Abstract
One of the major challenges for hypertension research is to identify the mechanisms that cause the comorbidities encountered in many hypertensive patients, as seen in the metabolic syndrome. An emerging body of evidence suggests that human and experimental hypertensives may exhibit uncontrolled activity of proteinases, including the family of matrix metalloproteinases, recognized for their ability to restructure the extracellular matrix proteins and to play a role in hypertrophy. We propose a new hypothesis that provides a molecular framework for the comorbidities of hypertension, diabetes, capillary rarefaction, immune suppression, and other cell and organ dysfunctions due to early and uncontrolled extracellular receptor cleavage by active proteinases. The proteinase and signaling activity in hypertensives requires further detailed analysis of the proteinase expression, the mechanisms causing proenzyme activation, and identification of the proteinase substrate. This work may open the opportunity for reassessment of old interventions and development of new interventions to manage hypertension and its comorbidities.
Keywords: Matrix metalloproteinase, MMP, ADAM, Metabolic syndrome, Hypertrophy, Insulin resistance, Capillary rarefaction, Immune suppression, Insulin receptor, Beta-adrenergic receptor, Vascular endothelial growth factor receptor, NF-kappaB, Spontaneously hypertensive rat, Essential hypertension, Microcirculation, Artery, Arteriole, Extracellular matrix protein, Proteinase inhibitor, Angiotensin-converting enzyme, Angiotensin, Hypertension, Metabolic syndrome, Pathogenesis
Introduction
A robust body of evidence derived from experimental models and patients indicates that the elevated arterial blood pressure in hypertensives is only a part of a more general set of cellular and organ complications. In many patients, the high blood pressure occurs within a cluster of other clinically significant cell and organ dysfunction [1], such as insulin resistance, insomnia, hypertrophy, capillary rarefaction, and immune suppression. We need a way of putting these many pathophysiologic phenomena under one conceptual roof and explaining the elevated arterial blood pressure at the same time. This issue is the focus of this review.
Oxygen free radical production in hypertensives could be argued to form a bridge between elevated blood pressure and vascular complications. Reactive oxygen species serve as signaling molecules for elevated arterial and arteriolar tone and central blood pressure by reacting with nitric oxide, and they are also involved in cell injury. But no molecular pathways have emerged in any model of hypertension that outline a mechanistic pathway by which free radicals cause the more specific cell dysfunctions that accompany hypertension.
Roles of Matrix Metalloproteinases in Hypertrophy and Hypertension
An emerging body of evidence reveals a role in hypertension and its comorbidities for unchecked activity of degrading proteinases, including the family of extracellular matrix metalloproteinases (MMPs) and A disintegrin and metalloproteinases (ADAMs) [2]. As proteinases that can cleave collagen and modulate the extracellular matrix proteins, the focus on MMPs in hypertension is justified by two general lines of existing evidence: (1) the role of MMPS in complications for which hypertension is a risk factor, such as stroke, atherosclerosis, renal disease, heart disease, and arterial aneurysm [3–9], and (2) the involvement of MMPs in cardiac hypertrophy and its transition into heart failure in the hypertensive state [10–14].
Vascular Wall Restructuring
MMPs are required during the restructuring of arteries and arterioles under pressure, from the earliest wall thickness change to the point of arterial aneurysm [15–17] and the formation of venous varicose vessels [18]. At elevated intraluminal pressure, endothelial and smooth muscle cells of carotid arteries (even isolated in culture) have increased gelatin zymographic activity by MMP-2. At higher pressures, activity of both MMP-2 and collagenolytic MMP-9 is induced, together with a shifting pressure-diameter curve [19]. This form of early MMP-2 involvement in the presence of mechanical stretch is consistent with the increasing body of evidence suggesting that it can be activated by mere mechanical stretch (see below).
The progressive rise of blood pressure with increasing age in the spontaneously hypertensive rat (SHR) is associated with increased elastinolytic MMP-2 activity in the aorta. The rise in MMP-2 expression and activity levels in older hypertensive rates—but not normotensive rats—can be influenced by an endothelin receptor antagonist [20]. In the SHR, MMP activation (e.g. by MMP-2) eventually leads to decreased cardiac tissue tensile strength and may cause systolic and diastolic dysfunction [21]. MMP-2 and MMP-9 are also activated in a behavioral model of elevated blood pressure by (air-jet) stressor, and endothelin-1 mediates the early events in vascular remodeling before the development of hypertension [22]. In hypertensive rats with chronic nitric oxide blockade, the MMP-2 activity in the aorta and the ratio of media thickness to lumen diameter is sustained, in contrast to the MMP activity in small mesentery arteries, which is transient and decreases over time, even though central blood pressure remains elevated [23]. In addition to MMP-2 and MMP-9, a marked increase in MMP-1 mRNA levels occurs with age in hypertrophied left ventricular tissue of SHRs, whereas normotensive Wistar-Kyoto rats show a progressive decrease, even though mRNA levels are lower in SHRs at a young age [24].
The cardiac hypertrophy in the stroke-prone SHR is accompanied by elevation of MMP-2 and MMP-9 activity and involves the peroxisome proliferator-activated receptor γ (PPARγ). PPARγ blockade serves to reduce the hypertrophy but does not affect the elevated blood pressure or the elevated production of oxygen free radicals [25].
