ±7.0. There was a significant inverse relation between BDI and FCWAY V/f1 ipsilateral to the patient’s epileptic focus r= 0.38 p<0.02 (figure 2). There was no difference between patients with a right or left temporal focus, or a relation between BDI and immediate or delayed auditory memory.
Figure 2.
Beck Depression Inventory Score showed a significant inverse relation with HT1A receptor plasma free-fraction corrected volume of distribution (V/f1) in the hippocampus on the side of the seizure focus.
Discussion
Our study suggests that reduced left hippocampal 5-HT1A receptor binding may play a role in memory impairment in patients with TLE. This effect remained after the side of the epileptic focus, based on ictal video-EEG monitoring, was included in the analysis. We also found that there was a significant relation between BDI score and hippocampal 5-HT1A receptor binding on the side of the focus, irrespective of side, confirming previous results. In this study the relation was weaker, perhaps because the patients had lower mean BDI. We found no relation between left hippocampal volume and BDI, or between BDI and memory performance. The relation between left hippocampal FCWAY V/f1 and immediate auditory memory was not significant. Moreover, the multicollinearity found between left hippocampal volume and FCWAY binding complicates interpretation of the multiple regression analysis.
Previous studies suggested that intractable TLE hippocampal volume predicts delayed verbal memory functioning in TLE (Stewart et al 2009). Hippocampal and parahippocampal volumes, but not glucose metabolism measured by 18FDG-PET were correlated with memory measures (Griffith et al 2004).
In our study, hippocampal volume and 5HT1A binding were both significant predictors of delayed auditory memory. On multiple regression, neither variable alone was significant but the interaction was. These results, as well as our use of a stringent partial volume correction procedure, suggest that the reduced 5HT1A binding has an independent effect on delayed auditory memory that interacts with hippocampal volume loss. Moreover, results were independent of the side of the epileptic focus, suggesting that structural atrophy alone cannot explain the finding. The presence of significant collinearity between the two variables is consistent not only with an effect of volume loss on receptor density, but also with data showing that 5-HT1A innervation itself may be important for maintenance of hippocampal neoneurogenesis and functional integrity (Chugani and Chugani 2003, Ogren et al 2008).
5-HT1A receptors play a role in learning and memory as well as mood (Nagai et al 2009). Cell bodies in the midbrain raphe have dense projections to hippocampus (King et al 2008). Chronically tryptophan-depleted rats with 40–50% reduced hippocampal 5HT had impaired object-recognition memory (Jenkins et al 2009). Knockout rats (SERT −/−) for the serotonin transporter (5-HTT) gene, with lower intracellular basal serotonin levels than controls in several brain regions showed impaired performance compared with wild-type controls on a delayed object recognition task (Olivier et al 2009). Several studies have suggested an association between 5-HTT polymorphisms and epilepsy (Kauffman et al 2009, Hecimovic et al 2010).
5-HT1A receptor PET studies in patients with SDAT show decreased binding in hippocampus and parahippocampal gyrus, while studies in patients with minimal cognitive impairment have shown both increases and decreases, suggesting the possibility of compensatory receptor upregulation early in the pathophyiologic process, followed by progressive loss of serotoninergic innervation (Kepe et al 2006, Truchot et al 2008). In patients with large vessel cerebrovascular disease, 5-HT1A binding in temporal cortex correlated positively with performance on several cognitive scales (Elliott et al 2009).
Acute tryptophan depletion in normal volunteers caused impaired verbal learning, delayed recall and delayed object relocation in a spatial task (Sambeth et al 2008). Although tryptophan depletion is not receptor subtype-specific, some data suggesting that acute tryptophan depletion impairs consolidation of episodic memory are consistent with 1A receptor effects on neurogenesis (Grabiec et al 2009, Mendelsohn et al 2009). Treatment with partial 5-HT1A agonists has been shown to improve some cognitive parameters in patients with schizophrenia (Meltzer and Sumiyoshi 2008, Piskulić et al 2009). 1A effects on cognitive function could be related to baseline 5-HT levels and receptor availability, and relative effects on postsynaptic versus presynaptic autoreceptors, modulated by dose and timing.
