Abstract
An approach toward the BCD-ring of atropurpuran via a sequence of allenic 1,3-H shift, 6π-electron pericyclic ring-closure, and intramolecular Diels-Alder cycloaddition of cyclic 2-amidodiene is described.
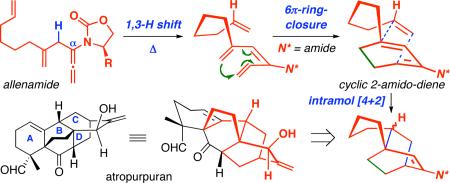
We recently1 reported a highly stereoselective tandem sequence consisting of allenic 1,3-hydrogen shift,2 6π-electron pericyclic ring-closure,3 and intramolecular Diels-Alder cycloaddition. This three-bond formation cascade provides a facile transformation of simple allenamide 14,5 into the bridged tricycle 4 with four new stereocenters through the intermediacy of 1,3,5-hexatriene 2 and the rare chiral cyclic 2-amido-diene 36-9 [Scheme 1]. While both the hexatriene 2 and the cyclic diene 36 are stable entities that could be intercepted and serve for the ensuing purpose, the entire sequence could proceed in tandem commencing with α-allylated allenamide 1. In addition, depending upon the substitution pattern of the hexatriene 2 [X = H or halogen], its pericyclic ring-closure could be rendered in a diastereoselective manner,10 thereby constituting a rather impressive long range 1,6-induction,11 while setting up stereochemically regulat ed intramolecular Diels-Alder cycloaddition [albeit 3a and 3b would converge to the same cycloadduct 4]. We have since been developing a potential application of this methodology to demonstrate its power as this tandem cascade. We wish to communicate here the possibility of employing this tandem sequence as an approach toward the BCD-ring of atropurpuran.
Scheme 1.
A Stereoselective Three-Bond Formation Cascade.
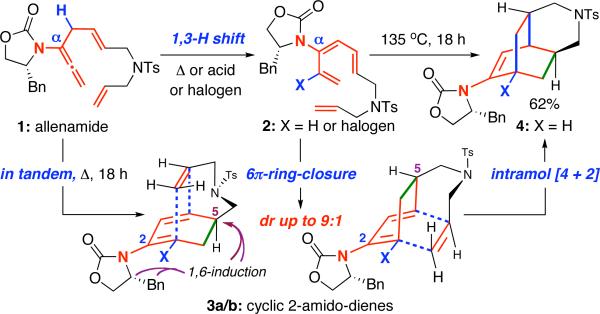
Wang et al. reported the isolation of diterpene atropurpuran [Scheme 2] from Aconitum hemsleyanum var. atropurpureum, unveiling an unusual pentacyclic motif that containing two contiguous bicyclo[2.2.2]octanes.12 Although atropurpuran represents the latest example of unique and brilliant structural topology that Aconitum genus has engineered, examples of diterpenes are rare. Most of them are C18, C19, and C20-diterpenoid alkaloids and possess a superb array of medicinal properties.13 Very recently, Kobayashi14 reported an elegant approach to the pentacyclic core of atropurpuran.
Scheme 2.
An Approach to the BCD-Ring of Atropurpuran.
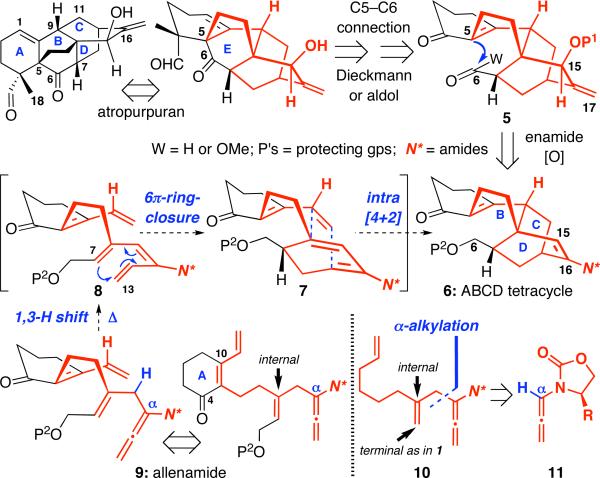
We have been drawn to the synthesis of the BCD-ring of atropurpuran with an intent to feature our chemistry. As shown in Scheme 2, our tandem sequence could allow transformation of allenamide 9, a significantly simplified retron, to the ABCD-tetracycle 6 in one operation. The ensuing steps in leading to 5 would involve enamide oxidation chemistry that was developed in our group15-18, and the final E-ring formation could be envisioned through the formation of C5-C6 bonds. While we are poised with the plan and have elected allenamide 10 to demonstrate the proof-of-concept, the challenge would be that unlike in allenamide 1, the dienophile for the Diels-Alder cycloaddition in allenamide 10 [or 9] is tethered in the internal olefinic position of the α-allyl group.