In contrast to the SHR, in the Dahl salt-sensitive rat, the stage of compensated left ventricular hypertrophy is followed by congestive heart failure; both endogenous tissue inhibitors of metalloproteinases (TIMPs) and MMP-2 remain inactive during hypertrophy but are activated during the transition to heart failure. At this transition time, the activation of MMP-2 surpasses that of TIMPs, possibly resulting in the extracellular matrix protein breakdown and progression of left ventricular expansion and wall thinning [10]. Compared with normotensive Lewis rats, the levels of MMP-9 in mature Dahl salt-sensitive rats are also reported to be higher and levels of TIMP are lower. To further test the hypothesis that left ventricular hypertrophy in Dahl salt-sensitive rats is due to high levels of MMP and low levels of TIMP, congenic translocation of a segment from chromosome 10 of the Lewis rat, containing an extracellular proteinase inhibitor gene, decreased blood pressure in Dahl salt-sensitive rats, associated with increase in proteinase inhibitor expression. The congenic transfer of TIMP ameliorates left ventricular hypertrophy and cardiac dysfunction [26].
Fluid shear stress also has a direct effect on MMP activation. MMP-2 and the membrane-bound membrane-type MMP (MT-MMP, also designated MMP-14) are upregulated in mesentery collateral arteries during elevated shear stress (produced by ligation of selected arteries) and are required for outward luminal expansion [27]. The same high-flow arteries (but not low-flow arteries) also have early (day 4) elevated MMP-9 activity, together with endothelial nitric oxide synthase (eNOS) overexpression, prior to the development of outward hypertrophic remodeling (after 14 days) [28].
The involvement of MMPs in hypertrophy of arteries is not limited to the systemic circulation. Pulmonary artery hypertrophy in association with cell proliferation and matrix accumulation is also mediated by proteinases and can be attenuated by serine elastase or MMP inhibition [29, 30].
Differential Regulation of Hypertension and Hypertrophy by MMPs
A spectrum of experimental approaches with different immunohistochemical and zymographic techniques, pharmacologic inhibition of activity, expression knockdown (e.g., by antisense oligodeoxynucleotides and RNA interference), and gene knockout are opening insights that point towards an MMP signaling network with separate controls over hypertension and hypertrophy.
Induction of acute hypertension by vasoactive peptides (e.g., catecholamines, angiotensin II, nitric oxide synthase inhibitor) requires activation of vascular MMP-7, which in turn is posttranscriptionally connected with ADAM-12. In SHRs, knockdown of MMP-7 reduces blood pressure and stops the development of cardiac hypertrophy. MMP-7 controls the transcription of ADAM-12, a metalloproteinase also implicated in cardiac hypertrophy [31•]. In mice with angiotensin II–induced hypertension and cardiac hypertrophy, myocardial ADAM-12 and downstream hypertrophy marker genes are overexpressed. Knockdown of MMP-7 attenuates hypertension, inhibits ADAM-12 overexpression, and prevents cardiac hypertrophy [31•].
After activation, MMPs and ADAMs also modulate vasoactive peptides (e.g., big endothelin, adrenomedullin), growth factor ligands (e.g., heparin-binding epidermal growth factor receptor), pro-inflammatory mediators (e.g., tumor necrosis factor [TNF]-α), and others, to produce arterial contraction and central hypertension via vasoconstrictor G protein–coupled receptors. For example, activation of the α1-adrenoreceptor by an agonist stimulates vasoconstriction in rat mesenteric arteries and thereby contributes to hypertension, but it also activates MMP-7. The MMP-7 sheds the heparin-binding epidermal growth factor and thereby activates the epidermal growth factor receptor [32], signaling growth that leads to cardiac hypertrophy (by an ERK1/2 and Akt pathway). MMP inhibition reduces SHR blood pressure and attenuates heparin-binding epidermal growth factor receptor shedding in mesenteric arteries of SHRs but not in normotensive rats [33].
In mice with angiotensin II–induced hypertension, MMPs are also part of the mechanisms responsible for the dual action on elevated blood pressure and cardiac wall hypertrophy and fibrosis. In this model, the signaling cascade involves TNF-α converting enzyme (TACE, also designated as ADAM-17) [34•] and MMP-7 [31•], and activation of MMP-7 and ADAM-17/TACE may be necessary for subsequent upregulation of MMP-2 and ADAM-12 transcription. Knockdown of TACE serves to partially attenuate MMP-2, and the combination of TACE and MMP-7 siRNA treatment prevents the MMP-2 activity. Simultaneous knockdown of TACE and MMP-7 attenuates blood pressure elevation as well as the development of cardiac hypertrophy and fibrosis in this model. In contrast, MMP-2 inhibition prevents a rise in blood pressure but does little to prevent hypertrophy. This evidence points towards a transcriptional regulation of MMP-2 by MMP-7 and TACE. Although angiotensin II–induced cardiovascular disease is signaled via multiple MMP pathways with unique physiological roles, MMP-2 appears to modulate only the blood pressure in this model of hypertension. MMP-7, which participates in a number of fibrotic processes, is able to modulate the cardiac hypertrophy but needs to activate MMP-2 to modulate blood pressure [35••].
The Variety of MMP Substrates
Introduction of proteomic techniques shows that in addition to extracellular matrix proteins (e.g., native collagens, elastin, proteoglycan, laminin, fibronectin, vitronectin, aggrecan, brevican, tenascin, decorin), a colorful variety of biologic proteins can be substrates for MMPs [36•]. For example, MMP-2 can cleave cytokines, molecules associated with growth factors, neurotransmitter substances and their receptors, pro-MMPs or proteinase inhibitors (e.g.,. MCP-3, Pro-TGF-β1, Pro-TNF-α, Pro-IL-1β IGFBP-3, IGFBP-5, FGFR-1, substance P, galectin-3, Pro-MMP-1, Pro-MMP-2, Pro-MMP-13, α1-proteinase inhibitor, α2-macroglobulin) [37, 38•], and many other proteins and peptides.