Patients with depression may have impairment on a variety of memory measures, even if hippocampal volume loss is not present (MacQueen et al 2003, Vythilingam et al 2004, O’Brien et al 2004, Douglas et al 2011). Some studies have shown reduced mesial temporal 5-HT1A binding in depression, paralleling TLE (Drevets et al, 2007). Successful treatment with antidepressants improved memory but did not affect hippocampal volume in patients with depression, implicating serotoninergic mechanisms rather than diffuse hippocampal dysfunction (Vythilingam et al 2004). A 5-HT1A receptor agonist impaired memory function in normal volunteers, but improved it in depressed patients (Ogren et al 2008). In patients with chronic stress, performance in attention, odor discrimination, and semantic memory tasks was impaired, correlating with 5-HT(1A) receptor binding potential (BP) measured with [(11)C]WAY100635 PET (Jovanovic et al 2011). Unfortunately, there have been no specific PET studies of 1A binding in relation to cognitive function in depression (Borg, 2008).
Reduced 5HT innervation may be related to other epilepsy comorbidities in addition to depression and cognitive impairment. Patients taking selective serotonin reuptake inhibitors had significantly fewer episodes of ictal oxygen desaturation during complex partial seizures (Bateman et al 2010). Our study supports previous work suggesting that serotoninergic pathways are potential therapeutic targets for the treatment of seizures and co-morbidities in TLE.
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
Supported by NINDS NIH Division of Intramural Research. We thank Dr Sungyoung Auh for statistical advice.
Footnotes
None of the authors has any conflict of interest to disclose.
References
- Bateman LM, Li CS, Lin TC, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictalhypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–4. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008;195:103–11. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Chugani DT, Chugani HC. Does serotonin have trophic effects in temporal lobe epilepsy? Neurology. 2003;60:736–7. doi: 10.1212/01.wnl.0000057384.83302.34. [DOI] [PubMed] [Google Scholar]
- Didelot A, Ryvlin P, Amélie Lothe A, Merlet I, Hammers A, Mauguiere F. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain. 2008;131:2751–2764. doi: 10.1093/brain/awn220. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Porter RJ, Knight RG, Maruff P. Neuropsychological changes and treatment response in severe depression. Br J Psychiatr. 2011;198:115–22. doi: 10.1192/bjp.bp.110.080713. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolkob EL, Julie Price J, Frank E, Kupfer DJ, Chester Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MS, Ballard CG, Kalaria RN, Perry R, Hortobágyi T, Francis PT. Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. 2009;132:1858–65. doi: 10.1093/brain/awp069. [DOI] [PubMed] [Google Scholar]
- Giovacchini G, Toczek MT, Bonwetsch MDR, Bagic A, Lang L, Fraser C, Reeves-Tyer P, Herscovitch P, Eckelman WC, Carson RE, Theodore WH. 5-HT 1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;46:1128 – 1135. [PMC free article] [PubMed] [Google Scholar]
- Giovacchini G, Conant S, Herscovitch P, Theodore WH. Using cerebral white matter for estimation of nondisplaceable binding of 5-HT1A receptors in temporal lobe epilepsy. J Nucl Med. 2009;50:1794–800. doi: 10.2967/jnumed.109.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiec M, Turlejski K, Djavadian RL. The partial 5-HT1A receptor agonist buspirone enhances neurogenesis in the opossum (Monodelphis domestica) Eur Neuropsychopharmacol. 2009;19:431–9. doi: 10.1016/j.euroneuro.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Pyzalski RW, Seidenberg M, Hermann BP. Memory relationships between MRI volumes and resting PET metabolism of medial temporal lobe structures. Epilepsy & Behavior. 2004;5:669–676. doi: 10.1016/j.yebeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Hecimovic H, Stefulj J, Cicin-Sain L, Demarin V, Jernej B. Association of serotonin transporter promoter (5-HTTLPR) and intron 2 (VNTR-2) polymorphisms with treatment response in temporal lobe epilepsy. Epilepsy Res. 2010;91:35–8. doi: 10.1016/j.eplepsyres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Ito S, Suharab T, Ito H, Yasuno F, Ichimiya T, Takanob A, Maeharad T, Matsuura M, Okubo Y. Changes in central 5-HT1A receptor binding in mesial temporal epilepsy measured by positron emission tomography with [11C]WAY100635. Epilepsy Research. 