Abstract
Lipin-1 is a protein that exhibits dual functions as a phosphatidic acid phosphohydrolase (PAP) enzyme in the triglyceride synthesis pathways and a transcriptional co-regulator. Our previous studies have shown that ethanol causes fatty liver by activation of sterol regulatory element-binding protein 1 (SREBP-1) and inhibition of hepatic AMP-activated kinase (AMPK) in mice. Here, we tested the hypothesis that AMPK-SREBP-1 signaling may be involved in ethanol-mediated up-regulation of lipin-1 gene expression. The effects of ethanol on lipin-1 were investigated in cultured hepatic cells and in the livers of chronic ethanol-fed mice. Ethanol exposure robustly induced activity of a mouse lipin-1 promoter, promoted cytoplasmic localization of lipin-1 and caused excess lipid accumulation both in cultured hepatic cells and in mouse livers. Mechanistic studies showed that ethanol-mediated induction of lipin-1 gene expression was inhibited by a known activator of AMPK or overexpression of a constitutively active form of AMPK. Importantly, overexpression of processed nuclear form of SREBP-1c (nSREBP-1c) abolished the ability of AICAR to suppress ethanol-mediated induction of lipin-1 gene expression level. Chromatin immunoprecipitation (ChIP) assays further revealed that ethanol exposure significantly increased association of acetylated Histone H3 at lysine 9 (Lys9) with the SRE-containing region in the promoter of the lipin-1 gene. In conclusion, ethanol-induced up-regulation of lipin-1 gene expression is mediated through inhibition of AMPK and activation of SREBP-1.
Keywords: Alcoholic fatty liver, signal transduction, lipid metabolism, acetylation, sumoylation
Introduction
Lipin-1, a mammalian Mg2+-dependent phosphatidate phosphatase (PAP), has recently been identified as a key regulator of lipid metabolism in several organs including liver.1 The gene encoding lipin-1 (LPIN1) was first identified by positional cloning of the mutant gene underlying lipodystrophy in the fatty liver dystrophy (fld) mouse in 2001.1,2
Lipin-1 exhibits two distinct functions in regulating lipid metabolism according to subcellular localization studies. In the cytoplasm, lipin-1 functions as a Mg2+-dependent PAP enzyme involved in the biosynthesis of triacylglycerol (TAG) and phospholipids by converting phosphatidate (PA) to diacylglycerol (DAG) at the endoplasmic reticulum (ER).1 In the nucleus, lipin-1 acts as a transcriptional-co-activator to increase the capacity of the liver for fatty acid oxidation by interacting with peroxisome proliferator-activated receptor α (PPARα) and coactivator-1α (PGC-1α).1,3 The nuclear-localized lipin-1 also suppresses the functions of sterol regulatory element binding protein-1 (SREBP-1), a master regulator of lipid metabolism.4 The subcellular localization of lipin-1 is highly regulated by post-translational modifications. Specifically, sumoylation promotes nuclear retention and transcriptional activity.5 Secondly, while there are numerous putative phosphorylation sites that may have accessory effects, serine phosphorylation promotes nuclear export and translocation to the ER membrane; whereas dephosphorylation promotes its cytosolic distribution.1,5
Clinically, alcoholic fatty liver disease (AFLD) is characterized by increased accumulation of fat in the livers of patients who have consumed excessive amounts of alcohol for prolonged periods. Considerable evidence has shown that increased fat accumulation in liver can progress to more harmful forms of liver injury such as fibrosis and cirrhosis in humans. The molecular and cellular mechanisms by which ethanol causes AFLD are multiple and still incompletely understood. Previously, we and several other groups have shown that ethanol induces lipid synthesis by activation of SREBP-1 in the livers of animals.6–9 Moreover, ethanol’s effect on SREBP-1 results partially from inhibition of AMP-activated kinase (AMPK).9 Hence, ethanol-mediated dysregulation of the AMPK-SREBP-1 signaling pathway contributes to the development of AFLD.
Prior to the identification of lipin-1, PAP activity was shown to be increased in the livers of human alcoholics and patients with AFLD in several studies.10–12 In parallel, ethanol-mediated activation of PAP was closely associated with the development of fatty liver in rodents and humans.10–12 Consistent with these studies, we recently reported that chronic ethanol feeding significantly increased lipin-1 mRNA and its cytosolic protein levels in the livers of mice, supporting the concept that up-regulation of lipin-1 by ethanol contributes to enhanced PAP activity and hepatic lipid accumulation in ethanol-fed mice.13 Nevertheless, the molecular mechanisms and signaling pathways affected by ethanol, which result in altering the gene and protein expression of lipin-1, are not fully understood. The present study was undertaken to investigate the underlying mechanisms by which ethanol regulates lipin-1 with a focus on the role of AMPK-SREBP-1 signaling.
Materials and Methods
Studies with Mouse AML-12 Hepatocytes
The immortalized mouse hepatocyte cell line (AML-12) was purchased from the American Type Culture Collection (Manassas, VA). Various in vitro assays using AML-12 cells exposed to ethanol or other reagents were performed as described in the Supporting information.
Animal Studies
Male C57BL/6J mice (6 to 8 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were fed a modified Lieber-DeCarli ethanol containing diet or a pair-fed control diet as described in the Supporting information.
Statistics
Data are presented as means±SD. All data were analyzed by two-way ANOVA followed by Tukey’s multiple comparison procedure with P<0.05 being considered significant.
Additional Materials and Methods are described in the Supporting information.
Results
Up-Regulation of Lpin1 Promoter by Ethanol in Mouse AML-12 Hepatocytes
Mouse AML-12 hepatocytes express sufficient levels of class I (low K m) alcohol dehydrogenase (ADH) and aldehyde dehydrogenase 2 (ALDH2) proteins, and efficiently metabolize ethanol (data not shown). However, AML-12 cells lack detectable immunoreactive protein CYP2E1 (Supporting Fig. 1A).