Peptide Cleavage
Directly relevant for arteriolar vasoconstrictor actions, cleavage of big endothelin or calcitonin gene-related peptide or adrenomedullin may serve as a substrate for MMP-2 that generates a series of cleaved peptides, which have in part vasoconstrictor activity [39]. MMP-7 also has a spectrum of extracellular matrix protein substrates, including the proteolytic processing of pro-hormones.
Renal Fibrosis
In addition to elastinolytic activity, several experimental results in the SHR and related models show an increasing complex picture of the involvement of MMP-2, MMP-9, and the TIMPs in renal hypertensive remodeling and fibrosis, all of which may contribute to glomerular injury [40–43].
Receptor Cleavage by MMPs and Cell Dysfunctions in the Metabolic Syndrome
In addition to hypertension, hypertrophy, and fibrosis, the uncontrolled proteinase activity in hypertensives may be more diverse and may in fact be the basis for a conceptual framework that can start to explain the multiple dysfunctions of organs and cells encountered in the metabolic syndrome. Indeed, a more extensive examination of the mature SHR shows MMP protein levels and MMP activity not only in wall of the arterioles, with elevated blood pressure, but also in the plasma (MMP-2, MMP-9, MMP-7), in circulating leukocytes (MMP-8, MMP-9) that pass periodically through high-pressure and low-pressure regions of the circulation, and also on the venular side of the microcirculation with low blood pressure (MMP-1, MMP-1/9, MMP-7, MMP-8) [33, 44•, 45, 46]. MMP-9 is especially prominent on the endothelium. In fact, even the Wistar Kyoto rat, the low-pressure control of the SHR, has elevated blood pressure and proteinase activity compared with the Wistar strain from which it was generated [44•]. The elevations of the proteinase activity in plasma are relatively mild and in part transient.
One of the consequences of such unchecked extracellular proteinase activity is that important proteins in the circulation and on cell surfaces may be proteolytically cleaved in the SHR. Among a number of proteins, an interesting candidate in this regard is the β2 adrenergic receptor. Upon agonist binding, this receptor normally stimulates vasodilation. But in SHR arterioles, the ability of the β2 adrenergic receptor to mediate vasodilation is compromised, as shown in agonist and antagonist studies [47•] and in line with the characteristically elevated arteriolar tone in the SHR [48]. Immunolabeling of the receptor with an antibody against the extracellular domain and, as control, with an antibody against the intracellular domain shows that the extracellular domain density is reduced in the SHR. Furthermore, application of fresh venous SHR plasma to naive endothelial cells for 30 min reduces the extracellular domain density of the β2 adrenergic receptor, an effect that is prevented by using MMP inhibitors. Direct application of MMP-9 to arterioles causes arteriolar vasoconstriction [47•]. Although there are likely other reasons for the elevated arteriolar tone in SHRs, the proteolytic cleavage of the β2 adrenergic receptor may be one of the contributors to the elevated arterial blood pressure.
Study of the peptide sequence of other membrane receptors shows that many have multiple theoretical cleavage sites on the extracellular domain for MMPs. Therefore, in light of the fact that the SHR exhibits insulin resistance [49], we tested its insulin receptor-α density on leukocytes and mesentery cells. This insulin receptor-α also shows evidence for cleavage of the extracellular domain by MMPs, which is associated with a reduced ability to transport glucose across its membranes. Chronic MMP inhibition alleviates the elevated blood glucose and glycosylation values in SHR plasma [45]. A similar cleavage of the extracellular domain of the insulin receptor can also be found in other models of insulin resistance [50].
Like other hypertensives and prehypertensives, the SHR exhibits capillary rarefaction, a loss of microvessels in many organs [51–53]. The loss of capillaries is caused by endothelial apoptosis and loss of pericytes. The apoptosis is also observed in larger vessels of the SHR [54–56]. The enhanced endothelial apoptosis is associated with enhanced cleavage of the vascular endothelial growth factor receptor 2 (VEGFR2) [44•, 46]. Just as in the case of the β2 adrenergic receptor and the insulin receptor-α, the cleavage occurs predominantly at the extracellular domain of the receptor and is attenuated by MMP inhibition. Impaired angiogenesis in the SHR is associated with decreased MMP-14 expression [57].
Other SHR membrane receptors are cleaved as well. The extracellular domain of the formyl peptide receptor (FPR, a G protein–coupled receptor, GPCR) on SHR circulating leukocytes has a reduced density and exhibits an attenuated response to chemotactic peptides such as N-formylmethionine-leucine-phenylalanine [58•]. The same receptor also serves as a mechanosensor for pseudopod projection and retraction in circulating leukocytes in response to fluid shear stress [59]. The extracellular domain of this receptor is in part cleaved in the SHR, a phenomenon that is associated with attenuated pseudopod retraction in its circulating pool of leukocytes [58•]. Compared with the Wistar-Kyoto normotensive control, the SHR has an increased number of circulating leukocytes with pseudopods, which in turn increases the capillary resistance and contributes to its overall increase in hemodynamic resistance [60].
Over its lifetime, the SHR also has an elevated circulating leukocyte count [61], in part because the leukocyte adhesion receptor, β2 integrin (CD18), is cleaved [45]. Its counter-receptor on the endothelium, intercellular adhesion molecule (ICAM)-1, can also be cleaved by MMPs and elastase but exhibits a reversed pattern in the SHR in a tissue-specific fashion. Extracellular fragments of ICAM-1 may accumulate in renal glomeruli [62].