2007;73:111–118. doi: 10.1016/j.eplepsyres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Elliott JJ, Ardis TC, Cahir M, Reynolds GP, Bell R, Cooper SJ. Tryptophan depletion impairs object-recognition memory in the rat: Reversal by risperidone. Behav Brain Res. 2009;208:479–83. doi: 10.1016/j.bbr.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Perski A, Berglund H, Savic I. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2010.12.060. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kauffman MA, Consalvo D, Gonzalez-Morón D, Aguirre F, D’Alessio L, Kochen S. Serotonin transporter gene variation and refractory mesial temporal epilepsy with hippocampal sclerosis. Epilepsy Res. 2009;85:231–4. doi: 10.1016/j.eplepsyres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci U S. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KC. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–92. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Liew CJ, Lim Y-M, Bonwetsch R, Shamim S, Sato S, Reeves-Tyer P, Herscovitch P, Dustin I, Bagic A, Giovacchini G, Theodore WH. 18F-FCWAY and 18F-FDG PET in MRI Negative Temporal Lobe Epilepsy. Epilepsia. 2009;50:234–9. doi: 10.1111/j.1528-1167.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, Ryvlin P. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PETstudy. Brain. 2008;131:2765–2782. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S. 2003;100:1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H, Sumiyoshi T. Does stimulation of 5-HT1A receptors improve cognition in schizophrenia? Behavioural Brain Research. 2008;195:98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33:926–52. doi: 10.1016/j.neubiorev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology (Berl) 2009;202:315–28. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Jans LA, Blokland A, Broers NJ, Homberg JR, Ellenbroek BA, Cools AR. Serotonin transporter deficiency in rats contributes to impaired object memory. Genes Brain Behav. 2009;8:829–34. doi: 10.1111/j.1601-183X.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- Piskulić D, Olver JS, Maruff P, Norman TR. Treatment of cognitive dysfunction in chronic schizophrenia by augmentation ofatypical antipsychotics with buspirone, a partial 5-HT(1A) receptor agonist. Hum Psychopharmacol. 2009;24:437–46. doi: 10.1002/hup.1046. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Riedel WJ, Tillie DE, Blokland A, Postma A, Schmitt JA. Memory impairments in humans after acute tryptophan depletion using a novel gelatin-based protein drink. J Psychopharmacol. 2009;23:56–64. doi: 10.1177/0269881108089577. [DOI] [PubMed] [Google Scholar]
- Savic I, Lindström P, Gulyás B, Halldin C, Andrée B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- Shamim S, Wiggs E, Heiss J, Sato S, Liew C, Solomon J, Theodore WH. Temporal Lobectomy: Resection Volume, Neuropsychological Effects and Seizure Outcome. Epilepsy and Behav. 2009;16:311–4. doi: 10.1016/j.yebeh.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CC, Griffith HR, Okonkwo OC, Martin RC, Knowlton RK, Richardson EJ, Hermann BP, Seidenberg M. Contributions of volumetrics of the hippocampus and thalamus to verbal memory in temporal lobe epilepsy patients. Brain Cogn. 2008;69:65–72. doi: 10.1016/j.bandc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore WH, Bonwetsch R, Bagic A, Giovacchini G, Reeves-Tyer P, Herscovitch P, Carson RE. The effect of antiepileptic drugs on 5-HT1A receptor binding measured by positron emission tomography. Epilepsia. 2006;47:499 – 503. doi: 10.1111/j.1528-1167.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Hasler G, Giovacchini G, Kelley K, Reeves-Tyer P, Herscovitch P, Drevets W. Reduced Hippocampal 5HT1A PET Receptor Binding and Depression in Temporal Lobe Epilepsy. Epilepsia. 2007;48:1526–30. doi: 10.1111/j.1528-1167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- Truchot L, Costes N, Zimmer L, Laurent B, Le Bars D, Thomas-Antérion C, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. Neuroimage. 2008;40:1251–6. doi: 10.1016/j.neuroimage.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatr. 2004;56:101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]