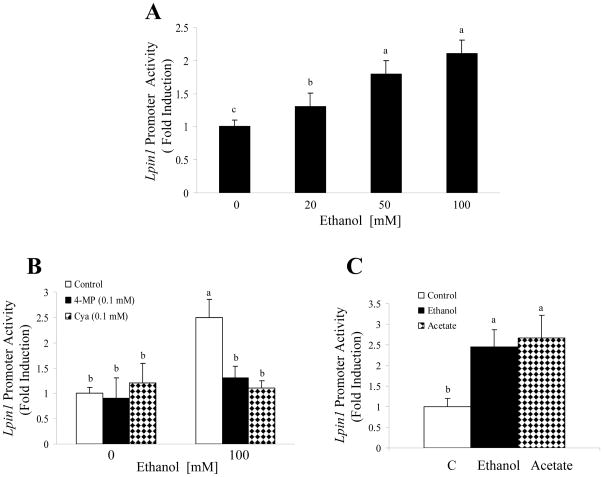
The AML-12 cells were transfected with a reporter gene (mouse Lpin1-luciferase) and an internal control plasmid (β-Galactosidase) and exposed to various concentrations of ethanol (20–100 mM) then harvested for assay of reporter enzymes. The Lpin1 reporter activity was significantly increased in a concentration-dependent manner by incubation with ethanol in AML-12 hepatocytes (Fig. 1A).
Figure 1. Up-regulation of Lpin1 by ethanol in cultured hepatic cell lines.
(A) AML-12 cells were transfected with a mouse Lpin1-luciferase reporter (10 μg) and β -galactosidase (2 μg; internal control). Ethanol was added for 36 h. Forty-eight hours after transfection, cells were harvested, and luciferase and β-galactosidase activities were determined. (B, C) AML-12 cells were transfected as described above with the Lpin1-luciferase reporter plasmid; the inhibitors with or without ethanol (100 mM) or acetate (10 mM) were added, and cells then were harvested for assay of the reports. Data are means±SD from 3–5 experiments. Means without a common letter differ, p< 0.05.
We determined whether ethanol metabolism was required for the ethanol-induced Lpin1 promoter activity by the use of inhibitors of ethanol metabolism. We used the ADH inhibitor 4-methylpyrazole (4-MP) and the ALDH2 inhibitor cyanamide (Cya). Treatment with each of these inhibitors alone had no effect on baseline Lpin1-luciferase levels, however, when the cells were exposed to ethanol, the inhibitors virtually abolished the ethanol dependent induction of Lpin1-luciferase (Fig. 1B). Moreover, acetate (10 mM), one of ethanol’s major metabolites, shared its ability to increase Lpin1 promoter activity in AML-12 cells (Fig. 1C).
Taken together, the results suggest that ethanol metabolism is necessary for the ability of ethanol to activate Lpin1 promoter activity.
Ethanol Promotes Cytoplasmic Localization of Lipin-1 in Mouse AML-12 Hepatocytes
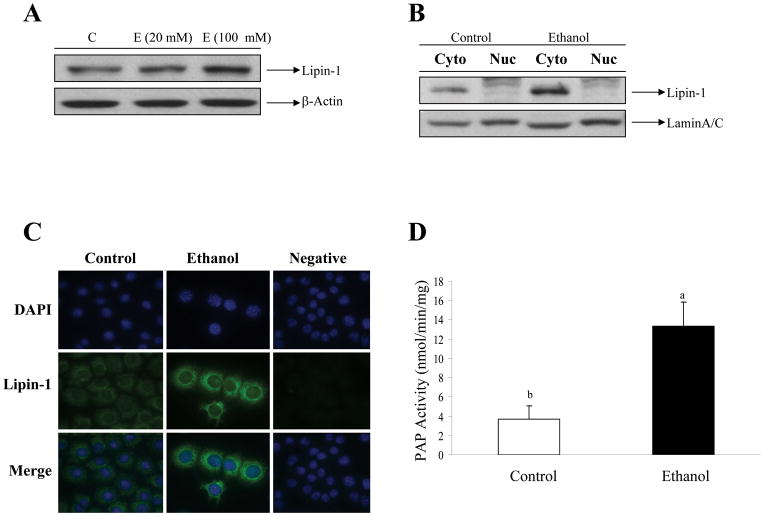
In AML-12 cells, ethanol increased the total lipin-1 protein levels (Fig. 2A). We assessed the subcellular localization of lipin-1 in response to ethanol treatment in AML-12 cells. AML-12 cells were cultured in the presence of ethanol, and extracts of these cells were fractionated into cytosol and nuclei followed by Western blot analysis. The increase in endogenous lipin-1 protein induced by ethanol was observed strictly in the cytosolic fractions (Fig. 2B).
Figure 2. Ethanol promotes cytoplasmic localization of lipin-1 in mouse AML-12 hepatocytes.
(A) Representative Western blot of AML-12 cells treated with ethanol (E) for 24 h. Lipin-1 was detected with an anti-lipin-1 antibody. (B) Western blot analysis of the lipin-1 protein expression levels in nucleus (Nuc) or cytoplasm (Cyto) of AML-12 cells treated with or without ethanol (100 mM) for 24 h. (C) Representative photomicrographs of immunofluorescence lipin-1 (green) or DAPI (blue) in AML-12 cells treated with or without ethanol (100 mM) for 24 h. Original magnification×200. (D) AML-12 cells were treated with ethanol (100 mM) for 24 hours. The cellular PAP activity assays were then performed. Data are means±SD from 3–5 experiments. Means without a common letter differ, p < 0.05.
Immunofluorescent staining of nuclei (DAPI staining) and lipin-1 confirmed that endogenous lipin-1 was present predominantly in the cytoplasm (Fig. 2C). Further, treatment with ethanol dramatically increased the intensity of lipin-1 staining in the cytoplasm as compared to controls, suggesting an increase of lipin-1 protein expression. Cellular PAP activity was significantly induced by ethanol treatment compared with the control in AML-12 cells (Fig. 2D).