Membrane protein cleavage is also detected on erythrocytes of the SHR. SHR plasma cleaves the glycocalyx of erythrocytes, causing swelling of the cells, and erythrocyte aggregation occurs in a MMP-dependent fashion [63]. A subpopulation of SHR erythrocytes with low mass density (during centrifugation) shows increased aggregation in fibrinogen. This same population of erythrocytes exhibits reduced adhesion to macrophages, indicating that glycocalyx core proteins are also cleaved in the SHR [63].
There are other indicators for receptor cleavage in the SHR. Tight junction proteins (occludin and claudins) in the SHR blood–brain barrier are degraded by MMPs during ischemia with elevated levels of MMP-14 and furin, which promote MMP-2 and MMP-9 activity. This degradation can be blocked by MMP inhibition [64]. The extracellular domain of the serotonin receptor 5HT-1A density is reduced in the SHR brain [65], a process that may be associated with the reduced sleep quality in the SHR [66].
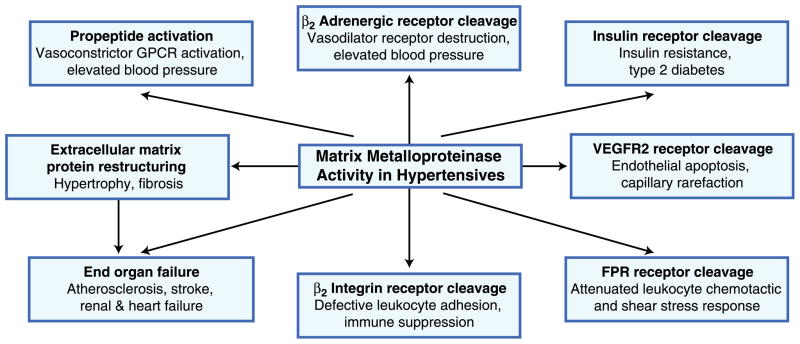
The combined evidence, though still preliminary, points toward an early form of proteolytic autodigestion in the form of receptor cleavage as the common denominator for these multiple cell dysfunctions in experimental models of hypertension (Fig. 1).
Fig. 1.
Schematic summary of evidence supporting a hypothesis for involvement of matrix metalloproteinase in hypertension, ventricular wall hypertrophy, insulin resistance, capillary rarefaction, attenuated leukocyte adhesion and shear stress response, and eventual organ failure. FPR formyl peptide receptor; GPCR G protein–coupled receptor; VEGFR2 vascular endothelial growth factor receptor 2
The Effect of Proteinase Inhibition
Uncontrolled proteolytic activity raises the issue of whether proteinase inhibition can serve to reduce cell dysfunction in hypertension and the metabolic syndrome. There are a number of indicators that this may be possible.
Chronic blockade of the MMPs in the SHR with doxycycline [67] and other MMP blockers interferes with the extensive cleavage of membrane receptors and restores normal membrane receptor densities. Such blockade reduces the arteriolar tone and central blood pressure [33, 47•], increases cellular glucose transport and normalizes blood glucose levels [45], reduces endothelial apoptosis and rarefaction of capillary blood vessels [44•, 46], normalizes blood counts of leukocytes [45] and their chemotactic and fluid shear stress response [58•], and restores normal serotonin receptor densities in the brain [65]. This evidence supports the notion that the multitude of cell dysfunctions may be derived from one set of extracellular proteolytic degrading processes and may form a common treatment target. Doxycycline also blocks vascular restructuring in two-kidney, one-clip hypertension [68].
It should be noted, however, that in light of the essential role of MMPs for development, reproduction, and tissue repair, a nuanced approach to proteinase inhibition is required, which should target predominantly the excessive and uncontrolled proteinase activity in hypertension, and ideally no other activity.
Furthermore, there is a need to understand the mechanisms that lead to the overexpression of MMPs in the first place. The promotor regions of MMPs have many transcription factor binding sites [69]. An interesting candidate is NF-κB, a major player in inflammation. The SHR overexpresses NF-κB [45]. Chronic inhibition of NF-κB with pyrrolidine dithiocarbamate reduces MMP-1, MMP-2, and MMP-9; reduces extracellular domain cleavage of the β2 adrenergic receptor; and normalizes central blood pressure in the SHR [70•]. Other transcription factors remain to be investigated in hypertension.
In this context, it is also important to recognize that existing treatments for hypertension affect MMP activity. Angiotensin-converting enzyme (ACE) inhibitors reduce MMP-2 and MMP-9 activity [71, 72], so their full inhibitory action needs to be determined in terms of a broader profile of degrading enzymes. ACE inhibitors can directly bind to MMPs. The ability to inhibit MMPs and attenuate insulin receptor cleavage may be responsible in part for their efficacy in reducing new-onset type II diabetes [73, 74] and complications in experimental diabetes [75]. Treatment of hypertensive patients with MMP-lowering drugs such as ACE inhibitors and angiotensin receptor blockers may have favorable effects on left ventricular hypertrophy and diastolic dysfunction. Plasma MMP-3 and MMP-9 concentrations have been observed to be higher in patients with left ventricular hypertrophy and left ventricular posterior wall thickness, and Doppler indices of diastolic dysfunction are correlated with MMP-3 and MMP-9 levels [76]. Other treatments for hypertension (e.g., calcium channel blockers, β adrenergic receptor blockers) also need to be investigated more closely in regard to their inhibitory impact on degrading proteinases.
MMP Activation
Since generated in a proform, MMPs need to be activated. The process of activation may depend on the specific MMP and the location where it is expressed. Many MMPs can be activated by serine proteinase (e.g., trypsin, thrombin, plasmin) [77, 78]. Some (e.g., MMP-2 and MMP-9) can be activated by mechanical stretch [79, 80], including cyclic stretch [81]. MMP-14 is inactive in the cell cytoplasm but can become activated when transported into the cell membrane, and in turn can activate other MMPs [82].