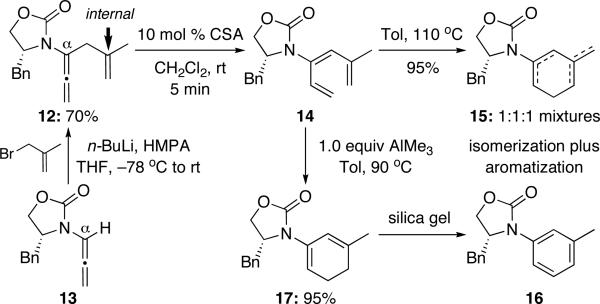
More specifically, we had found that when using an allenamide such as 12 [prepared from 1319,20] substituted at the internal olefinic position of the α-allyl group [Scheme 3], the ring-closure step from the triene 14 is problematic, and the resulting cyclic amido diene tends to isomerize and is also prone to oxidation, leading to a mixture of dienes 15 along with arene 16. The usage of 1.0 equiv AlMe3 proved to be useful in lowering the thermal activation barrier of the ring-closure,10,21 allowing a more clean isolation of the desired cyclic 2-amido diene 17. Nevertheless, air-oxidation and aromatization of 17 remained a problem.
Scheme 3.
Potential Impediment of an Internal Tethering.
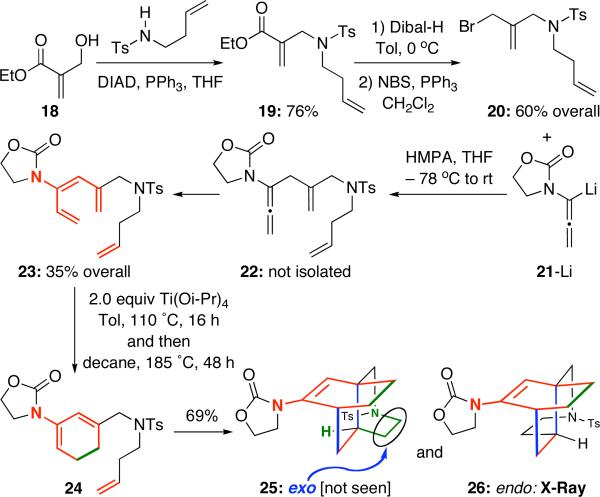
Intending to investigate these potential problems further, we proceeded to prepare achiral allenamide 22 from ester 1822 with nitrogen tethering at the internal olefinic carbon. We found that 1,3-H shift is actually very fast and had occurred during the allylation stage, thereby yielding the triene 23 directly from 21-Li [Scheme 4]. The ring-closure 23 proved to be challenging, as we initially failed when using high temperature conditions in toluene, xylene, or decane, or employing 1.0 equiv AlMe3. Only after switching the Lewis acid to Ti(O-i-Pr)4 in 2 equiv, we were able to effectively carry out the ring-closure in tandem with intramolecular Diels-alder cycloaddition, leading to the endo cycloadduct 26 as the only isomer. The relative position of CH2-NTs fragment defines the endo and exo sense.
Scheme 4.
A Successful Sequence with an Internal N-Tethering.