Collectively, these results suggest that ethanol promotes lipin-1 cytoplasmic accumulation, and induces its PAP activity.
Ethanol-Induced Triglyceride (TG) Accumulation is Mediated through Lipin-1 Induction in Mouse AML-12 Hepatocytes
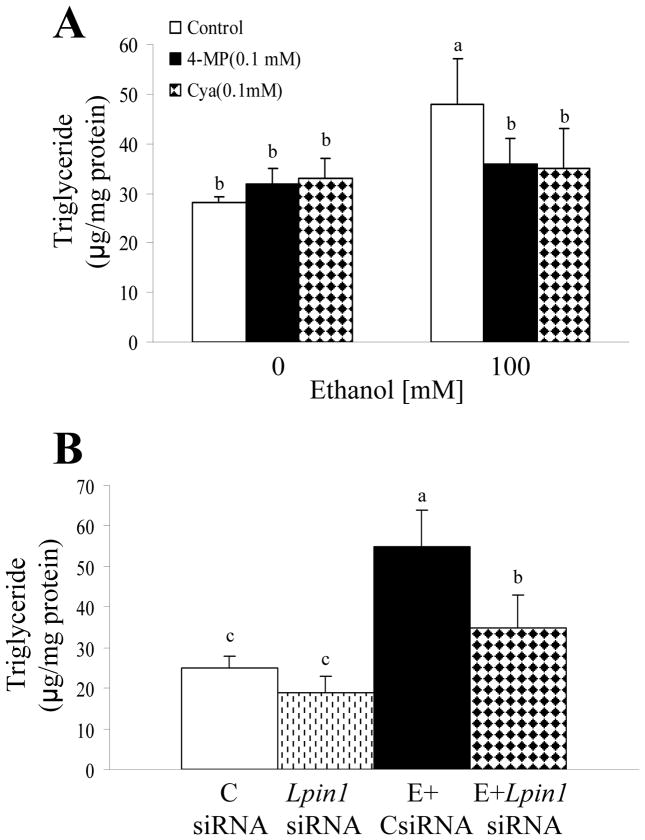
The effect of ethanol on the development of steatosis in AML-12 cells was determined by measuring cellular TG content with enzymatic assays using a triglyceride kit.14 There was a significant, concentration-dependant increase in the TG content of cells exposed to ethanol (Supporting Fig. 2). Moreover, treatment with either 4-MP or Cya essentially blocked the ability of ethanol to increase TG levels, indicating that ethanol metabolism is necessary for ethanol-mediated cellular lipid accumulation (Fig. 3A).
Figure 3. Ethanol-induced triglyceride (TG) accumulation is mediated through lipin-1 induction in AML-12 hepatocytes.
(A) AML-12 cells were treated with ethanol (100 mM) or inhibitors, 4-methylpyrazole (4-MP) and cyanamide (Cya), for 48 h and cellular TG levels were measured. (B) AML-12 cells were transfected with control siRNA or Lpin1siRNA. Forty-eight hours after transfection, ethanol (E; 100 mM) was added. After incubation for 48 h, cellular TG contents were measured. Data are means±SD from 3–5 experiments. Means without a common letter differ, p< 0.05.
To determine whether the increased lipin-1 induced by ethanol affects cellular TG synthesis, we examined the TG accumulation in AML-12 cells transfected with Lpin1siRNA or control siRNA in response to ethanol exposure. Knocking down lipin-1 with Lpin1siRNA largely eliminated the capacity of ethanol to induce TG accumulation (Fig. 3B). Note that lipin-1 protein levels were decreased ~70% after transfection with Lpin1siRNA (Supporting Fig. 1B). These results suggest that ethanol metabolism increases lipin-1 enzymatic activity and subsequently promotes TG accumulation.
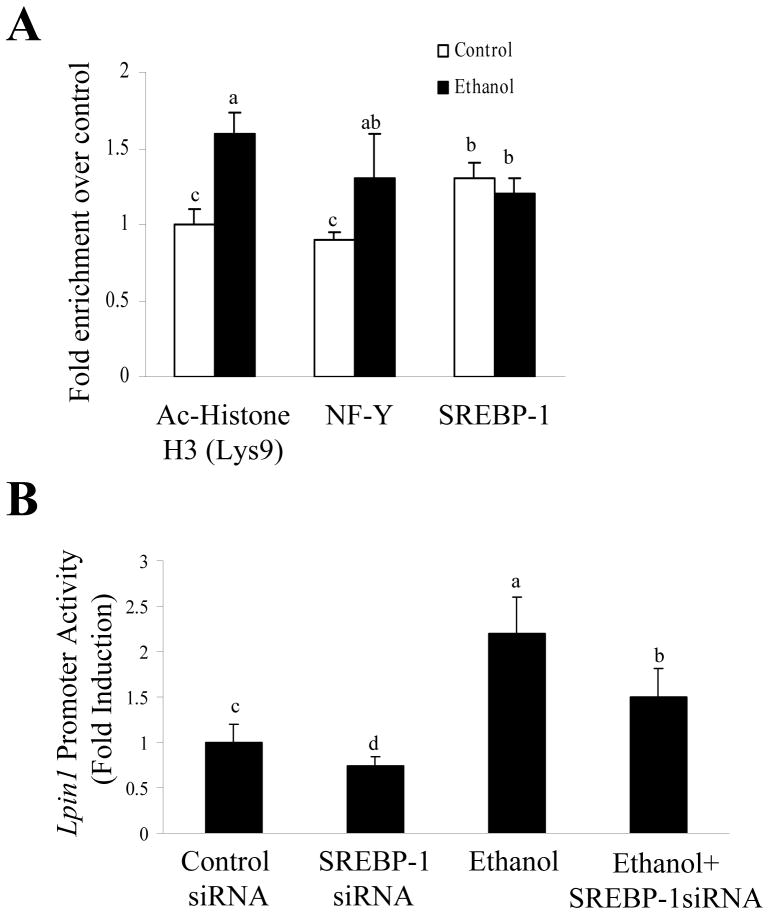
Ethanol Exposure Increased Association of Acetylated Histone H3-Lysine 9 (Lys 9) and NF-Y with Lpin1 Promoter in vitro
SREBP-1 functions together with NF-Y to transactivate the LPIN1 promoter through SRE and nuclear factor Y (NF-Y)-binding sites.15 The effect of ethanol on the binding of acetylated Histone H3-Lys9, SREBP-1 or NF-Y to the Lpin1-SRE binding site was determined using the ChIP assays. After the chromatin had been cross-linked in control and ethanol-treated AML-12 cells, it was sheared by sonication. DNA-protein complexes were immunoprecipitated with antibodies directed against acetylated Histone H3-Lys9, SREBP-1 or NF-Y. Immunoprecipitated DNA was amplified by real-time qPCR with primers for the Lpin1 promoter region containing SRE binding elements. Ethanol significantly increased the interaction of acetylated Histone H3-Lys9 and of NF-Y with the Lpin1-SRE promoter (Fig. 4A). The association of SREBP-1 with the Lpin1 promoter was not affected by ethanol. This maybe due to rapid proteasomal degradation of nuclear SREBP-1 protein.16
Figure 4. Ethanol exposure increased association of acetylated Histone H3-Lys 9 and NF-Y with Lpin1 promoter in AML-12 hepatocytes.