Guo et al. [83] proposed that mechanical stress due to pressure overload in the myocardium causes expression and activation of MMPs. The mechanical stress raises the cAMP levels, which in turn raises expression of MMP-2, MMP-13, and MMP-14, as well as MMP-2 and collagenase activity, causing the pathologic myocardial restructuring with disorganization of the N-cadherin/beta-catenin cell adhesion complex [83]. The effect is blocked by β-adrenergic blockers. Alternatively, plasminogen activator or the pro-protein convertase, furin, activate MMPs [84], and some reactive oxygen metabolites, such as hypochlorous acid, can activate MMP-8 without cleavage of the propeptide by disrupting a cysteine residue at the Zn++ catalytic site [85].
The degree to which endogenous serine proteinase inhibitors (serpins such as α1-antitrypsin and α2-macroglobulin) or MMP inhibitors (TIMPs) block proteinase activity in hypertension remains to be explored.
MMP Levels and Activation in Plasma of Hypertensive Patients
Quantitative measurements of the levels of MMPs and their activities in human hypertensives are still at an early stage [86], partly because multiple factors influence their values. Proteinase activities are not constant during the day; they depend on the level of endogenous inhibitors, and values in plasma may differ substantially from those on endothelial cells and around tissue parenchymal cells [44•, 46]. Measurement of proteinase activity in archived blood samples shows that the activity may not be elevated if patients are receiving medication (unpublished results in our laboratory). Details of the treatment status need to be regarded as an independent variable. In contrast to inflammatory markers such as the acute phase protein c-reactive protein or fibrinogen, which have minimal diurnal variations, proteinase protein levels and especially proteinase activities may fluctuate, so the time of day, meals, medication, or medical conditions other than hypertension must be considered. Resolution of this issue will require systematic in vivo measurements using improved zymographic techniques that can read nanomolar concentrations of fresh active enzymes with minimal sample preparation and storage [87]. Techniques to measure MMP activity in blood vessels of humans in vivo are also required.
Considering these limitations, it is not surprising that few agreements about MMP values in the plasma of hypertensive patients have yet been reached. Among five MMP proteins measured and detected in plasma (MMP-1, MMP-2, MMP-3, MMP-9, and MMP-10), elevated levels were found in patients with essential hypertension and in hypertensive patients with end-stage renal disease [88]. In hypertensives, elevated MMP-9 values (compared with normotensive controls) [88–90] correlate with systolic blood pressure [88] and with arterial pulse wave velocity [91–93]. A correlation between blood pressure and MMP-9 is also observed between normotensives and hypertensives with end-stage renal disease [88], pointing towards a direct influence of MMP-9 on arteriolar tone, as suggested by receptor cleavage and its impact on the arteriolar tone [47•]. Protein levels of MMP-2 and MMP-10 (a stromelysin) in plasma are also elevated in hypertensive patients with end-stage renal disease, suggesting that they may be involved in the development of renal injury once essential hypertension has been established. MMP-2 and MMP-9 exhibit a positive correlation, when considered across both essential hypertensives and controls, whereas MMP-1 levels are not elevated in these patients [88]. Hypertensive patients with left ventricular hypertrophy have elevated serum levels of MMP-1 [94] that correlate with ankle pulse wave velocity [92].
Other studies have reported no elevation of MMP-9 in patients with essential hypertension [95] and even depressed MMP-9 values [96, 97]. Even though in our own studies we have observed no difference in plasma levels of MMP-1 or MMP-2 [88], other studies have reported decreased levels of MMP-1 [98], decreased levels of MMP-2 [96], or increased levels of MMP-2 [90] in hypertensive patients versus normotensive controls. Much more investigation is required, especially at early stages of hypertension, before full involvement of secondary complications. Genetic polymorphisms in the MMP genes may modulate the susceptibility of hypertensive patients [99].
Conclusions
The multiple proteinase families, the diversity of individual proteinase family members, and the ability for proteinases to cleave propeptides, plasma proteins, and surface receptors make several of them potential candidates for involvement in hypertension. Specifically, although there is mounting evidence that members of the MMP family are overexpressed and activated during hypertrophy and during advanced cardiovascular complications for which hypertension is a risk factor, there is also initial evidence that MMPs may already be active in hypertensives at an early stage, before the development of overt organ failure. Extracellular proteinase activity and receptor cleavage may cause the many cell dysfunctions that accompany hypertension. Extracellular cleavage of a receptor causes loss of the associated receptor function, and many membrane receptors may be cleaved in the presence of extracellular MMPs. In vivo measurements are required to detect MMP activity and to identify their substrates. This hypothesis may explain why hypertension is often accompanied by diabetes and other cell dysfunctions and why multiple complications can be attenuated by treatment with the same class of proteinase inhibitors in some models of hypertension. The hypothesis also suggests that an elevated blood pressure may need to be regarded as one of several vascular dysfunctions derived from a more fundamental problem associated with proteinase activation and inadequate inhibition.
Acknowledgments
Funding NIH Grant HL 10881 and an unrestricted research gift from Leading Ventures.