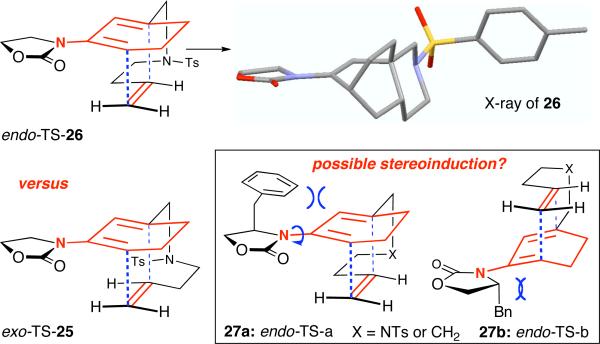
Relative stereochemistry of 26 was unambiguously assigned using X-ray structure of a single crystal [Figure 1]. It is noteworthy that it would appear that the proposed transition states for both exo-25 and endo-26 are equally feasible, but only the endo product was isolated. In addition, we were intrigued that based on the proposed endo-TS, if long-range stereochemical induction could be again observed here when using chiral amides as shown in cyclic 2-amido diene 27a [see inside the box of Figure 1]. However, we also recognize that 27 could exist as two possible C-N rotameric conformations a and b, which could impede or work unfavorably for such stereoinduction.
Figure 1.
X-Ray Structure of 26 and Proposed endo-TS.
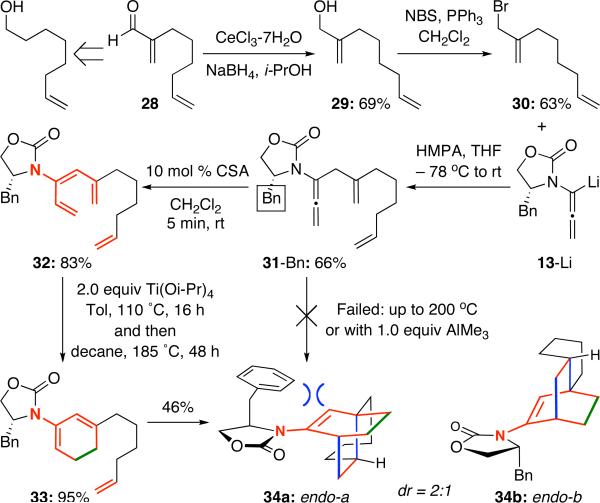
Consequently, we turned our attention to chiral allenamide 31-Bn, which was synthesized from enal 2823 [Scheme 5]. It was quickly evident that each of these internally tethered systems behaves quite differently. Initial attempts to directly take 31 to tricycle 34 complete failed under thermal conditions including the use of AlMe3. We then isomerized 31 to triene 32 using 10 mol % CSA, and found that with the use of 1.0 equiv AlMe3, 32 underwent almost quantitative ring-closure to give cyclic 2-amidodiene 33. However, the intramolecular Diels-Alder cycloaddition proved to be problematic here.24 To effectively achieve the synthesis of tricycle 34, the ultimate conditions would involve those adopted in Scheme 4. Tricycle 34 was attained on 46% yield as a mixture of two diastereomers, which would be the two possible endo-isomers. The modest ratio implies that achieving a long-range stereochemical induction is more challenging here based on the TS in Figure 1, or the two competing conformational positions of the amido group [see 27a versus 27b] preclude the possibility of having a favorable facial differentiation, although the specifics will await for future suitable computational analysis.
Scheme 5.
Success in an All-Carbon Internal Tethering.
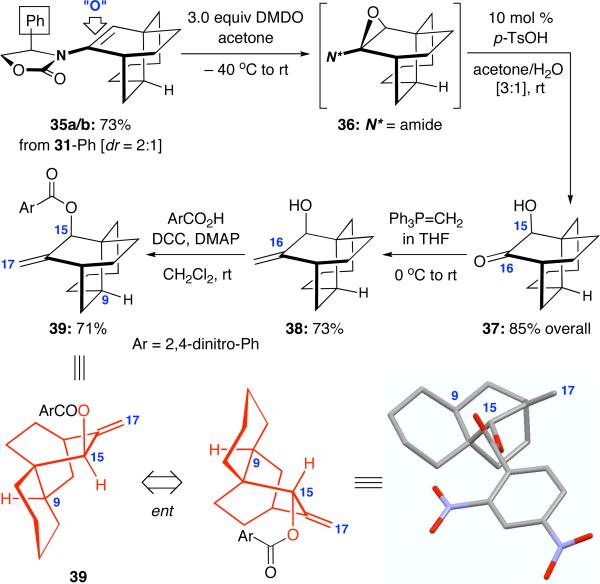
Changing the Bn substituent on the Evans auxiliary in 31-Bn to a Ph group did not improve the diastereoselectivity but gave the respective tricycle 35a/b in a significantly better overall yield [Scheme 6]. To both demonstrate the enamide oxidation chemistry and to concisely assign the relative stereochemistry, we elected to epoxidize 35a/b as a 2:1 isomeric mixture, and subsequent hydrolysis of the epoxy intermediate 36 unveiled hydroxy ketone 37 as single diastereomer in 85% yield over 2 steps. The fact that 37 being a single isomer further suggests that the 2:1 ratio represents the ratio of the two endo isomers and not that of endo:exo. It is also noteworthy that the DMDO epoxidation of 35a/b was completely stereoselective in favoring of the more accessible face of the tricyclic manifold. It is not clear at this point whether the chiral auxiliary plays a cooperative role in the selectivity of the oxidation.