(A) ChIP assays were performed using ethanol (100 mM) exposed AML-12 cells. Chromatin was immunoprecipitated with preimmune rabbit IgG, anti-acetyl (Ac)-Histone H3-Lys9, anti-SREBP-1, and anti-NF-YA antibody, respectively. Immunoprecipitates were subjected to PCR with a primer pair specific to the Lpin1-SRE promoter. (B) AML-12 cells were transfected with the Lpin1-luciferase reporter plasmid, along with expression plasmids for SREBP-1 siRNA or control siRNA with or without ethanol (100 mM). Cells then were harvested for assay of the Lpin1-luciferase activity. All data are given as means±SD from at least 3–5 experiments. Means without a common letter differ, p< 0.05.
SREBP-1 siRNA was found to be an effective inhibitor of SREBP-1 expression in AML-12 cells (Supporting Fig. 1C). Knocking down SREBP-1 with SREBP-1siRNA partially abrogated the ability of ethanol to stimulate Lpin 1 promoter activity (Fig. 4B).
Pharmacological or Genetic Activation of AMPK Prevents Enhanced Expression of Lipin-1 and Elevated Lipid Accumulation Induced by Ethanol
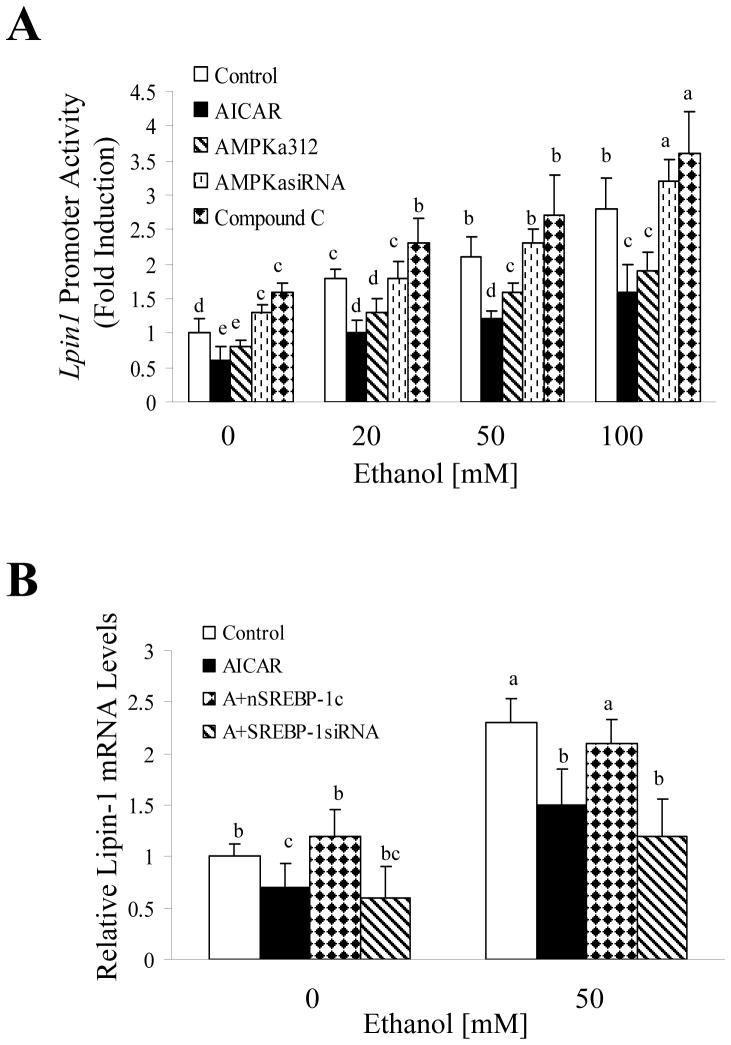
We further explored the role of AMPK-SREBP-1 signaling in the ethanol-mediated increase of Lpin1.9 While ethanol robustly increased Lpin1 promoter activity and mRNA, pre-treatment with AICAR or over-expression of a constitutively active form of AMPK (AMPKα1312) largely prevented ethanol-dependent increases in Lpin1 promoter activity and mRNA levels (Fig. 5A and Supporting Fig. 3). Conversely, pharmacological inhibition or epigenetic silencing of AMPK with either Compound C or AMPKα siRNA slightly augmented the effect of ethanol on Lpin1
Figure 5. Pharmacological or genetic activation of AMPK prevents enhanced expression of Lpin1 and elevated lipid accumulation induced by ethanol.