Footnotes
Disclosure Dr. Schmid-Schönbein is scientific advisor to Leading Ventures and owns interest in InhibeX, a company by Leading Ventures.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2010;41(2):271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–62. [PubMed] [Google Scholar]
- 4.Rodriguez JA, Orbe J, Martinez de Lizarrondo S, et al. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci. 2008;13:2916–21. doi: 10.2741/2896. [DOI] [PubMed] [Google Scholar]
- 5.Ronco P, Lelongt B, Piedagnel R, Chatziantoniou C. Matrix metalloproteinases in kidney disease progression and repair: a case of flipping the coin. Semin Nephrol. 2007;27(3):352–62. doi: 10.1016/j.semnephrol.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Thrailkill KM. Clay Bunn R, Fowlkes JL: Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35(1):1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8(1):82–9. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Moore L, Fan D, Basu R, et al. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. 2011 Jun 30; doi: 10.1007/s10741-011-9266-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Turu MM, Krupinski J, Catena E, et al. Intraplaque MMP-8 levels are increased in asymptomatic patients with carotid plaque progression on ultrasound. Atherosclerosis. 2006;187(1):161–9. doi: 10.1016/j.atherosclerosis.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Iwanaga Y, Aoyama T, Kihara Y, et al. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39(8):1384–91. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- 11.Shah BH, Catt KJ. Matrix metalloproteinase-dependent EGF receptor activation in hypertension and left ventricular hypertrophy. Trends Endocrinol Metab. 2004;15(6):241–3. doi: 10.1016/j.tem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Tayebjee MH, MacFadyen RJ, Lip GY. Extracellular matrix biology: a new frontier in linking the pathology and therapy of hypertension? J Hypertens. 2003;21(12):2211–8. doi: 10.1097/01.hjh.0000098178.36890.81. [DOI] [PubMed] [Google Scholar]
- 13.Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension. 2002;39(2 Pt 2):679–84. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- 14.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90(5):520–30. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 15.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139 (2):292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko H, Anzai T, Horiuchi K, et al. Tumor necrosis factor-alpha converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis. 2011;218(2):470–8. doi: 10.1016/j.atherosclerosis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–7. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 18.Raffetto JD, Khalil RA. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6(3):158–72. doi: 10.2174/157016108784911957. [DOI] [PubMed] [Google Scholar]
- 19.Lehoux S, Lemarie CA, Esposito B, et al. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109(8):1041–7. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 20.Spiers JP, Kelso EJ, Siah WF, et al. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23(9):1717–24. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 21.Mujumdar VS, Smiley LM, Tyagi SC. Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol. 2001;79(2–3):277–86. doi: 10.1016/s0167-5273(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 22.Ergul A, Portik-Dobos V, Giulumian AD, et al. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol. 2003;285(5):H2225–2232. doi: 10.1152/ajpheart.00133.2003. [DOI] [PubMed] [Google Scholar]
- 23.Bouvet C, Gilbert LA, Girardot D, et al. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension. 2005;45(3):432–7. doi: 10.1161/01.HYP.0000154680.44184.01. [DOI] [PubMed] [Google Scholar]
- 24.Seccia TM, Bettini E, Vulpis V, et al. Extracellular matrix gene expression in the left ventricular tissue of spontaneously hypertensive rats. Blood Press. 1999;8(1):57–64. doi: 10.1080/080370599438400. [DOI] [PubMed] [Google Scholar]
- 25.Shinzato T, Ohya Y, Nakamoto M, et al. Beneficial effects of pioglitazone on left ventricular hypertrophy in genetically hypertensive rats. Hypertens Res. 2007;30(9):863–73. doi: 10.1291/hypres.30.863. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez WE, Tyagi N, Deng AY, et al. Congenic expression of tissue inhibitor of metalloproteinase in Dahl-salt sensitive hypertensive rats is associated with reduced LV hypertrophy. Arch Physiol Biochem. 2008;114(5):340–8. doi: 10.1080/13813450802535978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas TL, Doyle JL, Distasi MR, et al. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol. 2007;293(4):H2429–2437. doi: 10.1152/ajpheart.00100.2007. [DOI] [PubMed] [Google Scholar]
- 28.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27(2):317–24. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105(1):21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Wang X, Chow FL, Oka T, et al. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119(18):2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. This article demonstrates that hypertension and hypertrophy depend on transcriptional and posttranscriptional mechanisms involving MMP-7 and transcriptional connection with ADAM-12. [DOI] [PubMed] [Google Scholar]
- 32.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 33.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94(1):68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 34•.Wang X, Oka T, Chow FL, et al. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54(3):575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. This article demonstrates that agonist-induced cardiac hypertrophy and fibrosis processes are signaled through TNF-α–converting enzyme by transcriptional regulation of ADAM-12 and MMP-2. [DOI] [PubMed] [Google Scholar]
- 35••.Odenbach J, Wang X, Cooper S, et al. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2010;57(1):123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. This article demonstrates that in the angiotensin II–induced hypertension model, MMP-7 and TNF-α–converting enzyme mediate fibrosis and hypertrophy and induce MMP-2 upregulation. The MMP-2 induces hypertension without hypertrophy. [DOI] [PubMed] [Google Scholar]