Scheme 6.
X-Ray Confirmation and Enamide Oxidation.
The ensuing Wittig olefination of the C16-carbonyl in 37 afforded allyl alcohol 38 in 73% yield. Preparation of the 2,4-dinitrobenzoyl ester derivative 39 from 38 as well as attaining its X-ray structure allowed us to unambiguously assign the complete carbon skeleton of 38, which would match that of the BCD-ring of atropurpuran. Given the endo nature of intramolecular Diels-Alder cycloaddition, it appears that to complete a synthesis of atropurpuran, a key epimerization at C9 would be required, which represents an achievable operation based on our actual synthetic plan.
We have described here the feasibility of a synthetic approach toward the BCD-ring of atropurpuran via a sequence of allenic 1,3-H shift, 6π–electron pericyclic ring-closure, and intramolecular Diels-Alder cycloaddition of cyclic 2-amidodiene. While the pericyclic ring-closure required the assistance of Lewis acid, the entire process is highly stereoselective in favor of the endo-cycloadduct. Total synthesis efforts toward atropurpuran as well as mechanistic understanding of the overall stereoinduction in this process are currently underway.
Supplementary Material
Acknowledgement
Authors thank NIH [GM066055] for financial support and Dr. Victor Young [University of Minnesota] for X-ray structural analysis.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, characterizations, and X-ray structural files are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.Hayashi R, Feltenberger JB, Hsung RP. Org. Lett. 2010;12:1152. doi: 10.1021/ol902821w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crandall JK, Paulson DR. J. Am. Chem. Soc. 1966;88:4302. For examples of allenamide isomerizations, see: Overman LE, Clizbe LA, Freerks RL, Marlowe CK. J. Am. Chem. Soc. 1981;103:2807.Trost BM, Stiles DT. Org. Lett. 2005;7:2117. doi: 10.1021/ol050395x.
- 3.For reviews, see: Marvell EN. Thermal Electrocyclic Reactions. Academic Press; New York: 1980. Okamura WH, de Lera AR. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Paquette LA, editors. Vol. 5. Pergamon Press; New York: 1991. pp. 699–750.
- 4.For a leading review on allenamide chemistry, see: Hsung RP, Wei L-L, Xiong H. Acc. Chem. Res. 2003;36:773. doi: 10.1021/ar030029i.
- 5.For general reviews on allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. Vol. 1. Vol. 2. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004.
- 6.a Hayashi R, Hsung RP, Feltenberger JB, Lohse AG. Org. Lett. 2009;11:2125. doi: 10.1021/ol900647s. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hayashi R, Feltenberger JB, Lohse AG, Walton MC, Hsung RP. Beil. J. Org Chem. 2011;7:410. doi: 10.3762/bjoc.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For reviews on chemistry of dienamides, see: Overman LE. Acc. Chem. Res. 1980;13:218.Petrzilka M. Synthesis. 1981:753.Campbell AL, Lenz GR. Synthesis. 1987:421.Krohn K. Angew. Chem. Int. Ed. Engl. 1993;32:1582.Enders D, Meyer O. Liebigs Ann. 1996:1023.
- 8.For a review on the synthesis of enamides, see: Tracey MR, Hsung RP, Antoline J, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Georg Thieme Verlag KG; p. 2005. Chapter 21.4.
- 9.For some examples cyclic amido-dienes, see: Martínez R, Jiménez-Vázquez HA, Delgado F, Tamariz J. Tetrahedron. 2003;59:481.Wallace DJ, Klauber DJ, Chen CY, Volante RP. Org. Chem. 2003;5:4749. doi: 10.1021/ol035959g.Wabnitz TC, Yu J-Q, Spencer JB. Chem. Eur. J. 2004;10:484. doi: 10.1002/chem.200305407.