(A) AML-12 cells were transiently transfected with expression plasmids for Lpin1-luciferase, along with or without expression plasmids for AMPKα312, control siRNA, or AMPKα siRNA (5 μg/each). Ethanol, AICAR (0.5 mM), or compound C (3μM) was then added. Cells then were harvested for assay of the Lpin1-luciferase activity. (B) AML-12 cells were transfected with AMPKα312, control siRNA, SREBP-1siRNA or nSREBP-1. Ethanol, AICAR (0.5 mM), or compound C (3μM) was then added. Seventy-two hours after transfection, qRT-PCR was used to estimate relative mRNA levels of lipin-1. All data are given as means±SD from at least 3–5 experiments. Means without a common letter differ, p< 0.05.
To determine whether SREBP-1 is involved in regulating the effects of AMPK on lipin-1, we stimulated SREBP-1 activity by overexpression of the active nuclear form of SREBP-1c (nSREBP-1) in AML-12 cells. Overexpression of nSREBP-1c abolished the ability of AICAR to suppress ethanol-mediated induction of lipin-1 gene expression (Fig. 5B). Conversely, inhibition of SREBP-1 expression by SREBP-1siRNA further augmented the effect of AICAR on Lpin 1 in AML-12 cells exposed to ethanol.
Collectively, these results suggest that inhibition of AMPK and activation of SREBP-1 by ethanol may be involved, at least in part, in the up-regulation of lipin-1.
It is important to note the effect of transfection with AMPKα312 and AMPKα siRNA on the levels of AMPKα protein as determined by Western blot analysis (Supporting Fig. 1D). Expression of AMPKα312 or AMPKα siRNA significantly increased or inhibited AMPK activity, respectively, in cultured hepatic cells.9 The alteration of AMPKα activity was accompanied by altered phosphorylation status of acetyl-CoA carboxylase (ACC), a downstream indicator of AMPK activity (Supporting Fig. 1D).
Effect of Chronic Ethanol Feeding on Lipin-1 in Mouse Livers
Feeding mice ethanol (29% of the total calories) via a modified Lieber-DeCarli liquid diet for 4 weeks led to development of fatty liver (Supporting Table I).
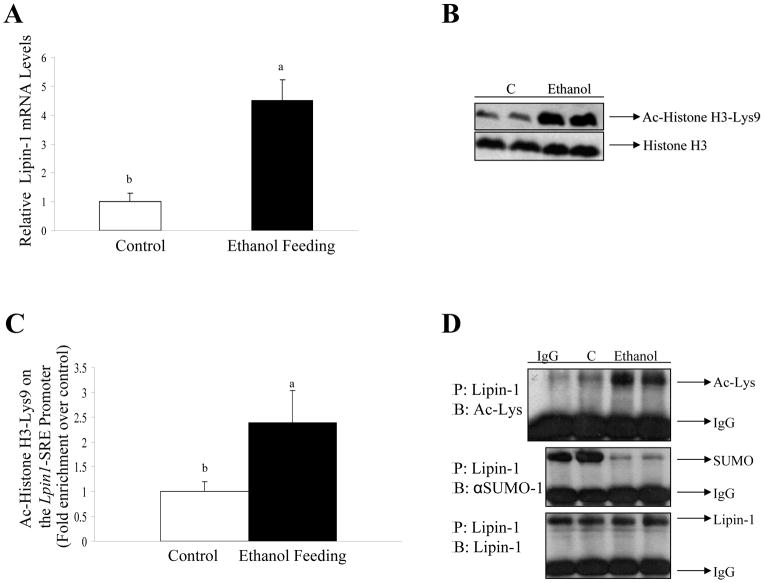
Ethanol feeding markedly increased total mRNA expression of hepatic lipin-1 in mice by nearly 4.5-fold compared to pair-fed controls (Fig. 6A). Note that there was no significant change in mRNA levels for lipin-2 and lipin-3 in the livers of ethanol-fed mice compared to controls (data not shown).
Figure 6. The effects of chronic ethanol feeding on lipin-1 in mouse livers.
(A) Relative levels of lipin-1 mRNA from mice fed a control diet with or without ethanol. (B) Acetylation of Histone H3-Lys9 was evaluated by Western blot using specific acetyl antibodies as indicated. (C) ChIP assays were performed. Immunoprecipitations (IP) were carried out using antibody directed against acetyl-Histone H3-lys9 from liver samples. Bound and input DNA was analyzed with primers for the Lpin1-SRE promoter by qRT-PCR. (D) Lipin-1 was immunoprecipitated from liver nuclear extracts of mice fed with control diet or ethanol and then immunoblotted with either an anti-acetylated lysine or anti-SUMO-1 antibody to determine the extent of lipin-1 acetylation or sumoylation or with a lipin-1 antibody to determine the total amount of lipin-1. IgG antibodies were used as a negative control. All data are expressed as means±SD; n=4–6 animals. Means without a common letter differ, p< 0.05
Acetylated Histone H3-Lys9 was drastically increased by ethanol feeding, whereas Histone H3 protein level was not affected by ethanol (Fig. 6B). Accordingly, the association of acetylated Histone H3-Lys9 with the Lpin1-SRE promoter was significantly enhanced ~2.4 fold by ethanol feeding compared with pair-fed control mice (Fig. 6C). Note that ChIP assays demonstrated that the association of acetylated Histone H3-Lys9 or glucocorticoid receptor (GR) with the Lpin 1-GRE site was not significantly affected by ethanol administration to mice compared with controls (data not shown).