- 36•.Butler GS, Dean RA, Morrison CJ, Overall CM. Identification of cellular MMP substrates using quantitative proteomics: isotope-coded affinity tags (ICAT) and isobaric tags for relative and absolute quantification (iTRAQ) Methods Mol Biol. 2010;622:451–470. doi: 10.1007/978-1-60327-299-5_26. This paper describes a quantitative proteomics and mass spectrometry technique to identify protease substrates in a cell. [DOI] [PubMed] [Google Scholar]
- 37.Overall CM, McQuibban GA, Clark-Lewis I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol Chem. 2002;383(7–8):1059–66. doi: 10.1515/BC.2002.114. [DOI] [PubMed] [Google Scholar]
- 38•.Prudova A, Auf dem Keller U, Butler GS, Overall CM. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol Cell Proteomics. 2010;9(5):894–911. doi: 10.1074/mcp.M000050-MCP201. This paper reports the development of a method for proteome-wide detection of protease-generated neo-N-termini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez A, Oh HR, Unsworth EJ, et al. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383(Pt. 3):413–8. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lekgabe ED, Kiriazis H, Zhao C, et al. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension. 2005;46(2):412–8. doi: 10.1161/01.HYP.0000171930.00697.2f. [DOI] [PubMed] [Google Scholar]
- 41.Tostes RC, Touyz RM, He G, et al. Endothelin A receptor blockade decreases expression of growth factors and collagen and improves matrix metalloproteinase-2 activity in kidneys from stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39(6):892–900. doi: 10.1097/00005344-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Xue H, Zhang YL, Liu GS, Wang H. A new ATP-sensitive potassium channel opener protects the kidney from hypertensive damage in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315(2):501–9. doi: 10.1124/jpet.105.089722. [DOI] [PubMed] [Google Scholar]
- 43.Camp TM, Smiley LM, Hayden MR, Tyagi SC. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J Hypertens. 2003;21(9):1719–27. doi: 10.1097/00004872-200309000-00022. [DOI] [PubMed] [Google Scholar]
- 44•.Tran ED, DeLano FA, Schmid-Schönbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47(5):423–431. doi: 10.1159/000281582. This paper demonstrates that extracellular-domain VEGFR-2 receptor cleavage by MMPs is a basis for endothelial apoptosis and capillary rarefaction in the SHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeLano FA, Schmid-Schönbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52(2):415–23. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran E, Yang M, Chen A, et al. Matrix metalloproteinase activity causes VEGFR-2 cleavage and microvascular rarefaction in rat mesentery. Microcirculation. 2011;18:1–10. doi: 10.1111/j.1549-8719.2011.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schönbein GW. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299(1):H25–35. doi: 10.1152/ajpheart.00620.2009. This article demonstrates that extracellular domain vasodilatory receptor cleavage by MMPs is a basis for arteriolar constriction and elevated blood pressure in the SHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid-Schönbein GW, Zweifach BW, DeLano FA, Chen P. Microvascular tone in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1987;9:164–71. doi: 10.1161/01.hyp.9.2.164. [DOI] [PubMed] [Google Scholar]
- 49.Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289 (2):H813–822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 50.Delano FA, Zhang H, Tran EE, et al. A new hypothesis for insulin resistance in hypertension due to receptor cleavage. Expert Rev Endocrinol Metab. 2010;5(1):149–58. doi: 10.1586/eem.09.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohlen HG. The microcirculation in hypertension. J Hypertens Suppl. 1989;7(4):S117–124. [PubMed] [Google Scholar]
- 52.Penna GL, Garbero Rde F, Neves MF, et al. Treatment of essential hypertension does not normalize capillary rarefaction. Clinics (Sao Paulo) 2008;63(5):613–8. doi: 10.1590/S1807-59322008000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noon JP, Walker BR, Webb DJ, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99(8):1873–9. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran ED, Schmid-Schönbein GW. An in-vivo analysis of capillary stasis and endothelial apoptosis in a model of hypertension. Microcirculation. 2007;14(8):793–804. doi: 10.1080/10739680701419992. [DOI] [PubMed] [Google Scholar]
- 55.Lim HH, DeLano FA, Schmid-Schönbein GW. Life and death cell labeling in the microcirculation of the spontaneously hypertensive rat. J Vasc Res. 2001;38(3):228–36. doi: 10.1159/000051051. [DOI] [PubMed] [Google Scholar]
- 56.Murfee WL, Schmid-Schönbein GW. Chapter 12. Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271–84. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Olszewski B, Rosebury W, et al. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem Biophys Res Commun. 2004;315(2):363–8. doi: 10.1016/j.bbrc.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 58•.Chen AY, DeLano FA, Valdez SR, et al. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am J Physiol Cell Physiol. 2010;299(6):C1441–1449. doi: 10.1152/ajpcell.00157.2010. This is a demonstration of extracellular domain chemotactic receptor cleavage in the SHR by MMPs as a contribution to attenuated chemotactic and fluid shear stress response in leukocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makino A, Prossnitz ER, Bünemann M, et al. G Protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633–9. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 60.Fukuda S, Yasu T, Kobayashi N, et al. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95(1):100–8. doi: 10.1161/01.RES.0000133677.77465.38. [DOI] [PubMed] [Google Scholar]
- 61.Schmid-Schönbein GW, Seiffge D, DeLano FA, et al. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–30. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 62.Tong S, Neboori HJ, Tran ED, Schmid-Schönbein GW. Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J Vasc Res. 2011;48:386–96. doi: 10.1159/000323474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pot C, Chen AY, Ha JN, Schmid-Schönbein GW. Proteolytic cleavage of the red blood cell glycocalyx in a genetic form of hypertension. Cell Mol Bioeng. 2011 doi: 10.1007/s12195-011-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 65.Valdez SR. MS Thesis. La Jolla, Univ. Calif; San Diego: 2010. Serotonin 5HT-1A Receptor Density in the Brain of Spontaneously Hypertensive Rats. [Google Scholar]