- 10.Hayashi R, Walton MC, Hsung RP, Schwab J, Yu X. Organic Lett. 2010;12:5768. doi: 10.1021/ol102693e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For some examples of 1,6-remote asymmetric inductions, see: Paterson I, Dlgado O, Florence GJ, Lyothier I, Scott JP, Sereinig N. Org. Lett. 2003;5:35. doi: 10.1021/ol0270780.Arai Y, Ueda K, Xie J, Masaki Y. Synlett. 2001:529.
- 12.Tang P, Chen Q-H, Wang F-P. Tetrahedron Lett. 2009;50:460. [Google Scholar]
- 13.For recent reviews, see: Wang F-P, Liang X-T. In: The Alkaloids. Cordell GA, editor. Vol. 59. Academic Press; San Diego, CA: 2002. pp. 1–280. Vol. 59.Atta-ur-Rahman, Choudhary MI. Diterpenoid and Steroidal Alkaloids. Nat. Prod. Rep. 1999;16:619. doi: 10.1039/a705715f.Nat. Prod. Rep. 1997;14:191. doi: 10.1039/np9971400191.Nat. Prod. Rep. 1995;12:361. doi: 10.1039/np9951200361.Yunuzov MS. Diterpenoid Alkaloids. Nat. Prod. Rep. 1993;10:471.
- 14.Suzuki T, Aya Sasaki A, Egashira N, Kobayashi S. Angew. Chem. Int. Ed. 2011;50:9177. doi: 10.1002/anie.201103950. [DOI] [PubMed] [Google Scholar]
- 15.Xiong H, Hsung RP, Shen L, Hahn JM. Tetrahedron Lett. 2002;43:4449. [Google Scholar]
- 16.Also see: Adam W, Bosio SG, Wolff BT. Org. Lett. 2003;5:819. doi: 10.1021/ol0273194.Sivaguru J, Saito H, Poon T, Omonuwa T, Franz R, Jockusch S, Hooper C, Inoue Y, Adam W, Turro NJ. Org. Lett. 2005;7:2089. doi: 10.1021/ol0502230.Koseki Y, Kusano S, Ichi D, Yoshida K, Nagasaka T. Tetrahedron. 2000;56:8855.
- 17.For a leading review on enamide chemistry, see: Carbery DR. Org. Biomol. Chem. 2008;9:3455. doi: 10.1039/b809319a.
- 18.Also see: Rappoport Z. The Chemistry of Enamines in The Chemistry of Functional Groups. John Wiley and Sons; New York: 1994. Whitesell JK, Whitesell MA. Synthesis. 1983:517.Hickmott PW. Tetrahedron. 1982;38:1975, 3363.
- 19.See Supporting Information.
- 20.For α−alkylation of allenamides, see: Xiong H, Hsung RP, Wei L-L, Berry CR, Mulder JA, Stockwell B. Org. Lett. 2000;2:2869. doi: 10.1021/ol000181+.
- 21.For recent examples using Lewis acid to mediate 6-π-electron electrocyclic ring-closures of 1,3,5-hexatrienes, see: Magomedov NA, Ruggiero PL, Tang YC. Org. Lett. 2004;6:3373. doi: 10.1021/ol048545b.Bishop LM, Barbarow JE, Bergmen RG, Trauner D. Angew. Chem. Int. Ed. 2008;47:8100. doi: 10.1002/anie.200803336.
- 22.Yu C, Liu B, Hu LJ. Org. Chem. 2001;66:5413. doi: 10.1021/jo015628m. [DOI] [PubMed] [Google Scholar]
- 23.a Snider BB, Zhou J. Org. Lett. 2006;8:1283. doi: 10.1021/ol052948+. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dai X, Davies HML. Adv. Synth. Catal. 2006;348:2449. [Google Scholar]
- 24.IMDA of cyclic 2-amidodiene 33 failed even at 200 °C and with 1.0 equiv AlMe3. When we attempted 10 mol % Rh(PPh3)3Cl and/or with AgSbF6 at 110 °C, these conditions were also not useful.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.