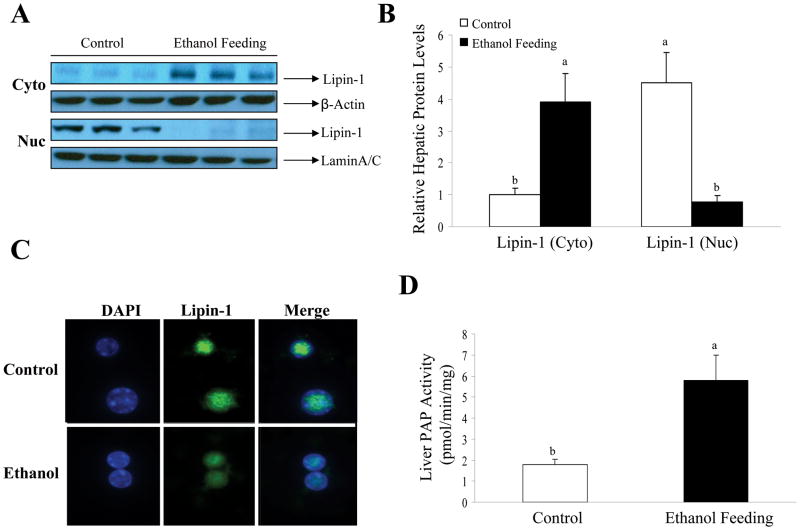
Ethanol feeding to mice significantly reduced sumoylation levels of hepatic lipin-1, while at the same time markedly increased its level of acetylation (Fig. 6D and Supporting Fig. 4). More importantly, ethanol feeding robustly increased the amount of lipin-1 in the cytoplasm and dramatically decreased it in the nucleus in the mouse livers (Fig. 7A, B). Accordingly, hepatic PAP activity was significantly increased in ethanol-fed mice compared with the pair-fed controls (Fig. 7D).
Figure 7. The effects of chronic ethanol feeding on lipin-1 in mouse livers.
(A) Representative Western blot analysis of the lipin-1 protein expression levels in nucleus (Nuc) or cytoplasm (Cyto) of mice fed control or ethanol diet. (B) Relative hepatic lipin-1 protein levels. (C) Isolated mouse liver nuclei of mice fed a control or ethanol diet immunofluorescence for lipin-1 (green) and DAPI (blue). Original magnification×200. (D) Hepatic PAP activities from liver extracts of mice fed with or without ethanol. All data are expressed as means±SD; n=3–6 animals. Means without a common letter differ, p< 0.05.
Taken together, our results clearly indicate that ethanol feeding increased hepatic lipin-1 gene expression and stimulated the cytoplasmic localization of lipin-1 in mouse livers.
Discussion
In the present study, we investigated the effects of ethanol on lipin-1 in cultured hepatic cells and in animal tissues and explored the underlying mechanisms. In cultured AML-12 hepatocytes, chronic ethanol exposure robustly enhanced activity of a mouse Lpin1 promoter and increased cytosolic lipin-1 protein levels as well as PAP activity. The ethanol-dependent up-regulation of lipin-1 was associated with elevated cellular TG accumulation in AML-12 cells. We also showed that acetate alone, a product of ethanol metabolism, produces many of these effects in AML-12 cells. Interestingly, ethanol-induced activation of the Lpin1 promoter and enhancement of lipin-1 mRNA levels were each inhibited by a known activator of AMPK (AICAR), as well as by over-expression of a constitutively active form of AMPK. Importantly, overexpression of nSREBP-1c largely abolished the ability of AICAR to suppress ethanol-mediated up-regulation of lipin-1, suggesting that AMPK lies upstream of the SREBP-1-lipin-1 axis. Consistent with the in vitro findings, feeding mice an ethanol-containing liquid diet resulted in a robust increase in the lipin-1 mRNA and cytosolic protein levels. Moreover, ChIP assays revealed that ethanol exposure significantly increased association of acetylated Histone H3-Lys9 with the SRE-containing region in the promoter of the lipin-1 gene both in vitro and in vivo. We also demonstrated for the first time that acetylation and sumoylation of lipin-1 displayed reciprocal patterns in livers of chronically ethanol-fed mice. Taken together, our findings suggest that chronic ethanol exposure up-regulates hepatic lipin-1 and that this effect may contribute to the development of AFLD. Importantly, we have shown that this effect is mediated, at least in part, by modulating AMPK-SREBP-1 signaling (Fig. 8).
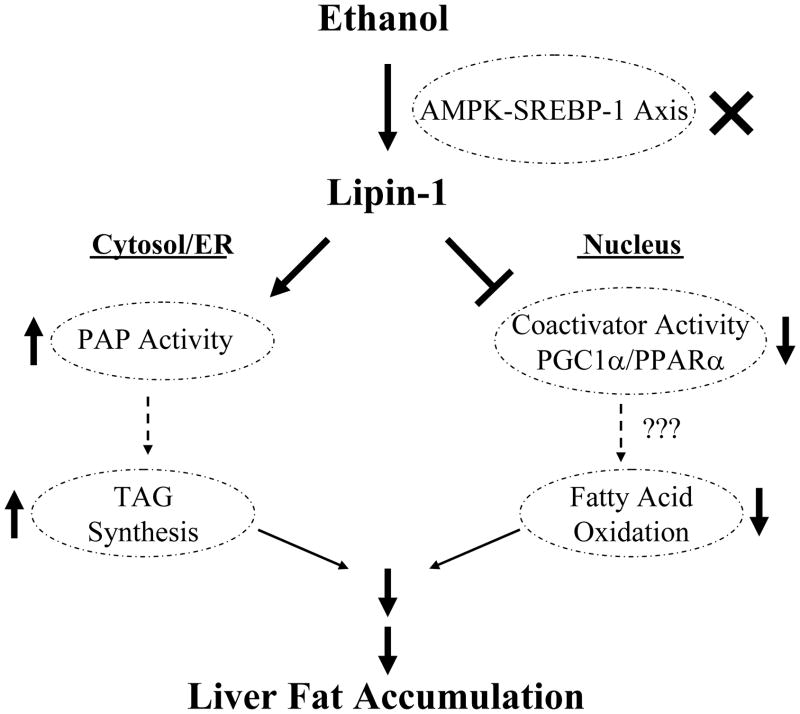
Figure 8. Proposed role of lipin-1 signaling in the development of alcoholic fatty liver.