- 66.Kuo TB, Shaw FZ, Lai CJ, et al. Changes in sleep patterns in spontaneously hypertensive rats. Sleep. 2004;27(3):406–12. doi: 10.1093/sleep/27.3.406. [DOI] [PubMed] [Google Scholar]
- 67.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev. 2004;9 (1):63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 68.Castro MM, Tanus-Santos JE, Gerlach RF. Matrix metalloproteinases: targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol Res. 2011 Apr 9; doi: 10.1016/j.phrs.2011.04.002. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 69.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40(6–7):1362–78. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 70•.Wu KI, Schmid-Schönbein GW. Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011;57(2):261–268. doi: 10.1161/HYPERTENSIONAHA.110.158709. This is a demonstration of NFκB-dependent MMP overexpression, vasodilatory receptor cleavage, and elevated blood pressure in the SHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liebetrau M, Burggraf D, Wunderlich N, et al. ACE inhibition reduces activity of the plasminogen/plasmin and MMP systems in the brain of spontaneous hypertensive stroke-prone rats. Neurosci Lett. 2005;376(3):205–9. doi: 10.1016/j.neulet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto D, Takai S. Pharmacological implications of MMP-9 inhibition by ACE inhibitors. Curr Med Chem. 2009;16 (11):1349–54. doi: 10.2174/092986709787846514. [DOI] [PubMed] [Google Scholar]
- 73.Pahor M, Psaty BM, Alderman MH, et al. Therapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetes. Diabetes Care. 2000;23(7):888–92. doi: 10.2337/diacare.23.7.888. [DOI] [PubMed] [Google Scholar]
- 74.Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28(9):2261–6. doi: 10.2337/diacare.28.9.2261. [DOI] [PubMed] [Google Scholar]
- 75.Ryan ME, Ramamurthy NS, Sorsa T, Golub LM. MMP-mediated events in diabetes. Ann N Y Acad Sci. 1999;878:311–34. doi: 10.1111/j.1749-6632.1999.tb07692.x. [DOI] [PubMed] [Google Scholar]
- 76.Saglam M, Karakaya O, Esen AM, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006;208(2):117–22. doi: 10.1620/tjem.208.117. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez-Patron C, Zhang Y, Radomski MW, et al. Rapid release of matrix metalloproteinase (MMP)-2 by thrombin in the rat aorta: modulation by protein tyrosine kinase/phosphatase. Thromb Haemost. 1999;82(4):1353–7. [PubMed] [Google Scholar]
- 78.Rosario HS, Waldo SW, Becker SA, Schmid-Schönbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164(5):1707–16. doi: 10.1016/S0002-9440(10)63729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asanuma K, Magid R, Johnson C, et al. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;284(5):H1778–1784. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- 80.Grote K, Flach I, Luchtefeld M, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92(11):e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 81.Cummins PM, von Offenberg Sweeney N, Killeen MT, et al. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: a force to be reckoned with. Am J Physiol Heart Circ Physiol. 2007;292(1):H28–42. doi: 10.1152/ajpheart.00304.2006. [DOI] [PubMed] [Google Scholar]
- 82.Deryugina EI, Ratnikov B, Monosov E, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263(2):209–23. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 83.Guo D, Kassiri Z, Basu R, et al. Loss of PI3Kgamma enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. 2010;107(10):1275–89. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- 84.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 85.Weiss SJ, Peppin G, Ortiz X, et al. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227 (4688):747–9. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- 86.Fontana V, Silva PS, Belo VA, et al. Consistent alterations of circulating matrix metalloproteinases levels in untreated hypertensives and in spontaneously hypertensive rats: a relevant pharmacological target. Basic Clin Pharmacol Toxicol. 2011;109(2):130–7. doi: 10.1111/j.1742-7843.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 87.Lefkowitz RB, Schmid-Schönbein GW, Heller MJ. Whole blood assay for elastase, chymotrypsin, matrix metalloproteinase-2, and matrix metalloproteinase-9 activity. Anal Chem. 2010;82 (19):8251–8. doi: 10.1021/ac101462c. [DOI] [PubMed] [Google Scholar]
- 88.Friese RS, Rao F, Khandrika S, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31(7):521–33. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papadopoulos DP, Makris TK, Krespi PG, et al. Changes in metalloproteinases in healthy normotensive patients with high-normal blood pressure. Eur Cytokine Netw. 2005;16(3):211–4. [PubMed] [Google Scholar]
- 90.Derosa G, D’Angelo A, Ciccarelli L, et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13(3):227–31. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 91.Tan J, Hua Q, Xing X, et al. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30(10):959–63. doi: 10.1291/hypres.30.959. [DOI] [PubMed] [Google Scholar]
- 92.Ishikawa J, Kario K, Matsui Y, et al. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 2005;28(12):995–1001. doi: 10.1291/hypres.28.995. [DOI] [PubMed] [Google Scholar]
- 93.Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(2):372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 94.Hirono O, Fatema K, Nitobe J, et al. Long-term effects of benidipine hydrochloride on severe left ventricular hypertrophy and collagen metabolism in patients with essential hypertension. J Cardiol. 2002;39(4):195–204. [PubMed] [Google Scholar]
- 95.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113 (17):2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 96.Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and –9 in essential hypertension. Am J Hypertens. 2004;17(3):273–6. doi: 10.1016/j.amjhyper.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Li-Saw-Hee FL, Edmunds E, Blann AD, et al. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy. Int J Cardiol. 2000;75(1):43–7. doi: 10.1016/s0167-5273(00)00274-6. [DOI] [PubMed] [Google Scholar]
- 98.Laviades C, Varo N, Fernandez J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98(6):535–40. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 99.Lacchini R, Jacob-Ferreira AL, Luizon MR, et al. Matrix metalloproteinase 9 gene haplotypes affect left ventricular hypertrophy in hypertensive patients. Clin Chim Acta. 2010;411(23–24):1940–4. doi: 10.1016/j.cca.2010.08.008. [DOI] [PubMed] [Google Scholar]