Chronic ethanol feeding downregulates hepatic AMPK signaling. This inhibition promotes SREBP-1 activity, leading to increased total lipin-1, and favors production of cytosolic lipin-1, resulting in increased PAP activity and TG synthesis in mouse livers. We further speculate that ethanol affects lipin-1’s role in regulating FA oxidation. The net consequence of these changes is to promote hepatic steatosis. AMPK, AMP-activated protein kinase; ER, endoplasmic reticulum; SREBP-1, sterol regulatory element binding protein1. PAP, phosphatidate phosphatase type; PPAR, peroxisome proliferator-activated receptor; PGC-1α, PPARγ co-activator-alpha; TG, triglyceride.
Our current data clearly suggest that ethanol metabolism through both ADH and ALDH2 are required for the effect of ethanol on lipin-1 in AML-12 cells. Paradoxically, our previous results suggest that acetaldehyde generated from ethanol is largely responsible for the ability of ethanol to activate SREBP-1 in hepatoma cell lines.6 This discrepancy may in part be explained by the use of different cell lines. Indeed, we have failed to establish cellular alcoholic steatosis models in two hepatoma cell lines (H4IIEC3 and McA-RH7777). On the other hand, while lipin-1 and SREBP-1 are both targets of ethanol, the underlying mechanisms for the observed effects could be entirely different between them. Interestingly, we and several other groups have recently shown that both acetaldehyde and acetate, two major metabolites of ethanol, are involved in alcoholic liver injury.17,18
Our current findings suggest that ethanol increased lipin-1 gene expression largely through activation of SREBP-1 and NF-Y. Conceivably, additional molecular mechanisms are also involved. For instance, several putative GREs in the LPIN1 promoter have been identified. Indeed, lipin-1 expression is directly regulated by GCs in liver and adipose tissue.19,20 This effect requires the GR and is mediated by binding of the receptor to GRE sites upstream of the LPIN1 gene. The GC-mediated effects are specific to lipin-1, (i.e.; not lipin-2 or -3). The involvement of GC in ethanol-induced increases in lipin-1 is supported by a previous study showing that ethanol-mediated PAP activity was attenuated in adrenalectomized rats.10 Surprisingly, we found the in vivo association of acetylated Histone H3-Lys9 or GR with the Lpin1-GRE site in response to ethanol exposure was not significantly induced. We recently demonstrated that lipin-1 exhibits reciprocal patterns of gene expression in livers and adipose tissues of chronically ethanol-fed mice, suggesting a mechanism largely independent of GC effects.13 The definitive involvement of GCs in ethanol-mediated up-regulation of lipin-1 may need to be further studied through use of genetically modified animal models -- such as liver-specific GR knockout mice. It is also tempting to speculate that ethanol may stabilize lipin-1 protein via enhanced lipin-1 acetyaltion and subsequently inhibition of lipin-1 degradation.
The molecular role of lipin-1 is dependant upon its subcellular localization. Nuclear compartmentalization of lipin-1 ensures that its role as a transcriptional co-activator predominates over its role as a PAP enzyme.1–5 Sumoylation of lipin-1α is required for its nuclear localization and transcriptional coactivator activity toward PGC-1α in cultured neuronal cells.5 Our current in vivo findings show that ethanol feeding markedly reduced hepatic lipin-1 sumoylation levels, which correlates with the observed dramatic reduction in its nuclear localization. In addition to inhibition of lipin-1 sumoylation, ethanol feeding also robustly increased acetylation of lipin-1 in mouse livers. Acetate, the principal hepatic metabolite of ethanol, may contribute to hepatic lipin-1 hyperacetyaltion in ethanol-fed mice. Moreover, a lysine acetylation/deacetylation-sumoylation switch has been implicated in the functional regulation of several important molecules.21,22 Ethanol inhibits sirtuin 1 (SIRT1), an NAD+-dependent class III protein deacetylase, both in cultured hepatic cells and in animals.13,23 It is possible that this ethanol-mediated hyperacetylation/hyposumoylation of lipin-1 may be a consequence of inhibition of SIRT1 by ethanol. Whether acetylation/sumoylation would serve as a molecular switch to control the nuclear localization and coactivator activity of lipin-1 in liver and how ethanol affects the functional relationship of SIRT1 and lipin-1 are currently under investigation in our laboratory.
Lipin-1 localizes to the nucleus, and is a component of a transcriptional complex with PPARα/PGC-1α which stimulates fatty acid oxidation in liver.3 Ethanol-mediated dysregulation of the hepatic PPARα/PGC-1α axis and subsequent incomplete stimulation of PPARα/PGC-1α target genes involved in fatty acid oxidation contributes to the development of alcoholic liver steatosis.24 Taken together with a recent study demonstrating that a high-fat diet-induced fatty liver is partially mediated by impairment of the PGC-1α-nuclear lipin-1-PPARα axis and fatty acid oxidation in mice, our current findings suggest that depletion of nuclear lipin-1 is likely to lead to impairment of the PPARα/PGC-1α axis and fatty acid oxidation in the livers of chronically ethanol-fed animals.25 Furthermore, lipin-1 subcellular localization regulates SREBP-1 signaling and governs the assembly and secretion of very low density lipoproteins (VLDL).1,4 It is tempting to speculate that ethanol-induced nucleocytoplasmic shuttling may activate SREBP-1 and impair VLDL secretion and subsequently contribute to hepatic fat accumulation.
Another major novel finding of the present study is that ethanol up-regulates lipin-1 largely through inhibition of AMPK and activation of SREBP-1. Our study provides evidence for the first time, to our knowledge, that AMPK is involved in the regulation of lipin-1 gene expression. However, the exact mechanism by which AMPK inhibition by ethanol leads to activation of SREBP-1 and subsequent inhibition of Lpin1 remains to be determined. Our earlier work showed that ethanol selectively increases hepatic SREBP-1 activity in rodent models through inhibition of AMPK.6,9 AMPK directly phosphorylates SREBP-1 and suppresses SREBP-1 activity in hepatocytes exposed to high glucose.26 Conceivably, ethanol-mediated inhibition of AMPK may cause reduced phosphorylation of SREBP-1, which in turn results in activation of proteolytic processing and transcriptional activity of SREBP-1 and ultimately increased lipin-1 gene expression. Moreover, several lines of evidence have suggested functional connections between SIRT1 and AMPK.27,28 The ethanol-mediated impairment of the SIRT1/AMPK axis and the ensuing enhancement of SREBP-1 activity has emerged as a central mechanism in the development of AFLD. Therefore, it is tempting to speculate that ethanol may up-regulate lipin-1 through these metabolic modulators.
In summary, we have demonstrated that ethanol feeding up-regulates hepatic lipin-1 mRNA and protein and promotes lipin-1 cytoplasmic localization which, in turn, may lead to increased lipogenesis, impaired fatty acid oxidation, and the development of steatosis in mice. Furthermore, we have identified lipin-1 as a vital downstream target of AMPK-SREBP-1 signaling in the action of ethanol in liver. Our study sheds new light on the multifactorial pathogenesis of AFLD and suggests that future studies to investigate the effects of nutritional or pharmacological inhibitors of SREBP-1, lipin-1, and/or activators of AMPK on its development are clearly warranted.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by National Institute on Alcoholism and Alcohol Abuse Grants AA-015951 and AA-013623 (to M. You) and NIDDK grant DK-078187 (to B. Finck).
We thank Dr. David N. Brindley (University of Alberta, Edmonton, Canada), and Drs. R. Kennedy Keller and Laura Flatow (University of South Florida) for their outstanding technical and intellectual contributions.
List of Abbreviations
- ACC
acetyl-CoA carboxylase
- AFLD
alcoholic fatty liver disease
- AICAR
5-aminoimidazole-4-carboxamide ribonucleoside
- ADH
alcohol dehydrogenase
- ALDH2
aldehyde dehydrogenase 2
- AMPK
AMP-activated protein kinase
- CYP2E1
cytochrome P4502E1
- ChIP
chromatin immunoprecipitation
- Ct
comparative threshold
- Cya
cyanamide
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GC
glucocorticoid
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- H2O2
hydrogen peroxide
- MP
methylpyrazole
- NF-Y
nuclear factor-Y
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- SIRT1
sirtuin 1
- SREBP-1
sterol regulatory element binding protein 1
- PAP
phosphatidate phosphatase type
- PA
phosphatidate
- PPAR
peroxisome proliferator-activated receptor
- PGC-1α
PPARγ co-activator-1 alpha
- TG
triglyceride
- VLDL
very low density lipoproteins
References
- 1.Harris TE, Finck BN. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab. 2011;22:226–33. doi: 10.1016/j.tem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 3.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–20. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu GH, Gerace L. Sumoylation regulates nuclear localization of lipin-1alpha in neuronal cells. PLoS One. 2009;4:e7031. doi: 10.1371/journal.pone.0007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element- binding protein (SREBP) J Biol Chem. 2002;277:29342–7. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 7.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–24. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Passeri MJ, Cinaroglu A, Gao C, Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–52. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Brindley DN, Cooling J, Burditt SL, Pritchard PH, Pawson S, Sturton RG. The involvement of glucocorticoids in regulating the activity of phosphatidate phosphohydrolase and the synthesis of triacylglycerols in the liver. Effects of feeding rats with glucose, sorbitol, fructose, glycerol and ethanol. Biochem J. 1979;180:195–199. doi: 10.1042/bj1800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savolainen MJ, Baraona E, Pikkarainen P, Lieber CS. Hepatic triacylglycerol synthesizing activity during progression of alcoholic liver injury in the baboon. J Lipid Res. 1984;25:813–820. [PubMed] [Google Scholar]
- 12.Simpson KJ, Venkatesan S, Martin A, Brindley DN, Peters TJ. Activity and subcellular distribution of phosphatidate phosphohydrolase (EC 3. 134) in alcoholic liver disease. Alcohol Alcohol. 1995;30:31–36. [PubMed] [Google Scholar]
- 13.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–74. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VW, et al. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology. 2010;138:694–704. doi: 10.1053/j.gastro.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 15.Ishimoto K, Nakamura H, Tachibana K, Yamasaki D, Ota A, Hirano K, et al. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J Biol Chem. 2009;284:22195–205. doi: 10.1074/jbc.M109.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendrick SF, O’Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988–97. doi: 10.1002/hep.23572. [DOI] [PubMed] [Google Scholar]
- 18.Park PH, Lim RW, Shukla SD. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1124–36. doi: 10.1152/ajpgi.00091.2005. [DOI] [PubMed] [Google Scholar]
- 19.Manmontri B, Sariahmetoglu M, Donkor J, Bou Khalil M, Sundaram M, Yao Z, et al. Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically. J Lipid Res. 2008;49:1056–67. doi: 10.1194/jlr.M800013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, O’Loughlin L, Brindley DN, Reue K. Regulation of lipin-1 gene expression by glucocorticoids during adipogenesis. J Lipid Res. 2008;49:1519–28. doi: 10.1194/jlr.M800061-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stankovic-Valentin N, Deltour S, Seeler J, Pinte S, Vergoten G, Guérardel C. An acetylation/deacetylation-SUMOylation switch through a phylogenetically conserved psiKXEP motif in the tumor suppressor HIC1 regulates transcriptional repression activity. Mol Cell Biol. 2007;27:2661–75. doi: 10.1128/MCB.01098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campagna M, Herranz D, Garcia MA, Marcos-Villar L, González-Santamaría J, Gallego P, et al. SIRT1 stabilizes PML promoting its sumoylation. Cell Death Differ. 2011;18:72–9. doi: 10.1038/cdd.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun. 2008;370:44–8. doi: 10.1016/j.bbrc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 25.Barroso E, Rodríguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, et al. The PPAR{beta}/{delta} Activator GW501516 Prevents the Down-Regulation of AMPK Caused by a High-Fat Diet in Liver and Amplifies the PGC-1α-Lipin 1-PPARα Pathway Leading to Increased Fatty Acid Oxidation. Endocrinology. 2011;152:1848–59. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–26. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.